Abstract
Background
An electrocardiogram (ECG)-based artificial intelligence (AI) algorithm has shown good performance in detecting hypertrophic cardiomyopathy (HCM). However, its application in routine clinical practice may be challenging owing to the low disease prevalence and potentially high false-positive rates.
Objective
Identify clinical characteristics associated with true- and false-positive HCM AI-ECG results to improve its clinical application.
Methods
We reviewed the records of the 200 patients with highest HCM AI-ECG scores in January 2021 at our institution. Logistic regression was used to create a clinical variable–based “Candidacy for HCM Detection (HCM-DETECT)” score, differentiating true-positive from false-positive AI-ECG results. We validated the HCM-DETECT score in an independent cohort of 200 patients with the highest AI-ECG scores from January 2022.
Results
In the 2021 cohort (median age 71 [interquartile range 58–80] years, 48% female), the rates of true-positive, false-positive, and indeterminate AI-ECG results for HCM detection were 36%, 48%, and 16%, respectively. In the 2022 cohort, the rates were 26%, 47%, and 27%, respectively. The HCM-DETECT score included age, coronary artery disease, prior pacemaker, and prior cardiac valve surgery, and had an area under the receiver operating characteristic curve of 0.81 (95% confidence interval 0.73–0.87) for differentiating true- vs false-positive AI results. When the 2022 cohort was limited to HCM detection candidates identified with the HCM-DETECT score, the false-positive AI-ECG rate was reduced from 47% to 13.5%.
Conclusion
Application of a clinical score (HCM-DETECT) in tandem with an AI-ECG model improved HCM detection yield, reducing the false-positive rate of AI-ECG more than 3-fold.
Keywords: Electrocardiography, Artificial intelligence, Hypertrophic cardiomyopathy, Diagnostic performance, Echocardiography
Key Findings.
-
•
An electrocardiogram (ECG)-based artificial intelligence (AI) algorithm for detecting hypertrophic cardiomyopathy (HCM) may result in high false-positive rates when applied to unselected patients in clinical practice, potentially owing to the relatively low disease prevalence.
-
•
A newly derived clinical variable–based score (HCM-DETECT score) including age, coronary artery disease, prior pacemaker, and prior cardiac valve surgery can help identify patients with a high AI-ECG score in whom HCM is likely.
-
•
Application of the clinical HCM-DETECT score in tandem with the AI-ECG model improved HCM detection yield by reducing the false-positive rate of AI-ECG more than 3-fold.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiomyopathy and is characterized by abnormal myocardium hypertrophy, frequently involving the septum. It affects 1 in 500 to 1 in 200 persons in the general population and is among the most common reasons for sudden cardiac death in the young.1,2 Cardiac imaging is essential for the diagnosis,3 but importantly, more than 90% of patients with HCM present with electrocardiogram (ECG) changes.4,5 However, 12-lead ECG changes associated with HCM, such as high QRS amplitudes and T-wave inversions, are often nonspecific.6
A completely automated deep learning artificial intelligence (AI) algorithm for the detection of HCM (HCM AI-ECG) from a standard ECG has shown promising results.7 However, the clinical application of this algorithm could be challenging owing to the low disease prevalence and potentially high false-positive rate when applied to unselected patients, particularly patients undergoing ECG for other clinical indications during routine care. Furthermore, the HCM AI-ECG algorithm, which was derived using a convolutional neural network (CNN), may be hard to implement because the features identified by the model are not readily apparent to a human interpreter. As such, clinicians cannot identify potential false-positive results based on the ECG or model output alone. Although these models are powerful, implementing these approaches has met some resistance owing to their limited interpretability.8
This study aimed to simulate the application of the HCM AI-ECG algorithm in existing samples of patients from real-world clinical practice and to identify potential patient characteristics associated with true- and false-positive AI results. This approach would create a clinical stratification scheme to identify patients in whom the HCM AI-ECG algorithm is likely to have a high diagnostic yield and result in targeted downstream cardiac imaging to confirm or refute the HCM diagnosis.
Methods
Study design
In this observational cohort study, we identified a cohort of 20,677 patients who had at least one 12-lead ECG for any indication in routine clinical practice at any Mayo Clinic enterprise site in the United States in January 2021. A separate, nonoverlapping cohort of 15,147 patients with an ECG in routine practice in January 2022 was also identified. The HCM AI-ECG algorithm was applied to each of these ECGs stored in Mayo Clinic’s digital data vault. The medical records of the 200 patients with the highest HCM AI-ECG scores in each cohort were reviewed in detail by 1 investigator (M.M.), including clinical notes and cardiac imaging, to determine whether they had one of the following:
-
(1)
Definite HCM (group A): HCM diagnosis was known and was clearly documented in the medical record before or after the index ECG.
-
(2)
Possible HCM (group B): HCM diagnosis was possible based on available clinical and imaging information, but the diagnosis had not been established in the patient’s record.
-
(3)
Likely non-HCM (group C): The patient had a well-characterized cardiovascular comorbidity that can mimic HCM features, such as hypertensive heart disease or aortic stenosis with left ventricular hypertrophy.
-
(4)
Indeterminate (group D): Insufficient information available in the medical record to make a designation into one of groups A-C.
The Mayo Clinic institutional review board approved the study, and all participants had provided a general informed consent for use of their data in research.
ECG acquisition and AI analysis
Resting 12-lead ECG data for each subject were collected from the local ECG database (MUSE data management system; GE Healthcare, Chicago, IL), acquired at a sampling rate of 500 Hz using a GE Marquette ECG machine (GE Marquette, Milwaukee, WI). The previously validated HCM AI-ECG score was calculated for all ECGs, as described previously.7 In brief, after converting ECGs to a matrix of 12 separate ECG leads, the AI CNN was developed using a Keras framework with a Tensorflow backend (Google, Mountain View, CA), performing convolutions across all 12 leads. The CNN model was trained in 1 dataset and validated in a separate dataset. An AI score threshold of 11%, reflecting probability of HCM, was found to offer the best combination of sensitivity and specificity (Youden index). Thus, patients with ECGs where the AI-ECG algorithm reports a higher than 11% probability of HCM are considered AI-positive for HCM. For the purpose of this study, the following measures were also documented from the automated ECG interpretation: heart rate, PR duration, QRS duration, QTc duration, and QRS axis.
Data collection
All patients received clinical care at Mayo Clinic, and information on clinical variables was collected with chart review of the entire available electronic health record. All input clinical variables of interest were labeled as “yes” or “no” for each patient in our dataset, without any missing data. The following baseline characteristics were collected: age, sex, race, HCM diagnosis status (groups A–D), coronary artery disease (CAD), hypertension, diabetes mellitus type 2, cardiovascular comorbidities, end-stage renal disease status, presence of pacemaker, implantable cardioverter-defibrillator, history of myectomy, coronary artery bypass graft (CABG) surgery, and cardiac valve surgery. For the variables to be included as input for the prediction score (all the above except HCM status), we only considered information known at the time of the index ECG in both cohorts, and we did not consider information that became known after the index ECG for each patient.
The clinical notes and the interpretation statements of available echocardiographic and cardiac magnetic resonance imaging (MRI) reports were reviewed to determine HCM diagnosis status. In equivocal cases, the primary imaging studies were also reviewed. Left ventricular ejection fraction and septum and posterior left ventricular wall thickness were collected from the report of the echocardiogram or MRI closest to the index ECG.
Statistical analysis
Baseline characteristics were described using median and interquartile range, or absolute numbers and percentages for continuous and categorical variables, respectively. Univariable logistic regression was used to identify clinical comorbidities that differed between definite/possible HCM (groups A+B, true-positive) from likely non-HCM (group C, false-positive) in the January 2021 cohort, and the odds ratio was calculated. Group D was excluded from statistical analyses owing to the uncertainty regarding HCM status in that group. Variables with statistically significant associations with the definite/possible vs likely non-HCM result were then included in a “Candidacy for HCM Detection (HCM-DETECT)” score that was derived using stepwise forward logistic regression for differentiating definite/possible HCM (group A+B) from likely non-HCM (group C). The final score ranges from 0 to 1 and represents the likelihood of an ECG belonging to a definite/possible HCM case. A higher score indicates that the subject may be a good candidate for application of AI-ECG for HCM determination. The adjusted odds ratio was calculated for each variable in the HCM-DETECT score. The area under the receiver operating characteristic curve (AUC) was bootstrapped 2000 times to obtain the 95% confidence intervals (CI). The Youden index9 was used to determine the cut-off of the score that optimized the diagnostic yield. The performance of the HCM-DETECT score in distinguishing definite/possible HCM vs likely non-HCM AI results was subsequently tested in the independent January 2022 cohort, and sensitivity, specificity, positive predictive value, negative predictive value, area under the precision-recall curve, and AUC were calculated. A P value <.05 was considered statistically significant. Statistical analysis was performed in R (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of the January 2021 (derivation) and January 2022 (testing) cohorts are presented in Table 1 and Table 2, respectively. Among the January 2021 patients (n = 200, age 71 [interquartile range 58–80] years, 48% female), 67 (34%) were definite HCM cases, 4 (2%) possible HCM cases, 96 (48%) likely non-HCM cases, and 33 (16%) indeterminate.
Table 1.
Patient characteristics in the January 2021 (derivation) cohort
| All | Definite HCM (group A) | Possible HCM (group B) | Definite/possible HCM (group A+B) | Likely non-HCM (group C) | Indeterminate (group D) | P value (group A+B vs croup C) | Odds ratio (95% CI) (group A+B vs group C) | |
|---|---|---|---|---|---|---|---|---|
| N (%) | 200 (100) | 67 (34) | 4 (2) | 71 (36) | 96 (48) | 33 (16) | ||
| Clinical characteristics and comorbidities | ||||||||
| Known HCM | 67 (34) | 67 (100) | 0 (0) | 67 (94) | 0 (0) | 0 (0) | <.001∗ | 2.61 (2.49–2.74)∗ |
| Age, y | 71 (58–80) | 63 (50–74) | 63 (56–67) | 63 (50–73) | 75 (67–82) | 72 (62–82) | <.001∗ | 0.99 (0.98–0.99)∗ |
| Female sex | 95 (47.5) | 36 (54) | 1 (25) | 37 (52) | 42 (44) | 16 (48) | .29 | 1.09 (0.93–1.27)∗ |
| CAD | 76 (38) | 14 (21) | 1 (25) | 15 (21) | 44 (46) | 17 (52) | <.001∗ | 0.77 (0.66–0.90)∗ |
| Hypertension | 137 (68.5) | 32 (48) | 3 (75) | 35 (49) | 74 (77) | 28 (84) | <.001∗ | 0.74 (0.63–0.87)∗ |
| DM type 2 | 50 (25) | 10 (15) | 3 (75) | 13 (18) | 24 (25) | 13 (39) | .31 | 0.91 (0.76–1.09) |
| Pacemaker | 49 (25) | 6 (9) | 0 (0) | 6 (8) | 43 (45) | 0 (0) | <.001∗ | 0.65 (0.56–0.76)∗ |
| ICD | 35 (18) | 21 (31) | 0 (0) | 21 (30) | 14 (16) | 0 (0) | .02∗ | 1.25 (1.04–1.5)∗ |
| Myectomy | 28 (14) | 27 (10) | 0 (0) | 27 (38) | 1 (0) | 0 (0) | <.001∗ | 1.91 (1.6–2.3)∗ |
| CABG | 28 (14) | 4 (6) | 1 (25) | 5 (7) | 20 (21) | 3 (9) | .01∗ | 0.77 (0.62–0.95)∗ |
| Cardiac valve surgery | 31 (16) | 6 (9) | 0 (0) | 6 (8) | 25 (26) | 0 (0) | .004∗ | 0.75 (0.62–0.91)∗ |
| Echocardiographic measures | ||||||||
| EF, % | 64 (55–69) | 69 (65–72) | 70.5 (67–73.5) | 69 (65–72) | 60 (47–65) | 55 (43–65) | <.001∗ | 1.02 (1.01–1.02)∗ |
| Septum, mm | 13 (11–17) | 17 (13–20) | 18 (16–19) | 17 (14–20) | 13 (11–14) | 12 (10–13) | <.001∗ | 1.05 (1.03–1.07)∗ |
| Posterior wall, mm | 12.0 (10.0–13.0) | 12 (10–14) | 13.5 (12.5–14.5) | 12 (10–14) | 12 (10–13) | 10 (9–12.5) | .10 | 1.03 (0.99–1.06) |
| ECG measures | ||||||||
| HR, min-1 | 64 (58–72) | 61 (57–69.5) | 71.5 (64–82) | 62 (57–70) | 66 (60–73) | 65 (55–73) | .2 | 1.00 (0.99–1.00) |
| PR, ms | 182 (160–212) | 178 (158–203) | 146 (137–155.5) | 174 (153–201) | 190 (170–222) | 174 (157–204.5) | .04∗ | 0.99 (0.99–0.99)∗ |
| QRS duration, ms | 119 (100–154) | 114 (96–148) | 103 (92–114) | 114 (95–147) | 139 (108–163) | 106 (88–116) | .001∗ | 0.99 (0.99–0.99)∗ |
| QTc, ms | 474.5 (454–506) | 473 (445–504.5) | 483.5 (474.5–490) | 474 (446–503) | 481 (459–512) | 468 (442–486) | .09 | 0.99 (0.99–1.00) |
| QRS axis, | -7 (-37 to 42) | -7 (-34 to 39) | 5.5 (-14 to 28) | -6 (-34 to 39) | -17.5 (-52.5 to 43) | 4 (-12 to 32) | .71 | 1.00 (0.99–1.00) |
Group A+B is compared to group C using univariable logistic regression.
Continuous data are given as number, n (%), or median (interquartile range), and compared between definite/possible HCM (group A+B) and likely non-HCM (group C) using univariable logistic regression.
Data marked with an asterisk (∗) represents a significance level of .05.
CABG = coronary artery bypass grafting; CAD = coronary artery disease; DM = diabetes mellitus type 2; ECG = electrocardiogram; EF = ejection fraction; HCM = hypertrophic cardiomyopathy; HR = heart rate; ICD = implantable cardioverter-defibrillator.
Table 2.
Patient characteristics in the January 2022 (testing) cohort
| All | Definite HCM (group A) | Possible HCM (group B) | Definite/possible HCM (group A+B) | Likely non-HCM (group C) | Indeterminate (group D) | |
|---|---|---|---|---|---|---|
| N (%) | 200 (100) | 41 (21) | 10 (5) | 51 (26) | 94 (47) | 55 (27) |
| Known HCM | 41 (21) | 41 (100) | 0 (0) | 41 (80) | 0 (0) | 0 (0) |
| Age, y | 69 (58–79) | 59 (48–69) | 74 (66–79) | 63 (49–72) | 75 (63–83) | 68 (58–75) |
| Female sex | 84 (42) | 12 (29) | 5 (50) | 17 (33) | 44 (47) | 23 (42) |
| CAD | 81 (41) | 8 (20) | 6 (60) | 14 (27) | 45 (48) | 22 (40) |
| Hypertension | 146 (73) | 19 (46) | 9 (90) | 28 (55) | 75 (80) | 43 (78) |
| DM type 2 | 49 (25) | 4 (10) | 5 (50) | 9 (18) | 25 (27) | 15 (27) |
| Pacemaker | 60 (30) | 3 (7) | 0 (0) | 3 (6) | 57 (31) | 0 (0) |
| ICD | 21 (11) | 8 (20) | 0 (0) | 8 (17) | 13 (14) | 0 (0) |
| Myectomy | 4 (2) | 4 (10) | 0 (0) | 4 (8) | 0 (0) | 0 (0) |
| CABG | 34 (17) | 3 (7) | 2 (20) | 5 (10) | 23 (21) | 6 (11) |
| Cardiac valve surgery | 34 (17) | 0 (0) | 1 (10) | 1 (2) | 26 (26) | 7 (13) |
| EF, % | 60 (51.5–65) | 68 (65–71.5) | 58 (56–64) | 67 (61–70) | 56 (44–62) | 60 (51–65) |
| Septum, mm | 12 (11–15) | 17 (14–19.5) | 14.5 (14–15) | 16 (14–18) | 12 (10–14) | 11 (10–12) |
| Posterior wall, mm | 11(10.00–13.00) | 12 (11–16) | 13.5 (12–15) | 13 (11–15) | 11 (10–13) | 11 (10–11) |
| HR, min-1 | 66 (60–74) | 64 (60–73) | 69 (59–75) | 64 (60–73.5) | 69 (61–75) | 65 (58–74) |
| PR duration, ms | 176 (152–202) | 175 (156–202.5) | 171(160.5–186.5) | 174 (153–198.5) | 178 (164–208) | 161 (143–194) |
| QRS duration, ms | 126 (102–166) | 104 (94–124) | 104 (90.5–121) | 104 (94–124) | 166 (134–186) | 110 (96–130) |
| QTc time, ms | 492 (458–520) | 456 (441–478) | 470 (461–486) | 461 (442–483.5) | 511 (492–534) | 465 (444–502) |
| QRS axis, | -11 (-52 to 27) | 12 (-19 to 48) | -2.5 (-19 to 12.5) | 9 (-21 to 46) | -145 (-69 to 0) | 0 (-20 to 48) |
CABG = coronary artery bypass grafting; CAD = coronary artery disease; DM = diabetes mellitus type 2; EF = ejection fraction; HCM = hypertrophic cardiomyopathy; HR = heart rate; ICD = implantable cardioverter-defibrillator.
In the January 2021 cohort, univariable logistic regression identified history of CABG surgery, cardiac valve surgery, presence of pacemaker, hypertension, CAD, and age to be inversely associated with definite or possible HCM status, as shown in Table 1. The presence of implantable cardioverter-defibrillator and history of myectomy were positively associated with a definite HCM adjudication. Race and end-stage renal disease were not statistically significant (results not shown). Because hypertension was very commonly present as a diagnosis and we were unable to consistently adjudicate from the medical record whether it was severe or uncontrolled (ie, potentially resulting in hypertensive heart disease as an HCM mimicker), we did not include that clinical variable in the stepwise logistic regression. Since myectomy is a surgical procedure for HCM, and patients who have undergone myectomy have definite HCM, it was not included in the stepwise logistic regression either.
The final logistic regression–based HCM-DETECT score included pacemaker, age, cardiac valve surgery, and CAD. The HCM-DETECT score had an AUC of 0.81 (95% CI 0.73–0.87) in the January 2021 derivation cohort. A binary cut point was established at the Youden index such that a HCM-DETECT score <0.40 classified patients as likely non-HCM (poor targets for HCM AI-ECG implementation). At that operating point, the HCM-DETECT score’s sensitivity and specificity were 89% and 70%, respectively, in the derivation cohort. Table 3 shows that the adjusted odds ratios for the 4 variables in the HCM-DETECT score are less than 1, indicating their association with the probability of not having HCM (ie, a false-positive AI-ECG adjudication).
Table 3.
Clinical variables in the HCM-DETECT score based on the stepwise forward logistic regression in the January 2021 cohort, and their adjusted odds ratio for association with definite/possible hypertrophic cardiomyopathy status
| Variable | Odds ratio | P value |
|---|---|---|
| Pacemaker | 0.12 (0.05–0.34) | <.001 |
| Age | 0.97 (0.95–0.99) | .017 |
| Cardiac valve surgery | 0.34 (0.12–0.99) | .043 |
| CAD | 0.45 (0.20–1.0) | .048 |
CAD = coronary artery disease.
In the testing cohort of 200 patients from January 2022 (age 69 [58–79] years, 42% female), manual chart review adjudicated each case as definite HCM (41/200, 21%), possible HCM (10/200, 5%), likely non-HCM (94/200, 47%), and indeterminate (55/200, 27%).
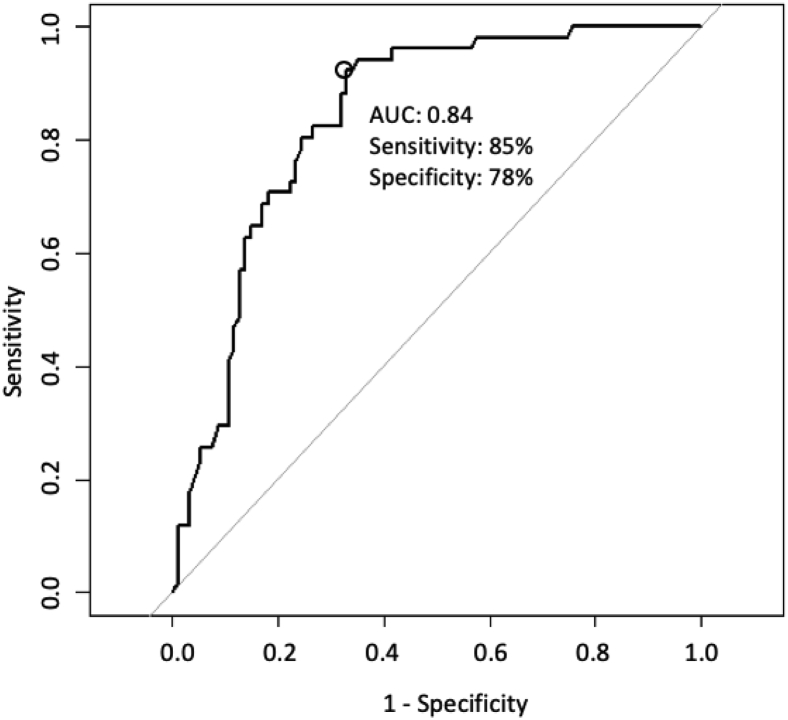
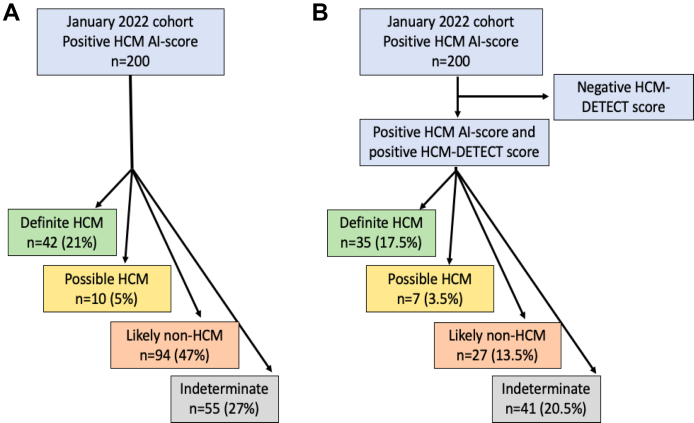
Applying the HCM-DETECT score to the 200 patients of the January 2022 cohort identified 90 (45%) as having a low clinical probability of HCM (poor candidates for HCM implementation) and 110 (55%) with a high probability of HCM. After exclusion of the patients with indeterminate HCM status (n = 55, insufficient data to adjudicate), in the remaining 145 patients the HCM-DETECT score identified 76 (52%) as having a low clinical probability of HCM (poor candidates for HCM implementation), and 69 (48%) patients with a high clinical probability of HCM for further evaluation. Among these 69 patients, 35 (51%) were definite HCM cases, 7 (10%) were possible HCM cases, and 27 (39%) were likely non-HCM cases. Based on these adjudications, the HCM-DETECT score had sensitivity of 85%, specificity of 78%, positive predictive value of 65%, negative predictive value of 91%, area under the precision-recall curve 0.66, and AUC 0.84 (95% CI 0.77–0.90) to distinguish definite/possible HCM from likely non-HCM in the January 2022 cohort, as shown in the receiver operating characteristic curve in Figure 1. From the starting 200 patients in the January 2022 cohort, application of the HCM-DETECT score resulted in 35 (17.5%) definite HCM, 7 (3.5%) possible HCM, 27 (13.5%) likely non-HCM, and 41 (20.5%) indeterminate cases, as shown in Figure 2.
Figure 1.
Area under the receiver operating characteristic curve (AUC) for the HCM-DETECT score in the testing cohort based on 145 patients with determinable hypertrophic cardiomyopathy status.
Figure 2.
Flow charts of the hypertrophic cardiomyopathy (HCM) artificial intelligence (AI) electrocardiography score in the January 2022 cohort stratifying patients as definite HCM, possible HCM, likely non-HCM, and indeterminate alone (A), and in tandem with the HCM-DETECT score (B).
Discussion
This study demonstrates that a clinical risk score can facilitate the application of an AI-ECG model to unselected patients in routine clinical practice by excluding patients who would not benefit from further HCM evaluation and reducing the false-positive AI-ECG detection rate (47% with AI-ECG alone vs 13.5% with AI-ECG plus HCM-DETECT score). These findings could guide the application of AI-ECG and other AI tools with limited direct interpretability to clinical medicine by allowing clinicians to target efforts to the higher-yield patients.
The estimated prevalence of diagnosed HCM is approximately 1 in 500 to 1 in 200, but the number of undiagnosed cases is unknown and difficult to estimate.10 Widespread application of AI-ECG to the general population would be the only way to identify all previously undetected cases, but this is too costly and impractical, since echocardiography and MRI are the gold-standard methods for the detection of HCM. It has previously been reported that AI-ECG can be effective in cardiovascular disease management.11 Still, it remains uncertain how to optimally deploy this tool to identify new HCM cases among patients undergoing ECG in routine clinical practice. Indeed, the HCM AI-ECG could be applied to larger patient groups for detection of previously unknown or missed HCM given its comparatively low cost and ease of data collection. However, given the low prevalence of HCM, this approach is likely to generate a substantial number of false-positive cases.
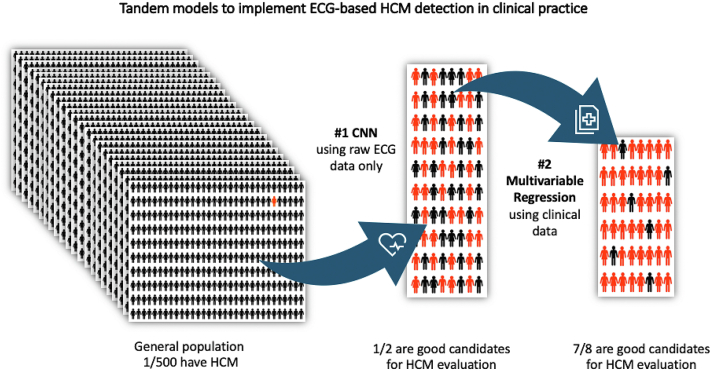
We propose that the HCM-DETECT model should be applied at the time of an ECG in tandem with the AI-ECG model using clinical data available at that time. We anticipate that by improving the yield of AI-ECG implementation by using 2 models in tandem to exclude patients who would not benefit from AI-ECG implementation, we could create a system to evaluate ideal candidates and reduce false-positive rates. Figure 3 shows a representation of the concept of the tandem deep learning and logistic regression models for ECG-based HCM detection in a clinical practice setting. We acknowledge that there is an undetermined group in our study where this tandem approach could not be applied. Patients were designated as indeterminate based on insufficient medical record information to adjudicate them as HCM or non-HCM. This would not apply to a prospective implementation of the tandem approach in clinical practice where a patient would be recommended to have or not to have confirmatory cardiac imaging based on the AI-ECG and HCM-DETECT score results.
Figure 3.
A representation of the concept of the tandem deep learning and logistic regression models for electrocardiography (ECG)-based hypertrophic cardiomyopathy (HCM) detection in routine clinical practice.
This study identified clinical comorbidities associated with a true-positive and false-positive HCM AI-ECG score. When the HCM AI-ECG score was derived, ECGs with left bundle branch block (LBBB) or pacing were excluded.7 Both LBBB and ventricular pacing increase the QRS duration, and can hinder ST-T-segment analysis. Complete bundle branch block is uncommon in HCM except in those with prior septal reduction interventions.12 For instance, septal myectomy often results in LBBB and alcohol septal ablation can result in right bundle branch block.12 Fine-tuning of the algorithm with training specifically to distinguish abnormalities such as LBBB in HCM vs non-HCM may be required. Similarities in ECG manifestations in patients with HCM and patients who had undergone cardiac surgery or had cardiac pacing may explain the association of those clinical features with probability for false-positive AI-ECG result. Furthermore, ST-T changes may present after myocardial infarction,13 and CAD and CABG were more prevalent among likely non-HCM cases in the January 2021 (derivation) cohort. ST-T changes are present in most patients with HCM,14 which could possibly mimic ischemic changes. Aging is associated with various ECG changes, including QRS widening, left ventricular hypertrophy patterns, and left axis deviation,15 also potentially mimicking ECG changes seen in HCM.12 The findings in the current study generated insights into the HCM AI-ECG model that could be used for model adjustments and improvements. To further improve HCM detection in clinical practice, the ECG could potentially be used in tandem with information from the electronic health record and with different imaging modalities including AI-enhanced MRI, when that becomes more widely available. The studied models are based on patients receiving routine clinical care. However, improving the accuracy of detection of HCM has important implications also in other groups, such as young athletes. The use of the HCM AI-ECG algorithm in the pre-participation screening setting is under investigation.
Study limitations
Our HCM status adjudication relied on medical record review. In some instances, the lack of incomplete clinical information reporting in the patient’s record could impact the accuracy of the adjudication process. Longer follow-up and more comprehensive and uniform diagnostic testing would be helpful but is not practical in an analysis of patients receiving routine clinical care for indications other than HCM. Not all patients had readily available cardiac imaging data, resulting in several cases being considered indeterminate. In the current study, we investigated an older population compared to the derivation cohort, which could affect the performance of the AI-ECG model, which appears to perform best in younger subgroups of patients.7,16 Besides the comorbidities investigated in the current study, other potential clinical comorbidities may affect the HCM AI-ECG score. The current study included patients at the top end of AI-ECG-predicted scores for HCM and cannot fully inform about the optimal AI score threshold above which patients should undergo HCM-DETECT assessment in clinical practice. That threshold is likely to differ across different health systems and remains to be defined in prospective implementation studies. Lastly, false-negative AI-ECG results were not evaluated in the current study, and such future studies are justified. However, given the high sensitivity of the algorithm and relatively low prevalence of HCM in most general clinical practices, the overall impact of false-negative AI-ECG results should be expected to be low.
Conclusion
Our study demonstrates a paradigm for AI-ECG implementation in routine clinical practice. AI-ECG could identify a large sample of patients in terms of probability of HCM and a second model derived from simple clinical data could narrow the results to the best AI-ECG evaluation candidates. The false-positive rate of AI-ECG was cut by two-thirds when a tandem AI-ECG and clinical score approach was used. This model may provide a framework for applying AI-ECG for HCM detection to patients undergoing ECG for any clinical indication during routine care.
Acknowledgments
Funding Sources
M.M. was supported by grants from the Swedish Heart Lung Foundation, Erik and Edith Fernström’s Foundation, and Karolinska Institutet.
Disclosures
Mayo Clinic along with Peter Noseworthy, Konstantinos Siontis, Michael Ackerman, Zachi Attia, and Paul Friedman have intellectual property related to the detection of hypertrophic cardiomyopathy with AI-ECG. This algorithm has been licensed to Anumana, Inc. The other authors have nothing to declare.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All participants had provided a general informed consent for use of their data in research.
Ethics Statement
The Mayo Clinic institutional review board approved the study. All participants had provided a general informed consent for use of their data in research.
Disclaimer
Given their role as Associate Editor, Zachi Attia had no involvement in the peer review of this article and has no access to information regarding its peer review.
References
- 1.Semsarian C., Ingles J., Maron M.S., Maron B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J., Gardin J.M., Flack J.M., Gidding S.S., Kurosaki T.T., Bild D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 3.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142 doi: 10.1161/CIR.0000000000000938. e533–e557. [DOI] [PubMed] [Google Scholar]
- 4.McLeod C.J., Ackerman M.J., Nishimura R.A., Tajik A.J., Gersh B.J., Ommen S.R. Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram. J Am Coll Cardiol. 2009;54:229–233. doi: 10.1016/j.jacc.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 5.Rowin E.J., Maron B.J., Appelbaum E., et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110:1027–1032. doi: 10.1016/j.amjcard.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Pelliccia A., Maron B.J., Culasso F., et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation. 2000;102:278–284. doi: 10.1161/01.cir.102.3.278. [DOI] [PubMed] [Google Scholar]
- 7.Ko W.Y., Siontis K.C., Attia Z.I., et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol. 2020;75:722–733. doi: 10.1016/j.jacc.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Yoon C.H., Torrance R., Scheinerman N. Machine learning in medicine: should the pursuit of enhanced interpretability be abandoned? J Med Ethics. 2022;48:581–585. doi: 10.1136/medethics-2020-107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youden W. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Maron M.S., Hellawell J.L., Lucove J.C., Farzaneh-Far R., Olivotto I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am J Cardiol. 2016;117:1651–1654. doi: 10.1016/j.amjcard.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 11.Siontis K.C., Noseworthy P.A., Attia Z.I., Friedman P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol. 2021;18:465–478. doi: 10.1038/s41569-020-00503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finocchiaro G., Sheikh N., Biagini E., et al. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm. 2020;17:142–151. doi: 10.1016/j.hrthm.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Mieszczanska H., Pietrasik G., Piotrowicz K., McNitt S., Moss A.J., Zareba W. Gender-related differences in electrocardiographic parameters and their association with cardiac events in patients after myocardial infarction. Am J Cardiol. 2008;101:20–24. doi: 10.1016/j.amjcard.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage D.D., Seides S.F., Clark C.E., et al. Electrocardiographic findings in patients with obstructive and nonobstructive hypertrophic cardiomyopathy. Circulation. 1978;58:402–408. doi: 10.1161/01.cir.58.3.402. [DOI] [PubMed] [Google Scholar]
- 15.Vicent L., Martínez-Sellés M. Electrocardiogeriatrics: ECG in advanced age. J Electrocardiol. 2017;50:698–700. doi: 10.1016/j.jelectrocard.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Siontis K.C., Liu K., Bos J.M., et al. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int J Cardiol. 2021;340:42–47. doi: 10.1016/j.ijcard.2021.08.026. [DOI] [PubMed] [Google Scholar]