Abstract
Left bundle branch pacing (LBBP) involves direct capture of left bundle fibers by placing the lead deep inside the interventricular septum. Several studies have shown the feasibility and efficacy of LBBP as an alternative modality for cardiac resynchronization therapy (CRT). This paper describes approach for providing cost effective CRT with defibrillator (CRT-D) by LBBP and dual chamber implantable cardioverter defibrillator (ICD) which we label as LBBP optimized ICD (LOT-ICD). LBBP was performed using C315 sheath and 3830 Selectsecure lead in all patients by premature ventricular complex guided approach. In patients with complete correction of conduction system disease, IS-1 connector plug of the IS-1/DF-1 lead was capped and 3830 lead connected to the dual chamber ICD pulse-generator at RV-P/S port. LOT-ICD provided stable R-wave sensing for arrhythmia monitoring and resulted in cost-effective resynchronization therapy at reduced fluoroscopy duration and radiation dose.
Keywords: Cardiac resynchronization therapy, Heart failure, Implantable cardioverter-defibrillator, Left bundle branch pacing, Left bundle branch pacing–optimized implantable cardioverter-defibrillator
Key Findings.
-
▪
Left bundle branch pacing–optimized implantable cardioverter-defibrillator (LOT-ICD) is a novel approach for providing cost-effective cardiac resynchronization therapy using a dual-chamber implantable cardioverter-defibrillator (ICD) and left bundle branch pacing.
-
▪
LOT-ICD was successful in 57.9% of the population eligible for cardiac resynchronization therapy–defibrillator with reduced fluoroscopy duration and radiation dose.
-
▪
The low success rate could be due to extensive scar burden in a highly selected population as detected by cardiac magnetic resonance imaging.
-
▪
Pacing parameters remained stable during mean follow-up of 13.9 ± 8.7 months, with no episodes of inappropriate ICD discharges.
Introduction
Conduction system pacing has been suggested as an alternative for overcoming chronic right ventricular (RV) pacing–related complications. Left bundle branch pacing (LBBP) involves direct capture of a broad fan of left bundle fibers on the left ventricular (LV) subendocardium by placing the lead deep inside the interventricular septum. Several nonrandomized multicenter studies have shown the feasibility and efficacy of LBBP as an alternative to biventricular pacing (BVP) in patients eligible for cardiac resynchronization therapy (CRT).1,2 In patients who are candidates for an implantable cardioverter-defibrillator (ICD) and have an indication for CRT, implantation of a cardiac resynchronization therapy–defibrillator (CRT-D) is recommended. CRT-D is associated with significant reduction in all-cause mortality in patients with ischemic cardiomyopathy. However, even though CRT-D provides significant reduction in mortality, the cost of the device therapy is a major concern that prevents its utilization in eligible patients, especially in developing countries. Left bundle branch pacing–optimized cardiac resynchronization therapy (LOT-CRT) has been shown to provide superior electrical resynchronization compared to BVP-CRT in patients with suboptimal resynchronization from BVP alone.3 This article describes a novel approach for cost-effective CRT-D by LBBP and dual-chamber ICD (left bundle branch pacing–optimized implantable cardioverter-defibrillator [LOT-ICD]).
Methods
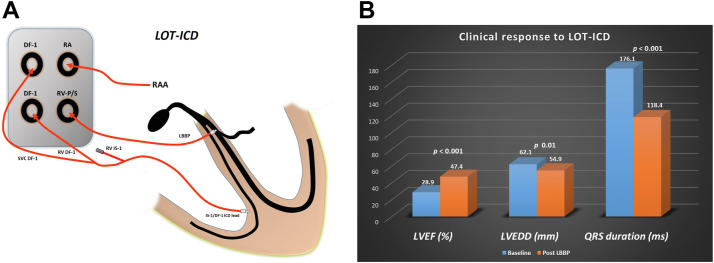
This was a prospective, observational, single-center study that included consecutive patients with an indication for CRT-D. The study was approved by the institutional review board and adhered to the guidelines of the Helsinki Declaration. Patients provided informed consent after understanding LBBP to be a nonstandard approach to CRT. Continuous monitoring of intracardiac electrograms and 12-lead electrocardiography (ECG) were performed using WorkMate Claris electrophysiology system (Abbott, Plymouth, MN). An IS-1/DF-1 ICD lead was deployed in the RV in all patients (Figure 1). LBBP was performed using the 3830 SelectsecureTM lead and C315-His sheath (Medtronic, Minneapolis, MN) using a premature ventricular complex–guided approach.4 LBB capture was confirmed as per previously described criteria.5 In patients with complete correction of conduction system disease, the IS-1 connector plug of the IS-1/DF-1 lead was capped, and the 3830 lead was connected to the dual-chamber ICD pulse generator at the RV-P/S port (Figure 1). The DF-1 connector plugs (superior vena cava and RV) of the IS-1/DF-1 lead were connected to the corresponding port in the pulse generator (Figure 1). In patients with incomplete correction of conduction system disease and in whom LBB capture could not be confirmed, conventional CRT-D using a coronary sinus (CS) lead was performed. Baseline characteristics, pacing parameters at the time of implantation, and ECG parameters were documented. Echocardiographic parameters before and after LBBP were recorded. Patients underwent follow-up at the device clinic after 15 days and 1 month, and every 3 months thereafter with clinical, ECG, and echocardiographic evaluation. Any arrhythmia detected by the device and heart failure hospitalizations were documented.
Figure 1.
Left bundle branch pacing (LBBP)–optimized implantable cardioverter-defibrillator (ICD) (LOT-ICD). A: IS-1/DF-1 lead was implanted in right ventricle (RV). IS-1 connector plug was capped, and 3830 lead was connected to the dual-chamber ICD pulse generator at the RV-P/S port. B: LOT-ICD resulted in significant reduction in QRS duration, as well as improvement in left ventricular ejection fraction (LVEF) and reduction in left ventricular end-diastolic diameter (LVEDD). RAA = right atrial appendage.
Results
LBBP was successful in 11 of 19 CRT-D–eligible patients (57.9% procedural success rate), who were included in the study. Extensive scar, inability to penetrate the septum, and incomplete QRS correction were the reasons for failure in 8 patients. Mean age of the study population was 58.2 ± 12.5 years (n = 11), and patients were predominantly male (72% [n = 8]). Of the 11 patients included in the study, 7 had coronary artery disease (CAD), 3 had nonischemic cardiomyopathy, and 1 had infiltrative cardiomyopathy. Baseline QRS morphology was left bundle branch block (LBBB) in 9 patients and right bundle branch block in 2 patients. Cardiac magnetic resonance imaging (cMRI) showed late gadolinium enhancement suggestive of scar in 7 patients (Figure 2). LBBP resulted in reduction of QRS duration from 176.1 ± 21.3 ms to 118.4 ± 18.7 ms (P <.001) (Figure 2), with mean R-wave peak time of 80.1 ± 12.8 ms. Echocardiography showed significant improvement in LV ejection fraction from 28.9% ± 3.7% to 47.4% ± 10.4% (P <.001), as well as reduction in LV end-diastolic-diameter from 62.1 ± 6.3 mm to 54.9 ± 5.8 mm (P = .01) (Figure 1). Mean fluoroscopy time was 14.6 ± 4.3 minutes, and the radiation dose as measured by dose–area product was 43.7 ± 34.6 Gy·cm2. Pacing threshold was 0.58 ± 0.36 V at 0.5-ms pulse width, sensed R-wave amplitude was 9.9 ± 5.8 mV, and unipolar pacing impedance was 669 ± 181 Ω. LBB capture could be confirmed in all 11 patients during mean follow-up of 13.9 ± 8.7 months (Figure 3). Pacing parameters remained stable (threshold 0.61 ± 0.3 V, P = .84; R wave 9.4 ± 5.9 mV, P = .84; impedance 572 ± 101 Ω, P = .13) during follow-up. New York Heart Association functional class improved from baseline 3.3 ± 0.5 to 1.8 ± 0.4 (P <.001). There was a significant reduction in N-terminal pro–brain natriuretic protein level from 2727 ± 2530 pg/mL to 732 ± 371 pg/mL (P = .01). One patient had an increase in LBB capture threshold from 0.5 V to 1.75 V, for which the pacing output was optimized. There were no episodes of lead dislodgment, loss of LBB capture, pocket infection, thromboembolism, or heart failure hospitalization during follow-up. One patient had atrial tachycardia and 1 had nonsustained atrial and ventricular tachycardia during follow-up, for which antiarrhythmic medications were optimized. No patient received inappropriate ICD discharge during follow-up.
Figure 2.
LOT-ICD for dilated cardiomyopathy with left bundle branch block. LBBP resulted in reduction in QRS duration from 156 ms (A) to 108 ms (D). Note the right bundle branch delay pattern due to LBBP along with T-wave memory. B: Left anterior oblique (LAO) view showing the positions of the IS-1/DF-1 and LBBP leads in the interventricular septum. C: Computed tomographic angiographic image showing the relationship of the LBBP and IS-1/DF-1 leads to the noncoronary cusp (NCC). Note the stent in the left circumflex artery (LCX) immediately behind the NCC. RA = right atrium; other abbreviations as in Figure 1.
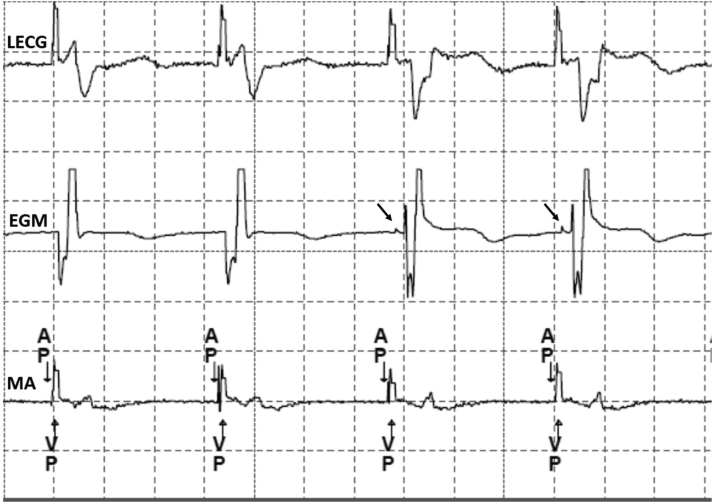
Figure 3.
Left bundle branch pacing lead electrogram (EGM) during follow-up showing nonselective to selective capture transition (arrowhead) at near-threshold output. AP = atrial paced; EGM = electrogram; LECG = lead electrocardiography; MA = marker; VP = ventricular paced.
Discussion
The main findings of our study were as follows: (1) LOT-ICD by LBBP was successful in 57.9% of CRT-D–eligible patients; (2) LBBP lead provided stable R-wave sensing for arrhythmia monitoring; and (3) LOT-ICD resulted in cost-effective resynchronization therapy at reduced fluoroscopy duration and radiation dose.
LBBP has been suggested as an effective alternative for CRT in patients with LBBB and non-LBBB morphology.1 In patients with suboptimal resynchronization by BVP, LOT-CRT provides greater electrical resynchronization. The success rate was low in our study due to a highly selected population with ICD indication and extensive myocardial scar as demonstrated by late gadolinium enhancement on cMRI (Figure 4). In addition, incomplete QRS correction by LBBP alone was not accepted, and patients received an additional CS lead to achieve effective resynchronization (LOT-CRT). In comparison with other studies,1,2 successful LBBP resulted in improved LV ejection fraction from 28.9% ± 3.7% to 47.4% ± 10.4% (P <.001) in our patients. Mean fluoroscopy duration and dose–area product measured in our study were less compared to data reported in the literature for BVP implantation.6 CRT trials have shown higher rates of CS lead dislodgment7 ranging from 2.9% to 10.6%, preventing its utilization for sensing ventricular arrhythmia. In our study, we could show stable lead position and sensed R wave during mean follow-up of 13.9 ± 8.7 months. One patient had appropriately detected nonsustained ventricular tachycardia for which antiarrhythmic drugs were optimized. Inappropriate ICD discharges were not observed during follow-up in the device clinic.
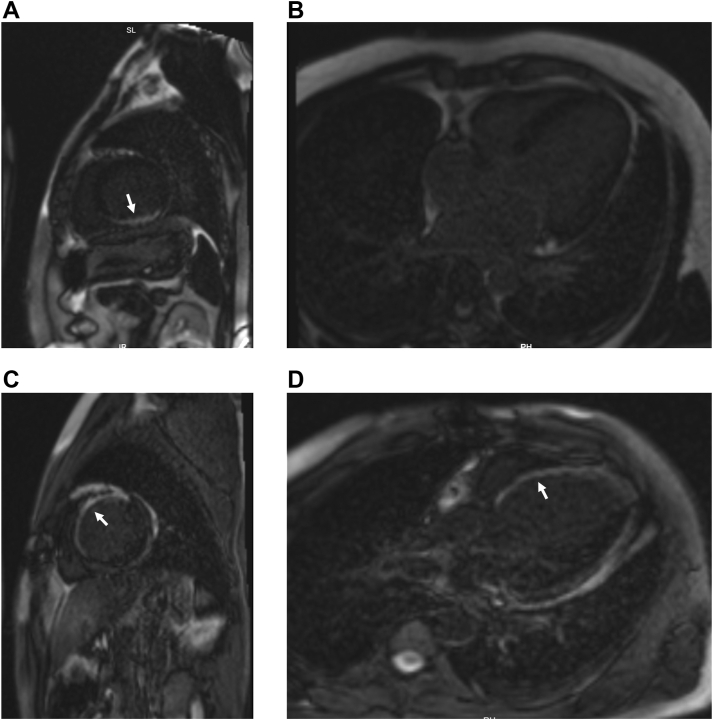
Figure 4.
A, B: Cardiac magnetic resonance imaging (cMRI) in a patient with successful left bundle branch pacing–optimized implantable cardioverter-defibrillator (LOT-ICD). Late gadolinium enhancement (LGE) was noted in the basal inferior and inferolateral wall of the left ventricle (white arrow). Basal septum at the site of lead deployment did not have scar. C, D: cMRI in a patient with unsuccessful LOT-ICD. Extensive scar was noted in the septum as evidenced by LGE (white arrow).
Because an ICD pulse generator was used instead of CRT-D in all 11 patients, we could reduce the cost of therapy by 30% without any compromise in clinical response. In developing countries with limited health care resources, reducing the cost of therapy without compromising the clinical outcome would benefit patients. In a large, long-term observational study of Indian heart failure patients, out of 471 enrolled patients only 24.6% opted for cardiac resynchronization therapy–pacemaker/CRT-D; in the remaining 75.4% of patients, financial constraints were the main reason for therapy refusal.8 With conduction system pacing providing complete correction of wide QRS, LBBP would obviate the need for CS lead placement. The additional cost, procedural duration, poor venous anatomy, phrenic nerve capture, high capture threshold, and lead dislodgments associated with CS lead placement could be addressed by complete correction of conduction system disease by LBBP. We could expect a surge in the adoption of conduction system pacing worldwide, especially in developing countries, if cost-effectiveness without compromise of clinical outcomes could be proved by multicenter studies. Randomized trials comparing LBBP with BVP will help in identifying a subset of patients for whom LBBP can be used as the primary approach for CRT.
Study limitations
The major limitation of the study was the nonrandomized study design involving a small number of patients with no head-to-head comparison with conventional CRT-D. The low success rate (57.9%) for LBBP could be due to extensive scar burden in a highly selected population as detected by cMRI (Figure 4) and crossover to CRT-D if QRS correction was incomplete.
Conclusion
The combination of IS-1/DF-1 ICD lead and LBBP with stable lead parameters could obviate the need for CRT-D pulse generator, and cost-effective resynchronization therapy could be achieved with LOT-ICD. Further large-scale randomized trials comparing LOT-ICD with conventional CRT-D are necessary.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Dr Ponnusamy reports receiving consultant fees from Medtronic. William Basil reports consultant fees from Medtronic. Dr Vijayaraman reports speaker, consultant, research, and fellowship support from Medtronic; consultant fees from Abbott, Biotronik, and Boston Scientific; and a patent for an HBP delivery tool.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Patients provided informed consent after understanding LBBP to be a nonstandard approach to CRT.
Ethics Statement
The study was approved by the institutional review board and adhered to the guidelines of the Helsinki Declaration.
References
- 1.Vijayaraman P., Ponnusamy S.S., Cano O., et al. Left-bundle-branch-area pacing for cardiac resynchronization therapy: results from international LBBAP collaborative study group. JACC Clin Electrophysiol. 2021;7:135–147. doi: 10.1016/j.jacep.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Huang W., Wu S., Vijayaraman P., et al. Cardiac resynchronization therapy in patients with nonischemic cardiomyopathy utilizing left bundle branch pacing. JACC Clin Electrophysiol. 2020;6:849–858. doi: 10.1016/j.jacep.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Jastrzebski M., Moskal P., Huybrechts W., et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm. 2022;19:13–21. doi: 10.1016/j.hrthm.2021.07.057. [DOI] [PubMed] [Google Scholar]
- 4.Ponnusamy S.S., Vijayaraman P. Left bundle branch pacing guided by premature ventricular complexes during implant. HeartRhythm Case Rep. 2020;6:850–853. doi: 10.1016/j.hrcr.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponnusamy S.S., Vijayaraman P. Evaluation of criteria for left bundle branch capture. Card Electrophysiol Clin. 2022;14:191–202. doi: 10.1016/j.ccep.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Butter C., Schau T., Meyhoefer J., et al. Radiation exposure of patient and physicians during implantation and upgrade of cardiac resynchronization devices. Pacing Clin Electrophysiol. 2010;33:1003–1012. doi: 10.1111/j.1540-8159.2010.02765.x. [DOI] [PubMed] [Google Scholar]
- 7.Rees J.B.V., Bie M.K.D., Thijssen J., et al. Implantation related complications of implantable cardioverter defibrillators and cardiac resynchronization therapy devices. J Am Coll Cardiol. 2011;58:995–1000. doi: 10.1016/j.jacc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Naik A., Singh B., Yadav R., et al. Cardiac resynchronization therapy is associated with improvement in clinical outcomes in Indian heart failure patients: results of a large, long-term observational study. Indian Heart J. 2018;70:S377–S383. doi: 10.1016/j.ihj.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]