Abstract
Background
Early self-detection of atrial fibrillation (AF) can help delay and/or prevent significant associated complications, including embolic stroke and heart failure. We developed a facial video technology, videoplethysmography (VPG), to detect AF based on the analysis of facial pulsatile signals.
Objective
The purpose of this study was to evaluate the accuracy of a video-based technology to detect AF on a smartphone and to test the performance of the technology in AF patients across the whole spectrum of skin complexion and under various recording conditions.
Methods
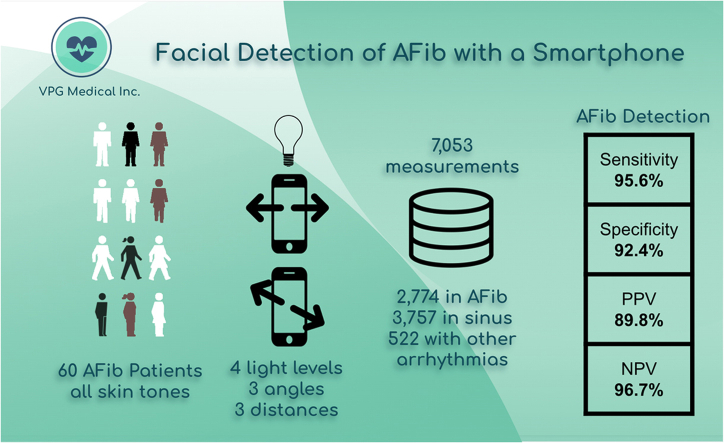
The performance of video-based monitoring depends on a set of factors such as the angle and the distance between the camera and the patient’s face, the strength of illumination, and the patient’s skin tone. We conducted a clinical study involving 60 subjects with a confirmed diagnosis of AF. A continuous electrocardiogram was used as the gold standard for cardiac rhythm annotation. The VPG technology was fine-tuned on a smartphone for the first 15 subjects. Validation recordings were then done using 7053 measurements collected from the remaining 45 subjects.
Results
The VPG technology detected the presence of AF using the video camera from a common smartphone with sensitivity and specificity ≥90%. The ambient level of illumination needs to be ≥100 lux for the technology to deliver consistent performance across all skin tones.
Conclusion
We demonstrated that facial video-based detection of AF provides accurate outpatient cardiac monitoring including high pulse rate accuracy and medical-grade performance for AF detection.
Keywords: Atrial fibrillation, Cardiac monitoring, Electrocardiogram, Photoplethysmography, Pulse rate, Videoplethysmography
Graphical abstract
Key Findings.
-
•
A smartphone is used as a contactless intermittent cardiac monitoring device. The HealthKam AFib monitoring solution uses the front camera to capture a plethysmography-like signal from the patient’s face. Then, the technology measures heart rate and detects the presence of atrial fibrillation or atrial flutter.
-
•
The proposed technology works in all normal daily levels of illumination and for all skin complexions.
-
•
In reference to electrocardiogram-based heart rate, the video-based measurement of pulse rate has an error below 1 bpm. The values of sensitivity and specificity for the detection of atrial fibrillation are above 90%.
Introduction
Videoplethysmography (VPG), also referred to as remote photoplethysmography (PPG) is a novel contactless video monitoring technology that enables measurements of pulse rate (PR)1,2 and detection of atrial fibrillation (AF).3,4 These methods rely on extracting photoplethysmographic-like signals from facial videos. Our pioneering work3 was followed by independent investigations confirming that VPG-based methods can accurately detect AF.3, 4, 5, 6 VPG provides a touch-free alternative solution to cardiac monitoring by utilizing the web cameras connected to laptops and desktop computers, as well as cameras embedded into smart devices such as smartphones and tablets. The arterial blood volume pulsations and associated changes in the volume of hemoglobin (Hb) modulate the absorption of ambient light,1 and VPG technologies capture these subtle changes in light absorption from the facial skin. VPG signal strength depends on the level of ambient light, the movement of the patient’s face (modifying reflective angle), and how the patient’s skin reflects light. In the visible range, one of the important chromophores of the human skin is melanin.
Melanin is primarily concentrated in the epidermis above the skin layers where microvascularization occurs (dermis).7 The photoprotective effect of melanin increases the skin absorption of ambient light, resulting in weaker facial VPG signals.8,9 This phenomenon has also been observed in PPG-based technologies, especially for SpO2 measurements.10 PPG technology resolved this issue using dynamic light intensity or multiwavelength light sources. VPG technologies require a different solution because they rely on ambient light sources and relatively simple RGB cameras. We have developed a VPG technology that automatically adjusts to the user’s skin complexion in order to preserve the AF detection performance of measurements across human skin tones. We present a unique study evaluating the performance of a specific VPG technology (HealthKam® AFib, VPG Medical Inc., Rochester, NY) developed to detect the presence of AF across all skin tones for various illumination levels. The technology was tested on an Android smartphone device (Samsung Galaxy S10) used by individuals with a confirmed diagnosis of paroxysmal, persistent, or permanent AF.
We conducted a clinical study designed to understand the impact of various technical, human, and demographic factors on the performance and accuracy of a VPG method to measure PR and detect AF in patients with a history of AF using a smartphone.
Methods
VPG-based application
Our objective was to evaluate the accuracy of VPG-based technology in detecting the presence of AF in a cohort of patients with different skin tones. The VPG technology utilized in this study is called “HealthKam AFib,” which has been described in multiple previous studies.11, 12, 13, 14, 15 This technology was tailored to effectively work in the S10 device using 25% of the study cohort. We reserved the remaining 75% of the study cohort for validation.
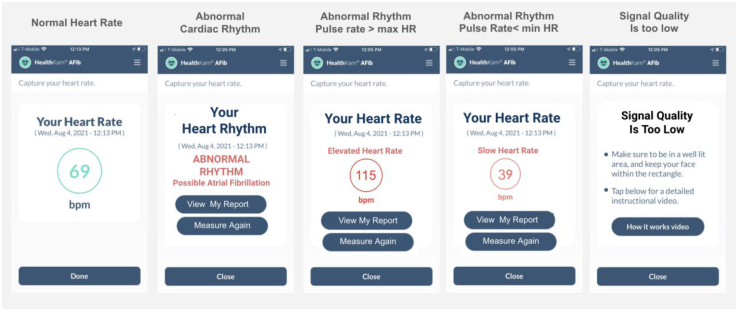
This tested smartphone has a camera with a 10-megapixel (MP) sensor and frame rate of 30 frames per second (fps). We evaluated the technology when used indoors with a level of illumination varying from a dark environment (50 lux) to a standard office space (500 lux). The VPG method captures signals of 25-second length. It provides the mean PR values when the PR rhythm is deemed to be normal (sinus origin) and notifies the user if an irregular rhythm is detected. The method is based on the analysis of the power spectrum density of the VPG signals. In general, the presence of a single dominant frequency peak defines the rhythm regularity, and its frequency sets the PR value. Further analysis of the spectral structure determines when the spectral peak is likely to reliably represent the PR or, conversely, when the spectral spread is likely to indicate abnormal rhythm associated with AF. If the spectral structure provides neither determination, a third determination is made, that is, the signal is of too low quality. When multiple frequency components are detected, a method is used to infer the presence of either an irregular rhythm (conclusive) or unsatisfactory environmental recording conditions (inconclusive). This method combines a series of information automatically extracted from the facial video recording. It includes, but is not limited to, the level of illumination, face movements, video recording stability, and darkness of the user’s skin.16 The possible outputs of a recording session are the presence of an “abnormal rhythm” (suggesting AF); reporting a “heart rate value” (sinus rhythm); or presenting a message informing the VPG signal is “too-low-quality” (TLQ) (Figure 1).
Figure 1.
Examples of the screens from the HealthKam AFib application after an active videoplethysmographic (VPG) recording is performed (selfie-like procedure). Message about bradycardia or tachycardia depends on patient settings of limits for maximum heart rate (max HR) and minimum heart rate (min HR) values. From left to right: Heart rate value is presented, message indicating that an abnormal rhythm was detected, elevated and slow heart rate values are reported, and finally notification that the recording conditions are not satisfactory and the VPG signal is too low quality (so-called inconclusive result).
Study design
The design of the experimental study mimicked the conditions under which AF patients would use their smartphones. Subjects were not restrained during the recordings but were asked to remain as still as possible. The angle between the user’s face and camera were set at 20° (almost flat), 60°, and 90° (frontal). The distance from the user’s face to the camera varied from 28 to 44 cm covering the distances observed in smartphone usage for adults with and without presbyopia.17 Three distinct distances were studied: close (30 cm), medium (35 cm), and far (40 cm). Two sources of lights were tested: light-emitting diode (LED) and incandescent lights. Four illumination levels were experimented: dark (∼50 lux), low (∼150 lux), medium (∼200 lux), and normal (∼500 lux). As a reference, the outdoor illumination level during the daytime is >1000 lux and is expected to be ∼500 lux in a typical office. The levels of illumination measured within the 4 categories were as follows: dark (56 ± 6 lux; N = 3047); low (115 ± 11 lux; N = 3091); medium (197 ± 15 lux; N = 3964); and normal (510 ± 26 lux; N = 3916). N represents the number of measurements collected in each illumination group. The lux values correspond to the mean and standard deviations (mean ± SD) of illumination within each illumination category.
Overall, each subject collected a maximum of 144 measurements at rest, including 2 replicates ∗ 2 types of light ∗ 3 distances ∗ 4 illuminations ∗ and 3 angles. Our protocol included additional measurements obtained after short bouts of physical exercise that stopped once baseline heart rate (HR) increased 30% over pre-exercise resting HR. A total of 24 video measurements were captured postexercise (2 replicates ∗ 2 types of light ∗ 1 distance ∗ 2 illuminations ∗ 3 angles). During these measurements, the subjects were asked to sit comfortably, facing toward the front of the devices without any physical constraints.
Study population
The study participants had a diagnosis of paroxysmal, persistent, or permanent AF. We enrolled adult patients aged ≥40 years. Patients who were unable to complete the Fitzpatrick skin type (FST) questionnaire, suffered from tremors and/or Parkinson disease, had blindness, needed to cover their face due to safety or religious reasons, or had known allergies to skin adhesives (electrocardiographic [ECG] electrodes) were excluded. The participants were required to be able to use an under-desk mini-cycle elliptical machine. The study was approved by an independent Institutional Review Board for Clinical Research. The subjects were enrolled after responding to an enrollment packet that included study information, a consent form, and a questionnaire about their cardiac history.
HR, PR, and cardiac rhythms
We used a single-lead ECG (M5 ECG patch, Global Instrumentations LLC, Manlius, NY) as a reference for HR and cardiac rhythms. We extracted 25-second ECG strips from the continuous recordings at the time of each VPG signal. The ECGs were annotated by a technician and reviewed by a cardiologist (BH). We formed 3 rhythm categories: category 1 for AF or atrial flutter with an ECG that may also contain other arrhythmias such as premature atrial contractions and ventricular premature contractions; category 2 for sinus rhythm and 100% normal sinus rhythm; and category 3 defined as sinus rhythm with at least a single occurrence of nonsinus beats. In addition to the ECG patch, we used a finger-based SpO2 sensor (MightySat Rx, Masimo, Irvine, CA) and recorded the PR from the sensor screen at the end of each recording.
Fitzpatrick classification for skin complexion
We asked each subject to respond to the FST questionnaire for a self-reported skin complexion rating.18 During enrollment, it became apparent that the Fitzpatrick survey was not a reliable means of classifying skin complexion, especially in the groups of subjects with dark skin. The scientific literature revealed that use of the FST survey as a tool to measure skin pigmentation has been strongly questioned for its lack of accuracy. For example, the Scientific and Technology branch of the U.S. Department of Homeland Security released a report concluding that “FST is known to be a generally unreliable estimator of skin pigmentation.”19 These observations led us to define a score based on a visual inspection of the subject’s skin. The visual FST score was recorded by an individual who was trained to classify the subject in 3 “visual FST” levels: I–II (vFST-1); III–IV (vFST-2); and V–VI (vFST-3). These visual FST categories were defined such that category vFST-1 was for white subjects; category vFST-2 was for subjects with tan and olive complexions; and category vFST-3 was for the darkest complexions.
Statistical analysis
The validation studies include repeated assessments on each subject as to whether their HR rhythm was classified as AF vs non-AF rhythms (rhythm category 1 vs category 2 + 3). These binary assessments will be paired, respectively, for the VPG-based method of measuring HR (Yvpg = 1 if VPG technology classifies the rhythm as AF; 0 otherwise) and the gold standard ECG-based method of measuring HR (Xecg = 1 if ECG AF; 0 otherwise). In general terms, the (average) sensitivity and specificity of the VPG-based approach to determining whether a heart rhythm is abnormal can be represented as follows: Sensitivity = P(Yvpg = 1 | Xecg = 1), and Specificity = P(Yvpg = 0 | Xecg = 0).These formulas assume that ECG is an error-free gold standard test as to whether a subject’s HR is abnormal. In addition, these formulas may be regarded as capturing the average sensitivity and specificity across a wide range of plausible measurement conditions and across various subjects. In the presence of repeated measures of Yvpg and Xecg on every subject, the average sensitivity and specificity can be estimated by fitting an appropriate regression model. In particular, using a logistic regression model representation [Logit(p) = ln(p/(1-p))], we have Logit P(Yvpg = 1 | Xecg) = β0 + β1Xecg, and one can then obtain estimates of both sensitivity and specificity, as well as confidence intervals (CIs) (or hypothesis tests), by estimating b0 and b1 using a technique known as generalized estimating equations.20 If b0 is the estimated value of β0 obtained from the training data, then the estimated specificity is 1 – Expit(b0), where Expit(b) = 1/(1+exp(–b)) is the inverse of the Logit transformation. Similarly, the estimated sensitivity is Expit(b0 + b1).
For the training data, we obtain b0 = –1.823 and b1 = 3.877 under a working independence correlation structure. This leads to an estimated specificity of 1 – Expit(–1.823) = 0.88 and sensitivity of expit(–1.823 + 3.877) = 0.89. There is a simple formula for the asymptotic variance of the estimated regression parameters assuming that all observations within a subject are equi-correlated and all subjects have the same number of observations.21 This formula can be used to construct a 100(1 – α)% 2 -sided CI for β0. The limits of this CI can then be transformed to the probability scale to generate a corresponding 100(1 – α)% 2 -sided CI for specificity. Suppose n represents the number of subjects and we assume there are (at least) m = 100 replicate observations with Xecg = 0 for each subject, with an associated conservative specification of within-subject correlation of 0.3. Then, we compute the expected (ie, average) performance of such an interval assuming that α = 0.045 (ie, 95.5% 2-sided CI) for the given number of subjects and that the actual specificity is 0.9 (or 90%). Use of the choice α = 0.045, rather than the more common choice of α = 0.05, constitutes a Bonferroni adjustment and intends to reserve 0.5% for justifying the validation set size for evaluating the accuracy of the reported HR. Our computation suggested that as few as 45 subjects would be needed in the validation set to ensure that the 95.5% CI is no wider than 10%, such that there is an 82% chance that the lower limit of this .CI exceeds 0.8 (ie, lower 95.5% confidence limit exceeds specificity of 0.8, or 80%, with probability 0.81).
Results
Demographics
Study enrollment started in January 2021 and was completed in March 2022. We enrolled a total of 60 subjects (age 67 ± 10 years; 44 men; body mass index [BMI] 31± 6 kg/m2; blood pressure 130 ± 15 mm Hg). Demographics for the validation cohort (N = 45; 32 men) and the number of subjects in the 3 categories of skin complexion (vFST-1 to vFST-3) are given in Table 1. Twenty-eight subjects were receiving beta-blockade therapy (metoprolol, atenolol, carvedilol), and 10 subjects were being treated with a rhythm control drug. Our subjects self-reported the presence of diseases or syndromes associated with a low level of Hb, including anemia (N = 5), chronic kidney disease (N = 3), irritable bowel syndrome (N = 1), lead poisoning (N = 0), leukemia (N = 0), hypothyroid (N = 5), iron deficiency (N = 8), ferritin deficiency (N = 1), and chronic cirrhosis (N = 0).
Table 1.
Demographics of the validation cohort
| Women | Men | All | |
|---|---|---|---|
| N | |||
| Age (y) | |||
| Height (m) | |||
| Weight (kg) | |||
| BMI (kg/m2) | |||
| BP (mm Hg) | |||
| Obese | 4 (15) | 9 (28) | 13 (29) |
| Hypotensive | 1 (8) | 2 (6) | 3 (7) |
| Hypertensive | 2 (15) | 9 (28) | 11 (24) |
| vFST 1 | 5 (39) | 13 (41) | 18 (40) |
| vFST 2 | 6 (46) | 13 (41) | 19 (42) |
| vFST 3 | 2 (15) | 6 (19) | 8 (18) |
Values are given as mean ± SD or n (%) unless otherwise indicated.
BMI = body mass index; BP = blood pressure; vFST = visual Fitzpatrick skin type.
∗P <.05 for t test between gender in the upper rows and for χ2 test in the lower rows.
Blood pressure, BMI, age, and gender distribution were in the expected ranges for a population with AF.22 The group of men was younger (P = .06) and significantly heavier (P <.01) than the enrolled women, but BMIs were similar between genders (P = .16). We studied the VPG technology in 8 AF patients with vFST-3 (N = 8 subjects), the largest cohort of dark skin subjects ever reported for video-based detection of AF.
VPG-based detection performance across rhythm categories
We collected 7060 VPG recordings in the validation set. Seven VPG signals had an ECG signal that was too noisy for HR extraction and/or rhythm annotation. Among the remaining 7053 recordings (6.7%), the number of recordings per vFST group were 2830, 2968, and 1255 for vFST-1 (N = 18 subjects), vFST-2 (N = 19 subjects), and vFST-3 (N = 5 subjects), respectively. The percentage of conclusive measurements was 93%, 92%, and 61% for the same groups, respectively (Table 2).
Table 2.
Number of recordings across skin tones and number of conclusive and inconclusive measurements within each group
| vFST-1 | vFST-2 | vFST-3 | N | |
|---|---|---|---|---|
| N | 2830 | 2968 | 1255 | 7053 |
| Conclusive | 2631 (93) | 2732 (92) | 770 (61) | 6133 (87) |
| Inconclusive | 199 (7) | 236 (8) | 485 (39) | 920 (13) |
Values are given as N or n (%).
vFST = visual Fitzpatrick skin type.
All VPG recordings had a corresponding 25-second ECG labeled with a rhythm interpretation: 1 (AF), 2 (sinus), or 3 (other rhythms). The number of VPG recordings in each ECG-based rhythm category across all HealthKam AF labeled “abnormal rhythm,” sinus rhythm (ie, providing a “pulse rate”), and “too-low-quality” (TLQ) results are given in Table 2. The percentage of TLQ recordings was 7.4% throughout the entire validation recording dataset. This included all the levels of illumination, light sources, camera angles, and distances from the face.
Among the conclusive recordings, sensitivity of the method to detect AF was 95.6% (2235/2339), and specificity was 92.4% (3090/3343). Positive predictive value was 89.8% (2235/2488), and negative predictive value was 96.7% (3090/3194). We combined rhythm categories 2 and 3 because we observed that the set of ECGs labeled as abnormal rhythm had 93% ± 7% sinus beats. Hence, most of these 25-second recordings contained a single nonsinus beat.
The accuracy of PR measurements was evaluated in the set of 3590 recordings from 40 subjects from the training set. Five subjects were constantly in AF and did not have PRs reported during their recordings. The mean error of PR, in reference to HR, was –0.1 bpm, with upper and lower bounds of –4 bpm and +4 bpm, respectively. We found 2 measurements with an error >60 bpm. These 2 recordings were associated with pulse deficit. The PPG-based readings for these 2 recordings were similar to the ones from the VPG measurements. We observed 8 measurements (0.2%) with an error >10 bpm. There were 38 measurements (1.0%) with an error >5 bpm. Most of these PR measurements were collected during undetected AF rhythm (false negative). A confusion matrix for PR reporting is given in Table 3.
Table 3.
Number of VPG recordings per VPG labels across the 3 ECG rhythm categories
| Rhythm category/VPG labels | 1 (AF) | 2 (sinus) | 3 (other) | n∗ |
|---|---|---|---|---|
| Abnormal rhythm | 2235 | 253 | 55 | 2543 |
| Pulse rate | 104 | 3090 | 396 | 3590 |
| Inconclusive | 435 | 414 | 71 | 920 |
| N∗ | 2774 | 3757 | 522 | 7,053 |
AF = atrial fibrillation; ECG = electrocardiogram; VPG = videoplethysmography.
N is the number of recordings across rhythm categories, and n is the number of recordings across VPG labels.
Performance across skin complexions and other environmental factors
A summary of the performance and accuracy of the method across the study demographic characteristics including gender, obesity, and blood pressure status (hypertensive, normotensive, and hypotensive subjects) is given in Table 4. We look at the mean measurement error (in bpm) and its Bland-Altman 95% limits of agreement (LoA), that is, upper and lower boundaries of the errors adjusted for replicated measurements.23 In the lower section of Table 4, we provided the method performance for AF detection in these subgroups. We note that the mean errors in these subgroups were never larger than 1.5 bpm, and the largest LoA boundary was 3.5 bpm. Specificity and sensitivity of the method remained at the 90% level across most groups. Min and max sensitivities were 91% and 98%, and 88% and 93%, respectively, for specificity.
Table 4.
Summary of performances for VPG-based detection of AF and accuracy in pulse rate measurements across population demographic characteristics
| Gender |
BMI |
BP |
|||||
|---|---|---|---|---|---|---|---|
| Subsets |
Men |
Women |
Obese |
Not obese |
Hypertensive |
Normotensive |
Hypotensive |
| Pulse rate accuracy | |||||||
| N (subjects) | 27 | 12 | 18 | 18 | 17 | 14 | 4 |
| N (datapoints) | 2300 | 1219 | 1326 | 1964 | 1746 | 930 | 594 |
| Mean error (bpm) | –1.1 | –1.0 | –1.4 | –0.9 | –0.6 | 1.0 | –0.1 |
| Upper bound (bpm) | 0.2 | 1.5 | 0.7 | 0.7 | 0.6 | 0.8 | 0.0 |
| Lower bound (bpm) | –2.5 | –3.4 | –3.5 | –2.4 | –1.8 | 0.0 | –0.3 |
| AF detection performance | |||||||
| N (subjects) | 32 | 13 | 19 | 22 | 21 | 15 | 0 |
| N (datapoints) | 4272 | 1861 | 2705 | 2967 | 2818 | 2108 | — |
| Sensitivity (%) | 96 | 95 | 91 | 97 | 98 | 94 | — |
| Specificity (%) | 89 | 92 | 90 | 93 | 92 | 88 | - |
| PPV (%) | 97 | 84 | 72 | 94 | 85 | 90 | - |
| NPV (%) | 86 | 98 | 97 | 97 | 99 | 92 | - |
We did not observe differences in AF detection performance and accuracy when comparing the 2 different types of light source—LED vs incandescent light. However, the percentage of inconclusive VPG recordings was lower in measurements collected with LED than with incandescent lights: 13.1% vs 31.6% (P <.0001) at 50 lux. This difference progressively decreased with increasing level of illumination. At 500 lux, these percentages were not statistically different: 7.6% vs 9.6% (P = .2) for LED and incandescent light sources, respectively.
The accuracy and performance results across vFST categories, level of illumination, distances, and angles are given in Table 5. Accuracy was consistent across all subgroups, with a mean error inferior to 1 bpm. The larger LoA was found in the vFST-1 group (–0.1 to –5.5 bpm) driven by the outliers due to pulse deficit. Other subgroups had LoA with limits inferior to –2.2 bpm. The highest detection performance was found in the vFST-1 group (sensitivity 99%; specificity 94%), and the lowest performance was associated with the vFST-3 group (sensitivity 97%; specificity 81%). We performed an additional analysis of performance in the subgroup vFST-3 considering data collected for levels of illumination >50 lux only, that is, we eliminated the lowest illumination condition from the analysis (N = 8 subjects; n = 618 recordings). The resulting sensitivity and specificity were 96% of 90%, respectively, with positive predictive value of 89 and negative predictive value of 96%.
Table 5.
Summary of performances for VPG-based detection of AF and accuracy in pulse rate measurements across recording conditions and skin tones.
| vFST |
Illumination (lux) |
Distance |
Angle (°) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ≥50 | ≥100 | ≥200 | >200 | Close | Medium | Far | 20 | 60 | 90 | |
| Pulse rate accuracy | |||||||||||||
| N (subjects) | 16 | 18 | 5 | 39 | 37 | 36 | 35 | 32 | 35 | 35 | 35 | 36 | 38 |
| N (datapoints) | 1555 | 1691 | 342 | 3588 | 2927 | 2177 | 1121 | 1072 | 1505 | 1011 | 1149 | 1203 | 1236 |
| Mean error (bpm) | –2.7 | –0.2 | 0.3 | –1.1 | –0.5 | –0.3 | –0.5 | –0.2 | –0.9 | –0.2 | –0.7 | –0.6 | –0.6 |
| Upper bound (bpm) | –0.1 | 0.1 | 1.2 | 0.0 | 0.4 | 0.4 | 0.2 | 0.0 | 0.2 | 0.7 | 0.2 | 0.1 | 0.5 |
| Lower bound (bpm) | –5.5 | –0.4 | –0.5 | –2.2 | –1.5 | –1.0 | –1.2 | –0.5 | –2.1 | –1.1 | –1.7 | –1.3 | –1.6 |
| AF detection performance | |||||||||||||
| N (subjects) | 18 | 19 | 8 | 45 | 45 | 45 | 45 | 45 | 45 | 43 | 45 | 45 | 45 |
| N (datapoints) | 2631 | 2732 | 770 | 6133 | 4918 | 3600 | 1809 | 1836 | 2547 | 1750 | 1945 | 2076 | 2112 |
| Sensitivity (%) | 99 | 92 | 97 | 96 | 95 | 94 | 93 | 98 | 94 | 96 | 96 | 96 | 95 |
| Specificity (%) | 94 | 93 | 81 | 92 | 93 | 94 | 94 | 93 | 92 | 92 | 92 | 91 | 92 |
| PPV (%) | 90 | 88 | 82 | 88 | 89 | 90 | 91 | 90 | 87 | 88 | 88 | 87 | 89 |
| NPV (%) | 99 | 95 | 97 | 97 | 97 | 97 | 96 | 99 | 96 | 97 | 97 | 97 | 97 |
Discussion
In the past, facial video-based monitoring technologies have been primarily used for monitoring pulse and respiratory rates,24 but the technology is rapidly evolving due, in part, to the increased availability of good-quality video cameras (>5 MP) in consumer products. In this study, we present an application of video monitoring that goes beyond the measurements of vital signs with the ability to detect AF, which is one of the most prevalent clinical cardiac arrhythmias with significant associated morbidity.
Our work reveals this technology can detect the presence of AF utilizing video cameras from a common smartphone with sensitivity and specificity ≥90% when the right recording conditions are present. These conditions include an ambient illumination of at least 100 lux, a distance from the camera between 28 and 44 cm, and an angle between 20° (almost flat) and 90° (facing). The tested range of distances and angles between the patients’ faces and the camera corresponds to how individuals would be expected to use their smartphone. This technology may need further tuning if a different type of camera is used.
Melanin plays its photoprotective role and reduces the amount of light reflected from the face of subjects with the darkest skin pigmentation. In our study, we report a larger number of TLQ recordings in the group of subjects with vFST-3 compared to other vFST categories, especially at low illumination. However, the technology delivered a similar accuracy and performance across all skin types when the ambient illumination was >50 lux. We observed similar AF detection performance and PR accuracy between LED and incandescent lights, but a lower number of rejected VPG recordings was reported for the LED light source, especially at low illumination. LEDs generally emit more intensity in the green wavelength (∼550 nm) than incandescent lights, which is the wavelength primarily absorbed by oxygenated Hb.
VPG technology enables a new paradigm for cardiac monitoring that does not provide continuous monitoring but addresses important limitations of existing monitoring solutions. The clinical benefit of continuous monitoring for the purpose of detecting AF has recently been questioned by 2 important studies. The LOOP study (Atrial Fibrillation Detected by Continuous ECG Monitoring Using Implantable Loop Recorder to Prevent Stroke in High-risk Individuals) compared continuous monitoring utilizing implantable cardiac monitors vs usual care.25 Systemic anticoagulation was recommended for any detection of AF in both groups. There was no significant difference in the reduction of the risk of stroke or systemic embolism between the 2 monitoring strategies.25 The STROKESTOP (Systematic ECG Screening for Atrial Fibrillation Among 75 Year Old Subjects in the Region of Stockholm and Holland, Sweden) trial utilized a handheld single-lead ECG recorder for 30 seconds twice daily for 30 days to screen for AF in a large cohort of elderly individuals in 2 communities in Sweden and compared them to controls who were not screened.26 There was a 4% reduction in the screened subjects for the combined primary endpoint of stroke, systemic embolism, hospitalization for severe bleeding, and death.26 These studies suggest that continuous monitoring often detects a higher percentage of short-duration episodes of AF that likely are associated with a lower risk of stroke and other comorbid conditions and do not necessarily require further treatment. Intermittent monitoring, as occurs with VPG technology, may be superior at detecting longer-duration episodes of AF for which clinical intervention can have a meaningful impact. In addition, the proposed technology may be an attractive alternative for patients who are not compliant with wearable technologies. The inconvenience, skin irritation, and extra effort of using, wearing, and maintaining a monitoring device do not exist for the VPG method, so it avoids the problem of device attrition. Device attrition is not a negligible challenge, especially in individuals with chronic diseases. Shaw et al27 reported that 24% of healthy individuals abandoned wearable devices after a month, whereas 84% of patients living with chronic diseases stopped using their devices during the same time period. Smartphones are owned by the majority of people in countries with advanced economies (81% in the United States).28 The VPG technology can be leveraged to easily deploy cardiac monitoring services to subjects by simply downloading an application to their personal device. Furthermore, the VPG monitoring technology requires the patient to remain still; therefore, it primarily collects information at rest, avoiding the challenge of data overload posed by continuous monitoring technologies capturing HR across all daily activities.
Study limitations
Integrating a video-based monitoring technology into a patient's everyday life may raise concerns about privacy and cybersecurity. It is worth noting that the technology tested in this study was developed with Health Insurance Portability and Accountability Act (HIPAA) and privacy requirements in mind. The technology was designed so that it does not record or store any video/image of the patient’s face. More importantly, the camera is used as a sensor, extracting VPG signal from each frame without the need for an intermediary step involving the storage of any images.
VPG technology measures the changes in volume and concentration of Hb underneath the skin. Because we did not have access to direct measurement of Hb concentration, we surveyed the cohort for the presence of diseases and conditions such as anemia, chronic kidney disease, irritable bowel syndrome, lead exposure, iron and ferritin deficiency, leukemia, hypothyroid, and cirrhosis. We found that 2 subjects had at least 3 of these factors. One subject (68-year-old black woman) had persistent AF, and the second subject (68 year-old white man) remained in sinus rhythm during the study. The results from these 2 individuals were similar to those of the study population as a whole. Future studies should include Hb measurements to clarify whether abnormal Hb is associated with a loss of monitoring performance.
Conclusion
We investigated a video-based method that captures a pulsatile signal from the face of patients with a diagnosis of AF. The method provides a level of accuracy and performance that is equivalent to existing medical-grade, pulse-based technologies. We believe that video-based monitoring will be an attractive solution for telemedicine platforms because it enables vital signs and cardiac rhythm monitoring without the need to ship dedicated devices to patients. Its deployment is made easy by it being a pure software solution.
Acknowledgments
We would like to thank Thuan Pham, Sheetal Kashid, Ketaki Barde, Gulnar Raman, Kim Wells, and Kelley Cename for their support in conducting the clinical study described in this article.
Funding Sources
This research did not receive any specific grant from funding agencies. The work described in this paper was funded by VPG Medical Inc.
Disclosures
All authors were employed by VPG Medical during the study. Drs Couderc, Tsouri, and Hall have a significant financial interest in VPG Medical Inc., a company commercializing the VPG technology under the name HealthKam® AFib.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided written informed consent.
Ethics Statement
The authors designed the study, gathered, and analyzed the data according to the Helsinki Declaration guidelines on human research. The research protocol used in this study was reviewed and approved by the institutional review board.
References
- 1.Verkruysse W., Svaasand L.O., Nelson J.S. Remote plethysmographic imaging using ambient light. Opt Express. 2008;16:21434–21445. doi: 10.1364/oe.16.021434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takano C., Ohta Y. HR measurement based on a time-lapse image. Med Eng Phys. 2007;29:853–857. doi: 10.1016/j.medengphy.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Couderc J.-P., Kyal S., Mestha L.K., et al. Detection of atrial fibrillation using contactless facial video monitoring. Heart Rhythm. 2015;12:195–201. doi: 10.1016/j.hrthm.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y., Yang Y.Y., Wu B.J., et al. Contactless facial video recording with deep learning models for the detection of atrial fibrillation. Sci Rep. 2022;12:281. doi: 10.1038/s41598-021-03453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan B.P., Lai W.H.S., Chan C.K.Y., et al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol. 2020;5:105–107. doi: 10.1001/jamacardio.2019.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J., Alikhani I., Li X., Yu Z., Seppänen T., Zhao G. Atrial fibrillation detection from face videos by fusing subtle variations. IEEE Trans Circuits Syst Video Technol. 2020;30:2781–2795. [Google Scholar]
- 7.Zonios G., Bykowski J., Kollias N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J Invest Dermatol. 2001;117:1452–1457. doi: 10.1046/j.0022-202x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 8.Nowara E.M., McDuff D., Veeraraghavan A. A meta-analysis of the impact of skin tone and gender on non-contact photoplethysmography measurements. 2020. IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW) 2020:1148–1155. [Google Scholar]
- 9.Fallow B.A., Tarumi T., Tanaka H. Influence of skin type and wavelength on lightwave reflectance. J Clin Monit Comput. 2013;27:313–317. doi: 10.1007/s10877-013-9436-7. [DOI] [PubMed] [Google Scholar]
- 10.Feiner J.R., Severinghaus J.W., Bickler P.E. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: the effects of oximeter probe type and gender. Anesth Analg. 2007;105(6 Suppl):S18–S23. doi: 10.1213/01.ane.0000285988.35174.d9. [DOI] [PubMed] [Google Scholar]
- 11.Couderc J.-P., Page A., Xia J., et al. Pulse harmonic strength of facial video signal for the detection of atrial fibrillation. Comput Cardiol. 2014;2014:661–664. [Google Scholar]
- 12.Couderc J.-P., Tsouri G.R., Savur C., et al. Monitoring atrial fibrillation patients using active contactless videoplethysmography implemented on a personal device. Heart Rhythm. 2021;18(Suppl):S265. [Google Scholar]
- 13.Couderc J.-P., Page A., Xia J., et al. Heart Rhythm Conference; Boston, Massachusetts: 2020. AI-based method for monitoring pulse rate using facial videoplethysmography recorded with mobile devices. (Abstract) [Google Scholar]
- 14.Dautov C.P., Dautov R., Couderc J.P., Tsouri G.R. Machine learning approach to detection of atrial fibrillation using high quality facial video. IEEE EMBS International Conference on Biomedical and Health Informatics (BHI) 2021 [Google Scholar]
- 15.Guzman J., Couderc J.P., Tsouri G.R. Accurate hemodynamic sensing using video plethysmography with high quality cameras. IEEE International Symposium on Medical Information and Communication Technology (ISMICT) June 2019 [Google Scholar]
- 16.Ly B.C.K., Dyer E.B., Feig J.L., Chien A.L., Del Bino S. Research techniques made simple: cutaneous colorimetry: a reliable technique for objective skin color measurement. J Invest Dermatol. 2020;140:3–12.e1. doi: 10.1016/j.jid.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Boccardo L. Viewing distance of smartphones in presbyopic and non-presbyopic age. J Optom. 2021;14:120–126. doi: 10.1016/j.optom.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzpatrick T. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 19.Homeland Security Science and Technology; Howard J, Sirotin Y, Tipton J, Vemury A. Revisiting the Fitzpatrick Scale and Face Photo-based Estimates of Skin Phenotypes. October 29, 2020. https://pages.nist.gov/ifpc/2020/presentations/36_Fitzpatrick_IFPC2020_Final.pdf. Accessed February 19, 2022
- 20.Leisenring W., Pepe M.S., Longton G. A marginal regression modelling framework for evaluating medical diagnostic tests. Stat Med. 1997;16:1263–1281. doi: 10.1002/(sici)1097-0258(19970615)16:11<1263::aid-sim550>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Pan W. Sample size and power calculations with correlated binary data. Contr Clin Trials. 2001;22:211–227. doi: 10.1016/s0197-2456(01)00131-3. [DOI] [PubMed] [Google Scholar]
- 22.Singleton M.J., German C.A., Carnethon M., Soliman E.Z., Bertoni A.G., Yeboah J. Race, body mass index, and the risk of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carstensen B., Simpson J., Gurrin L.C. Statistical models for assessing agreement in method comparison studies with replicate measurements. Int J Biostatist. 2008;4:16. doi: 10.2202/1557-4679.1107. [DOI] [PubMed] [Google Scholar]
- 24.Molinaro N., Schena E., Silvestri S., et al. Contactless vital signs monitoring from videos recorded with digital cameras: an overview. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.801709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svendsen J.H., Diederichsen S.Z., Højberg S., et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507–1516. doi: 10.1016/S0140-6736(21)01698-6. [DOI] [PubMed] [Google Scholar]
- 26.Svennberg E., Friberg L., Frykman V., Al-Khalili F., Engdahl J., Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet. 2021;398:1498–1506. doi: 10.1016/S0140-6736(21)01637-8. [DOI] [PubMed] [Google Scholar]
- 27.Shaw R.J., Steinberg D.M., Bonnet J., et al. Mobile health devices: will patients actually use them? J Am Med Inform Assoc. 2016;23:462. doi: 10.1093/jamia/ocv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott M., Coventry A. Signs of patient monitoring. Br J Nurs. 2012;21:621–625. doi: 10.12968/bjon.2012.21.10.621. [DOI] [PubMed] [Google Scholar]