Abstract
Coronary artery disease and heart failure are leading causes of morbidly and mortality, resulting in a substantial economic burden globally. Guidelines from the European Society of Cardiology and American Heart Association place adherence to medication and healthy lifestyle behaviors at the core of cardiovascular disease primary and secondary prevention strategies. The growing collective burden of cardiovascular disease is likely to eventually outgrow the available resources allocated for traditional care provision, such as nurse-led outreach services. Novel strategies are required to address this growing need. Worldwide, more than 6.5 billion people own smartphones and opportunities to deliver healthcare digitally for patients with cardiac conditions are expanding exponentially. Multiple randomized controlled trials have now demonstrated that various modes of noninvasive digital health technology, including teleconsultations, smartphone applications (apps), wearables, remote monitoring, and predictive analytics can influence patient behaviors in both the primary and secondary prevention of coronary artery disease and prevention and management of heart failure. The purpose of this narrative review is to critically analyze pivotal trials and discuss examples of successfully deployed mobile digital technology in the prevention of heart failure hospitalizations, and in the primary and secondary prevention of coronary artery disease.
Keywords: Digital health technology, mHealth, Telehealth, Heart failure, Coronary artery disease
Key Findings.
-
•
Multiple forms of noninvasive mobile digital technology are now available to assist in the optimal management of heart failure patients, such as teleconsultations, SMS systems, smartphone applications, wearables, and remote monitoring systems. Digital technology that incorporates clinical data recording, combined with clinician feedback and structured follow-up, appear to be more efficacious and have been proven to reduce hospital readmissions.
-
•
There is growing evidence of the effectiveness of mobile digital health technology in reducing risk factors for coronary artery disease; whether this translates to a reduction in clinical endpoints is yet to be determined. Further higher-powered studies with longer follow-up are needed to prove a reduction in hard endpoints such as mortality and repeat cardiovascular events.
-
•
Many challenges still exist that are potentially limiting factors to the widespread uptake of digital health in cardiology, including equitable access and usability of digital health technology for patients; system integration and workflow; and technology costs and healthcare utilization.
Introduction
Cardiovascular disease is the leading cause of mortality worldwide, with an estimated 17.9 million deaths (32% of all global deaths) in 2019.1 Guidelines from the European Society of Cardiology and American Heart Association place adherence to medication and healthy lifestyle behaviors at the core of cardiovascular disease primary and secondary prevention strategies.2,3 In practice, an exceedingly small proportion of patients achieve target goals across all measures, suggesting there is a critical unmet need for patient behavioral change to optimize modifiable risk factors such as hypertension, dyslipidemia, tobacco use, inactivity, obesity, and medication nonadherence. The growing collective burden of disease, particularly of heart failure (HF), is likely to eventually outgrow the available resources allocated for traditional care provision, such as nurse-led HF outreach services. Novel strategies are required to address this growing need.
Telemedicine refers to the provision of healthcare by means of any telecommunication technology. Traditionally, telemedicine required the provision of home-based specialized monitoring equipment to patients. However, smartphones, mobile phones, and wearable technology offer tremendous potential for monitoring health through phone calls, text messages, data recording, and activity monitoring.4 This technology is at the heart of digital health, which is defined as the use of digital, mobile, and wireless technologies to support the achievement of health objectives.5 mHealth is a branch of digital health that is specific to the use of mobile devices such as smartphones and tablets.
The potential of digital health has become even more relevant during the COVID-19 pandemic, in which social isolation has boosted the need for rapid proliferation of digital medicine.6 Worldwide, more than 6.5 billion people own smartphones and opportunities to deliver healthcare digitally for patients with cardiac conditions are expanding exponentially.7
Multiple studies have underlined several advantages of using digital health to reduce inequalities in cardiovascular outcomes8 and improve care for patients with HF and coronary artery disease.9 Several of these novel models of healthcare delivery are cost-effective, accessible, patient-centric, and focused on patient behavior change.10
The importance of digital health solutions during the recent COVID-19 pandemic has been reflected by an increase in publications on this important topic; however, many studies are underpowered with short follow-up owing to difficulty sourcing funding and the relative short lifespan of digital health interventions as technology advances. This narrative review will critically analyze large, adequately powered pivotal randomized controlled trials (RCTs) (summarized in Table 1) and discuss examples of successfully deployed noninvasive mobile digital technology in the prevention of HF hospitalizations, and in the primary and secondary prevention of coronary artery disease.
Table 1.
Pivotal trials on the use of digital health technology in the prevention of heart failure and coronary artery disease
| First author, year | Study design, study population | Number of patients, follow-up | Intervention | Control | Main results |
|---|---|---|---|---|---|
| Koehler, 201119 | Multicenter RCT, heart failure | 710 patients, 12 months | Telemonitoring system. No linkage with usual healthcare providers. | Standard care | No difference in all-cause mortality, cardiovascular death, or HF hospitalization |
| Chow, 201522 | 2-center RCT, coronary artery disease | 710 patients, 6 months | Text messages | Standard care | Reduction in LDL cholesterol, systolic blood pressure, and smoking rates |
| Ong, 201620 | Multicenter RCT, heart failure | 1437 patients, 180 days | Health coaching phone calls and telemonitoring. No linkage with usual healthcare providers. Poor patient adherence. | Standard care | No difference in all-cause mortality or readmission for any cause |
| Koehler, 201818 | Multicenter RCT, heart failure | 1571 patients, 393 days | Telemonitoring system, patient education, monthly phone calls | Standard care | Reduction in days lost to unplanned hospital admission or mortality |
| Chen, 201921 | Single-center RCT, heart failure | 767 patients, 180 days | 2 arms; structured telephone support and SMS-based support system | Inpatient nurse heart failure education | Reduced readmission rates and improved self-care (medication compliance) in SMS group vs control |
| Tekkeşin, 202123 | Single-center RCT, high-cardiovascular-risk patients | 483 patients, 12 months | Smartphone app + remote monitoring devices + motivational text messages | Standard care | Reduction in 10-year cardiovascular risk |
| Li, 202224 |
Single-center, RCT, coronary artery disease | 290 patients, 12 months | Smartphone self-management app + remote monitoring device | Standard care | Increase in use of guideline-directed medical therapy, reduction in blood pressure and LDL cholesterol |
HF = heart failure; RCT = randomized controlled trial.
Heart failure
HF is a major public health concern, owing to its morbidity, mortality, and increasing prevalence among aging populations. HF is the most common hospital discharge diagnosis among older adults in the United States, and one-fifth of HF patients are readmitted within 30 days of discharge.11 It is estimated that by 2030, more than 8 million American adults will be living with HF, representing a 50% increase in its prevalence compared to 2012.12 Despite the availability of effective evidence-based treatment options, hospitalization rates are universally increasing13 and the prognosis of HF remains poor, with almost half of patients dying within 5 years of initial diagnosis.14 Noninvasive digital health technology, which encompasses teleconsultations, smartphone applications (apps), wearables, remote monitoring, and predictive analytics, holds promise to improve HF care and management. HF is typically characterized by acute decompensations with an otherwise steady decline in cardiac function. Acute decompensated HF is often a result of medication nonadherence,15 failure to implement positive lifestyle changes, and lack of detection of subacute deteriorations, which may manifest with increasing body weight or changes in blood pressure (BP) and heart rate. All of the above can be addressed by digital health technologies. Smartphone apps and SMS-based systems can remind patients to take medications and can educate them on positive self-care habits, and telemonitoring systems, which involve the collection of physical parameters, can be used to warn clinicians of impending clinical deteriorations, which can be treated early and thus reduce or prevent rehospitalization.16
Multiple meta-analyses have demonstrated benefits of digital health on the prevention of HF hospitalizations. A systematic review and meta-analysis in 2020 investigated the benefit of mobile phone technologies in the management of ischemic heart disease, HF, and hypertension.10 A total of 6 RCTs assessed the efficacy of mobile phone interventions in the management of HF against standard care. These interventions were associated with a significantly lower rate of hospitalizations (244/792, 30.8% vs 287/803, 35.7%; n = 1595; odds ratio 0.77, 95% confidence interval [CI] 0.62–0.97; P = .03; I2 = 0%) in relation to both total admissions and HF admissions, with no significant difference in mortality rates between the groups. More recently, a systematic review and meta-analysis published by Kitsiou and colleagues17 in 2021 investigated the use of interventions including mobile phones, smartphones, tablets, and remote patient monitoring devices in the management of HF. They included 16 RCTs comprising 4389 patients that found that these technologies, in comparison to usual care, reduced the risk of all-cause mortality (risk ratio [RR] 0.80; 95% CI 0.65–0.97; absolute risk reduction [ARR] 2.1%), cardiovascular mortality (RR 0.70; 95% CI 0.53–0.91; ARR 2.9%), and HF hospitalizations (RR 0.77; 95% CI 0.67–0.88; ARR 5%) but had no effect on all-cause hospitalizations. Results were mainly driven by telemonitoring interventions, where parameters such as weight and BP were reviewed and alerted if predefined data thresholds were exceeded. In addition, a systematic review and meta-analysis by Coorey and colleagues16 in 2018 investigated the effect of smartphone apps on HF self-management. A total of 28 articles assessing 23 apps, and a total of 1397 participants, were included. The most common app features were weight monitoring, symptom monitoring, and vital sign monitoring; however, only a quarter of the apps provided all guideline-defined core components of HF self-management programs: education, symptom monitoring, medication support, and physical activity support. From these meta-analyses, it is clear that not all mobile health interventions have equal efficacy, and the reported benefits depend on the type of technology used, the presence of organizational support and feedback, and the level of care provided to control groups. Interventions need to be easy to use, be able to cope with large amounts of data, and be integrated into existing models of care involving the patient’s usual healthcare providers.
An example of the above was a pivotal RCT on the efficacy of a telemedicine intervention in patients with HF (TIM-HF2), published by Koehler and colleagues18 in 2018. The study was a prospective, multicenter RCT conducted in Germany on 1571 patients with New York Heart Association class II–III dyspnea, an ejection fraction below 45%, and at least 1 hospital admission in the preceding 12 months. Patients were randomized 1:1 to receive usual care or a telemonitoring system consisting of daily transmission of body weight, systolic and diastolic BP, heart rate, analysis of the heart rhythm via ECG, peripheral capillary oxygen saturation, and a self-rated health status to the telemedical center. This was combined with patient education and structured monthly telephone interviews with cooperation between the telemedical center and the patient’s general practitioner and cardiologist. The monthly structured telephone interviews combined with the daily data transmissions allowed the patient’s clinical and symptomatic status and concomitant medications to be assessed. The number of days lost to unplanned cardiovascular hospital admissions and all-cause death was lower in the intervention group at 17.8 days (95% CI 16.6–19.1 days) per year vs 24.2 days (22.6–26.0 days) per year for patients assigned to usual care. The all-cause death rate was 7.96 (95% CI 6.1–10.1) per 100 person-years of follow-up in the remote patient management group compared with 11.3 (9.2–14.0) per 100 person-years of follow-up in the usual care group (hazard ratio [HR] 0.70, 95% CI 0.50–0.96; P = .0280). Cardiovascular mortality was not significantly different between the 2 groups (HR 0.67, 95% CI 0.45–1.01; P = .056). This study was more effective than its predecessor (TIM-HF),19 which was a prospective RCT of 710 chronic HF patients randomized to usual care or to a remote telemonitoring system consisting of remote ECG, BP, and body weight monitoring paired via Bluetooth to a personal digital assistant, which transmitted the data via a mobile phone service to a central data monitoring unit. One of the major differences in this earlier trial was that the central monitoring unit did not involve the patient’s usual healthcare providers in clinical decision-making, which may have contributed to this trial finding no difference in mortality (15% in both groups, 54/354 intervention vs 55/356 in the control group) or hospitalizations over 12 months of follow-up. Similarly, a large prospective multicenter RCT (BEAT-HF) of 1437 patients published by Ong and colleagues20 in 2016 compared a combination of regularly scheduled telephone coaching and home telemonitoring of weight, BP, heart rate, and symptoms to usual care in HF patients. They found no difference between 180-day readmission rates (363/715, 50.8% vs 355/722, 49.2%, adjusted HR 1.03; 95% CI 0.88–1.20; P = .74) among patients admitted to hospital for treatment of decompensated HF and also did not involve the patient’s usual healthcare providers in clinical decision-making. This trial also experienced poor patient adherence, with only 55% of patients adherent to telephone calls and telemonitoring within the first 30 days. These 2 negative trials support the concept that the processes that support decision-making on remote data are as important as the data and the monitoring tools themselves.
A large RCT published by Chen and colleagues21 in 2019 demonstrated a successful SMS based support system. The trial randomized 767 patients admitted to a tertiary hospital in China with HF into 3 arms: structured telephone support, an SMS-based support system, or a control group. Patients in the control group received inpatient nurse-led HF education. Patients in the structured telephone support group received 1 phone call from research nurses within 30 days after discharge. The SMS system consisted of daily educational messages for 10 days—for example, how to monitor for symptoms of HF. This was followed by weekly reminder messages—for example, medication and weighing reminders. These messages were automated, were not personalized, and could not be replied to. A comparison of the SMS group with the control group over 180 days of follow-up demonstrated significantly lower readmission rates (33.7% vs 42.7%, odds ratio 0.790; CI 0.632–0.988; P = .037) as well as improved self-care behavior, with higher rates of medication adherence and fluid restriction adherence. There was no significant difference in mortality or quality of life.
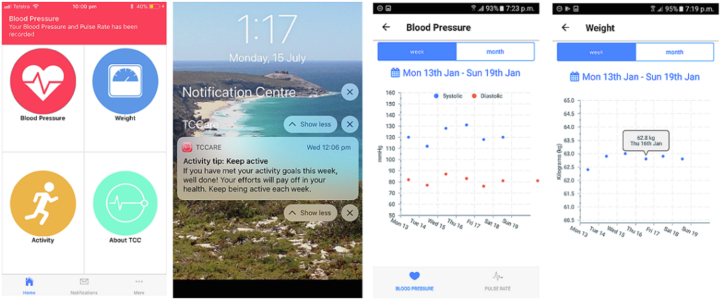
A 2-center RCT published by Indraratna and colleagues4 randomized 164 patients admitted with HF or an acute coronary syndrome to a smartphone app–based model of care applied at discharge (TeleClinical Care [TCC]) (Figures 1 and 2), with control arm patients receiving usual care alone. Patients were enrolled during the index admission and required a compatible smartphone to be included. Patients assigned to the intervention arm received a digital sphygmomanometer, a weighing scale, and a fitness band, with instructions to perform physiological measurements daily. Readings were automatically transmitted from the peripheral devices to the smartphone app via Bluetooth and subsequently to a web-based server. If a reading returned outside of the defined limits, an alert was delivered by email to the monitoring team. Upon reviewing an alert, the monitoring clinician would decide whether to contact the patient and, upon doing so, assess whether the alert required escalation to the patient’s general practitioner or cardiologist. The app also provided educational push notifications 3 times per week to promote healthy behavior choices, including dietary advice, physical exercise, and smoking cessation. Over a mean follow-up of 193 days, the intervention was associated with a significant reduction in unplanned hospital readmissions (21 in TCC vs 41 in the control arm; P = .02), including cardiac readmissions (11 in TCC vs 25 in the control arm; P = .03) and higher completion rates of traditional face-to-face cardiac rehabilitation (20/51, 39% vs 9/49, 18%; P = .03) and medication adherence (57/76, 75% vs 37/74, 50%; P < .002). It was surmised that the use of Bluetooth technology to transmit recordings, as well as the use of an alert system to automatically identify abnormal readings, contributed to the success of the program, as the work burden for both patients and clinicians was streamlined.
Figure 1.
An example of the TeleClinical Care (TCC) smartphone app. From left to right: The TCC app home screen; the appearance of an educational notification; weekly record of blood pressure recordings and weight recordings.
Figure 2.
Examples of Bluetooth-enabled peripheral devices used in TeleClinical Care. From left to right: Sphygmomanometer (A&D Medical UA-651BLE), weighing scale (A&D Medical UC-352BLE), and activity monitor (Xiaomi MiBand2).
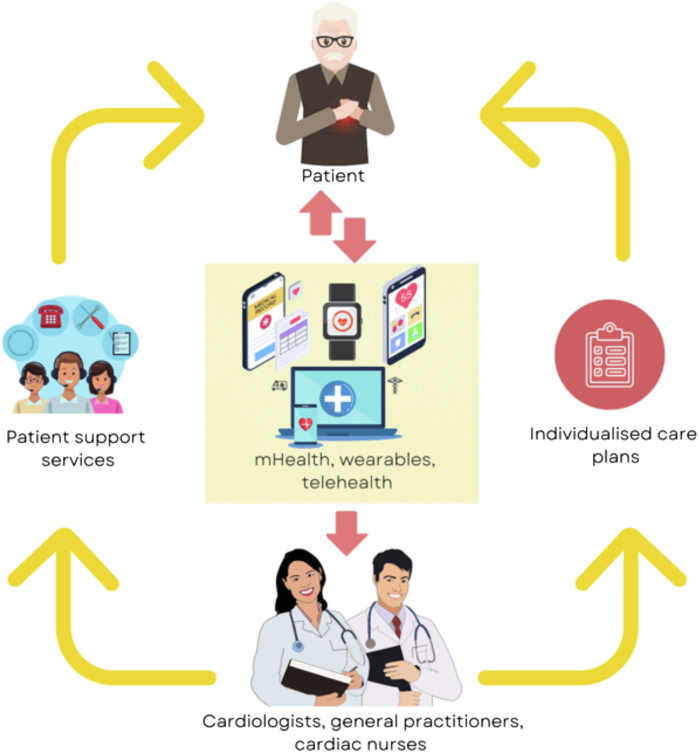
Multiple forms of noninvasive mobile digital technology are now available to assist in the optimal management of HF patients, such as teleconsultations, SMS systems, smartphone applications, wearables, and remote monitoring systems. Digital technology that incorporates clinical data recording, combined with clinician feedback and structured follow-up, appear to be more efficacious. These interventions have promise as part of the ecosystem of HF care, integrated into existing models of care, linked with a patient’s usual HF nurses, general practitioners, and cardiologists rather than as standalone interventions (Figure 3).
Figure 3.
The digital health ecosystem, illustrating the interaction between cardiac patients, digital technology, and clinicians.
Coronary artery disease
Coronary artery disease remains a leading cause of morbidity and mortality and a substantial economic burden globally. mHealth technology has huge potential in targeting behavior change in the primary and secondary prevention of coronary artery disease. However, compared to HF, evidence supporting a reduction in major adverse cardiac events, hospital readmission, and mortality is relatively lacking.
An early pivotal RCT on mobile phone technology in targeting risk factor modification in the prevention of coronary artery disease was the Tobacco, Exercise and Diet Messages (TEXT ME) trial published by Chow and colleagues in 2015.22 A total of 352 patients received 4 motivational text messages per week during daylight hours over a 6-month period. The messages focused on secondary prevention strategies. The primary endpoint was low-density lipoprotein cholesterol levels, which were lower in the intervention group (79 mg/dL vs 84 mg/dL; P = .04). Several other endpoints were examined, and significant improvements in systolic BP (−7.6 mm Hg; P < .001), physical activity, and smoking rates (88/339, 25.9% vs 152/354, 42.9%; P < .001) were noted. Differences in mortality and myocardial infarction were not measured. The majority of patients found the intervention motivational, educational, and useful.
More recently, in 2021, a single-center RCT assessed the effect of lifestyle intervention using mobile technology on 483 patients with high cardiovascular risk as indicated by a 10-year ASCVD (atherosclerotic cardiovascular disease) risk score ≥7.5%.23 Patients were randomly allocated in a 1:1 ratio to either the intervention plus usual care or the usual care arm. The participants randomized to the intervention group received a set of smart devices, namely a smartphone, wristband, scale, and BP monitor. The participants entered daily data regarding their diet, BP, weight, and step count in an application compatible with their smartphones. Motivational messages were delivered daily to encourage patients toward a healthy lifestyle. The entered data were tracked from the main server, and automatic messages were sent to noncompliant patients to invite them for outpatient review. After 1-year follow-up, the intervention reduced the ASCVD score by 2.7% (adjusted treatment effect -2.7, 95% CI -2.2 to -3.3; P < .0001). An improvement was observed in favor of the intervention arm in the majority of the prespecified secondary endpoints, including smoking cessation, BP, body mass index, blood lipids, triglycerides, and glycated hemoglobin (HbA1c) levels. Again, clinical endpoints such as myocardial infarction, mortality, and hospital readmission were not assessed.
Mobile digital technology has also been found to be effective in risk factor management in the secondary prevention of coronary artery disease. A recent single-center RCT published in 202224 randomized 290 patients with coronary artery disease to receive conventional care or conventional care and a smartphone app for self-management. Patients were enrolled during admission to a tertiary hospital in China and required a diagnosis of coronary artery disease, as defined by a previous myocardial infarction, coronary artery bypass graft surgery, percutaneous coronary intervention, or ≥50% stenosis in at least 1 major epicardial vessel on coronary angiography. The intervention group received a self-management mobile app that contained 3 modules. The first was a discharge module, where medication, patient education material and instructions, lifestyle intervention plan, and follow-up plans were integrated into the app according to diagnosis prior to discharge. The second module was the home management module. An electronic sphygmomanometer was given to all participants, with BP and heart rate data transferred through Bluetooth connection to the app. In addition, an automatic alarm was set up in the patient portal to help manage the patient’s daily medication regimen. The third module was the follow-up module, which had a dynamic design and dashboard overview displaying the latest discharge summaries, vital signs, symptoms, and medications, allowing physicians to update medication and lifestyle plans during each follow-up. At 12 months follow-up there was a statistically significant improvement in the percentage of all guideline-recommended medications in the intervention group compared with the control group (RR 1.34, 95% CI 1.12–1.61; P < .001). The intervention group also had a significantly higher proportion of patients achieving BP targets (systolic BP <140 mm Hg and diastolic BP <90 mm Hg, RR 1.45, 95% CI 1.22–1.72; P < .001) and low-density lipoprotein targets <1.8 mmol/L (RR 1.40, 95% CI 1.11–1.75; P = .004) at 12 months.
Unlike remote monitoring in HF, which focuses on the collection of physical parameters to warn clinicians of impending clinical deteriorations, allowing early treatment and the prevention of hospitalization,16 remote monitoring in coronary artery disease has focused mainly on risk factor modification. A potential reason for this is that coronary artery disease often progresses over a longer time period, without the development of physiological changes that can be easily detected by remote monitoring until an acute event occurs. Also, the likelihood of repeat acute coronary syndrome is much lower than that of repeat HF exacerbation requiring hospitalization. Repeat acute coronary syndrome occurs after many years of suboptimal BP, exercise, cholesterol, and diabetes control, and therefore the yield has been lower in terms of reducing cardiovascular events and hospitalization in trials thus far that have had relatively short follow-up. Although recent trials demonstrate there is growing evidence of the effectiveness of mobile digital health technology in reducing risk factors for coronary artery disease, whether this translates to a reduction in clinical endpoints is yet to be determined. Further adequately powered studies with longer follow-up are needed to support the routine use of mobile health–based interventions in secondary preventative care. A recently published study protocol25 outlines such a trial, which aims to recruit a total of 2820 patients and follow them for 3 years to assess whether mHealth-based individualized interventions could reduce the incidence of major cardiovascular events in patients with established coronary artery disease.
Future directions
Successful integration of mHealth into routine HF and ischemic heart disease care presents many challenges. Technology innovation has outpaced the ability of clinicians and health systems to incorporate the infrastructure for optimal use of data. Although many mHealth technologies may generate sufficient data for clinical action, challenges remain associated with collating, analyzing, interpreting, and responding to data.26 Some of these challenges require significant investments that are not possible for all health systems and will limit generalizability. For example, data from digital health tools are infrequently operable with current electronic health records.27 Future research agendas should consider access and usability of digital health technology for patients; system integration and harmonization for provider workflow; and technology costs and healthcare utilization.24 The longest minimum follow-up period for the above-mentioned studies in both HF and coronary artery disease study was 12 months. A longer duration of monitoring may have allowed for a difference in readmission rates and mortality to have been identified, particularly for patients with acute coronary syndromes, which may take many years to recur after suboptimal risk factor management. As telehealth is a rapidly evolving field, it is hypothesized that many studies were published with a short follow-up period to avoid publication when the intervention is outdated or obsolete. Further vigorous high-quality large RCTs with longer-term follow-up on clinical endpoints such as hospital admissions, mortality, and major cardiovascular events, as well as evidence of cost-effectiveness, will be needed before there is widespread government and private insurance reimbursement for novel digital technologies. Owing to the heterogeneity in the field, published RCTs should also be accompanied by process evaluations that demonstrate the mechanism of any perceived clinical benefit, and also may allow similar models of care to be adapted to different healthcare systems. Despite these shortcomings, the recent COVID-19 pandemic has created unprecedented challenges to healthcare delivery resulting in an urgent need to find digital health solutions to patient management that do not require direct physical patient-clinician interaction. This has resulted in a recent rapid proliferation in digital medicine as well as increased acceptance of mHealth by patients, clinicians, and payers, with some insurance providers in the United States now offering incentives for purchasing wearables such as Apple iWatch and Fitbit. As technology and innovation continues, the integration of artificial intelligence software and personal voice assistants (Alexa by Amazon or Siri by Apple) and interactive social robots (Jibo) into mobile apps has the potential to influence the collecting, collating, and analyzing of data as well as improved usability of mHealth technology for patients in the future. As smartphone and device hardware becomes more advanced and the mobile interface becomes more intuitive and immersive, it is possible that mHealth utilization to supplement traditional in-person care may eventually become the norm rather than the exception. This poses important future considerations, as rural and homeless patients are less likely to have reliable internet access28 and both older patients and people of lower socioeconomic status are less likely to own smartphones29 and have the digital literacy to use the technology, highlighting the importance of healthcare policy overcoming barriers to equitable access to digital healthcare in the future to minimize the risk of this contributing to a further healthcare divide.
Conclusion
Multiple RCTs have now demonstrated that various modes of digital health technology can influence patient behaviors in both the primary and secondary prevention of coronary artery disease and prevention and management of HF. As technologies continues to develop, there will be further opportunity to develop and deliver novel models of remote healthcare to cardiac patients that are cost-effective, widely accessible, and individualized. These same systems will also be able to promote and support patient self-care through empowering the user with information pertaining to their health condition. The integration of digital health solutions into existing models of care, such as collaborative shared-care models between cardiac nurses, general practitioners, and cardiologists,25 remains the key to improving acceptability, increasing reach, and improving system efficiencies and cost-effectiveness. These facets are the key to the widespread adoption of digital health solutions.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
References
- 1.World Health Organization The Top 10 Causes of Death. 2019. https://www.who.int/news-room/fact-sheets/detail/ the-top-10-causes-of-death
- 2.Piepoli M., Hoes A., Agewall S., et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnett D., Blumenthal R., Albert M., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Indraratna P., Biswas U., McVeigh J., et al. A smartphone-based model of care to support patients with cardiac disease transitioning from hospital to the community (TeleClinical Care): pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10 doi: 10.2196/32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monitoring and Evaluating Digital Health Interventions: A Practical Guide to Conducting Research and Assessment. World Health Organization; Geneva: 2016. [Google Scholar]
- 6.Keesara S., Jonas A., Schulman K. COVID-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82. doi: 10.1056/NEJMp2005835. [DOI] [PubMed] [Google Scholar]
- 7.Statista Number of smartphone users worldwide from 2016 to 2022 (in billions) https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/
- 8.Wade V., Stocks N. The use of telehealth to reduce inequalities in cardiovascular outcomes in Australia and New Zealand: a critical review. Heart Lung Circ. 2017;26:331–337. doi: 10.1016/j.hlc.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Kotb A., Cameron C., Hsieh S., Wells G. Comparative effectiveness of different forms of telemedicine for individuals with heart failure: a systematic review and network meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indraratna P., Tardo D., Yu J., et al. Mobile phone technologies in the management of ischemic heart disease, heart failure, and hypertension: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmarajan K., Wang Y., Lin Z., Normand S., Ross J., Horwitz L. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318:270–278. doi: 10.1001/jama.2017.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virani S., Alonso A., Benjamin E., Bittencourt M., Callaway C., Carson A. Heart disease and stroke statistics - 2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal M., Fonarow G., Ziaeian B. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol. 2021;6:952–956. doi: 10.1001/jamacardio.2020.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roger V., Weston S., Redfield M., et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 15.Granger B., Ekman I., Hernandez A., Sawyer T., Bowers M.T., DeWald T.A. Results of the chronic heart failure intervention to improve medication adherence study: a randomized intervention in high-risk patients. Am Heart J. 2015;169:539–548. doi: 10.1016/j.ahj.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coorey G.M., Neubeck L., Mulley J., Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol. 2018;25:505–521. doi: 10.1177/2047487317750913. [DOI] [PubMed] [Google Scholar]
- 17.Kitsiou S., Vatani H., Paré G., et al. Effectiveness of mobile health technology interventions for patients with heart failure: systematic review and meta-analysis. Can J Cardiol. 2021;37:1248–1259. doi: 10.1016/j.cjca.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Koehler F., Koehler K., Deckwart O., et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–1057. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 19.Koehler F., Winkler S., Schieber M., et al. Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 20.Ong M., Romano P., Edgington S., et al. Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition -- Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C., Li X., Sun L., et al. Post-discharge short message service improves short-term clinical outcome and self-care behaviour in chronic heart failure. ESC Heart Fail. 2019;6:164–173. doi: 10.1002/ehf2.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow C., Redfern J., Hillis G., Thakkar J., Santo K., Hackett M. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. J Am Med Assoc. 2015;314:1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 23.Tekkeşin A., Hayıroğlu M., Çinier G., et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: a pragmatic randomised clinical trial. Atherosclerosis. 2021;319:21–27. doi: 10.1016/j.atherosclerosis.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Gong Y., Zheng B., et al. Effects on adherence to a mobile app-based self-management digital therapeutics among patients with coronary heart disease: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10 doi: 10.2196/32251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y., Ji M., Wu Y., Deng Y., Wu F., Lu Y. Individualized mobile health interventions for cardiovascular event prevention in patients with coronary heart disease: study protocol for the iCARE randomized controlled trial. BMC Cardiovasc Disord. 2021;21:340. doi: 10.1186/s12872-021-02153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVore A., Wosik J., Hernandez A. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7:922–932. doi: 10.1016/j.jchf.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Narla A., Paruchuri K., Natarajan P. Digital health for primary prevention of cardiovascular disease: promise to practice. Cardiovasc Digit Health J. 2020;1:59–61. doi: 10.1016/j.cvdhj.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsetty A., Adams C. Impact of the digital divide in the age of COVID-19. J Am Med Inform Assoc. 2020;27:1147–1148. doi: 10.1093/jamia/ocaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indraratna P., Magdy J., Li J., et al. Patterns and predictors of smartphone ownership in a cardiology inpatient population. Eur Heart J. 2021;42 ehab724.3111. [Google Scholar]