Abstract
Schizophrenia is a severe neuropsychiatric syndrome with psychotic behavioral abnormalities and marked cognitive deficits. It is widely accepted that genetic and environmental factors contribute to the onset of schizophrenia. However, the etiology and pathology of the disease remain largely unexplored. Recently, the synaptopathology and the dysregulated synaptic plasticity and function have emerging as intriguing and prominent biological mechanisms of schizophrenia pathogenesis. Synaptic plasticity is the ability of neurons to change the strength of their connections in response to internal or external stimuli, which is essential for brain development and function, learning and memory, and vast majority of behavior responses relevant to psychiatric diseases including schizophrenia. Here, we reviewed molecular and cellular mechanisms of the multiple forms synaptic plasticity, and the functional regulations of schizophrenia-risk factors including disease susceptible genes and environmental alterations on synaptic plasticity and animal behavior. Recent genome-wide association studies have provided fruitful findings of hundreds of risk gene variances associated with schizophrenia, thus further clarifying the role of these disease-risk genes in synaptic transmission and plasticity will be beneficial to advance our understanding of schizophrenia pathology, as well as the molecular mechanism of synaptic plasticity.
Keywords: Synaptic plasticity, Neurotransmission, Schizophrenia, Neuropsychiatric disease
1. Introduction
Neurons are the structural and functional units of the central nervous system, which connect and communicate with each other through synapse, a intercellular junction transferring information from presynaptic neurons to postsynaptic cells (Südhof, 2018). This kind of connection is not invariable, but can be modulated, which called synaptic plasticity. Synaptic plasticity describes the morphological and functional changes in preexisting synapses of the nervous system in response to internal and external environmental stimuli (Citri and Malenka, 2008, Cramer et al., 2011). These activity-dependent modifications are essential for brain development, neuronal circuits establishment and synaptic transmission. Dysregulated synaptic plasticity has been involved in pathophysiology of numerous neuropsychiatric disorders (Stampanoni Bassi et al., 2019), such as schizophrenia, bipolar disorder, and major depressive disorder.
Schizophrenia is a severe psychiatric disorder affecting basic brain processes, such as thinking, perception and emotion (Kahn et al., 2015, Marder and Cannon, 2019). Patients with schizophrenia suffer from positive symptoms (hallucination, delusion, confused thoughts, disorganized speech, trouble of concentration), negative symptoms (anhedonia, alogia, social withdrawal, blunted affect, avolition), and abnormal cognition (Birnbaum and Weinberger, 2017). Typically, symptoms emerge at adolescence or early adulthood.
Researches on schizophrenic pathology mechanisms have been focused on brain connectivity alteration, anatomically manifested as structural changes of nerve fibers, functionally manifested as synaptic plasticity dysregulation. It is acknowledged that synaptic plasticity is involved in the onset of schizophrenia (Stephan et al., 2006), but the neuropathophysiology is not quite clear yet. In this review, we focus on the relationship between synaptic plasticity and pathology of schizophrenia. We hope to unravel the neurobiological basis of schizophrenia by summarizing multiple forms and mechanisms of synaptic plasticity, and schizophrenia animal models with deficient synaptic function and plasticity.
2. Etiology of schizophrenia
Genetics and epidemiological investigations have confirmed that both genetic and environmental factors contribute to schizophrenia etiology. Twin studies of schizophrenia suggest that genetic factors account for about 85 % of schizophrenia (Tsuang, 2000). However, schizophrenia is not caused by a single risk gene (Trubetskoy et al., 2022), and it does not show a simple pattern of inheritance. Each genetic variances result in only a small increase in risk. Until the threshold level is reached and clinical symptoms appear. Moreover, environmental influences during prenatal and postnatal brain development or across adulthood, such as uterine infection or pregnancy complications, psychosocial causes, amphetamine abuse, autoimmune disease and other brain trauma, also affect the risk of schizophrenia.
3. Synaptic plasticity deficit is common pathological mechanism in schizophrenia
Heterogenous and complexity of schizophrenia pathology poses a great challenging to diagnosis and therapeutic intervention of the disorder. Unlike Alzheimer's and Parkinson’s disease that display pathological inclusion and neuron loss, schizophrenia lacks notable characteristic and hallmark in pathological change. Imaging studies (CT and Magnetic resonance examination) revealed the decrease in neocortical and hippocampal volumes, as well as enlarged ventricles in patients with schizophrenia (Harrison, 1999, Woods et al., 1996). Although structural abnormalities such as neuron loss in several brain regions have been revealed by postmortem studies, the results are fairly in consistence. For example, cortical loss is at least partly due to a loss of nerve fibers, including synapses, rather than neuron loss (Harrison, 1999).
Thus far, several lines of evidence have suggested that synaptic deficits including abnormal synaptic transmission and plasticity might be essential neuropathological feature of schizophrenia. Earlier neurochemical and recent genetic findings have implicated that glutamate, dopamine and GABA neurotransmitter dysfunction underlies the neurobiological basis of schizophrenia (Birnbaum and Weinberger, 2017), thereby leading to the hypothesis that excess or insufficient synaptic transmission might be related to pathology of the illness. Neuro-anatomical analysis of postmortem brain also shows that density of dendritic spines that are essential postsynaptic structure of neurons, is lower in cortical tissues from schizophrenia patients than that in controls, especially in layer 3 of the neocortex (Glausier and Lewis, 2013). Despite synapse loss, molecular alterations related to disturbance in synaptic function and plasticity have been implicated in schizophrenia. Indeed, large-scale gene expression examination indicates that presynaptic protein synaptophysin levels are decreased in the hippocampus, frontal cortex, and cingulate cortex in schizophrenia patients, while synaptic protein SNAP − 25, synapsin, rab3A, and PSD-95 are also reduced in the hippocampus in schizophrenia patients (Osimo et al., 2019). Therefore, understanding the disease-associated synaptic deficiency and the underlying mechanism are critical to elucidate the pathogenesis of schizophrenia and to develop new therapeutic interventions for the illness.
4. Molecular and cellular mechanism of synaptic plasticity
Synaptic plasticity, one of the most important properties of brain, refers to the ability of neurons to modify their connections in response to extrinsic stimuli. Neurons connect and exchange information through synapses, which are consist of presynaptic terminal, postsynaptic specialization or dendrite or cell (neuron) and a synaptic cleft between them. From birth to adulthood, the development of the brain involves the activity-dependent refinement of synapses and the construction of synaptic circuitry (Forrest et al., 2018). These modifications of the strength or efficacy of synaptic transmission during development and aging play central roles in brain functions such as thinking, perception, learning and memory, and have been widely recognized as a fundamental mechanism by which information is encoded and stored in the brain. Synaptic plasticity includes Hebbian plasticity that describes co-activity between neuron networks and homeostatic plasticity that acts as a compensative process to counterbalance the alteration of neuron network (Andersen et al., 2017, Zenke et al., 2017).
4.1. Short-term synaptic plasticity
Short-term synaptic plasticity lasts from milliseconds to minutes, which is important for the formation of short-term memory and transient behavioral response to environment (Citri and Malenka, 2008, Zucker and Regehr, 2002). Paired-pulse facilitation and depression, that are induced by delivery of two stimuli within a short interval, as well as post-tetanic potentiation (PTP) and depression, that are trigged by trains of stimulation, are several major forms of short-term synaptic plasticity. The key event for induction of facilitation in most forms of short-term plasticity is the rapid increase of presynaptic calcium level, which alters the probability of neurotransmitter release (Citri and Malenka, 2008). Transient depletion of readily releasable pool of synaptic vesicles or inactivation of voltage-dependent sodium or calcium channels might contribute to the depression at many synapses. In addition, desensitization of post-synaptic ligand-gated receptors and glia-neuron interaction are also involved in the mechanism of some forms of short-term synaptic plasticity (Araque et al., 1998, Haydon, 2001, Otis et al., 1996, Trussell et al., 1993).
4.2. Long-term synaptic plasticity
Long-term potentiation (LTP) and long-term depression (LTD) are two forms of Hebbian plasticity that biologically correlates for Hebb’s algorithm about network connection and contributes to structural and functional plasticity. In contrast to short-term synaptic plasticity, these two types of synaptic plasticity can last for hours or even days, and are recognized as essential mechanism for long-term memory storage.
LTP is the persistent increase of synaptic strength between neurons (Bliss and Gardner-Medwin, 1973). The most extensively studied forms of synaptic plasticity is NMDAR-dependent LTP (NMDAR-LTP) that can be induced by various protocols, such as high-frequency tetanic stimulation or ‘pairing protocol’ in which postsynaptic depolarization is coupled with low-frequency synaptic activation to stimulate pre-synaptic neurons, increasing the efficiency of synaptic transmission and excitability of post-synaptic neurons (Abraham and Huggett, 1997, Bliss and Lomo, 1973). LTP induction leads to calcium influx through NMDAR and an increase of calcium in dendritic spines, thereby activating intracellular signaling pathways and enrichment of AMPARs into the postsynaptic membrane surface (Diering and Huganir, 2018), which is vital for long-term learning (Lu et al., 2017, Malleret et al., 2010, Penn et al., 2017), memory consolidation and cognitive processes (Machado et al., 2018, Ren et al., 2020, Zhang et al., 2018). The activation and accompanied phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) are key molecule processes mediating post-synaptic mechanism of NMDAR-LTP. Firstly, phosphorylated CaMKII (pCaMKII) activates protein kinase A to stimulate the new synthesis of AMPARs through the cAMP-response-element-binding (CREB) protein pathway (Barco et al., 2002, Lisman et al., 2018, Wu et al., 2007). Secondly, pCaMKII phosphorylates postsynaptic scaffold protein and enhances endophilin A1 binding to the membrane, promoting the polymerization of actin to initiate the expansion of dendritic spines (Yang et al., 2021), which establish harbors for AMPARs. Thirdly, pCaMKII catalyzes the phosphorylation of AMPAR at residue Ser831 and Ser845 of the GluA1 subunit, increases its conductance and single channel open–probability, promotes AMPAR to locate to membrane surface (Lisman and Raghavachari, 2015, Lisman et al., 2012, Sanhueza et al., 2011). LTP is considered to be strongly facilitated with learning behavior, while is suppressed when exposed to a hazardous environment associated with impaired learning and memory performance (Jorgenson et al., 2005, Xiao et al., 2016, Zhang et al., 2021).
LTD is an prolonged decrease of synaptic transmission between neurons which can be triggered by repetitive low-frequency stimulations (LFS) (Mulkey and Malenka, 1992). NMDAR-dependent LTD induced at hippocampal Schaffer collateral-CA1 synapses is a major form of LTD that has been studied most extensively. The canonical mechanism of NMDAR-LTD induction in hippocampus involves calcium influx, activation of NMDAR, and the subsequent activation of a calcium-dependent protein phosphatase cascade. Moderate Ca2+ enters through NMDAR to activate CaM-dependent serine/threonine phosphatase calcineurin (CaN, also known as PP2B), which rescinds the inhibition to inhibitor-1 and in turn activates protein phosphatase 1 (PP1) (Collingridge et al., 2010, Mulkey et al., 1993). PP1 dephosphorylates various substrates crucial for expression of NMDAR-LTD, such as Ser845 of the GluA1 subunit and Ser295 of postsynaptic density protein 95 (PSD95) (Collingridge et al., 2010, Kim et al., 2007, Lee et al., 2000). Contrary to LTP, the main mechanism of LTD is CaN-mediated removal and endocytosis of AMPAR (Diering and Huganir, 2018). In addition, researches prove that transient recruitment and removal of Ca2+-permeable AMPA receptors mediated by AKAP150, PKA and CaN are necessary to LTD (Sanderson et al., 2016). LTD is known as a mechanism mediating synapse weakening in order to reconstruct useful synapse connection for LTP then to restore new information (Malleret et al., 2010, Stanton, 1996). LTD in hippocampus has been linked to cognitive process (Collingridge et al., 2010, Durkee et al., 2021, Navarrete et al., 2019). Compared with LTP, there are lots of blind spots to be figured out about how LTD regulate learning and memory.
4.3. Homeostatic plasticity
Homeostatic plasticity refers to that neuron has the ability to maintain an appropriate excitability to adapt to sustained alteration of network activity (Surmeier and Foehring, 2004). Scaling up and scaling down are two forms of homeostatic plasticity, which are thought to counterbalance LTP and LTD in a negative feedback loop. Activity-dependent accumulation or dispense of AMPA receptors has been recognized as important mechanism of postsynaptic expression of homeostatic plasticity. Scaling up increases AMPARs expression and location while scaling down reduces AMPARs on the membrane (Diering and Huganir, 2018), both of which rely on PSD95- and MAGUKs (membrane-associated guanylate kinases)-mediated protein-protein interaction (Sun and Turrigiano, 2011) and phosphorylation of synaptic proteins that involved in cytoskeleton remodeling (Desch et al., 2021, Diering et al., 2017, Martin et al., 2019).
5. Schizophrenia risk genes play essential roles in synaptic plasticity
Earlier candidate risk gene studies have identified several schizophrenia susceptibility genes, which have been extensively investigated for their functional roles in synaptic plasticity and function. These large body of works have significantly advanced our understanding of the pathological mechanism of the disease. Moreover, accumulating data obtained from genome wide association studies (GWAS) continuously decode schizophrenia risk genes, resulting in the discovery of hundreds of disease-associated variants (Schizophrenia Working Group of the Psychiatric Genomics, C., 2014, Trubetskoy et al., 2022). Here we reviewed neurobiological studies that dissecting the roles of these schizophrenia risk genes in synaptic plasticity and function, as well as in animal behavior, hoping to provide mechanistic insight into schizophrenia pathophysiology.
5.1. 22q11.2 microdeletion
22q11.2 microdeletion syndrome (22q11.2DS) is caused by the hemizygous deletion of a 1.5–3 Mb region on chromosome 22, which carries one of the most significant genetic risk of schizophrenia (Karayiorgou et al., 1995, Karayiorgou et al., 2010). Individuals with 22q11.2DS display a spectrum of cognitive deficits, and ∼30 % of the patients develop schizophrenia in adolescence or early adulthood (Swillen et al., 2000). Several animal models have been generated to mimic 22q11.2DS, which carries hemizygous deletion of different subset of genes in the mouse chromosome 16 that are syntenic to human 22q11.2 region. These mice display core behavioral abnormalities observed in schizophrenia patients, such as impaired sensorimotor gating function and learning and memory (Devaraju et al., 2017, Fénelon et al., 2013, Paylor and Lindsay, 2006, Paylor et al., 2001, Stark et al., 2008). Moreover, abnormal or abnormally enhanced short-term and long-term plasticity in hippocampus and prefrontal cortex are frequently observed in these animal models (Devaraju et al., 2017, Diamantopoulou et al., 2017, Earls et al., 2010).
It is worth noting that studies have revealed essential effective genes contributing to the abnormal synaptic plasticity in 22q11.2DS animal models. For example, pharmacological inhibition of SERCA, a sarco(endo)plasmic reticulum Ca(2 +) ATPase, restores the enhanced LTP in hippocampus of Df(16)1+/− and Df(16)2/+/− mice (Earls et al., 2010, Earls et al., 2012); loss of function mutation of Mirta22, which rescues abnormal short-term potentiation (STP) and LTP in prefrontal cortex of Df(16)A+/− mice (Diamantopoulou et al., 2017); hemizygous deletion of Mrpl40 contributes to STP and working memory deficits in Df(16)5+/− mice, another animal model of 22q11.2DS, through dysregulation of mitochondria calcium (Devaraju et al., 2017). In addition, Dgcr8 (a gene disrupted by the 22q11.2 microdeletion) heterozygous mutant mice display smaller spines in the basal dendrites of layer 5 pyramidal neurons of the prefrontal cortex, but enhanced short-term synaptic depression (STD) in the same region (Fenelon et al., 2011). DGCR8 is essential for microRNA biogenesis. Earls et al. reported that haploinsufficiency of Dgcr8 leads to upregulation of SERCA and increased LTP, which were restored by presynaptic expression of miR-25 and miR-185 that regulates SERCA expression (Earls et al., 2012). Another 22q11.2 DS mouse model, Lgdel+ /− , display embryonic-premature alterations in the neuronal chloride cotransporters and show dysregulated network homeostatic plasticity and synchronous activity in hippocampus that might be attribute by the excitatory/inhibitory imbalance (Amin et al., 2017).
5.2. Neuregulin1
Neuregulin 1 (NRG1) is a trophic factor that has an epidermal growth factor (EGF)-like domain signaling through ErbB receptor (Mei and Xiong, 2008). Genes encoding NRG1 and its predominant receptor on neuron, ErbB4 both confer risk of schizophrenia (Harrison and Weinberger, 2005). NRG1 generates many kinds of isoforms, several of which have been associated with schizophrenia-like behavior in animal model study. For instance, reduced type II neuregulin-1 in rat causes impaired visuospatial learning and working memory; whereas overexpression of type III neuregulin-1, whose expression level is increased in schizophrenia patients, in mice forebrain neurons results in decreased PPI and impaired fear memory (Olaya et al., 2018, Taylor et al., 2012). Moreover, Papaleo et al. reported that neuronal-specific overexpressing a human NRG1-IV isoform, which is increased in brain of schizophrenia individual, leads to schizophrenia-like behaviors, such as impaired PPI, object location memory, and social interaction (Papaleo et al., 2016).
In the past two decades, the major progress has been accomplished in understanding the function of NRG1 in neurotransmission and synaptic plasticity. A serial of elegant works presented that an optimized level of NRG1 signaling is critical for synaptic plasticity. Heterozygous deletion of NRG1 leads to impaired theta-burst LTP in hippocampus (Bjarnadottir et al., 2007). However, bath application of NRG1 on hippocampal slices suppressed tetanus stimulation-induced LTP (Chen et al., 2010). Consistently, Agarwal et al. reported that loss of NRG1 in cortical projection neurons disrupts LTP in hippocampus, while overexpression of the major brain isoform of NRG1, a cysteine-rich domain (CRD)-NRG1 also causes reduced LTP (Agarwal et al., 2014). GABAergic neurotransmission is involved in the regulation of NRG1 on synaptic plasticity. NRG1 deficiency or CRD-NRG1 overexpression in cortical projection neurons results in enhanced GABAergic inhibitory neurotransmission, thereby causing imbalance of excitatory-Inhibitory neurotransmission and altered LTP (Agarwal et al., 2014). Nevertheless, one possibility that NRG1 regulates LTP through an excitatory neuron-intrinsic mechanism cannot be excluded, as a recent study reported that overexpression of NRG1 in forebrain excitatory neuron in ctoNrg1 mice that mimic the upregulated level of NRG1 in schizophrenic patients, results in decreased spine density and impaired glutamatergic neurotransmission in hippocampus CA1 and PFC via activation of LIMK1 and subsequent inactivation of p-cofilin (Chen et al., 2021), which are essential for AMPAR stabilization at synapse and various forms of synaptic plasticity (Ben Zablah et al., 2021, Zhou et al., 2021).
The effect of NRG1 on inhibitory neurotransmission and LTP at hippocampus might be mediated by signaling through its receptor ErbB4 on PV-positive interneurons. ErbB4 mutation in parvalbumin (PV)-positive interneurons or acutely blocking ErbB4 enhances hippocampal LTP, suggesting that ErbB4 is a suppressor of LTP (Pitcher et al., 2008, Shamir et al., 2012). Moreover, deletion of ErbB4 in PV-positive interneurons eliminates the effect of NRG1 on increased inhibitory postsynaptic currents and suppression of LTP, while ablation of ErbB4 in pyramidal neurons has no effect (Chen et al., 2010). In addition, neuronal vesicular protein calcyon is a potent activator of NRG1 ecto-domain cleavage and overexpression of calcyon stimulates NRG1 cleavage, resulting in enhanced GABA transmission; conversely, NRG1 cleavage, ErbB4 activity and GABA transmission are decreased in calcyon null mice (Yin et al., 2015). Collectively, NRG1 activates ErbB4 in PV-positive interneurons and provokes GABA release, thereby increasing inhibitory neurotransmission and leading to diminished hippocampal LTP.
The above studies on NRG1/ErbB4 regulation of LTP mainly focused on PV-positive interneurons, however, the effect of NRG1/ErbB4 signaling on other types of interneurons remain largely elucidated. Endocannabinoids (eCBs) are implicated in the regulation of synaptic plasticity, cognition, and emotion (Castillo et al., 2012, Zanettini et al., 2011); eCBs system dysfunction is involved in schizophrenia (D'Souza et al., 2005, Giuffrida et al., 2004). The type 1 cannabinoid receptor (CB1R) is expressed in Cholecystokinin (CCK)-, but not PV-positive interneurons, suggesting an unique function of eCBs signaling in CCK-positive interneurons. Du et al. revealed that prolonged treatment of cultured rat hippocampal slices with NRG1 increased the expression of monoacylglycerol lipase (MGL), resulting in degradation of 2-arachidonolyglycerol (2-AG, one of the major eCBs) and impaired 2-AG-dependent long-term depression of inhibitory synapses (Du et al., 2013). In addition to hippocampus and PFC, amygdala is important brain region for emotional behavior and memory associated with emotion. Jiang et al. investigated the role of NRG1 in cortical glutamatergic inputs onto pyramidal neurons in basolateral nucleus of the amygdala, and found that NRG1 is also essential for multiple forms of synaptic plasticity in cortico-amygdala circuits (Jiang et al., 2013).
5.3. Disrupted-in-schizophrenia1
Disrupted-in-schizophrenia 1 (DISC1) was first identified in a large Scottish family with a high loading of major mental illness (St Clair et al., 1990). The t(1; 11)(q42;q14.3) chromosomal translocation that disrupts DISC1 gene cosegregates with schizophrenia and a wide range of major mental disorders (Blackwood et al., 2001). Several mouse models carrying different DISC1 mutations have been generated to explore the function of DISC1 on schizophrenia pathology. Disc1Tm1Kara mice, which contain a truncation of murine Disc1 that mimic the effects of the (1;11) translocation exhibits working memory deficits, a behavioral feature observed in individuals with schizophrenia (Kvajo et al., 2008, Kvajo et al., 2011). These mice showed aberrant short-term plasticity, but not LTP at Schaffer collateral and mossy fiber circuit in hippocampus and medial prefrontal cortex (Crabtree et al., 2017, Kvajo et al., 2008, Kvajo et al., 2011), which might be largely attributed by dysregulated PDE4, cAMP, and voltage dependent potassium channel subunit Kv1.1 (Crabtree et al., 2017, Kvajo et al., 2011). It is known that DISC1 binds directly with Pde4b and Gsk3β, two molecules involved in schizophrenia pathology. DISC1 L100P mutation affects its interaction with these two proteins, leading to deficits in working memory and object recognition memory in mice (Clapcote et al., 2007, Cui et al., 2016). Decreased synaptic transmission at DG and diminished LTP at CA1 were observed in DISC1 L100P homozygous mice (Cui et al., 2016). Moreover, Disc1 L100P mutants have defects to reorganize cortical circuitry in vivo and have impaired functional reorganization of cortical neurons in vitro in response to stimulation (Tropea et al., 2016). In addition, transiently disruption of DISC1’s interaction with signaling molecules Lis1 and Nudel during early development causes decreased AMPA/NMDA ratio and inhibited LTP in cortical layer 2 and 3 in adulthood (Greenhill et al., 2015). N-methyl-D-aspartate receptors (NMDAR) play a critical role in synaptic plasticity and cognition. It has been shown that DISC1 interacts with GluN1 subunit to regulate dendritic NMDAR motility in cultured mice neurons (Malavasi et al., 2018). Notably, DISC1 might interact with other schizophrenia susceptibility factors. it was shown that NRG1 and DISC1 link directly into a common pathway mediated by Erb (ErbB2 and ErbB3) receptors and P13K/AKT1 signaling (Seshadri et al., 2010). These findings suggest that NRG1, ErbBs and DISC1 synergistically regulates neurodevelopment to contribute to schizophrenia pathology.
5.4. Dystrobrevin binding protein1
DTNBP1 (dystrobrevin binding protein 1) encodes a coiled-coil-containing protein commonly called dysbindin-1, one of the subunits of biogenesis of lysosome-related organelles complex 1 (BLOC-1) involved in protein trafficking. DTNBP1 is associated with increased risk of schizophrenia and its mRNA and protein level are reduced in postmortem brain from schizophrenia patient (Straub et al., 2002, Talbot et al., 2004, Weickert et al., 2008). Majority of the functional investigations on DTNBP1 were performed on Sandy (Sdy) mice that have a nature loss of function mutation in DTNBP1 gene resulting in no dysbindin-1 protein expression. Sdy mice showed an overactivation of dopamine D2 receptor (D2R), and the subsequent spine deficiency, dysconnectivity in the entorhinal-hippocampal circuit and impaired working memory (Jia et al., 2013). Moreover, loss of dysbindin-1 in Sdy mice leads to impaired group 1 metabotropic glutamate receptor (mGluRI)-extracellular signal regulated kinase 1/2 (ERK1/2) signaling and reduced mGluRI-dependent LTD at CA1 synapses (Bhardwaj et al., 2015). Notably, enhancing mGluR5 transmission with CDPPB ameliorates deficits in short-term novel object recognition memory in Sdy mice (Bhardwaj et al., 2015). Sdy mice also displayed increased surface expression of NR2A in hippocampal neurons and enhanced hippocampal LTP, but intact basal synaptic transmission, presynaptic properties, and LTD (Tang et al., 2009). In addition, dysbindin also contributes to cortical interneuron development and network activity. Loss of dysbindin-1 resulted in increased D2 signaling and decrease in the excitability of fast-spiking GABAergic interneurons in layer V of PFC (Ji et al., 2009); dysbindin-1 mutation reduced the exocytosis of BDNF from cortical excitatory neurons, decreasing inhibitory synapse numbers formed on excitatory neurons (Yuan et al., 2016). Double knockout of DTNBP1 and COMT (catechol-O-methyl transferase, another schizophrenia risk gene) causes working memory deficits in a discrete paired-trial T-maze task, which is highly associated with dopamine function in PFC (Papaleo et al., 2014). However, increased expression of dysbindin-1A in pyramidal neurons inhibits NMDAR function and blocks induction of LTP in hippocampal organotypic slices (Jeans et al., 2011).
5.5. miR-137
In addition to risk alleles locate at coding region, non-coding genes are also implicated in schizophrenia, such as microRNAs that play important roles in regulating gene expression at posttranscriptional level. miR-137 gain-of-function in vivo with lentiviral-mediated expression of miR-137 in dentate gyrus resulted in changed synaptic vesicle pool distribution, impaired induction of mossy fiber-LTP and deficits in hippocampus-dependently learning and memory (Siegert et al., 2015); while lentiviral expressing miR-137 ‘sponge’ that sequester miR-137 reverses the effect of miR-137 gain of function on LTP and behavior. Pre-synaptic mechanism might underly the regulation of miR-137 on mossy fiber-LTP and memory, as overexpression of miR-137 leads to downregulation of presynaptic targets genes including Complexin-1 (Cplx1) and Synaptotagmin-1 (Syt1) and impaired vesicle release (Siegert et al., 2015). Moreover, Chen et al. observed that complete loss of miR-137 in mice leads to postnatal lethality and miR-137 heterozygous conditional knockout mice exhibit inhibited Schaffer collateral LTP and impaired learning and social behavior, which is mediated by miR-137 target phosphodiesterase 10a (Pde10a) (Cheng et al., 2018).
5.6. SATB2
SATB2 is a schizophrenia risk gene encodes a DNA-binding protein (Schizophrenia Working Group of the Psychiatric Genomics, C., 2014). SATB2 is highly enriched in pyramidal neurons in cerebral cortex and the hippocampal CA1, two brain regions significant for memory formation (Jaitner et al., 2016). Deletion of SATB2 in forebrain of adult mice causes deficits in maintenance of Schaffer collateral LTP and impairs long-term fear and object discrimination memory (Jaitner et al., 2016). The underlying mechanism of SATB2 in synaptic plasticity remains complicated, as SATB2 is involved in a complex gene regulation network. SATB2 regulates a large amount of miRNAs in the hippocampal, many of which are involved in synaptic plasticity and memory formation (Jaitner et al., 2016). Meanwhile, interaction between SATB2 and the inner nuclear membrane protein LEMD2 alters expression of numerous genes that are related to schizophrenia etiology in pyramidal neurons (Feurle et al., 2021). Another gene set enrichment analysis showed that genes functionally related to or targeted by SATB2 are enriched for genes associated with schizophrenia (Whitton et al., 2018).
Growing evidence has shown that many other genes associated both with schizophrenia and synaptic plasticity, like SNAP25 (Baca et al., 2013), GRIN3B (Hornig et al., 2017), NRN1 (Fatjo-Vilas et al., 2016), NRGN (Hwang et al., 2021) and so on. However, there is still a very tough and long way to dissect the role of risk genes on synaptic plasticity and unveil the pathogenesis of schizophrenia and other kinds of mental disorder.
6. Environmental factors and synaptic plasticity disturbance
Many environmental factors, including prenatal immune activation, contribute to the onset of schizophrenia. The interference of early life infection on brain development is consistent with the neurodevelopmental hypothesis of schizophrenia, that is, abnormal early brain development is one of the causes of schizophrenia. Viral infections of central nervous system during perinatal (Brown, 2006) and childhood (Khandaker et al., 2012) increase the risk of mental illness in adulthood. A Finnish study showed that children with central nervous system infections were five times more likely to develop schizophrenia than the control group (Jones, 1998). Injection with viral analogue PolyI:C on gestation day 9 of pregnancy caused global DNA hypomethylation and enrichment of differentially methylated genes related to synaptic plasticity in mice offspring (Basil et al., 2018), implying that maternal immune activation affects synaptic plasticity in adult offspring.
MAM (Methylazoxymethanol) is an anti-mitotic agent that methylates DNA. It has been reported that MAM-exposure in embryonic day 17 (E17) contributes to schizophrenia-like behavior both in mice and rats (Huo et al., 2018, Moore et al., 2006). Intriguingly, MAM treatment in E17 mice leads to gender specific deficits in PPI and social recognition, as well as many differentially expressed genes relevant to synaptic plasticity (Huo et al., 2018). Moreover, disruption of one-carbon metabolism by increasing methionine daily intake in pregnant mice during the last week of gestation causes schizophrenia-like behavior including impaired social recognition, sensorimotor gating function, and working memory in offspring (Alachkar et al., 2018). However, electrophysiological examination of different forms synaptic plasticity in detail in these animal models are needed to characterize how schizophrenia-related environmental changes affect synaptic plasticity. (Fig. 1 and Table 1).
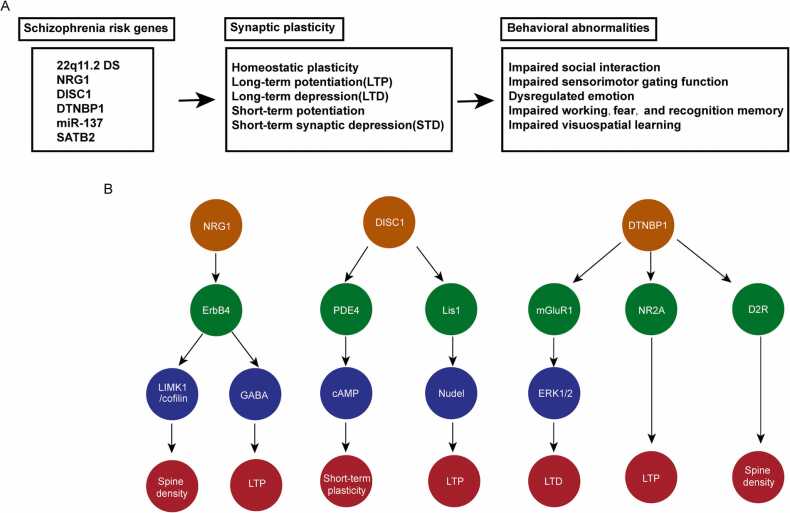
Fig. 1.
Mechanisms of synaptic plasticity in schizophrenia. A. Summary of the role and mechanism of synaptic plasticity in schizophrenia pathology. B. A schematic illustration for the function of selected schizophrenia risk genes.
Table 1.
Summary of selected schizophrenia risk genes or allele, and their roles in synaptic plasticity.
| Schizophrenia risk gene or allele | Mice or cell model | Deficits in synaptic plasticity | References |
|---|---|---|---|
| 22q11.2DS | Df(16)1 + /− , hemizygous deletion of 23 genes in the 22q11DS-related region of mouse chromosome 16 | enhanced PPF and LTP in hippocampus in mature animal | Earls et al., 2010 |
| Df(16)2 + /− , hemizygous deletion of 15 genes in the 22q11DS-related region of mouse chromosome 16 | increased LTP in hippocampus in mature animal | Earls et al., 2012 | |
| Df(16)5 + /− , hemizygous deletion of 6 genes in the 22q11DS-related region of mouse chromosome 16 | abnormal short-term potentiation (STP) in hippocampus | Devaraju et al., 2017 | |
| Df(16)A+ /-, hemizygous deletion of 27 genes in the 22q11DS-related region of mouse chromosome 16 | abnormal short-term potentiation (STP) and LTP in prefrontal cortex | Diamantopoulou et al., 2017 | |
| Lgdel+ /− , hemizygous deletion of 24 genes in the 22q11DS-related region of mouse chromosome 16 | dysregulated network homeostatic plasticity | Amin et al., 2017 | |
| DGCR8 heterozygous mutant mice | enhanced short-term synaptic depression in prefrontal cortex, increased LTP in hippocampus | Fenelon et al., 2011;Earls et al., 2012 | |
| NRG1 | Heterozygous deletion of NRG1 | impaired theta-burst LTP in hippocampus | Bjarnadottir et al., 2007 |
| bath application of NRG1 on hippocampal slices | suppressed tetanus stimulation-induced LTP in hippocampus | Chen et al., 2010 | |
| conditional deletion of NRG1 in forebrain pyramidal neuron | reduced short-term potentiation (STP) and LTP in hippocampus | Agarwal et al., 2014 | |
| overexpression of a cysteine-rich domain (CRD)-NRG1 | reduced LTP in hippocampus | Agarwal et al., 2014 | |
| prolonged treatment of cultured rat hippocampal slices with NRG1 | impaired 2-AG-dependent long-term depression of inhibitory synapses | Du et al., 2013 | |
| overexpression of human NRG1-IV isoform | impaired PPI, object location memory, and social interaction | Papaleo et al., 2016 | |
| DISC1 | Disc1Tm1Kara mice, a truncation of murine DISC1 | aberrant short-term plasticity at mossy fiber–CA3 synapses in hippocampus | Kvajo et al., 2011 |
| DISC1 L100P homozygous | diminished LTP in hippocampus | Cui et al., 2016 | |
| transiently disruption of DISC1’s interaction with signaling molecules Lis1 and Nudel | inhibited LTP in cortical layer 2 and 3 | Greenhill et al., 2015 | |
| DTNBP1 | Sandy mice | reduced mGluRI-dependent LTD at CA1 synapses in hippocampus | Bhardwaj et al., 2015 |
| Sandy mice | decreased inhibitory synapse numbers formed on excitatory neurons | Yuan et al., 2016 | |
| Sandy mice | increase expression of NR2A in hippocampal neurons,enhanced hippocampal LTP | Tang et al., 2009 | |
| Sandy mice | decreased inhibitory input to pyramidal neurons in layer V of PFC | Ji et al., 2009 | |
| increased expression of dysbindin-1A in pyramidal neurons | inhibited induction of LTP in hippocampal organotypic slices | Jeans et al., 2011 | |
| knockout of DTNBP1 and COMT(catechol-O-methyl transferase, another schizophrenia risk gene) | working memory deficits in a discrete paired-trial T-maze task | Papaleo et al., 2014 | |
| miR-137 | lentiviral-mediated expression of miR-137 in dentate gyrus | impaired induction of mossy fiber LTP | Siegert et al., 2015 |
| lentiviral expressing miR-137 ‘sponge’ that causes miR-137 sequestration in dentate gyrus | enhanced induction of mossy fiber LTP | Siegert et al., 2015 | |
| heterozygous conditional knockout of miR-137 in nervous system | inhibited Schaffer collateral LTP in hippocampus | Cheng et al., 2018 | |
| SATB2 | Deletion of SATB2 in forebrain pyramidal neuron | deficits in maintenance of Schaffer collateral LTP | Jaitner et al., 2016 |
7. Conclusion
Schizophrenia is a deliberating mental illness affecting 1 % human populations. Dysregulated synaptic transmission and plasticity have been implicated in the pathogenesis of the illness, although the underlying mechanism has yet determined. Synaptic plasticity plays a very significant role in normal brain function and development including the formation of correct circuit connection. In this review article, firstly, we discussed molecular mechanisms of various types of synaptic plasticity. LTP is important for memory formation and preservation and LTD is necessary for the reconstruction of synapse connection for LTP. Meanwhile, homeostatic plasticity keeps neuron activity in a moderate range. Secondly, we summarized the susceptibility gene and related animal models of schizophrenia, with the emphasis on risk genes that are known to modulate synaptic plasticity. In addition to genetic factors, environmental factors like viral infection, autoimmune disease or social isolation during childhood also contribute to the onset of schizophrenia. Thirdly, we proposed that environmental alterations might also lead to schizophrenia-like behavior and deficits in synaptic plasticity. As we reviewed here, each kind of plasticity plays a very important role in maintaining the normal function of the nervous system. Any imbalance in plasticity will pose a risk of mental disorder including schizophrenia. Given an rapidly expanded list of schizophrenia risk genes characterized by GWAS, functional studies to further dissect the roles of these genes in regulating synaptic plasticity and function are urgently needed to uncover the neuropathological mechanism of schizophrenia and develop new targets for clinical interventions.
Conflict of interest
The authors declare no conflict of interest
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82171506 and 31872778), the Discipline Innovative Engineering Plan (111 Program) of China (B13036), a Key Laboratory grant from Hunan province (2016TP1006), the Department of Science and Technology of Hunan Province (grant 2021DK2001, and Innovative Team Program 2019RS1010), the innovation-driven team project from Central South University (2020CX016), and Hunan Hundred Talents Program for Young Outstanding Scientists.
References
- Abraham W.C., Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Zhang M., Trembak-Duff I., Unterbarnscheidt T., Radyushkin K., Dibaj P., Martins de Souza D., Boretius S., Brzózka M.M., Steffens H., Berning S., Teng Z., Gummert M.N., Tantra M., Guest P.C., Willig K.I., Frahm J., Hell S.W., Bahn S., Rossner M.J., Nave K.A., Ehrenreich H., Zhang W., Schwab M.H. Dysregulated expression of neuregulin-1 by cortical pyramidal neurons disrupts synaptic plasticity. Cell Rep. 2014;8:1130–1145. doi: 10.1016/j.celrep.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Alachkar A., Wang L., Yoshimura R., Hamzeh A.R., Wang Z., Sanathara N., Lee S.M., Xu X., Abbott G.W., Civelli O. Prenatal one-carbon metabolism dysregulation programs schizophrenia-like deficits. Mol. Psychiatry. 2018;23:282–294. doi: 10.1038/mp.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin H., Marinaro F., De Pietri Tonelli D., Berdondini L. Developmental excitatory-to-inhibitory GABA-polarity switch is disrupted in 22q11.2 deletion syndrome: a potential target for clinical therapeutics. Sci. Rep. 2017;7:15752. doi: 10.1038/s41598-017-15793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen N., Krauth N., Nabavi S. Hebbian plasticity in vivo: relevance and induction. Curr. Opin. Neurobiol. 2017;45:188–192. doi: 10.1016/j.conb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Araque A., Parpura V., Sanzgiri R.P., Haydon P.G. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur. J. Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Baca M., Allan A.M., Partridge L.D., Wilson M.C. Gene-environment interactions affect long-term depression (LTD) through changes in dopamine receptor affinity in Snap25 deficient mice. Brain Res. 2013;1532:85–98. doi: 10.1016/j.brainres.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A., Alarcon J.M., Kandel E.R. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Basil P., Li Q., Gui H., Hui T.C.K., Ling V.H.M., Wong C.C.Y., Mill J., McAlonan G.M., Sham P.C. Prenatal immune activation alters the adult neural epigenome but can be partly stabilised by a n-3 polyunsaturated fatty acid diet. Transl. Psychiatry. 2018;8:125. doi: 10.1038/s41398-018-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Zablah Y., Zhang H., Gugustea R., Jia Z. LIM-Kinases in synaptic plasticity, memory, and brain diseases. Cells. 2021;10 doi: 10.3390/cells10082079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S.K., Ryan R.T., Wong T.P., Srivastava L.K. Loss of dysbindin-1, a risk gene for schizophrenia, leads to impaired group 1 metabotropic glutamate receptor function in mice. Front. Behav. Neurosci. 2015;9:72. doi: 10.3389/fnbeh.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum R., Weinberger D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017;18:727–740. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M., Misner D.L., Haverfield-Gross S., Bruun S., Helgason V.G., Stefansson H., Sigmundsson A., Firth D.R., Nielsen B., Stefansdottir R., Novak T.J., Stefansson K., Gurney M.E., Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/- knock-outs compared with wild-type mice. J. Neurosci.: Off. J. Soc. Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood D.H., Fordyce A., Walker M.T., St, Clair D.M., Porteous D.J., Muir W.J. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V., Gardner-Medwin A.R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S. Prenatal infection as a risk factor for schizophrenia. Schizophr. Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P.E., Younts T.J., Chavez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Jing H., Xiong M., Zhang Q., Lin D., Ren D., Wang S., Yin D., Chen Y., Zhou T., Li B., Fei E., Pan B.X. Spine impairment in mice high-expressing neuregulin 1 due to LIMK1 activation. Cell Death Dis. 2021;12:403. doi: 10.1038/s41419-021-03687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.J., Zhang M., Yin D.M., Wen L., Ting A., Wang P., Lu Y.S., Zhu X.H., Li S.J., Wu C.Y., Wang X.M., Lai C., Xiong W.C., Mei L., Gao T.M. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc. Natl. Acad. Sci. USA. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wang Z.M., Tan W., Wang X., Li Y., Bai B., Li Y., Zhang S.F., Yan H.L., Chen Z.L., Liu C.M., Mi T.W., Xia S., Zhou Z., Liu A., Tang G.B., Liu C., Dai Z.J., Wang Y.Y., Wang H., Wang X., Kang Y., Lin L., Chen Z., Xie N., Sun Q., Xie W., Peng J., Chen D., Teng Z.Q., Jin P. Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat. Neurosci. 2018;21:1689–1703. doi: 10.1038/s41593-018-0261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A., Malenka R.C. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Clapcote S.J., Lipina T.V., Millar J.K., Mackie S., Christie S., Ogawa F., Lerch J.P., Trimble K., Uchiyama M., Sakuraba Y., Kaneda H., Shiroishi T., Houslay M.D., Henkelman R.M., Sled J.G., Gondo Y., Porteous D.J., Roder J.C. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Collingridge G.L., Peineau S., Howland J.G., Wang Y.T. Long-term depression in the CNS. Nat. Rev. Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Crabtree G.W., Sun Z., Kvajo M., Broek J.A., Fénelon K., McKellar H., Xiao L., Xu B., Bahn S., O'Donnell J.M., Gogos J.A. Alteration of neuronal excitability and short-term synaptic plasticity in the prefrontal cortex of a mouse model of mental illness. J. Neurosci.: Off. J. Soc. Neurosci. 2017;37:4158–4180. doi: 10.1523/JNEUROSCI.4345-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S.C., Sur M., Dobkin B.H., O'Brien C., Sanger T.D., Trojanowski J.Q., Rumsey J.M., Hicks R., Cameron J., Chen D., Chen W.G., Cohen L.G., deCharms C., Duffy C.J., Eden G.F., Fetz E.E., Filart R., Freund M., Grant S.J., Haber S., Kalivas P.W., Kolb B., Kramer A.F., Lynch M., Mayberg H.S., McQuillen P.S., Nitkin R., Pascual-Leone A., Reuter-Lorenz P., Schiff N., Sharma A., Shekim L., Stryker M., Sullivan E.V., Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Sun W., Yu M., Li N., Guo L., Gu H., Zhou Y. Disrupted-in-schizophrenia1 (DISC1) L100P mutation alters synaptic transmission and plasticity in the hippocampus and causes recognition memory deficits. Mol. Brain. 2016;9:89. doi: 10.1186/s13041-016-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza D.C., Abi-Saab W.M., Madonick S., Forselius-Bielen K., Doersch A., Braley G., Gueorguieva R., Cooper T.B., Krystal J.H. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol. Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Desch K., Langer J.D., Schuman E.M. Dynamic bi-directional phosphorylation events associated with the reciprocal regulation of synapses during homeostatic up- and down-scaling. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraju P., Yu J., Eddins D., Mellado-Lagarde M.M., Earls L.R., Westmoreland J.J., Quarato G., Green D.R., Zakharenko S.S. Haploinsufficiency of the 22q11.2 microdeletion gene Mrpl40 disrupts short-term synaptic plasticity and working memory through dysregulation of mitochondrial calcium. Mol. Psychiatry. 2017;22:1313–1326. doi: 10.1038/mp.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulou A., Sun Z., Mukai J., Xu B., Fenelon K., Karayiorgou M., Gogos J.A. Loss-of-function mutation in Mirta22/Emc10 rescues specific schizophrenia-related phenotypes in a mouse model of the 22q11.2 deletion. Proc. Natl. Acad. Sci. USA. 2017;114:E6127–E6136. doi: 10.1073/pnas.1615719114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering G.H., Huganir R.L. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering G.H., Nirujogi R.S., Roth R.H., Worley P.F., Pandey A., Huganir R.L. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017;355:511–515. doi: 10.1126/science.aai8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Kwon I.K., Kim J. Neuregulin-1 impairs the long-term depression of hippocampal inhibitory synapses by facilitating the degradation of endocannabinoid 2-AG. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33:15022–15031. doi: 10.1523/JNEUROSCI.5833-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee C., Kofuji P., Navarrete M., Araque A. Astrocyte and neuron cooperation in long-term depression. Trends Neurosci. 2021;44:837–848. doi: 10.1016/j.tins.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls L.R., Bayazitov I.T., Fricke R.G., Berry R.B., Illingworth E., Mittleman G., Zakharenko S.S. Dysregulation of presynaptic calcium and synaptic plasticity in a mouse model of 22q11 deletion syndrome. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30:15843–15855. doi: 10.1523/JNEUROSCI.1425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls L.R., Fricke R.G., Yu J., Berry R.B., Baldwin L.T., Zakharenko S.S. Age-dependent microRNA control of synaptic plasticity in 22q11 deletion syndrome and schizophrenia. J. Neurosci.: Off. J. Soc. Neurosci. 2012;32:14132–14144. doi: 10.1523/JNEUROSCI.1312-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatjó-Vilas M., Prats C., Pomarol-Clotet E., Lázaro L., Moreno C., González-Ortega I., Lera-Miguel S., Miret S., Muñoz M.J., Ibáñez I., Campanera S., Giralt-López M., Cuesta M.J., Peralta V., Ortet G., Parellada M., González-Pinto A., McKenna P.J., Fañanás L. Involvement of NRN1 gene in schizophrenia-spectrum and bipolar disorders and its impact on age at onset and cognitive functioning. World J. Biol. Psychiatry. 2016;17:129–139. doi: 10.3109/15622975.2015.1093658. [DOI] [PubMed] [Google Scholar]
- Fenelon K., Mukai J., Xu B., Hsu P.K., Drew L.J., Karayiorgou M., Fischbach G.D., Macdermott A.B., Gogos J.A. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2011;108:4447–4452. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénelon K., Xu B., Lai C.S., Mukai J., Markx S., Stark K.L., Hsu P.K., Gan W.B., Fischbach G.D., MacDermott A.B., Karayiorgou M., Gogos J.A. The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33:14825–14839. doi: 10.1523/JNEUROSCI.1611-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurle P., Abentung A., Cera I., Wahl N., Ablinger C., Bucher M., Stefan E., Sprenger S., Teis D., Fischer A., Laighneach A., Whitton L., Morris D.W., Apostolova G., Dechant G. SATB2-LEMD2 interaction links nuclear shape plasticity to regulation of cognition-related genes. EMBO J. 2021;40 doi: 10.15252/embj.2019103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest M.P., Parnell E., Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nat. Rev. Neurosci. 2018;19:215–234. doi: 10.1038/nrn.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A., Leweke F.M., Gerth C.W., Schreiber D., Koethe D., Faulhaber J., Klosterkotter J., Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Glausier J.R., Lewis D.A. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill S.D., Juczewski K., de Haan A.M., Seaton G., Fox K., Hardingham N.R. Adult cortical plasticity depends on an early postnatal critical period. Science. 2015;349:424–427. doi: 10.1126/science.aaa8481. [DOI] [PubMed] [Google Scholar]
- Harrison P.J. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison P.J., Weinberger D.R. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Haydon P.G. GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hornig T., Gruning B., Kundu K., Houwaart T., Backofen R., Biber K., Normann C. GRIN3B missense mutation as an inherited risk factor for schizophrenia: whole-exome sequencing in a family with a familiar history of psychotic disorders. Genet Res. 2017;99 doi: 10.1017/S0016672316000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo C., Liu X., Zhao J., Zhao T., Huang H., Ye H. Abnormalities in behaviour, histology and prefrontal cortical gene expression profiles relevant to schizophrenia in embryonic day 17 MAM-Exposed C57BL/6 mice. Neuropharmacology. 2018;140:287–301. doi: 10.1016/j.neuropharm.2018.07.030. [DOI] [PubMed] [Google Scholar]
- Hwang H., Szucs M.J., Ding L.J., Allen A., Ren X., Haensgen H., Gao F., Rhim H., Andrade A., Pan J.Q., Carr S.A., Ahmad R., Xu W. Neurogranin, encoded by the schizophrenia risk gene NRGN, bidirectionally modulates synaptic plasticity via calmodulin-dependent regulation of the neuronal phosphoproteome. Biol. Psychiatry. 2021;89:256–269. doi: 10.1016/j.biopsych.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitner C., Reddy C., Abentung A., Whittle N., Rieder D., Delekate A., Korte M., Jain G., Fischer A., Sananbenesi F., Cera I., Singewald N., Dechant G., Apostolova G. Satb2 determines miRNA expression and long-term memory in the adult central nervous system. eLife. 2016;5 doi: 10.7554/eLife.17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeans A., Malins R., Padamsey Z., Reinhart M., Emptage N. Increased expression of dysbindin-1A leads to a selective deficit in NMDA receptor signaling in the hippocampus. Neuropharmacology. 2011;61:1345–1353. doi: 10.1016/j.neuropharm.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Ji Y., Yang F., Papaleo F., Wang H.X., Gao W.J., Weinberger D.R., Lu B. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc. Natl. Acad. Sci. USA. 2009;106:19593–19598. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.M., Zhao J., Hu Z., Lindberg D., Li Z. Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nat. Neurosci. 2013;16:1627–1636. doi: 10.1038/nn.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Emmetsberger J., Talmage D.A., Role L.W. Type III neuregulin 1 is required for multiple forms of excitatory synaptic plasticity of mouse cortico-amygdala circuits. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33:9655–9666. doi: 10.1523/JNEUROSCI.2888-12.2013. (The Journal of neuroscience: the official journal of the Society for Neuroscience) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. The new epidemiology of schizophrenia. Psychiatr. Clin. North Am. 1998;21:1–25. doi: 10.1016/s0193-953x(05)70358-0. [DOI] [PubMed] [Google Scholar]
- Jorgenson L.A., Sun M., O'Connor M., Georgieff M.K. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Sommer I.E., Murray R.M., Meyer-Lindenberg A., Weinberger D.R., Cannon T.D., O'Donovan M., Correll C.U., Kane J.M., van Os J., Insel T.R. Schizophrenia. Nat. Rev. Dis. Prim. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M., Morris M.A., Morrow B., Shprintzen R.J., Goldberg R., Borrow J., Gos A., Nestadt G., Wolyniec P.S., Lasseter V.K. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc. Natl. Acad. Sci. USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M., Simon T.J., Gogos J.A. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat. Rev. Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zimbron J., Dalman C., Lewis G., Jones P.B. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr. Res. 2012;139:161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Futai K., Jo J., Hayashi Y., Cho K., Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Kvajo M., McKellar H., Arguello P.A., Drew L.J., Moore H., MacDermott A.B., Karayiorgou M., Gogos J.A. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc. Natl. Acad. Sci. USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M., McKellar H., Drew L.J., Lepagnol-Bestel A.M., Xiao L., Levy R.J., Blazeski R., Arguello P.A., Lacefield C.O., Mason C.A., Simonneau M., O'Donnell J.M., MacDermott A.B., Karayiorgou M., Gogos J.A. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. Proc. Natl. Acad. Sci. USA. 2011;108:E1349–E1358. doi: 10.1073/pnas.1114113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Barbarosie M., Kameyama K., Bear M.F., Huganir R.L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lisman J., Cooper K., Sehgal M., Silva A.J. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat. Neurosci. 2018;21:309–314. doi: 10.1038/s41593-018-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Raghavachari S. Biochemical principles underlying the stable maintenance of LTP by the CaMKII/NMDAR complex. Brain Res. 2015;1621:51–61. doi: 10.1016/j.brainres.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W.H., Yeh N.H., Huang Y.S. CPEB2 Activates GRASP1 mRNA translation and promotes AMPA receptor surface expression, long-term potentiation, and memory. Cell Rep. 2017;21:1783–1794. doi: 10.1016/j.celrep.2017.10.073. [DOI] [PubMed] [Google Scholar]
- Machado I., Schiöth H.B., Lasaga M., Scimonelli T. IL-1β reduces GluA1 phosphorylation and its surface expression during memory reconsolidation and α-melanocyte-stimulating hormone can modulate these effects. Neuropharmacology. 2018;128:314–323. doi: 10.1016/j.neuropharm.2017.09.041. [DOI] [PubMed] [Google Scholar]
- Malavasi E.L.V., Economides K.D., Grünewald E., Makedonopoulou P., Gautier P., Mackie S., Murphy L.C., Murdoch H., Crummie D., Ogawa F., McCartney D.L., O'Sullivan S.T., Burr K., Torrance H.S., Phillips J., Bonneau M., Anderson S.M., Perry P., Pearson M., Constantinides C., Davidson-Smith H., Kabiri M., Duff B., Johnstone M., Polites H.G., Lawrie S.M., Blackwood D.H., Semple C.A., Evans K.L., Didier M., Chandran S., McIntosh A.M., Price D.J., Houslay M.D., Porteous D.J., Millar J.K. DISC1 regulates N-methyl-D-aspartate receptor dynamics: abnormalities induced by a Disc1 mutation modelling a translocation linked to major mental illness. Transl. Psychiatry. 2018;8:184. doi: 10.1038/s41398-018-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret G., Alarcon J.M., Martel G., Takizawa S., Vronskaya S., Yin D., Chen I.Z., Kandel E.R., Shumyatsky G.P. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30:3813–3825. doi: 10.1523/JNEUROSCI.1330-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder S.R., Cannon T.D. Schizophrenia. N. Engl. J. Med. 2019;381:1753–1761. doi: 10.1056/NEJMra1808803. [DOI] [PubMed] [Google Scholar]
- Martin S.C., Monroe S.K., Diering G.H. Homer1a and mGluR1/5 Signaling in Homeostatic Sleep Drive and Output. Yale J. Biol. Med. 2019;92:93–101. [PMC free article] [PubMed] [Google Scholar]
- Mei L., Xiong W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H., Jentsch J.D., Ghajarnia M., Geyer M.A., Grace A.A. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol. Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey R.M., Herron C.E., Malenka R.C. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey R.M., Malenka R.C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Navarrete M., Cuartero M.I., Palenzuela R., Draffin J.E., Konomi A., Serra I., Colié S., Castaño-Castaño S., Hasan M.T., Nebreda Á.R., Esteban J.A. Astrocytic p38α MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat. Commun. 2019;10:2968. doi: 10.1038/s41467-019-10830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya J.C., Heusner C.L., Matsumoto M., Sinclair D., Kondo M.A., Karl T., Shannon Weickert C. Overexpression of Neuregulin 1 Type III Confers Hippocampal mRNA Alterations and Schizophrenia-Like Behaviors in Mice. Schizophr. Bull. 2018;44:865–875. doi: 10.1093/schbul/sbx122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Beck K., Reis Marques T., Howes O.D. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry. 2019;24:549–561. doi: 10.1038/s41380-018-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis T., Zhang S., Trussell L.O. Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. J. Neurosci.: Off. J. Soc. Neurosci. 1996;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F., Burdick M.C., Callicott J.H., Weinberger D.R. Epistatic interaction between COMT and DTNBP1 modulates prefrontal function in mice and in humans. Mol. Psychiatry. 2014;19:311–316. doi: 10.1038/mp.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F., Yang F., Paterson C., Palumbo S., Carr G.V., Wang Y., Floyd K., Huang W., Thomas C.J., Chen J., Weinberger D.R., Law A.J. Behavioral, neurophysiological, and synaptic impairment in a transgenic Neuregulin1 (NRG1-IV) murine schizophrenia model. J. Neurosci.: Off. J. Soc. Neurosci. 2016;36:4859–4875. doi: 10.1523/JNEUROSCI.4632-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R., Lindsay E. Mouse models of 22q11 deletion syndrome. Biol. Psychiatry. 2006;59:1172–1179. doi: 10.1016/j.biopsych.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Paylor R., McIlwain K.L., McAninch R., Nellis A., Yuva-Paylor L.A., Baldini A., Lindsay E.A. Mice deleted for the DiGeorge/velocardiofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum. Mol. Genet. 2001;10:2645–2650. doi: 10.1093/hmg/10.23.2645. [DOI] [PubMed] [Google Scholar]
- Penn A.C., Zhang C.L., Georges F., Royer L., Breillat C., Hosy E., Petersen J.D., Humeau Y., Choquet D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549:384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher G.M., Beggs S., Woo R.S., Mei L., Salter M.W. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Hu Z., Chen Q., Jaffe A., Li Y., Sadashivaiah V., Zhu S., Rajpurohit N., Heon Shin J., Xia W., Jia Y., Wu J., Lang Qin S., Li X., Zhu J., Tian Q., Paredes D., Zhang F., Wang K.H., Mattay V.S., Callicott J.H., Berman K.F., Weinberger D.R., Yang F. KCNH2-3.1 mediates aberrant complement activation and impaired hippocampal-medial prefrontal circuitry associated with working memory deficits. Mol. Psychiatry. 2020;25:206–229. doi: 10.1038/s41380-019-0530-1. [DOI] [PubMed] [Google Scholar]
- Sanderson J.L., Gorski J.A., Dell'Acqua M.L. NMDA Receptor-Dependent LTD requires transient synaptic incorporation of Ca²⁺-Permeable AMPARs mediated by AKAP150-Anchored PKA and calcineurin. Neuron. 2016;89:1000–1015. doi: 10.1016/j.neuron.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M., Fernandez-Villalobos G., Stein I.S., Kasumova G., Zhang P., Bayer K.U., Otmakhov N., Hell J.W., Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci.: Off. J. Soc. Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S., Kamiya A., Yokota Y., Prikulis I., Kano S., Hayashi-Takagi A., Stanco A., Eom T.Y., Rao S., Ishizuka K., Wong P., Korth C., Anton E.S., Sawa A. Disrupted-in-Schizophrenia-1 expression is regulated by beta-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc. Natl. Acad. Sci. USA. 2010;107:5622–5627. doi: 10.1073/pnas.0909284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir A., Kwon O.B., Karavanova I., Vullhorst D., Leiva-Salcedo E., Janssen M.J., Buonanno A. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J. Neurosci.: Off. J. Soc. Neurosci. 2012;32:2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S., Seo J., Kwon E.J., Rudenko A., Cho S., Wang W., Flood Z., Martorell A.J., Ericsson M., Mungenast A.E., Tsai L.H. The schizophrenia risk gene product miR-137 alters presynaptic plasticity. Nat. Neurosci. 2015;18:1008–1016. doi: 10.1038/nn.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D., Blackwood D., Muir W., Carothers A., Walker M., Spowart G., Gosden C., Evans H.J. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Stampanoni Bassi M., Iezzi E., Gilio L., Centonze D., Buttari F. Synaptic plasticity shapes brain connectivity: implications for network topology. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20246193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P.K. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stark K.L., Xu B., Bagchi A., Lai W.S., Liu H., Hsu R., Wan X., Pavlidis P., Mills A.A., Karayiorgou M., Gogos J.A. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Baldeweg T., Friston K.J. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Straub R.E., Jiang Y., MacLean C.J., Ma Y., Webb B.T., Myakishev M.V., Harris-Kerr C., Wormley B., Sadek H., Kadambi B., Cesare A.J., Gibberman A., Wang X., O'Neill F.A., Walsh D., Kendler K.S. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am. J. Hum. Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. Towards an understanding of synapse formation. Neuron. 2018;100:276–293. doi: 10.1016/j.neuron.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Turrigiano G.G. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J. Neurosci.: Off. J. Soc. Neurosci. 2011;31:6800–6808. doi: 10.1523/JNEUROSCI.5616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D.J., Foehring R. A mechanism for homeostatic plasticity. Nat. Neurosci. 2004;7:691–692. doi: 10.1038/nn0704-691. [DOI] [PubMed] [Google Scholar]
- Swillen A., Vogels A., Devriendt K., Fryns J.P. Chromosome 22q11 deletion syndrome: update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am. J. Med. Genet. 2000;97:128–135. doi: 10.1002/1096-8628(200022)97:2<128::aid-ajmg4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Talbot K., Eidem W.L., Tinsley C.L., Benson M.A., Thompson E.W., Smith R.J., Hahn C.G., Siegel S.J., Trojanowski J.Q., Gur R.E., Blake D.J., Arnold S.E. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J. Clin. Investig. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T.T., Yang F., Chen B.S., Lu Y., Ji Y., Roche K.W., Lu B. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc. Natl. Acad. Sci. USA. 2009;106:21395–21400. doi: 10.1073/pnas.0910499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.R., Taylor S.B., Koenig J.I. The involvement of Type II Neuregulin-1 in rat visuospatial learning and memory. Neurosci. Lett. 2012;531:131–135. doi: 10.1016/j.neulet.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D., Molinos I., Petit E., Bellini S., Nagakura I., O'Tuathaigh C., Schorova L., Mitchell K.J., Waddington J., Sur M., Gill M., Corvin A.P. Disrupted in schizophrenia 1 (DISC1) L100P mutants have impaired activity-dependent plasticity in vivo and in vitro. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubetskoy V., Pardiñas A.F., Qi T., Panagiotaropoulou G., Awasthi S., Bigdeli T.B., Bryois J., Chen C.Y., Dennison C.A., Hall L.S., Lam M., Watanabe K., Frei O., Ge T., Harwood J.C., Koopmans F., Magnusson S., Richards A.L., Sidorenko J., Wu Y., Zeng J., Grove J., Kim M., Li Z., Voloudakis G., Zhang W., Adams M., Agartz I., Atkinson E.G., Agerbo E., Al Eissa M., Albus M., Alexander M., Alizadeh B.Z., Alptekin K., Als T.D., Amin F., Arolt V., Arrojo M., Athanasiu L., Azevedo M.H., Bacanu S.A., Bass N.J., Begemann M., Belliveau R.A., Bene J., Benyamin B., Bergen S.E., Blasi G., Bobes J., Bonassi S., Braun A., Bressan R.A., Bromet E.J., Bruggeman R., Buckley P.F., Buckner R.L., Bybjerg-Grauholm J., Cahn W., Cairns M.J., Calkins M.E., Carr V.J., Castle D., Catts S.V., Chambert K.D., Chan R., Chaumette B., Cheng W., Cheung E., Chong S.A., Cohen D., Consoli A., Cordeiro Q., Costas J., Curtis C., Davidson M., Davis K.L., de Haan L., Degenhardt F., DeLisi L.E., Demontis D., Dickerson F., Dikeos D., Dinan T., Djurovic S., Duan J., Ducci G., Dudbridge F., Eriksson J.G., Fañanás L., Faraone S.V., Fiorentino A., Forstner A., Frank J., Freimer N.B., Fromer M., Frustaci A., Gadelha A., Genovese G., Gershon E.S., Giannitelli M., Giegling I., Giusti-Rodríguez P., Godard S., Goldstein J.I., González Peñas J., González-Pinto A., Gopal S., Gratten J., Green M.F., Greenwood T.A., Guillin O., Gülöksüz S., Gur R.E., Gur R.C., Gutiérrez B., Hahn E., Hakonarson H., Haroutunian V., Hartmann A.M., Harvey C., Hayward C., Henskens F.A., Herms S., Hoffmann P., Howrigan D.P., Ikeda M., Iyegbe C., Joa I., Julià A., Kähler A.K., Kam-Thong T., Kamatani Y., Karachanak-Yankova S., Kebir O., Keller M.C., Kelly B.J., Khrunin A., Kim S.W., Klovins J., Kondratiev N., Konte B., Kraft J., Kubo M., Kučinskas V., Kučinskiene Z.A., Kusumawardhani A., Kuzelova-Ptackova H., Landi S., Lazzeroni L.C. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–508. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L.O., Zhang S., Raman I.M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol. Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Weickert C.S., Rothmond D.A., Hyde T.M., Kleinman J.E., Straub R.E. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr. Res. 2008;98:105–110. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton L., Apostolova G., Rieder D., Dechant G., Rea S., Donohoe G., Morris D.W. Genes regulated by SATB2 during neurodevelopment contribute to schizophrenia and educational attainment. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods B.T., Yurgelun-Todd D., Goldstein J.M., Seidman L.J., Tsuang M.T. MRI brain abnormalities in chronic schizophrenia: one process or more? Biol. Psychiatry. 1996;40:585–596. doi: 10.1016/0006-3223(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Wu H., Zhou Y., Xiong Z.Q. Transducer of regulated CREB and late phase long-term synaptic potentiation. FEBS J. 2007;274:3218–3223. doi: 10.1111/j.1742-4658.2007.05891.x. [DOI] [PubMed] [Google Scholar]
- Xiao H., Liu B., Chen Y., Zhang J. Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int. J. Dev. Neurosci. 2016;48:38–49. doi: 10.1016/j.ijdevneu.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Yang Y., Chen J., Chen X., Li D., He J., Wang S., Zhao S., Yang X., Deng S., Tong C., Wang D., Guo Z., Li D., Ma C., Liang X., Shi Y.S., Liu J.J. Endophilin A1 drives acute structural plasticity of dendritic spines in response to Ca2+/calmodulin. J. Cell Biol. 2021;220 doi: 10.1083/jcb.202007172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D.M., Chen Y.J., Liu S., Jiao H., Shen C., Sathyamurthy A., Lin T.W., Xiong W.C., Li B.M., Mei L., Bergson C. Calcyon stimulates neuregulin 1 maturation and signaling. Mol. Psychiatry. 2015;20:1251–1260. doi: 10.1038/mp.2014.131. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Yang F., Xiao Y., Tan S., Husain N., Ren M., Hu Z., Martinowich K., Ng J.S., Kim P.J., Han W., Nagata K.I., Weinberger D.R., Je H.S. Regulation of brain-derived neurotrophic factor exocytosis and gamma-aminobutyric acidergic interneuron synapse by the schizophrenia susceptibility gene Dysbindin-1. Biol. Psychiatry. 2016;80:312–322. doi: 10.1016/j.biopsych.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Zanettini C., Panlilio L.V., Alicki M., Goldberg S.R., Haller J., Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke F., Gerstner W., Ganguli S. The temporal paradox of Hebbian learning and homeostatic plasticity. Curr. Opin. Neurobiol. 2017;43:166–176. doi: 10.1016/j.conb.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang C., Vincent J., Zala D., Benstaali C., Sainlos M., Grillo-Bosch D., Daburon S., Coussen F., Cho Y., David D.J., Saudou F., Humeau Y., Choquet D. Modulation of AMPA receptor surface diffusion restores hippocampal plasticity and memory in Huntington's disease models. Nat. Commun. 2018;9:4272. doi: 10.1038/s41467-018-06675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhu M., Sun Y., Tang B., Zhang G., An P., Cheng Y., Shan Y., Merzenich M.M., Zhou X. Environmental noise degrades hippocampus-related learning and memory. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2017841117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., He G., Zhang X., Lv X., Zhang X., Liu A., Xia S., Xie H., Dang R., Han L., Qi J., Meng Y., Yu S., Xie W., Jia Z. NGPF2 triggers synaptic scaling up through ALK-LIMK-cofilin-mediated mechanisms. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109515. [DOI] [PubMed] [Google Scholar]
- Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]