Abstract
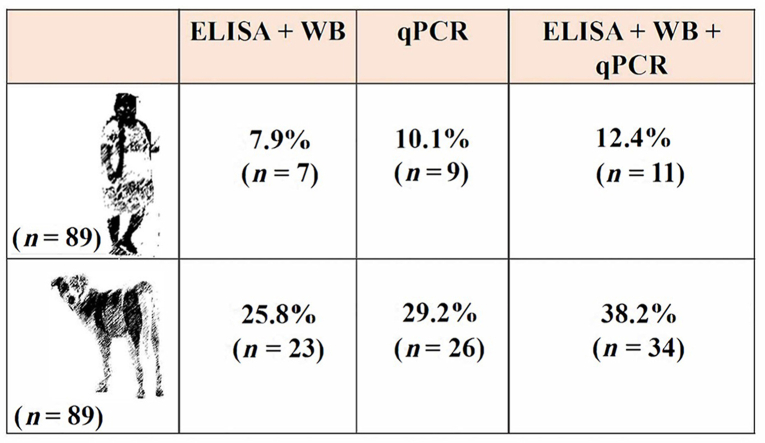
In this study, the prevalence of T. cruzi infection was estimated in dogs and their owners from a rural community in Mexico using serological techniques for chronic infection cases, qPCR for acute phase cases, and a combination of both techniques to detect chronic and acute infections. Eighty-nine blood samples were collected from owners and their dogs for obtaining serum and parasite DNA. Prevalence was calculated using (i) positive cases detected in a serological test (ELISA and Western blot), (ii) positive cases detected in a qPCR test, and (iii) positive cases detected by both techniques. Sensitivity, specificity, and predictive values were determined individually for serology, qPCR and for both techniques used simultaneously. The prevalence observed varied: for serology, 25.8% of the dogs and 7.9% of the owners were seropositive, while for qPCR 29.2% of the dogs and 10.1% of the owners were identified as positive. Combination of serological and molecular techniques resulted in a prevalence of 38.2% for dogs and 12.4% for their owners. The sensitivity, specificity and predictive values calculated for both techniques improved when both techniques were used simultaneously (sensitivity of 92.4% and specificity of 100% for infected dogs and sensitivity of 93.4% and specificity of 100% for infected owners). Combined use of serological tests and qPCR allowed identifying a greater number of positive cases in dogs and their owners. This strategy may help implement adequate and timely epidemiological surveillance of American trypanosomiasis in order to prevent the appearance of new cases of Trypanosoma cruzi infections in endemic zones.
Keywords: Trypanosoma cruzi, Parasite load, Dogs, Humans, Zoonosis
Graphical abstract
Highlights
-
•
Prevalence of Trypanosoma cruzi infection was estimated in dogs and their owners from a rural community in Mexico.
-
•
Serological techniques (ELISA and western blot), qPCR, and a combination of both techniques were applied.
-
•
Combined use serological and molecular techniques resulted in a prevalence of 38.2% for dogs and 12.4% for their owners.
-
•
Simultaneous use of both techniques improved the sensitivity and specificity of screening in both humans and dogs.
-
•
The combined use of serological and molecular techniques allows an adequate and timely diagnosis of American trypanosomiasis.
1. Introduction
Chagas disease, also known as American trypanosomiasis, is a potentially life-threatening illness caused by the protozoan parasite Trypanosoma cruzi. Chagas disease has been reported in humans and domestic and peridomestic reservoirs mainly from rural and peri-urban areas. Dogs, cats and synanthropic rodents are capable to maintain the life-cycle of T. cruzi. These animals and particularly dogs, can play a key role as amplifier hosts of T. cruzi in domestic transmission cycles covering a broad diversity of ecoregions and triatomine species, throughout the Americas (Gürtler & Cardinal, 2015). Chagas disease in Mexico is poorly understood because there are no official programmes for vector control in all of the endemic areas of the country (SSA, 2013). The techniques proposed for the diagnosis are based on the detection of antibodies to determine previous contact with the parasite (OPS, 2018). Molecular techniques such as PCR may help detecting T. cruzi in the acute phase of the disease where trypomastigotes are present in blood samples. A combination of serological tests and molecular tools may help improving the accuracy of the epidemiological surveillance of this parasitic disease in endemic areas such as the rural communities of the Neotropical region of Mexico. Most rural communities have abundant populations of stray dogs which could act as reservoirs of T. cruzi (Ortega-Pacheco et al., 2007; Jiménez-Coello et al., 2008, 2010b, 2015; Travi, 2019). Studies of T. cruzi seropositivity have demonstrated a direct correlation between the human infection and dog infection in the states of Mexico and Sonora (Estrada-Franco et al., 2006; Arce-Fonseca et al., 2017). However, such apparent association has not been confirmed in other parts of Mexico (Jiménez-Coello et al., 2010a). Combination of serological techniques for the detection of individuals in the chronic phase of the disease (antibodies), and molecular tools for the detection of the parasite in the acute phase (parasitemia) may help identifying correctly individuals infected with T. cruzi in rural communities from endemic zones.
The aim of this study was to determine the prevalence of T. cruzi in dogs and their owners using serological techniques for detection of chronic cases, qPCR tests for detection of acute infection cases, and a combination of both techniques to simultaneously detect chronic cases and acute infections.
2. Materials and methods
2.1. Study population and blood sample collection
A cross-sectional study was performed in the rural community of Molas, located at a distance of 16 km (10 miles) from Merida City, Yucatán, Mexico (20°48′57.96″N, 89°37′54.12″W). The climate of the region is humid subtropical, with a rainy season during the summer (Flores-Guido & Espejel-Carvajal, 1994). Blood samples were collected from 89 dogs by venipuncture of the jugular vein and from their respective owners by venipuncture of the cubital vein. Two blood samples were taken from each dog and owner: one using tubes without anticoagulant to obtain serum and the other to preserve DNA from whole blood (PAXgene Blood DNA Tubes, Qiagen, Hilden, Germany). Samples were stored at −20 °C until processed in the laboratory.
2.2. Obtaining of serum and DNA samples for the assays
Blood samples without anticoagulant were centrifuged at 440× g for 10 min to obtain the serum. For the PCR assay, DNA was obtained from whole blood, using a commercial kit (DNeasy Blood & Tissue Kit®, Qiagen), applying the modification described by Jalal et al. (2004). Genomic DNA was analyzed for quality and quantity using a spectrophotometer (Nanodrop® 2000, ThermoFisher Scientific, Massachusetts, USA).
2.3. Serological analysis (ELISA and Western blot)
The commercial ChagaTest® kit (enzyme-linked immunosorbent assay-ELISA, Wiener lab., Rosario, Argentina) was used as a screening diagnostic test. The kit contains six recombinant antigens of specific proteins from epimastigote and tripomastigote stages of different strains of T. cruzi.
Human serum samples were examined to detected T. cruzi-specific IgG antibody using the ChagaTest® following the manufacturerʼs instructions. Canine serum samples were tested using the ChagaTest® but modified using a second antibody anti-dog IgG conjugated with HRP (Jimenez-Coello et al., 2018). The ELISA procedure is described briefly: canine serum was used in a dilution of 1:100 with kit diluents and placed in ELISA plates. Then, a second species-specific antibody conjugated to the enzyme horseradish peroxidase (HRP) (goat anti-dog lgG-HRP, Cat. No. sc-2433, Santa Cruz Biotechnology, Texas, USA) was added using a dilution of 1:5000. The color was developed with tetramethylbenzidine and hydrogen peroxide substrates and reactions were stopped by acidification of the reaction medium. Plates were analyzed using an automatic ELISA reader (VICTORX3 Multilabel Plate Reader, PerkinElmer, Massachusetts, USA) and the optical density of the samples was measured with a 450 nm filter. Dog serum samples previously validated as positive or negative for T. cruzi infection were used as ELISA controls. Samples positive in the ELISA test were confirmed with the Western blot technique (WB).
For the WB technique, total protein extracts of T. cruzi epimastigotes of H4 strain were used as antigens (Laemmli, 1970). Total protein extracts were separated by electrophoresis in denatured polyacrylamide gel at 10% (SDS-PAGE). Proteins were then transferred to nitrocellulose membranes (Merck Millipore ®, Massachusetts, USA) (Towbin et al., 1979). An absorber of transference 1× (0.25 mM Tris-HCl, 190 mM glicine, 0.1% SDS, 20% methanol) was used at 80 V for 30 min in a cold buffer. The membranes were blocked with TBST/milk (0.05% Tween 20, 10 mM Tris-HCl, pH 8, 150 mM NaCl, and 1% skimmed milk) for the subsequent immunodetection (Towbin et al., 1979). Membranes were coated with positive serum samples detected in the ELISA assay at a dilution of 1:100 in TBST/milk (0.05% Tween 20, 10 mM Tris-HCl, pH 8, 150 m MNaCl, and 1% skimmed milk). Then, a second IgG antibody species-specific for dog, conjugated to alkaline phosphatase (AP) (Cat. No. sc-2434, Santa Cruz Biotechnology) at a dilution of 1:5000 in TBST-milk was incubated. The strips were visualized using the Alkaline Phosphatase Conjugate Substrate commercial kit (Bio Rad, Cat. No. 170-6432). Positive and negative for T. cruzi infection dog serum samples were used as controls. Additional negative control using only the second antibody conjugated to AP was incubated to discard nonspecific bindings. Samples were considered positive for the WB technique when at least one band different from the negative controls was observed (Ramos-Ligonio et al., 2006).
2.4. Detection of T. cruzi through a real-time quantitative PCR
GAPDH gene was amplified by qPCR before the use of a DNA sample in order to discard the presence of endogenous PCR inhibitors in each sample. For detection of T. cruzi DNA qPCR assay was performed using the primers TCZ-F (5′-GCT CTT GCC CAC AMG GGT GC-3′) and TCZ-R (5′-CCA AGC GGA TAG TTC AGG-3′) in a real-time thermocycler (CFX96TM, Bio-Rad, California, USA). The primers used detected a 195-bp repeat sequence (satellite) present in T. cruzi DNA (Gonzalez et al., 1984; Schijman et al., 2011). Each PCR reaction contained 1× QuantiTect SYBR-Green PCR Master Mix (Qiagen), 0.3 mM of the specific primers (TCZ-F and TCZ-R), 2 μl of template DNA and nuclease-free water to obtain a final volume of 20 μl. The amplification was conducted under the following cycling conditions: denaturation at 95 °C for 15 min, followed by 50 cycles (95 °C for 10 s, 55 °C for 15 s and 72 °C for 10 s). After amplification, a melt curve was generated at 74–85 °C raising the temperature by 0.5 °C at each step. The melting temperature (Tm) expected for T. cruzi amplicon was 81 °C. DNA from dogs negative for T. cruzi infection and a master mix without DNA were included as negative controls. qPCR assays were carried out in triplicates and T. cruzi load was estimated by the absolute quantification method. The standard curve was done using serial dilutions (1:10) of T. cruzi epimastigotes of the H4 strain (genomic DNA) (1–1 × 107 parasite equivalents/ml). Data were analyzed using the software Bio-Rad® Manager 2.0 to determine the parasite load in the blood samples.
2.5. Statistical analysis
In order to perform an analysis in the absence of a reference standard (gold standard), all known positive cases detected by the serological (ELISA/WB) and/or qPCR techniques were combined to construct a reference standard outcome (Rutjes et al., 2007). With the construct of this reference standard, the sensitivity, specificity, positive and negative predictive values, and accuracy of the serological techniques and qPCR were calculated employing contingency tables in the WinEpiscope 2.0 software (Thrusfield et al., 2001). The contingency tables were used to determine the significance of the differences in the estimated prevalence between the serological and molecular techniques.
3. Results
3.1. Serological and molecular techniques
The prevalence estimated by the serological techniques was 25.8% (23/89) for dogs and 7.9% (7/89) for their owners. Trypanosoma cruzi DNA was found in 29.2% (26/89) and 10.1% (9/89) of the blood samples from dogs and owners, respectively. The combined prevalence calculated with positive samples detected by ELISA/WB and/or qPCR was 38.2% for dogs and 12.4% for owners (Table 1). No significant differences were found in the prevalence calculated using positive cases detected by ELISA/WB, qPCR and both techniques for both dogs and their owners (P > 0.05); however, dogs presented a higher number of positive cases compared to their owners (P < 0.001).
Table 1.
Prevalence and number of Trypanosoma cruzi infection cases diagnosed by ELISA and confirmed by Western blot (WB) technique (chronic cases) and real-time PCR (qPCR) (acute cases) in dogs and their owners from a rural community in southern Mexico
| Chronic and acute cases detected by serological and molecular techniques | Dogs | Humans |
|---|---|---|
| No. of samples examined (N) | 89 | 89 |
| No. of positive cases (ELISA/WB) | 8 | 2 |
| No. of positive cases (qPCR) | 11 | 4 |
| No. of positive cases (ELISA/WB + qPCR) | 15 | 5 |
| Prevalence calculated for ELISA/WB results, % (n/N) | 25.8 (23/89)a | 7.9 (7/89)b |
| Prevalence calculated for qPCR results, % (n/N) | 29.2 (26/89)a | 10.1 (9/89)b |
| Prevalence calculated for ELISA/WB + qPCR results, % (n/N) | 38.2 (34/89)a | 12.4 (11/89)b |
Note: Different superscript letters (a and b) in a row indicate significant differences (P ≤ 0.05).
Abbreviations: n, no. of positive cases; N, no. of samples examined.
The overall mean parasite load in dogs was 14.8 ± 11.1 parasite equivalents/ml (range: 2.5–32.7 parasite equivalents/ml). Only 15 of 26 qPCR-positive dog samples were found to be positive by serological techniques, the remaining 11 cases in the acute phase (with circulating parasites) had no detectable antibodies. In the case of their owners, the overall mean parasite load was 13.7 ± 8.4 parasite equivalents/ml (range: 5.4–33.0 parasite equivalents/ml) (Table 2). Regarding the qPCR-positive samples, 5 of 9 samples were positive by serology and the remaining 4 cases in the acute phase were not detected by ELISA/WB (Table 2). In this study, it was observed that serological techniques present a good detection threshold for cases of trypanosomiasis in humans and dogs, as long as the parasite loads are higher (>21 parasite equivalents/ml). In cases with low (1–10 parasite equivalents/ml) and medium (11–20 parasite equivalents/ml) levels of parasitemia, the serological tests showed deficiencies in detecting most of the positive cases.
Table 2.
Parasite load of Trypanosoma cruzi (parasite equivalents/ml) in blood samples of dogs and owners from a rural community from southern Mexico that were diagnosed as positive cases using real-time PCR (qPCR) and the proportion of these cases detected by ELISA and Western blot (WB) technique
| Parasite load levela | qPCR positive cases | Parasite loadb | Cases detected by ELISA/WB (%) |
|---|---|---|---|
| Dogs | |||
| Low | 14 | 2.5–8.7 (5.2 ± 1.7) | 5 (35.7) |
| Medium | 2 | 19.6–20.1 (19.9 ± 0.4) | 1 (50.0) |
| High | 10 | 22.9–32.7 (27.4 ± 3.5) | 9 (90.0) |
| Overall | 26 | 2.5–32.7 (14.8 ± 11.1) | 15 (57.7) |
| Humans | |||
| Low | 3 | 5.4–7.6 (6.6 ± 1.1) | 0 (0) |
| Medium | 5 | 10.0–18.1 (14.0 ± 3.1) | 4 (80.0) |
| High | 1 | 33.0 | 1 (100) |
| Overall | 9 | 5.4–33.0 (13.7 ± 8.4) | 5 (55.6) |
Low, 1–10 parasite equivalents/ml; medium, 11–20 parasite equivalents/ml; high, >21 parasite equivalents/ml.
Range (Mean ± SD) parasite equivalents/ml.
3.2. Comparation between techniques
Table 3 provides the sensitivity (%), specificity, positive and negative predictive values (%), and accuracy (%), and their respective 95% confidence intervals (95% CI) for the use of serology (ELISA/WB), qPCR and both techniques to identify dogs and owners infected with T. cruzi in the rural community studied. The sensitivity observed for each technique varied between dogs and humans. The main variation on the sensitivity between both diagnostic techniques was observed in humans, the values for qPCR being higher. The specificity and positive predictive values were equal for both techniques in both species (100%). For the false negative cases, a higher variation was also observed in human samples and the qPCR resulted in a lower value. When both techniques were used combined for the detection of positive cases, all of the calculated values improved with respect to the values obtained from the techniques used individually. The negative predictive values (true negatives) were better estimated than those obtained with each technique individually.
Table 3.
Sensitivity (%), specificity (%), positive and negative predictive values (%), and accuracy (%) and their respective 95% confidence intervals resulting from the use of serological techniques (ELISA + Western blot, ELISA/WB), real-time PCR (qPCR) and both techniques to identify chronic and acute cases of dogs and owners infected with Trypanosoma cruzi in a rural community from southern Mexico
| Serological techniques (ELISA/WB) | qPCR | Combined | |
|---|---|---|---|
| Dogs | |||
| Sensitivity (%) | 67.6 (51.9–83.4) | 76.5 (62.2–90.7) | 92.4 |
| Specificity (%) | 100 | 100 | 100 |
| False positive (%) | 0 | 0 | – |
| False negative (%) | 32.4 (19.1–49.2) | 23.5 (12.4–40.0) | – |
| Predictive value (positive) (%) | 100 | 100 | 100 |
| Predictive value (negative) (%) | 83.3 (74.3–92.3) | 87.3 (79.1–95.5) | 95.5 |
| True prevalence (%) | 38.2 (28.1–48.3) | 38.2 (28.1–48.3) | – |
| Apparent prevalence (%) | 25.8 (16.7–34.9) | 29.2 (19.8–38.7) | – |
| Accuracy (%) | 87.6 (80.8–94.5) | 91.0 (85.1–97.0) | 96.6 |
| Humans | |||
| Sensitivity (%) | 63.6 (35.2–92.1) | 81.8 (59.0–100) | 93.4 |
| Specificity (%) | 100 | 100 | 100 |
| False positive (%) | 0 | 0 | – |
| False negative (%) | 36.4 (15.2–64.6) | 18.2 (5.1–47.7) | – |
| Predictive value (positive) (%) | 100 | 100 | 100 |
| Predictive value (negative) (%) | 95.1 (90.5–99.8) | 97.5 (94.1–100) | 99.1 |
| True prevalence (%) | 12.4 (5.5–19.2) | 12.4 (5.5–19.2) | – |
| Apparent prevalence (%) | 7.9 (2.3–13.5) | 10.1 (3.8–16.4) | – |
| Accuracy (%) | 95.5 (91.2–99.8) | 97.8 (94.7–100) | 98.9 |
4. Discussion
In the present study, we compared the prevalence of T. cruzi estimated with the combined use of serological (ELISA/WB) and a molecular detection technique (qPCR) compared with both methods used in isolation, to identify positive cases of T. cruzi infection in dogs and their owners.
Most studies exploring the prevalence of T. cruzi in humans, dogs or other parasite reservoirs have been based on serological tests such as WB, immunofluorescence antibody test (IF), ELISA, indirect hemagglutination test (IHA) (Jiménez-Coello et al., 2015; Ballesteros-Rodea et al., 2018). However, one of the limitations of serological tests in humans and dogs is that they only detect exposure to the parasite (chronic phase) and do not provide evidence regarding possible recent infections in which it is not possible to detect an immune response (acute phase) (Bahia et al., 2002; Basso, 2013; Cardillo et al., 2015). The acute phase cases can be diagnosed with qualitative techniques in which the presence of the parasites in the blood is confirmed under light microscope in stained samples of blood or by concentration techniques, such as examination of microhematocrit (CFSPH, 2017). The main limitation is the threshold of detection, as infections with low parasite loads may not be identified by the laboratory technician leading to a false negative result. Likewise, the clinical signs are unnoticed by the infected person, so diagnosing new cases of infection can be difficult. The diagnosis in the acute phase of the infection would provide timely treatment that would help eliminate the parasite and reduce cardiac complications associated with severe acute infections (Menezes et al., 2011).
The simultaneous use of serological tests (ELISA/WB) and qPCR allowed identifying a higher prevalence of individuals infected with T. cruzi (both dogs and their owners) in comparison with cases detected using either serology or qPCR. The use of both approaches allowed the detection of chronic infection cases, which present antibodies (serology), as well as acute-phase infection cases (with parasitemia). ELISA/WB and the qPCR had high specificity to detect T. cruzi, first by identifying antibodies that recognize different polypeptide fractions in the complex antigenic mixtures of parasite antigens, and secondly by amplification of DNA of parasites present in the blood samples (Brasil et al., 2010; Torres-Gutierrez et al., 2015). However, the sensitivity of these techniques is variable because positive results are influenced by the stage of infection of the individual sampled. Some variability in the sensitivity values between serological techniques and qPCR was observed in the present study (Table 3); however, when the results from both techniques were combined, the sensitivity improved up to 93.4% for humans and 92.4% for dogs. The use of both techniques increases the probability to identify correctly a non-infected dog (95.5%) and a non-infected human (99.1%). This improvement in the detection was observed in 11 dog samples and 4 human samples that were negative for the ELISA/WB techniques, but positive for the qPCR technique. The latter suggests that these individuals were in the acute phase of the disease, therefore have not yet developed a measurable immune response (IgG) (Kierszenbaum et al., 1993). The same situation occurs in the case of the molecular technique, where 8 dog samples and 2 human samples that were negative for the qPCR test were positive for serology, indicating chronic infection of the hosts and thus, the lack of circulating parasites in the peripheral blood.
The prevalence of infection in dogs estimated in this study is similar to the prevalence of 29.9% reported by Carrillo-Peraza et al. (2014) using a rapid serological test in the same rural community (Molas). However, the prevalence reported here is higher than that reported in other studies conducted in the same region of Mexico. Thus, the prevalence calculated using the results of WB, IFI and PCR endpoint in dogs of another rural community (Tunkas) was 9.8%, while in the urban area of Capital city of Yucatan, the prevalence was 17.3% (Jiménez-Coello et al., 2008). In other countries, such as Argentina and Panama, the prevalence of infection in dogs was 60% and 11.1%, respectively (Gürtler et al., 2007; Pineda et al., 2011).
The present study showed that the prevalence of T. cruzi in dogs and their owners is similar to those reported for other parts of Yucatán and other states of México. Jiménez-Coello et al. (2010a) reported a prevalence of 34% in dogs and 8% in their owners from the south of Mérida, Yucatan, using IFI and WB. Similar prevalence relation, i.e., 21% in dogs and 7.1% in their owners was reported by Estrada-Franco et al. (2006) in central Mexico using ELISA, IFI and IHA. In Venezuela, the seroprevalence of infected dogs and infected people living in the same household were 83.2% and 17.8%, respectively (Crisante et al., 2006), and although several dogs were classified as acute cases, no molecular examination was carried out to confirm this status in the study of Crisante et al. (2006). The above data suggest that dogs play an important role in the maintenance and transmission of T. cruzi to people living in close association with dogs, and that complementary molecular studies would provide a better epidemiological picture of the situation.
Although the seroprevalence in most of studies that include dogs and/or humans may be of epidemiological importance, the real prevalence may be underestimated because the acute phase (new infections and reinfections) has not been detected by serological techniques. The use of PCR as a complementary test for the diagnosis of Chagas disease is important due to its capacity for the detection of T. cruzi DNA (Gilber et al., 2013; Moreira et al., 2013; Abras et al., 2018). The combination of this technique with the serological tests allows the detection of cases in which parasitaemia is very low or when the infection is recent, thus improving the epidemiological surveillance of Chagas disease (Piron et al., 2007; Schijman et al., 2011; Seiringer et al., 2017; Rodrigues-dos-Santos et al., 2018). Parasite load can explain the efficacy of serological and molecular detection of the parasite, and it is advisable to include whenever possible this analytical evaluation in epidemiological surveys. The importance of this combination could be observed in the present study when 11 infected dogs were false negative for serology, which can contribute to the persistence of T. cruzi by becoming reservoirs of this parasite and more worrying in the case of four people which were infected but were not identified by serological tests, which, due to not being treated promptly, would suffer the effects of this parasite in the medium- and long-term. Therefore, it is advisable to include, whenever possible, the use of molecular tests alongside serological tests in epidemiological surveys.
Although the differences in prevalence estimated in the present study based on the results of the serological tests, qPCR and combined techniques were not statistically significant, the detection of higher number of infected human cases in the community using both techniques should be an encouragement to the health center services of countries to adopt this approach to improving the surveillance system in order to provide a timely medical service and reduce the impact of American trypanosomiasis on the health of the human population. This improvement in surveillance may help evaluate the effectiveness of official programmes aimed at reducing the presentation of new cases of this disease.
5. Conclusions
The combined use of serological techniques and qPCR allowed us to identify highest prevalence of T. cruzi in humans and dogs compared to the use of only one of these screening tools. The larger number of cases can be the result of the combined detection of cases in the acute phase (qPCR) and in the chronic phase (ELISA/WB) of the disease. In the acute phase cases, low parasite load was associated with negative serology. The use of ELISA/WB and qPCR tests in combination improved the sensitivity and specificity of the screening method for humans and dogs. This approach may allow improving the quality of the epidemiological studies in both hosts.
Funding
This research was partially funded by the Programa de Desarrollo del Personal Docente (PRODEP) and Cell Biology Laboratory funds CIR-UADY.
Ethical approval
Written informed consent was obtained from all participants in the study (Secretaria de Salud, NOM-012-SSA3-2012). Written informed consent was also obtained from the owners to allow blood samples to be taken from the dogs.
CRediT author statement
José I. Chan-Pérez: formal analysis, methodology, supervision, validation, writing - review & editing. Juan F.J. Torres-Acosta: data curation, formal analysis, writing - review & editing. Antonio Ortega-Pacheco: conceptualization, investigation, methodology, resources, supervision, writing–original draft, writing - review & editing. Ivonne B. Hernández-Cortazar: methodology, investigation, validation, Nohemi Cigarroa-Toledo: formal analysis, methodology, writing - review & editing. Matilde Jiménez-Coello: conceptualization, funding acquisition, investigation, project administration, supervision, writing - review & editing. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the “Centro Integrador de Bienestar” from the community of Molas, Yucatan, for their help in contacting people and samplings.
References
- Abras A., Ballart C., Llovet T., Roig C., Gutiérrez C., Tebar S., et al. Introducing automation to the molecular diagnosis of Trypanosoma cruzi infection: A comparative study of sample treatments, DNA extraction methods and real-time PCR assays. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce-Fonseca M., Carrillo-Sánchez S.C., Molina-Barrios R.M., Martínez-Cruz M., Cedillo-Cobián J.R., Henao-Díaz Y.A., Rodríguez-Morales O. Seropositivity for Trypanosoma cruzi in domestic dogs from Sonora, Mexico. Infect. Dis. Poverty. 2017;6:120. doi: 10.1186/s40249-017-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia M.T., Tafuri W.L., Caliari M.V., Veloso V.M., Carneiro C.M., Machado-Coelho G.L.L., de Lana M. Comparison of Trypanosoma cruzi infection in dogs inoculated with blood or metacyclic trypomastigotes of Berenice-62 and Berenice-78 strains via intraperitoneal and conjunctival routes. Rev. Soc. Bras. Med. Trop. 2002;35:339–345. doi: 10.1590/s0037-86822002000400010. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Rodea G., Martínez-Cuevas T.I., Jímenez-Ramos B., Campos A.A. Chagas disease: An overview of diagnosis. J. Microbiol. Exp. 2018;6:151–157. [Google Scholar]
- Basso B. Modulation of immune response in experimental Chagas disease. World J. Exp. Med. 2013;3:1–10. doi: 10.5493/wjem.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P.E.E.A., De Castro L., Hasslocher-Moreno A.M., Sangenis L.H.C., Braga J.U. ELISA versus PCR for diagnosis of chronic Chagas disease: Systematic review and meta-analysis. BMC infect. Dis. 2010;10:337. doi: 10.1186/1471-2334-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo F., de Pinho R.T., Antas P.R., Mengel J. Immunity and immune modulation in Trypanosoma cruzi infection. Pathog. Dis. 2015;73 doi: 10.1093/femspd/ftv082. ftv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Peraza J.R., Manrique-Saide P., Rodríguez-Buenfil J.C., Escobedo-Ortegón J.F., Rodríguez-Vivas R.I., Bolio-González M.E., et al. Estudio serológico de la tripanosomiasis Americana y factores asociados en perros de una comunidad rural de Yucatán, México. Arch. Med. Vet. 2014;46:75–81. [Google Scholar]
- CFSPH . Center for Food Security and Public Health, Iowa State University; Ames, IA, USA: 2017. American trypanosomiasis. Factsheets.http://www.cfsph.iastate.edu/Factsheets/pdfs/trypanosomiasis_american.pdf [Google Scholar]
- Crisante G., Rojas A., Teixeira M.M.G., Anez N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop. 2006;98:247–254. doi: 10.1016/j.actatropica.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Estrada-Franco J.G., Bhatia V., Diaz-Albiter H., Ochoa-Garcia L., Barbabosa A., Vazquez-Chagoyan J.C., et al. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg. Infect. Dis. 2006;12:624–630. doi: 10.3201/eid1204.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Guido J.S., Espejel-Carvajal I. Universidad Autónoma de Yucatán; Mérida, Yucatán, México: 1994. Tipos de vegetación de la península de Yucatán: Etnoflora yucatanense. [Google Scholar]
- Gilber S.R., Alban S.M., Gobor L., Bescrovaine J., de O., Myiazaki M.I., Thomaz-Soccol V. Comparison of conventional serology and PCR methods for the routine diagnosis of Trypanosoma cruzi infection. Rev. Soc. Bras. Med. Trop. 2013;46:310–315. doi: 10.1590/0037-8682-0046-2013. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Prediger E., Huecas M.E., Nogueira N., Lizardi P.M. Minicromosomal repetitive DNA in Trypanosoma cruzi: Its use in a high-sensitivity parasite detection assay. Proc. Natl. Acad. Sci. USA. 1984;81:3356–3360. doi: 10.1073/pnas.81.11.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler R.E., Cardinal M.V. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015;151:32–50. doi: 10.1016/j.actatropica.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Gürtler R.E., Cecere M.C., Lauricella M.A., Cardinal M.V., Kitron U., Cohen J.E. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal S., Nord C., Lappalainen M., Evengard B. Rapid and sensitive diagnosis of Toxoplasma gondii infections by PCR. Clin. Microbiol. Infect. 2004;10:922–950. doi: 10.1111/j.1469-0691.2004.00948.x. [DOI] [PubMed] [Google Scholar]
- Jiménez-Coello M., Acosta-Viana K., Guzmán-Marín E., Bárcenas-Irabién A., Ortega-Pacheco A. American tripanosomiasis and associated risk factors in owned dogs from the major city of Yucatan, Mexico. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:1–4. doi: 10.1186/s40409-015-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Coello M., Guzmán-Marin E., Ortega-Pacheco A., Acosta-Viana K.Y. Serological survey of American tripanosomiasis in dogs and their owners from an urban area of Merida, Yucatan, Mexico. Transbound. Emerg. Dis. 2010;57:33–36. doi: 10.1111/j.1865-1682.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- Jiménez-Coello M., Ortega-Pacheco A., Guzmán-Marin E., Guiris-Andrade D.M., Martínez-Figueroa L., Acosta-Viana K.Y. Stray dogs as reservoirs of the zoonotic agents Leptospira interrogans, Trypanosoma cruzi, and Aspergillus spp. in an urban area of Chiapas in Southern Mexico. Vector Borne Zoonotic Dis. 2010;10:135–141. doi: 10.1089/vbz.2008.0170. [DOI] [PubMed] [Google Scholar]
- Jiménez-Coello M., Poot-Cob M., Ortega-Pacheco A., Guzmán-Marin E., Ramos-Ligonio A., Sauri-Arceo C., Acosta-Viana K. American trypanosomiasis in dogs from an urban and rural area of Yucatan, Mexico. Vector Borne Zoonotic Dis. 2008;8:755–761. doi: 10.1089/vbz.2007.0224. [DOI] [PubMed] [Google Scholar]
- Jimenez-Coello M., Shelite T., Castellanos-Gonzalez A., Saldarriaga O., Rivero R., Ortega-Pacheco A., et al. Efficacy of recombinase polymerase amplification to diagnose Trypanosoma cruzi infection in dogs with cardiac alterations from an endemic area of Mexico. Vector Borne Zoonotic Dis. 2018;18:417–423. doi: 10.1089/vbz.2017.2258. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Moretti E., Sztein M.B. Molecular basis of Trypanosoma cruzi-induced immunosuppression. Altered expression by activated human lymphocytes of molecules which regulate antigen recognition and progression through the cell cycle. Biol. Res. 1993;26:197–207. [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Menezes C., Costa G.C., Gollob K.J., Dutra W.O. Clinical aspects of Chagas disease and implications for novel therapies. Drug. Dev. Res. 2011;72:471–479. doi: 10.1002/ddr.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira O.C., Ramírez J.D., Velázquez E., Melo M.F., Lima-Ferreira C., Guhl F., et al. Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: A sub study from the BENEFIT trial. Acta Trop. 2013;125:23–31. doi: 10.1016/j.actatropica.2012.08.020. [DOI] [PubMed] [Google Scholar]
- OPS . Guía para el diagnóstico y el tratamiento de la enfermedad de Chagas. Organización Panamericana de la Salud; Washington, D.C., USA: 2018. https://iris.paho.org/bitstream/handle/10665.2/49653/9789275320433_spa.pdf [Google Scholar]
- Ortega-Pacheco A., Rodriguez-Buenfil J., Bolio-Gonzalez M., Sauri-Arceo C., Jiménez-Coello M., Linde-Forsberg C. A survey of dog populations in urban and rural areas of Yucatan, Mexico. Anthrozoös. 2007;20:261–274. [Google Scholar]
- Pineda V., Saldaña A., Monfante I., Santamaría A., Gottdenker N.L., Yabsley M.J., et al. Prevalence of trypanosome infections in dogs from Chagas disease endemic regions in Panama, Central America. Vet. Parasitol. 2011;178:360–363. doi: 10.1016/j.vetpar.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Piron M., Fisa R., Casamitjana N., López-Chejade P., Puig L., Vergés M., et al. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Ramos-Ligonio A., Ramírez-Sánchez M.E., González-Hernández J.C., Rosales-Encina J.L., López-Monteon A. Prevalencia de anticuerpos contra Trypanosoma cruzi en donadores de sangre del IMSS, Orizaba, Veracruz, México. Sal. Pub. Mex. 2006;48:13–21. doi: 10.1590/s0036-36342006000100004. [DOI] [PubMed] [Google Scholar]
- Rodrigues-dos-Santos Í., Melo M.F., de Castro L., Hasslocher-Moreno A.M., do Brasil P.E.A.A., Silvestre de Sousa A., et al. Exploring the parasite load and molecular diversity of Trypanosoma cruzi in patients with chronic Chagas disease from different regions of Brazil. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutjes A.W.S., Reitsma J.B., Coomarasamy A., Khan K.S., Bossuyt P.M.M. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol. Assess. 2007;11:1–86. doi: 10.3310/hta11500. [DOI] [PubMed] [Google Scholar]
- Schijman A.G., Bisio M., Orellana L., Sued M., Duffy T., Mejia-Jaramillo A.M., et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Trop. Dis. 2011;5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiringer P., Pritsch M., Flores-Chaves M., Marchisio E., Helfrich K., Mengele C., et al. Comparison of four PCR methods for efficient detection of Trypanosoma cruzi in routine diagnostics. Diagn. Microbiol. Infec. Dis. 2017;88:225–232. doi: 10.1016/j.diagmicrobio.2017.04.003. [DOI] [PubMed] [Google Scholar]
- SSA Programa Sectorial de Salud: Prevención y control de la enfermedad de Chagas 2013-2018. Secretaría de Salud de Mexico, Mexico City, Mexico. 2013 http://www.cenaprece.salud.gob.mx/descargas/pdf/PAEPrevencionControlEnfermedadChagas2013.2018.pdf [Google Scholar]
- Thrusfield M., Ortega C., de Blas I., Noordhuizen J.P., Frankena K. WinEpiscope 2.0: Improved epidemiological software for veterinary medicine. Vet. Rec. 2001;148:567–572. doi: 10.1136/vr.148.18.567. [DOI] [PubMed] [Google Scholar]
- Torres-Gutierrez E., Barrios-Palacios D., Ruiz-Hernández A.L., Cabrera-Bravo M., Guevara-Gómez Y., Rojas-Wastavino G., et al. Identification of immunodominant components of an isolate of Trypanosoma cruzi by immunoblot and its standardization for diagnostic purposes. Gac. Med. Mex. 2015;151:6–13. [PubMed] [Google Scholar]
- Towbin H.T., Stahelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocelulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travi B.L. Considering dogs as complementary targets of Chagas disease control. Vector Borne Zoonotic Dis. 2019;19:90–94. doi: 10.1089/vbz.2018.2325. [DOI] [PubMed] [Google Scholar]