Abstract
Species of the genus Pelecitus Railliet & Henry, 1910 the most widely distributed avian filariae in Africa and South America. Zoonotic cases in humans were reported in South America. While investigating the filarial fauna of wild animals in Malaysia, we discovered an undescribed filaria from the swollen footpad of the left leg of Copsychus malabaricus (Scopoli) in Pahang, Peninsular Malaysia. Adults of both sexes have a corkscrew-shaped body. Based on comparison of their morphological characteristics (i.e. pre-oesophageal cuticular ring distinct, oesophagus divided, vulva protuberant and situated at the level of anterior half of oesophagus, spicules strongly sclerotized and left spicule with broad blade) with other Pelecitus species, they are here described as Pelecitus copsychi Uni, Mat Udin & Martin n. sp. Multi-locus sequence analyses based on seven genes (12S rDNA, cox1, 18S rDNA, 28S rDNA, MyoHC, rbp1 and hsp70) were performed to determine the phylogenetic position of the new species. The calculated p-distance between the cox1 gene sequences for P. copsychi n. sp. and Pelecitus fulicaeatrae (Diesing, 1861) was 14.1%. Intraspecific genetic variation between two individuals of the new species was 0.4%. In both the Bayesian inference and maximum-likelihood trees, P. copsychi n. sp. was positioned in the second clade of ONC5, containing three genera of the subfamily Dirofilariinae (Foleyella Seurat, 1917, Pelecitus and Loa Stiles, 1905). Immunostaining and molecular analyses remained negative for the presence of Wolbachia endosymbionts. Our findings corroborate the division of the subfamily Dirofilariinae into ONC3 with Dirofilaria Railliet & Henry, 1911 and ONC5 with Pelecitus.
Keywords: Dirofilariinae, Foleyella candezei, Multi-locus sequence analyses, Indomalaya, Pelecitus fulicaeatrae
Graphical abstract
Highlights
-
•
Pelecitus copsychi n. sp. is described from Copsychus malabaricus in Malaysia.
-
•
The filariae occur free and cause swelling in the footpad of the host.
-
•
Copsychus malabaricus is a new host record for Pelecitus.
-
•
Molecular analysis positioned the new species in clade ONC5 of the Onchocercidae.
-
•
Molecular analyses corroborate division of the Dirofilariinae into ONC3 and ONC5.
1. Introduction
Filarial nematodes within the family Onchocercidae Leiper, 1911 that infect avian hosts are an important taxonomic group. Indeed, based on morpho-anatomical criteria, more than one third of the approximately 600 described filarial species are avian filariae, divided into 16 genera (Anderson & Bain, 1976; Bain, 2002; Bartlett, 2008). Among them, only the genus Pelecitus Railliet & Henry, 1910 belongs to the subfamily Dirofilariinae Sandground, 1921, which includes the human filaria Loa loa (Cobbold, 1864) and the canine filaria Dirofilaria immitis (Leidy, 1856) (Anderson & Bain, 1976). The host spectrum of all avian filarial genera is limited to birds, except for Pelecitus, which includes three species that parasitize mammals (Bartlett & Greiner, 1986; Bartlett, 2008). The genus Pelecitus is cosmopolitan, with currently 20 species considered valid. Most of these are distributed in Africa and South America, except for Pelecitus fulicaeatrae (Diesing, 1861), which has been reported worldwide (Vanderburgh et al., 1984; Bartlett & Greiner, 1986) (see Supplementary Table S1 for details).
Bartlett & Greiner (1986) revised the genus Pelecitus. They retained 14 species as valid and also transferred two filarial species parasitic in mammals that had previously been placed in the genus Dirofilaria Railliet & Henry, 1911 to Pelecitus. Since then, a further two species, Pelecitus spiralis (Annett, Dutton & Elliott, 1901) from Ploceus aurantius (Vieillot) (Passeriformes) and Pelecitus major (Annett, Dutton & Elliott, 1901) from Ploceus nigricollis brachypterus Swainson in Nigeria, were assigned to Pelecitus (see Bartlett & Anderson, 1987a). Furthermore, two new congeners were described. In Mexico, Pelecitus meridionaleporinus Jiménez-Ruiz, Gardner, Cervantes & Lorenzo, 2004 was described from Lepus flavigularis Wagner (Lagomorpha) (Jiménez-Ruiz et al., 2004), and in Australia, Pelecitus bartneri Spratt, 2010 was described from Psephotus chrysopterygius Gould (Psittaciformes) (Spratt, 2010; Supplementary Table S1).
The pathology caused by Pelecitus spp. in their avian hosts includes swollen joints, lameness and chronic inflammatory tenosynovitis (Greve et al., 1982; Allen et al., 1985; Bartlett, 2008). Adult worms are generally localized in the tendons of the legs and feet, sometimes in the muscles of the legs or in the articulations of the tibia or knee joint (Bartlett & Greiner, 1986; Supplementary Table S1). Recently, Muñoz-García et al. (2018) reported a severe tenosynovitis caused by Pelecitus sp. found in swollen nodules on both legs of a crested caracara Caracara cheriway (von Jacquin) with signs of pain in Mexico. Rare cases of zoonotic infections caused by Pelicitus sp. have been described in humans (Bortero et al., 1984; Bain et al., 2011). Bain et al. (2011) reported Pelecitus sp. from the iris of a man in Brazil and suggested that specimens that had been recovered from the anterior chamber of the eye of a patient in Colombia and identified as Loaina sp. by Bortero et al. (1984) belonged to Pelecitus as well.
In Indomalaya, Pelecitus ceylonensis Dissanaike, 1967 was recovered from two naturally infected crows (Passeriformes) as well as from experimentally infected ash doves (Columbiformes) and chickens (Galliformes) in Sri Lanka (Dissanaike, 1967; Supplementary Table S1). In addition, Pelecitus galli Dissanaike & Fernando, 1974 was described from Gallus gallus spadiceus (Bonnaterre) (Galliformes) in Tasek Bera, Pahang, Malaysia (Dissanaike & Fernando, 1974). Although 36 species of filarial parasites from 22 genera have previously been recorded in vertebrates in Malaysia (Yen, 1983; Gibbons, 2010; Uni et al., 2017, 2020), P. galli is the only species of Pelecitus reported in Malaysia to date.
During a scientific inventory of the Krau Wildlife Reserve, Pahang, Malaysia, organized by the Department of Wildlife and National Parks, Malaysia in 2013, we collected nematodes from the swollen footpad of the leg of a white-rumped shama Copsychus malabaricus (Scopoli) (Passeriformes) caught in the primary forest. Herein, we describe the specimens as Pelecitus copsychi Uni, Mat Udin & Martin n. sp. and provide their molecular characterisation, revealing a close association with P. fulicaeatrae from birds and Foleyella candezei (Fraipont, 1882) from squamates in Africa (Bartlett, 1986).
A recent study on the phylogenetic relationships within the family Onchocercidae included few species of avian filariae, namely Aproctella alessandroi Bain, Petit, Kozek & Chabaud, 1981, Cardiofilaria pavlovskyi Strom, 1937 and P. fulicaeatrae (see Lefoulon et al., 2015). In the present study, newly generated as well as previously published partial sequences of two mitochondrial (12S rDNA and cox1) and five nuclear genes (18S rDNA, 28S rDNA, the myosin heavy chain (MyoHC), RNA polymerase II large subunit (rbp1) and 70 kDa heat-shock proteins (hsp70)) were used to study the phylogenetic interrelationships within the Onchocercidae, including a wider range of avian filariae.
2. Materials and methods
2.1. Collection of hosts and parasites
A scientific inventory, organized by the Institute of Biodiversity, Department of Wildlife and National Parks, Malaysia, in Sungai Teris (3°36′42.6″N, 102°06′55.9″E), Krau Wildlife Reserve, Pahang, Malaysia, was undertaken between 21 May 2013 and 27 May 2013. During this inventory, a white-rumped shama (ID no. A8) with a swollen footpad of the left leg was examined for parasites. The swollen footpad was dissected under a stereomicroscope and 16 filarial parasites (12 females and 4 males) removed for subsequent morphological and molecular analyses.
2.2. Morphological study
Adult worms (8 females and 4 males) were fixed in 70% ethanol for morphological examination. Specimens were cleared in lactophenol (R & M Chemicals, Essex, UK) and drawn under a compound microscope equipped with a camera lucida (Olympus U-DA, Olympus, Tokyo, Japan). Measurements are in micrometres unless otherwise indicated and are given as the range; body length was measured following the helical coils. Thick blood smears were made and stained with 3% Giemsa solution (pH 7.4). One female fixed in 70% ethanol was embedded in paraffin, and sections were stained with haematoxylin and eosin (HE).
2.3. Molecular analysis of filarial nematodes
Four females were transferred directly into 80% ethanol and stored for molecular analysis. DNA was extracted from two female fragments (ID nos. 168-4 and 268-5) using the QIAamp DNA Micro Kit (Qiagen, Hilden, Germany). Samples were incubated at 56 °C with proteinase K for 4 h. Polymerase chain reaction (PCR) DNA amplification targeted partial sequences of seven genes: two mitochondrial genes, 12S rDNA (c.450 bp) and cox1 (c.600 bp); and five nuclear genes, 18S rDNA (c.740 bp), 28S rDNA (c.900 bp), MyoHC (c.785 bp), rbp1 (c.640 bp) and hsp70 (c.610 bp). PCR reactions were processed in a final volume of 20 μl under the conditions summarised in Supplementary Table S2 (Casiraghi et al., 2001, 2004; Lefoulon et al., 2015).
Samples were sequenced at the Muséum National d’Histoire Naturelle (MNHN), using a MiSeq Illumina. Libraries were prepared following the protocol of Meyer & Kircher (2010). The reads were paired-ends with a theoretical size of 250 bp. For each gene, reads were mapped against the P. fulicaeatrae sequence (Supplementary Table S2) using Bowtie2 v2.4.1 (Langmead & Salzberg, 2012) with the following parameters: seed length of 18 bp; “very sensitive local” mode; mixed or discordant pair-end reads not allowed. The mapped reads were excluded if their length was inferior to 75 bp, or if more than 1% of their length was soft-clipped or if their edit distance was superior to 5% of their length. The remaining reads were assembled against their sequence of reference using IGV v2.9.4 (Robinson et al., 2011).
2.4. Filarial cox1 gene analysis
A DNA barcoding approach based on the cox1 marker was used to identify the specimens of Pelecitus from C. malabaricus. The cox1 marker was chosen over the 12S rDNA marker for its greater robustness (fewer indels, less susceptible to dataset variation) (Ferri et al., 2009). The cox1 sequence divergence was estimated by the number of base differences per site between two sequences (p-distance) using MEGA version 11 (Tamura et al., 2021). Cox1 sequences generated during the present study and those from the literature were analysed. Pairwise comparisons between the selected 48 cox1 sequences were processed, with each sequence representing a species (Supplementary Table S3).
2.5. Phylogenetic analyses
Sequences generated during the present study and those from the literature (Supplementary Table S3) were aligned using SATe v2.2.7 (Liu et al., 2009). Fifty-one sequences for 50 species belonging to 27 genera were included in the analyses and their phylogenetic relationships inferred using Bayesian inference as implemented in MrBayes 3 (Ronquist & Huelsenbeck, 2003). A partitioned model was implemented to estimate evolution parameters separately for each gene. For the analyses, the weighted average of the best-fit evolution models in the GTR+G landscape was used for each gene. Two runs were performed using five million steps with four chains, with tree sampling every 1000 generations; the first 25% of the trees were discarded as “burn-in” and posterior probabilities were calculated from the remaining trees.
In addition, a phylogeny based on the maximum-likelihood (ML) was implemented. For each gene, the best-fitting substitution model was determined using the corrected version of the Akaike Information Criterion (AICc) in JModelTest v2.1.10 analyses (Guindon & Gascuel, 2003). The TPM1uf+I+G was the best fit for 12S rDNA; TPM1+I+G for 18S rDNA; TVM+I+G for 28S rDNA; GTR+I+G for cox1; TPM2uf+I+G for hsp70; TrN+G for MyoHC and TPM3uf+G for rbp1. To root the trees, two species were included as the outgroup: Filaria latala Chabaud & Mohammad, 1989 (Spirurida: Filariidae) and Protospirura muricola Gedoelst, 1916 (Spirurida: Spiruridae). The phylogenetic relationships of the Onchocercidae were inferred by the ML on the partitioned concatenated dataset. The program was executed by generating 10 random start trees with 1000 bootstraps using RaxML-NG (Kozlov et al., 2019).
2.6. Wolbachia detection
Sections of a female of P. copsychi n. sp. were stained with a rabbit polyclonal antiserum raised against the surface protein of Wolbachia from Brugia pahangi (Buckley & Edeson, 1956) (1:2000 dilution) as described by Kramer et al. (2003) and Ferri et al. (2011).
In addition, we screened for Wolbachia endosymbionts by nested PCR covering six genes (16S rDNA, ftsZ, dnaA, coxA, fbpA and gatB), as described by Lefoulon et al. (2016).
3. Results
3.1. Pelecitus copsychiUni, Mat Udin & Martin n. sp.
3.1.1. Taxonomic summary
Type-host: Copsychus malabaricus (Scopoli) (Passeriformes: Muscicapidae), white-rumped shama.
Type-locality: Sungai Teris (3°36′42.6″N, 102°06′55.9″E), Krau Wildlife Reserve, Pahang, Malaysia.
Type-material: The holotype female (MNHN-IN-107YT) and allotype male (MNHN-IN-108YT) were deposited in the Muséum National d’Histoire Naturelle, Paris, France. Paratypes (7 females: Pf8-1–7; 3 males: Pm8-9–11) were deposited in the Institute of Biological Sciences, Universiti Malaya, Malaysia. Collection date: 24 May 2013.
Site in host: Adult worms occurred free in the swollen footpad of the left leg.
Representative DNA sequences: Sequence data for P. copsychi n. sp. were deposited in the GenBank database as follows: cox1 (OK480041 and OK480043), 12S rRNA (OK480976 and OK480977), 18S rRNA (OK481072 and OK481073), MyoHC (OK493401 and OK493402), hsp70 (OK493398 and OK493399), rbp1 (OK493403 and OK493404) and 28S rRNA (OK481079 and OK481080).
ZooBank registration: To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:17738217-2468-49CF-A6CA-2208134E5209. The LSID for the new name Pelecitus copsychi Uni, Mat Udin & Martin n. sp. is urn:lsid:zoobank.org:act:8BF6174D-DE38-4F83-9F64-6768E8F8287C.
Etymology: The specific epithet is derived from the genus name of the type-host.
3.1.2. Description
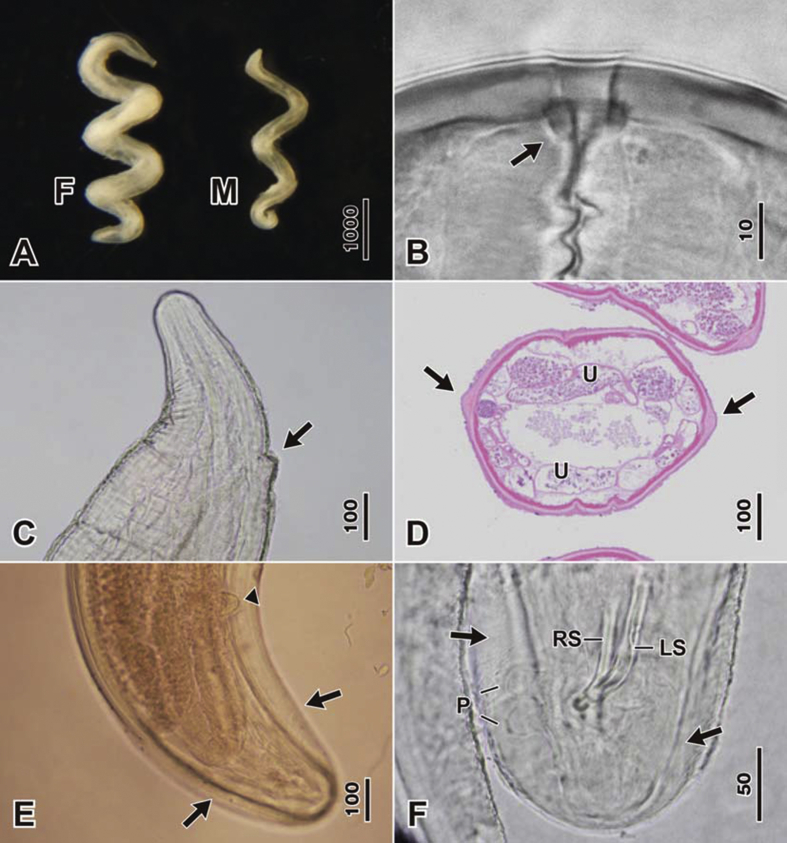
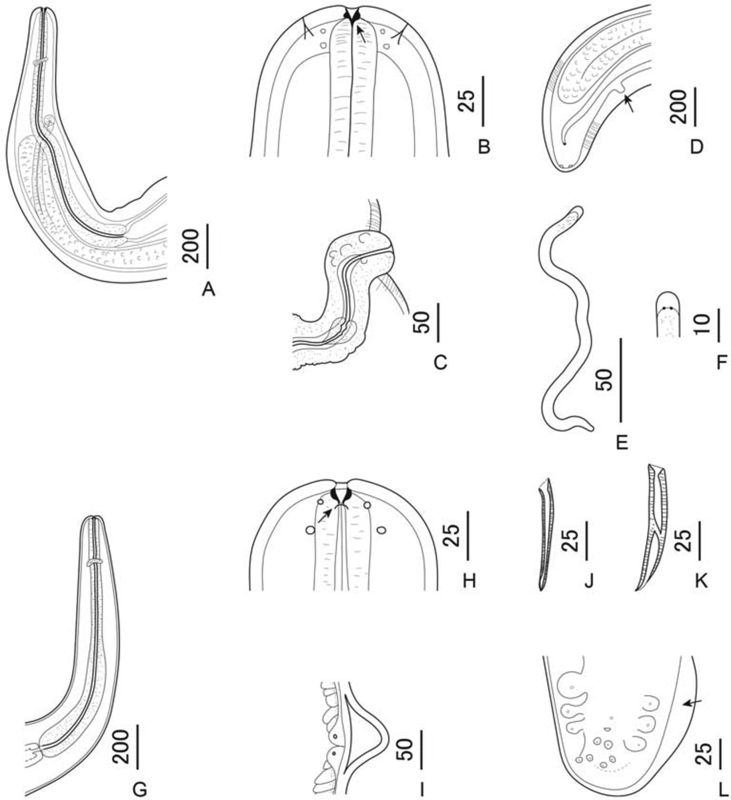
General. [Table 1; Fig. 1, Fig. 2.] Body corkscrew-shaped in both sexes, in form of dextral helix with 3–4 rotations (Fig. 1A). Body width uniform over most of length but tapering gradually at both ends. Cephalic papillae arranged in four submedian pairs, not markedly protuberant. Amphids small, lateral, not salient. Pre-oesophageal cuticular ring present and distinct (Fig. 1, Fig. 2B). Oesophagus clearly divided into anterior muscular and posterior glandular portions (Fig. 2A, G). Both sexes with rounded posterior extremity. Lateral alae present, broadening towards posterior extremity in both sexes (Fig. 1D–F). Cuticle thick, with conspicuous, closely-spaced, transverse striations.
Table 1.
Comparative morphometric data for Pelecitus copsychi n. sp. and congeners recorded from avian hosts in Indomalaya and Australia
| Species | Pelecitus copsychi n. sp.a | Pelecitus ceylonensis Dissanaike, 1967 | Pelecitus galli Dissanaike & Fernando, 1974 | Pelecitus bartneri Spratt, 2010 |
|---|---|---|---|---|
| Host | Copsychus malabaricus (Muscicapidae) | Ash dove (Columbidae), chicken (Phasianidae), crow (Corvidae) | Gallus gallus spadiceus (Phasianidae) | Psephotus chrysopterygius (Psittacidae) |
| Locality | Sungai Teris, Pahang, Malaysia | Colombo, Sri Lanka | Tasek Bera, Pahang, Malaysia | Brisbane, Queensland, Australia |
| Reference | Present study | Dissanaike (1967) | Dissanaike & Fernando (1974) | Spratt (2010) |
| Female | (n = 8) | |||
| Body length (mm) | 8.0 (4.5–8.7) | 6.0–9.6 | 15.6–20.4 | 13.3–15.5 |
| Body width at midbody | 570 (390–570) | 320–460 | 550–650 | 530–901 |
| Nerve-ring from anterior extremity | 193 (168–213) | 120–155 | 170–190 | 159–186 |
| Vulva from anterior extremity | 420 (330–500) | 335–440 | 670–780 | 594–1113 |
| Muscular oesophagus length | 450 (375–610) | Not divided | Not divided | 280–345 |
| Glandular oesophagus length | 570 (530–800) | – | – | 450–504 |
| Oesophagus length | 1020 (1020–1250) | 580–700 | 850–870 | 797 (mean)b |
| Tail length | 138 (80–138) | 50–88 | 150–180 | 150–169 |
| Microfilaria | (n = 10) | |||
| Body length | 158–183 | 190–260 | 180–295 | 207–212 |
| Body width | 3–5 | 5–6 | 6.7–8.2 | 4–5 |
| Male | (n = 4) | |||
| Body length (mm) | 4.6 (4.5–4.6) | 3.55–5.3 | 9–11 | 5.9–7.3 |
| Body width at midbody | 350 (280–380) | 185–300 | 400–500 | 344–466 |
| Nerve-ring from anterior extremity | 175 (163–175) | 125–150 | 140–160 | 158–170 |
| Muscular oesophagus length | 480 (310–600) | Not divided | Not divided | 260–292 |
| Glandular oesophagus length | 670 (650–710) | – | – | 345–413 |
| Oesophagus length | 1150 (980–1150) | 460–600 | 720–825 | 641 (mean)b |
| Left spicule length | 80 (80–90) | 68–82 | 85–95 | 83–94 |
| Right spicule length | 73 (62–73) | 55–70 | 70–90 | 67–77 |
| Tail length | 55 (55–68) | 42–55 | 40–48 | 48–58 |
Measurements of the holotype female and allotype male of Pelecitus copsychi n. sp. are present first, followed by the range, including the type-specimen, in parentheses.
Fig. 1.
Light micrographs of Pelecitus copsychi n. sp. A Corkscrew-shaped body of female (F) and male (M). B Pre-oesophageal cuticular ring (arrow) of female. C Anterior part of female, lateral view showing the protruding vulva (arrow). D Transverse section of female at midbody showing the lateral alae (arrows) and uteri with microfilariae (U) (HE staining). E Posterior part of female, ventral view showing post-deirid (arrowhead) and lateral alae (arrows). F Posterior part of male, ventral view showing lateral alae (arrows), pedunculate papillae (P), left spicule (LS) and right spicule (RS). Scale-bars are in micrometres.
Fig. 2.
Line drawings of Pelecitus copsychi n. sp. Females (A–D), microfilariae (E, F) and males (G–L). A Anterior region, right sublateral view. B Anterior extremity, dorsoventral view. Note the two pairs of cephalic papillae and amphids and the pre-oesophageal cuticular ring (arrow). C Protruding vulva, right lateral view. D Posterior extremity, ventral view with post-deirid arrowed. E Microfilaria with sheath and blunt tail. F Head with loose sheath. G Anterior region, lateral view. H Anterior extremity, lateral view. Note amphid (arrow). I Lateral ala at posterior end. J Right spicule, dorsoventral view. K Left spicule, dorsoventral view. L Male posterior extremity, ventral view. Note caudal ala (arrow). Scale-bars are in micrometres.
Female. [Based on 8 gravid specimens.] Vulva located at level of anterior half of oesophagus, roughly in region of junction between muscular and glandular oesophagus; ratio of distance of vulva from anterior extremity to total oesophagus length: 0.31–0.43. Vulva situated on protuberant cone of tissue (Fig. 1, Fig. 2C). Vagina directed posteriorly. Post-deirid at 533–620 from caudal extremity, on left side (Fig. 1, Fig. 2D).
Microfilaria. [Based on 10 specimens from anterior parts of uteri.] Loose sheath present, extending past anterior extremity of body. Extremities bluntly rounded (Fig. 2E and F). Microfilariae not found in thick blood smears of infected host.
Male. [Based on 4 specimens.] Caudal alae present (Fig. 1, Fig. 2); hyaline or granular inclusions not observed within caudal alae. Spicules well-sclerotized, unequal, dissimilar; right spicule short and slender, left spicule short and broad with well-defined handle and lamina (Fig. 1, Fig. 2J, K). Caudal papillae arranged in two groups: 3 pairs of large pedunculate papillae, first two pairs precloacal, third pair adcloacal; and 3 pairs of small sessile postcloacal papillae. A single ventral precloacal papilla present (Fig. 2L). Post-deirids not observed.
3.1.3. Remarks
The present specimens were assigned to the genus Pelecitus (Onchocercidae: Dirofilariinae) as defined by Anderson & Bain (1976), Bartlett & Greiner (1986), Bartlett & Anderson (1987b) and Gibbons (2010), because of their corkscrew-shaped body, the presence of a pre-oesophageal cuticular ring and lateral alae that extend from the cervical region to the caudal extremity. Although not observed in males, a single post-deirid was found in females. Microfilariae are bluntly rounded at both extremities and possess a loose sheath. As is typical for the genus, the present specimens were associated with tendons and muscles near the leg joints of their host. To date, 17 species of Pelecitus have been recorded from birds (Bartlett & Greiner, 1986; Bartlett & Anderson, 1987a; Spratt, 2010; Supplementary Table S1).
We compared the morphological characteristics and morphometrics of the present specimens with three congeners reported in Indomalaya and Australia: P. ceylonensis in Sri Lanka, P. galli in Malaysia, and P. bartneri in Australia (Table 1). Pelecitus copsychi n. sp. is distinct from P. galli and P. bartneri in that its females are shorter, only reaching half the length of the latter two species. In contrast, both males and females of P. copsychi n. sp. possess a longer oesophagus when compared to the remaining three species. In addition, the oesophagus is clearly divided into a muscular and glandular portion in the new species, but not in P. ceylonensis or P. galli. While in the present specimens, the vulva is located at the level of the anterior half of the oesophagus (Fig. 2A), it is located at the level of the posterior half in the remaining species (Table 1). Tail length of the present females is greater than that of P. ceylonensis, but shorter than that of P. galli and P. bartneri. Furthermore, the microfilariae of P. copsychi n. sp. are somewhat shorter than those of the remaining species.
The present species can be further differentiated from P. galli in that its males are shorter and more slender at midbody, but with a tail that is longer than that of P. galli. In P. copsychi n. sp., granular or hyaline inclusions were not seen in the caudal alae of males, but such inclusions are present in the caudal alae of P. bartneri (see Spratt, 2010).
Pelecitus fulicaeatrae has a wide host and geographical range. The species has been recorded in Fulica atra L. (Gruiformes) and various other hosts from England, Germany, Russia, Japan, France, Australia, Canada and Madagascar (Supplementary Table S1). However, P. copsychi n. sp. can be readily distinguished from this cosmopolitan species in that in P. fulicaeatrae, the pre-oesophageal ring is delicate, the oesophagus is not divided, and the vulva is situated at the level of the posterior half of the oesophagus, close to or posterior to the oesophago-intestinal junction (Vanderburgh et al., 1984; Bartlett & Greiner, 1986; Spratt, 2010).
The present species is further distinguished from the remaining 13 congeners parasitizing avian hosts by a combination of characters (Bartlett & Greiner, 1986; Bartlett & Anderson, 1987a). With the exception of Pelecitus vuylstekae Vuylsteke, 1957 and P. copsychi n. sp., in which the vulva in females is at the level of the anterior half of the oesophagus, the vulva is positioned at the level of the posterior half of the oesophagus in the remaining species. However, P. vuylstekae can be distinguished from the new species in that its females (19–25 vs 4.5–8.7 mm) and males (8–9 vs 4.5–4.6 mm) are longer, its spicules are not strongly sclerotized, the blade of the left spicule is slender, and inclusions are occasionally present in the caudal alae (Bartlett & Greiner, 1986). In the pre-oesophageal ring being delicate or absent, Pelecitus andersoni Bartlett & Greiner, 1986, Pelecitus chabaudi Bartlett & Greiner, 1986, Pelecitus circularis (Molin, 1860), Pelecitus helecinus (Molin, 1860), Pelecitus polamaetus Vuylsteke, 1957 and Pelecitus tubercauda Vanderburgh, Anderson & Stock, 1984 differ from the new species in which the pre-oesophageal ring is readily apparent (Bartlett & Greiner, 1986). In addition, the vulva is only slightly or not protuberant in P. andersoni, P. chabaudi and P. helicinus, while it is markedly protuberant in P. copsychi n. sp. Furthermore, caudal inclusions are present in P. andersoni, P. helecinus, P. polamaetus and P. tubercauda (see Bartlett & Greiner, 1986), but absent in the present specimens. Additional distinguishing features between these congeners and the new species are the oesophagus being undivided or only indistinctly divided in P. chabaudi, P. circularis and P. tubercauda and the weakly sclerotized spicules in P. polamaetus, with a narrow blade seen in the left spicule (Bartlett & Greiner, 1986), as opposed to a clearly divided oesophagus and well-sclerotized spicules with a broad left blade in P. copsychi n. sp. Pelecitus anhingae Vuylsteke, 1957 and Pelecitus tercostatus (Molin, 1860) are similar to the new species in that the pre-oesophageal ring is readily apparent and the oesophagus is divided (Bartlett & Greiner, 1986). However, in the former two species, the spicules are not well-sclerotized and the left spicule has a narrow blade (Bartlett & Greiner, 1986). A further congener in birds, Pelecitus armenica Chertkova, 1945 can be distinguished from the new species by females being longer (15.5 mm) and males having longer spicules (left spicule 150 μm and right spicule 110 vs 80–90 μm and 62–73 μm, respectively) (Bartlett & Greiner, 1986). Bartlett & Greiner (1986) considered Pelecitus barusi Coy Otero, 1982 morphologically very close to P. galli, but postponed a decision on their synonymy until more material of the former species could be examined. Pelecitus barusi differs from the new species in the females being larger (15–20 mm long) and the oesophagus being undivided or indistinctly divided (Bartlett & Greiner, 1986).
Little information is available on P. major and P. spiralis from Passeriformes in Nigeria. However, given their host spectrum (Ploceidae) and geographical distribution in Africa (Bartlett & Anderson, 1987a), it is unlikely that they should be conspecific with our material collected from a bird of the Muscicapidae in Malaysia.
Bartlett & Greiner (1986) transferred to Pelecitus two filarial species parasitic in mammals, previously placed in the genus Dirofilaria: Pelecitus roemeri (von Linstow, 1905) in the knee region of Macropus giganteus Shaw (Macropodidae) in Australia and Pelecitus scapiceps (Leidy, 1886) in the ankle region of Sylvilagus floridanus (Allen) (Leporidae) in North America. Later, P. meridionaleporinus was described from the subcutaneous tissue at the ears of L. flavigularis in Mexico (Jiménez-Ruiz et al., 2004). Pelecitus roemeri can be clearly distinguished from the new species in that its females (23–110 mm long; from M. giganteus) are larger, its spicules are not strongly sclerotized and the left blade is slender (Bartlett & Greiner, 1986; Jiménez-Ruiz et al., 2004; Spratt, 2011). Contrary to P. copsychi n. sp., in P. scapiceps the pre-oesophageal ring is not readily apparent, the oesophagus is indistinctly divided, and the vulva is situated posterior to the oesophago-intestinal junction. In addition, females of P. scapiceps are larger (25–30 mm long) (Sonin, 1975; Bartlett & Greiner, 1986; Jiménez-Ruiz et al., 2004). Pelecitus meridionaleporinus differs from the present species in having larger females (12.5–28 mm long) and microfilariae (210–285 vs 158–183 μm long) as well as males with longer spicules (left spicule102–119 vs 80–90 μm, right spicule 75–102 vs 62–73 μm) (Jiménez-Ruiz et al., 2004).
Based on the morphological and morphometric differences outlined above, we consider the present specimens as separate from their three congeners in Indomalaya and Australia as well as from the remaining 17 congeners in avian and mammalian hosts worldwide (Supplementary Table S1).
3.2. Molecular identification
A p-distance threshold has to be established to conclude if two sequences represent the same or different species. The optimum threshold (OT) is the p-distance value minimizing the false-positive and false-negative errors in the intraspecific and interspecific assignments (Ferri et al., 2009). Owing to the lack of intraspecific data in the present dataset, the OT could not be estimated. However, Ferri et al. (2009) previously estimated an OT for the cox1 marker of the Onchocercidae. Their p-distance threshold, set at 4.8%, was used in the present study. The mean interspecific distance established for the present dataset was 14.67% (S.E. = 2.15%; range = 2.76–23.03%), which falls within the range of other similar studies (Ferri et al., 2009; Lefoulon et al., 2017).
The calculated p-distance for cox1 gene sequences between P. copsychi n. sp. and closely related species was 11.2% for F. candezei, 11.2% for L. loa and 14.1% for P. fulicaeatrae. The genetic variation between the present two specimens (ID nos. 168-4 and 268-5) obtained from the swollen footpad of the host’s left leg was 0.4%. In Onchocerca spp., cox1 intraspecific genetic distance is lower than 2% (Lefoulon et al., 2017). Therefore, our molecular results corroborate that P. copsychi n. sp. is distinct from P. fulicaeatrae at the species level, whereas the genetic variation between the two specimens from C. malabaricus corresponds to intraspecific differences.
3.3. Molecular phylogeny
Overall, the concatenated phylogeny was well resolved and strongly supported. The Bayesian inference analysis resulted in a tree with five monophyletic Onchocercidae clades (ONC1–5) as previously proposed by Lefoulon et al. (2015). Most genera included in this study appeared as monophyletic (Fig. 3). The subfamily Dirofilariinae was divided into two clades: the genus Dirofilaria was placed in ONC3, whereas Pelecitus, including P. copsychi n. sp., Foleyella Seurat, 1917 and Loa Stiles, 1905 were placed in ONC5 (Fig. 3). Pelecitus is the only genus in this clade whose members parasitize birds and mammals, while species of Foleyella infect squamates and L. loa only infects humans (Bartlett, 1986; Bain et al., 1998). Notably, the genus Pelecitus presented as paraphyletic in that P. copsychi n. sp. together with F. candezei formed a well-supported clade as sister group to P. fulicaeatra within the Bayesian tree (Fig. 3). Affiliations within the ML phylogeny were similar to those in the Bayesian tree, although deeper nodes were less strongly supported (Supplementary Figure S1).
Fig. 3.
Clades of the Onchocercidae (ONC1–5) based on partitioned concatenated datasets of 12S rDNA, cox1, rbp1, hsp70, myoHC, 18S rDNA and 28S rDNA sequences. The total length of the dataset is c.3979 bp. Fifty onchocercid sequences (representing 49 species) were analysed. Filaria latala and Protospirura muricola were used as the outgroups. The topology was inferred using Bayesian inference on two runs of five million generations, with the first 25% of the trees removed as “burn-in”. The onchocercid subfamilies are indicated by colour: blue for Onchocercinae, dark green for Dirofilariinae, purple for Splendidofilariinae, pale green for Setariinae, yellow for Waltonellinae, orange for Icosiellinae and red for Oswaldofilariinae. The red triangles indicate the sequences generated in this study.
3.4. Wolbachia detection
Following immunohistological staining, Wolbachia endosymbionts could neither be detected in the genital system nor in the lateral chords of sections of a female P. copsychi n. sp. Similarly, nested PCR analysis failed to detect the presence of Wolbachia in the two specimens screened.
4. Discussion
Bartlett (1986) and Bartlett & Anderson (1987b) stated that although species of Pelecitus from birds exhibit a close morphological resemblance to Foleyella from squamates, many species of Pelecitus have a corkscrew-shaped body, whereas all adults of Foleyella spp. are straight or gently curved. Consequently, based on the corkscrew-shaped body in adults of both sexes as well as the predilection site in the host and the host taxon itself, we assigned the present specimens to Pelecitus.
Bain et al. (1993) suggested that the anterior position of the vulva in Onchocerca ramachandrini Bain, Wahl & Renz, 1993 from Phacochoerus africanus (Gmelin) (Suidae: Phacochoerinae) in the Afrotropical realm represented one of the ancestral characteristics within members of the genus Onchocerca Diesing, 1841, while a short, undivided oesophagus was considered an evolved character. In the Oswaldofilariinae Chabaud & Choquet, 1953, a long oesophagus was viewed as an ancestral characteristic (Chabaud & Bain, 1994). If these hypotheses are correct, we propose that P. copsychi n. sp. has some ancestral characteristics in comparison to its congeners P. ceylonensis, P. galli and P. bartneri from Indomalaya and Australia. The position of the vulva is anterior in P. copsychi n. sp., situated at the level of the anterior half of the oesophagus, not the posterior half, and the oesophagus is longer. In addition, the oesophagus is divided in the new species (as well as in P. bartneri), but undivided in P. ceylonensis and P. galli (Table 1).
With regard to the geographical distribution of Pelecitus spp. from birds, many species have been recorded in Africa and South America and only three species have been reported to date from Indomalaya and Australia. In the present study, we describe P. copsychi n. sp. from C. malabaricus in the primary forest of Pahang, Peninsular Malaysia. This bird has not been listed as host of previously described species of Pelecitus (see Bartlett & Greiner, 1986; Spratt, 2011; Supplementary Table S1), and thus constitutes a new host record for this genus. Copsychus malabaricus is distributed in the southern Thai-Malay Peninsula (Rasmussen & Anderson, 2005). Although P. copsychi n. sp. was discovered in close geographical vicinity to P. galli, its host, C. malabaricus, belongs to the order Passeriformes, whereas the host of P. galli, G. g. spadiceus, belongs to the order Galliformes (Dissanaike & Fernando, 1974).
Microfilariae were not found in the blood smears of C. malabaricus, suggesting skin-inhabiting microfilariae. According to Bartlett & Anderson (1987b), the skin-inhabiting microfilariae of P. fulicaeatrae are extraordinarily rare, and skin-inhabiting microfilariae have not previously been reported for any of the more than 140 known species of avian filariae. In future studies, skin snips should be taken from birds to test this hypothesis.
Regarding zoonotic infections, Bain et al. (2011) suggested that an intraocular worm from a patient in Brazil belonged to the genus Pelecitus. Similarly, an intraocular worm from a patient in Colombia was originally identified as Loaina sp. (Botero et al., 1984), but Bartlett & Greiner (1986) considered it more likely that the worm belonged to a species of Pelecitus parasitic in birds. Bain et al. (2011) supported their opinion. While we conclude that Pelecitus spp. of avian origin may be of zoonotic importance in South America, evidence of the zoonotic potential of the four Pelecitus spp. (including P. copsychi n. sp.) from avian hosts in Indomalaya and Australia has yet to be found. Bartlett & Greiner (1986) suggested that Pelecitus spp. of mammals represent “capture” of avian filariae by mammals. In other words, Pelecitus spp. in mammals could have originated from avian filariae through host-switching events facilitated by vectors.
The vector of P. ceylonensis in birds is a mosquito, namely Coquillettidia crassipes (van der Wulp) (syn. Mansonia crassipes (van der Wulp)) (Diptera) (Dissanaike, 1967), and development of P. fulicaeatrae was recorded in the chewing louse Pseudomenopon pilosum (Scopoli) (Mallophaga) (Bartlett & Anderson, 1987b). In mammalian hosts, the vectors of P. scapiceps are mosquitoes and those of P. roemeri tabanids (Highby, 1943; Spratt, 1972). Therefore, Pelecitus species possess the most heterogeneous vector range within the Onchocercidae. Although we did not find any potential vectors for P. copsychi n. sp. in the present study, we speculate that, like its congeners, the new species is transmitted by haematophagous arthropods.
The division of the subfamily Dirofilariinae is not only well-supported from a molecular standpoint (Lefoulon et al., 2015; this paper), but can also be observed in certain biological particularities. First, the third moult occurs as early as day 2–3 post-inoculation in Dirofilaria spp. (similar to Onchocerca spp.), but at a later stage, approximately one week post-inoculation, in the remaining genera of this subfamily (Bain et al., 1998). Secondly, the genus Dirofilaria shares morphological characteristics with the genus Onchocerca: buccal capsule normally developed in infective larvae but reduced in adults; a cylindrical tail with rounded extremity and tiny caudal lappets in infective larvae (Bain et al., 2008).
Based on the redescriptions of F. candezei and Foleyella furcata (von Linstow, 1899), Bartlett (1986) suggested that the close morphological resemblance between Foleyella and Pelecitus indicates their close phylogenetic affinities. The present phylogenetic analysis revealed Pelecitus as paraphyletic (Fig. 3). Two hypotheses may be put forward. Pelecitus may have a complex evolutionary history, possibly owing to its worldwide geographical distribution and a broad host spectrum at genus level (11 orders, representing more than half of the avian orders and two mammalian families) (Bartlett & Greiner, 1986; Jiménez-Ruiz et al., 2004). Alternatively, the current limited set of molecular data (two species out of 21 known species, including P. copsychi n. sp.) might not be inclusive enough to support reliable conclusions about the phylogenetic relationships of this genus. Adding molecular data for species of Pelecitus representing a wider host spectrum and geographical range may in future provide clarification. In the present phylogeny, Pelecitus formed a clade with Foleyella and Loa (second clade of ONC5). This clade presents the most heterogeneous host range within all onchocercid clades, comprising reptiles, birds and mammals.
Regarding the phylogenetic relationships between Wolbachia supergroups and clades of the Onchocercidae, an ancestral absence of Wolbachia, horizontal transfer events, secondary losses and local coevolution with host filariae have been discussed (Bain et al., 2008; Ferri et al., 2011; Lefoulon et al., 2012, Lefoulon et al., 2016; Uni et al., 2020). In this study, neither immunohistochemical staining nor molecular screening detected Wolbachia endosymbionts in P. copsychi n. sp. Bain et al. (2008) and Lefoulon et al. (2016) suggested that ancestral filarial subfamilies such as Setariinae Yorke & Maplestone, 1926, Waltonellinae Bain & Prod’hon, 1974 and Oswaldfilariinae have no acquisition of Wolbachia, whereas Dirofilariinae and Onchocercinae Leiper, 1911 acquired Wolbachia supergroup C. Subsequently, secondary losses occurred in P. fulicaeatrae, F. candezei and L. loa in the Dirofilariinae; as well as in Onchocerca flexuosa (Wedl, 1856), Acanthocheilonema spp., Cercopithifilaria spp. (except for Cercopithifilaria japonica (Uni, 1983) with Wolbachia supergroup F (Ferri et al., 2011)) and some other species in the Onchocercinae. We attribute the absence of Wolbachia endosymbionts in P. copsychi n. sp. to a secondary loss.
To date, five species of avian filariae have been tested for Wolbachia endosymbionts and have been found Wolbachia-free: Chandlerella quiscali (Linstow, 1904), A. alessandroi and C. pavlovskyi in the Splendidofilariinae Chabaud & Choquet, 1953 well as P. fulicaeatrae and P. copsychi n. sp. in the Dirofilariinae (McNulty et al., 2012; Lefoulon et al., 2016; this study). One might hypothesize that avian filariae in general are devoid of Wolbachia. However, compared to the total number of filarial species infecting birds (c.170 according to Bartlett (2008)), the number of tested species is still too limited to support a reliable conclusion. In addition, L. loa and F. candezei do not harbour any Wolbachia endosymbionts either (McGarry et al., 2003; Lefoulon et al., 2016), suggesting that this entire sub-clade within ONC5 may be free of Wolbachia (Fig. 3; Supplementary Figure S1).
5. Conclusions
Pelecitus copsychi was described as a new species from C. malabaricus in Peninsular Malaysia, integrating morphological as well as molecular characteristics. The filariae occurred free in the footpad of the host and caused swelling at the site of infection. Copsychus malabaricus represents a new host record for the genus Pelecitus. Phylogenetic analysis based on seven genes (two mitochondrial genes, 12S rDNA and cox1; and five nuclear genes, 18S rDNA, 28S rDNA, MyoHC, rbp1 and hsp70), revealed the new species as closely related to P. fulicaeatrae from birds and F. candezei from squamates in Africa. However, cox1 analysis supported the new species as being distinct from P. fulicaeatrae at the species level. In the Bayesian and maximum-likelihood inferences, P. copsychi n. sp. clustered with other members of the Dirofilariinae as well as some genera of the Onchocercinae and Splendidofilariinae in a well-supported clade named ONC5, sister to all other taxa evaluated within the Onchocercidae (Lefoulon et al., 2015). The present study corroborates the division of the Dirofilariinae into two distinct groups, linking Dirofilaria spp. on one side and P. fulicaeatrae, P. copsychi n. sp., F. candezei and L. loa on the other side.
Funding
This study was supported by the Ministry of Higher Education, Malaysia (FRGS FP020-2012A).
Ethical approval
Culling of animals and all experimental procedures were carried out in strict compliance with the policy and protocols approved by the Institutional Animal Care and Use Committee, Universiti Malaya, Kuala Lumpur, Malaysia (protocol No. S/15102018/31082018–02/R). The surveys were carried out in accordance with the conservation and control policies of the Department of Wildlife, Malaysia.
CRediT author statement
Shigehiko Uni, Ahmad Syihan Mat Udin, Jules Rodrigues, Coralie Martin, Kerstin Junker: conceptualization, data curation, formal analysis, funding acquisition, methodology, resources, writing - original draft, writing - review & editing. Poai Ean Tan, Takeshi Agatsuma, Van Lun Low, Yvonne Ai-Lian Lim, Weerachai Saijuntha, Hasmazaiti Omar, Nur Afiquah Zainuri, Masako Fukuda, Daisuke Kimura, Makoto Matsubayashi, Shoji Uga, Hiroyuki Takaoka, Mohd Sofian Azirun, Rosli Ramli: methodology, resources, writing - review & editing. All authors read and approved the final manuscript.
Data availability
The newly generated gene sequences are deposited in the GenBank database under the accession numbers OK480041, OK480043 (cox1), OK480976-OK480977 (12S rRNA), OK481072-OK481073 (18S rRNA), OK493401-OK493402 (MyoHC), OK493398-OK493399 (hsp70), OK493403-OK493404 (rbp1) and OK481079-OK481080 (28S rRNA) for P. copsychi n. sp. Type-material is deposited in the MNHN, Paris, France, under accession numbers MNHN-IN-107YT for the holotype female of P. copsychi n. sp. and MNHN-IN-108YT for the allotype male. The paratypes are deposited in the Institute of Biological Sciences, Universiti Malaya, Malaysia, under accession numbers Pf8-1–7 and Pm8-9–11.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to Academician Dr Yong Hoi Sen, Senior Fellow of the Academy of Science Malaysia and Professor Emeritus of the Universiti Malaya, who warmly encouraged us in our research efforts. We thank the late Dr Lim Boo Liat and the staff of the Department of Wildlife and National Parks, Malaysia, who conducted the Scientific Inventory in Sungai Teris, Krau Wildlife Reserve.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2022.100078.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Summary of valid species of Pelecitus Railliet & Henry, 1910, with type-host, host order, site in host and type location.
Primers and PCR programs used in this study. Abbreviations: Step 1: denaturation; Step 2: annealing; Step 3: elongation; T: temperature (°C); D: duration (s); N: number of cycles. An asterisk indicates the primers designed for nested PCR.
GenBank accession numbers for filarioids. Accession numbers in bold for Pelecitus copsychi n. sp. represent sequences generated in the present study. Abbreviations: ext., samples from other studies; Ø, no sequences.
Supplementary Figure S1.
Onchocercid clades based on partitioned concatenated datasets of 12S rDNA, cox1, rbp1, hsp70, myoHC, 18S rDNA and 28S rDNA sequences using maximum-likelihood analysis. The total length of datasets is approximately 3979 bp. Fifty-one onchocercid specimens (representing 50 species) were analysed. Filaria latala and Protospirura muricola were used as the outgroup. The topology was inferred using 1000 bootstraps. The onchocercid subfamilies are indicated by colour: blue for Onchocercinae, dark green for Dirofilariinae, purple for Splendidofilariinae, pale green for Setariinae, yellow for Waltonellinae, orange for Icosiellinae and red for Oswaldofilariinae. The red triangles indicate the sequences generated in this study.
References
- Allen J.L., Kollias G.V., Greiner E.C., Boyce W. Subcutaneous filariasis (Pelecitus sp.) in a yellow-collared macaw (Ara auricollis) Avian Diseases. 1985;29:891–894. [PubMed] [Google Scholar]
- Anderson R.C., Bain O. In: CIH keys to the nematode parasites of vertebrates. Anderson R.C., Chabaud A.G., Willmott S., editors. Commonwealth Agricultural Bureaux International (CABI), Farnham Royal; 1976. Keys to genera of the order Spirurida, Part 3, Diplotriaenoidea, Aproctoidea and Filarioidea; pp. 59–116. [Google Scholar]
- Bain O. In: World Class Parasites, The Filaria, Klei T.R., Rajan T.V., editors. Vol. 5. Kluwer Academic Publishers; Dordrecht: 2002. Evolutionary relationships among filarial nematodes; pp. 21–29. [Google Scholar]
- Bain O., Casiraghi M., Martin C., Uni S. The Nematoda Filarioidea: Critical analysis linking molecular and traditional approaches. Parasite. 2008;15:342–348. doi: 10.1051/parasite/2008153342. [DOI] [PubMed] [Google Scholar]
- Bain O., Otranto D., Diniz D.G., dos Santos J.N., de Oliveira N.P., de Almeida I.N.F., et al. Human intraocular filariasis caused by Pelecitus sp. nematode, Brazil. Emerg. Inf. Dis. 2011;17:867–869. doi: 10.3201/eid1705.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain O., Wahl G., Renz A. Onchocerca ramachandrini n. sp. from the warthog in Cameroon. Ann. Parasitol. Hum. Comp. 1993;68:139–143. [Google Scholar]
- Bain O., Wanji S., Enyong P., Petit G., Noireau F., Eberhard M.L., Wahl G. New features on the moults and morphogenesis of the human filaria Loa loa by using rodent host consequences. Parasite. 1998;5:37–46. doi: 10.1051/parasite/1998051037. [DOI] [PubMed] [Google Scholar]
- Bartlett C.M. The reptilian filarioid genus Foleyella Seurat, 1917 (Onchocercidae: Dirofilariinae) and its relationship to other dirofilariine genera. Syst. Parasitol. 1986;9:43–56. [Google Scholar]
- Bartlett C.M. In: Parasitic diseases of wild birds. Atkinson C.T., Thomas N.J., Hunter D.B., editors. Wiley-Blackwell; Hoboken, New Jersey: 2008. Filarioid nematodes; pp. 439–462. [Google Scholar]
- Bartlett C.M., Anderson R.C. Additional comments on species of Pelecitus (Nematoda: Filarioidea) from birds. Can. J. Zool. 1987;65:2813–2814. [Google Scholar]
- Bartlett C.M., Anderson R.C. Pelecitus fulicaeatrae (Nematoda: Filarioidea) of coots (Gruiformes) and grebes (Podicipediformes): Skin-inhabiting microfilariae and development in Mallophaga. Can. J. Zool. 1987;65:2803–2812. [Google Scholar]
- Bartlett C.M., Greiner E.C. A revision of Pelecitus Railliet & Henry, 1910 (Filarioidea, Dirofilariinae) and evidence for the “capture” by mammals of filarioids from birds. Bull. Mus. Natn. Hist. Nat., Paris, 4è sér. 1986;8:47–99. [Google Scholar]
- Botero D., Aguledo L.M., Uribe F.J., Esslinger J.H., Beaver P.C. Intraocular filaria, a Loaina species, from man in Colombia. Am. J. Trop. Med. Hyg. 1984;33:578–582. doi: 10.4269/ajtmh.1984.33.578. [DOI] [PubMed] [Google Scholar]
- Casiraghi M., Anderson T.J., Bandi C., Bazzocchi C., Genchi C. A phylogenetic analysis of filarial nematodes: Comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. doi: 10.1017/s0031182000007149. [DOI] [PubMed] [Google Scholar]
- Casiraghi M., Bain O., Guerrero R., Martin C., Pocacqua V., Gardner S.L., et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: Evidence for symbiont loss during evolution. Int. J. Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Chabaud A.G., Bain O. The evolutionary expansion of the Spirurida. Int. J. Parasitol. 1994;24:1179–1201. doi: 10.1016/0020-7519(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Dissanaike A.S. Pelecitus ceylonensis n. sp., from the chick and ash-dove experimentally infected with larvae from Mansonia crassipes, and from naturally-infected crows in Ceylon. Ceylon J. Sci. (Biol. Sci.) 1967;7:96–105. [Google Scholar]
- Dissanaike A.S., Fernando M.A. Pelecitus galli n. sp. from the Malayan jungle fowl Gallus gallus spadiceus. J. Helminthol. 1974;48:199–203. doi: 10.1017/s0022149x00022847. [DOI] [PubMed] [Google Scholar]
- Ferri E., Bain O., Barbuto M., Martin C., Lo N., Uni S., et al. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri E., Barbuto M., Bain O., Galimberti A., Uni S., Guerrero R.A., et al. Integrated taxonomy: Traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda) Front. Zool. 2009;6:1. doi: 10.1186/1742-9994-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons L.M. CAB International; Wallingford: 2010. Keys to the nematode parasites of vertebrates. Supplementary volume. [Google Scholar]
- Greve J.H., Graham D.L., Nye R.R. Tenosynovitis caused by Pelecitus calamiformis (Nematoda: Filarioidea) in the legs of a parrot. Avian Dis. 1982;26:431–436. [PubMed] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Highby P.R. Vectors, transmission, development, and incidence of Dirofilaria scapiceps (Leidy, 1886) (Nematoda) from the snowshoe hare in Minnesota. J. Parasitol. 1943;29:253–259. [Google Scholar]
- ICZN International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull. Zool. Nomencl. 2012;69:161–169. [Google Scholar]
- Jiménez-Ruiz F.A., Gardner S.L., Cervantes F.A., Lorenzo C. A new species of Pelecitus (Filarioidea: Onchocercidae) from the endangered Tehuantepec jackrabbit Lepus flavigularis. J. Parasitol. 2004;90:803–807. doi: 10.1645/GE-213R1. [DOI] [PubMed] [Google Scholar]
- Kozlov A.M., Darriba D., Flouri T., Morel B., Stamatakis A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum-likelihood phylogenetic inference. Bioinformatics. 2019;35:4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L.H., Passeri B., Corona S., Simoncini L., Casiraghi M. Immunohistochemical/immunogold detection and distribution of the endosymbiont Wolbachia of Dirofilaria immitis and Brugia pahangi using a polyclonal antiserum raised against WSP (Wolbachia surface protein) Parasitol. Res. 2003;89:381–386. doi: 10.1007/s00436-002-0765-6. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefoulon E., Bain O., Bourret J., Junker K., Guerrero R., Cañizales I., et al. Shaking the tree: Multi-locus sequence typing usurps current onchocercid (filarial nematode) phylogeny. PLoS Negl. Trop. Dis. 2015;9:e0004233. doi: 10.1371/journal.pntd.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefoulon E., Bain O., Makepeace B.L., d'Haese C., Uni S., Martin C., Gavotte L. Breakdown of coevolution between symbiotic bacteria Wolbachia and their filarial hosts. PeerJ. 2016;4 doi: 10.7717/peerj.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefoulon E., Gavotte L., Junker K., Barbuto M., Uni S., Landmann F., et al. A new type F Wolbachia from Splendidofilariinae (Onchocercidae) supports the recent emergence of this supergroup. Int. J. Parasitol. 2012;42:1025–1036. doi: 10.1016/j.ijpara.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Lefoulon E., Giannelli A., Makepeace B.L., Mutafchiev Y., Townson S., Uni S., et al. Whence river blindness? The domestication of mammals and host-parasite co-evolution in the nematode genus Onchocerca. Int. J. Parasitol. 2017;47:457–470. doi: 10.1016/j.ijpara.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Liu K., Raghavan S., Nelesen S., Linder C.R., Warnow T. Rapid and accurate large-scale coestimation of sequence alignments and phylogenetic trees. Science. 2009;324:1561–1564. doi: 10.1126/science.1171243. [DOI] [PubMed] [Google Scholar]
- McGarry H.F., Pfarr K., Egerton G., Hoerauf A., Akue J.-P., Enyong P., et al. Evidence against Wolbachia symbiosis in Loa loa. Filaria J. 2003;2:9. doi: 10.1186/1475-2883-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty S.N., Fischer K., Mehus J.O., Vaughan J.A., Tkach V.V., Weil G.J., Fischer P.U. Absence of Wolbachia endobacteria in Chandlerella quiscali: an avian filarial parasite. J. Parasitol. 2012;98:382–387. doi: 10.1645/GE-2879.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harbour Protocols. 2010 doi: 10.1101/pdb.prot5448. pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- Muñoz-García C.I., López-Díaz O., Osorio-Sarabia D., Martínez-Hernández F., Villalobos G., Isaak-Delgado A.B., et al. New insights into the clinico-histopathological and molecular features of Pelecitus (Filarioidea: Onchocercidae) from a raptor bird. Parasitol. Res. 2018;117:3319–3325. doi: 10.1007/s00436-018-6009-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen P.C., Anderson J.C. Smithsonian Institute & Lynx Editions; Barcelona: 2005. Birds of South Asia: The Ripley Guide; pp. 395–396. [Google Scholar]
- Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nature Biotech. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sonin M.D. Vol. 24. Nauka Publishers; Moscow: 1975. Filariata of animals and man and diseases caused by them. Part 3, Filariidae, Onchocercinae. (Fundamentals of nematology). Translated from Russian, published for the US Department of Agriculture, and the National Science Foundation, Washington, D.C., by New Delhi; Amerind Publishing Co. Pvt. Ltd., 1985. pp. 328–332. [Google Scholar]
- Spratt D.M. Natural occurrence, histopathology and developmental stages of Dirofilaria roemeri in the intermediate host. Int. J. Parasitol. 1972;2:201–208. doi: 10.1016/0020-7519(72)90007-0. [DOI] [PubMed] [Google Scholar]
- Spratt D.M. Pelecitus bartneri sp. nov. (Nematoda: Filarioidea) from the subcutaneous tissues of the leg of Psephotus chrysopterygius Gould, 1858 (Psittaciformes) Trans. Roy. Soc. South Australia. 2010;134:172–176. [Google Scholar]
- Spratt D.M. New records of filarioid nematodes (Nematoda: Filarioidea) parasitic in Australasian monotremes, marsupials and murids, with descriptions of nine new species. Zootaxa. 2011;2860:1–61. [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni S., Mat Udin A.S., Agatsuma T., Junker K., Saijuntha W., Bunchom N., et al. Description, molecular characteristics and Wolbachia endosymbionts of Onchocerca borneensis Uni, Mat Udin & Takaoka n. sp. (Nematoda: Filarioidea) from the Bornean bearded pig Sus barbatus Müller (Cetartiodactyla: Suidae) of Sarawak, Malaysia. Parasit. Vectors. 2020;13:50. doi: 10.1186/s13071-020-3907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni S., Mat Udin A.S., Agatsuma T., Saijuntha W., Junker K., Ramli R., et al. Morphological and molecular characteristics of Malayfilaria sofiani Uni, Mat Udin & Takaoka n. g., n. sp. (Nematoda: Filarioidea) from the common treeshrew Tupaia glis Diard & Duvaucel (Mammalia: Scandentia) in Peninsular Malaysia. Parasit. Vectors. 2017;10:194. doi: 10.1186/s13071-017-2105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderburgh D.J., Anderson R.C., Stock T.M. Pelecitus tubercauda n. sp. (Nematoda: Filarioidea) from Geothlypis trichas L. And a redescription of P. fulicaeatrae (Diesing, 1861) López-Neyra, 1956. Can. J. Zool. 1984;62:362–367. [Google Scholar]
- Yen P.K.F. In: Mak J.W., editor. Vol. 19. Institute for Medical Research; Kuala Lumpur: 1983. Taxonomy of Malaysian filarial parasites; pp. 17–35. (Filariasis). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of valid species of Pelecitus Railliet & Henry, 1910, with type-host, host order, site in host and type location.
Primers and PCR programs used in this study. Abbreviations: Step 1: denaturation; Step 2: annealing; Step 3: elongation; T: temperature (°C); D: duration (s); N: number of cycles. An asterisk indicates the primers designed for nested PCR.
GenBank accession numbers for filarioids. Accession numbers in bold for Pelecitus copsychi n. sp. represent sequences generated in the present study. Abbreviations: ext., samples from other studies; Ø, no sequences.
Data Availability Statement
The newly generated gene sequences are deposited in the GenBank database under the accession numbers OK480041, OK480043 (cox1), OK480976-OK480977 (12S rRNA), OK481072-OK481073 (18S rRNA), OK493401-OK493402 (MyoHC), OK493398-OK493399 (hsp70), OK493403-OK493404 (rbp1) and OK481079-OK481080 (28S rRNA) for P. copsychi n. sp. Type-material is deposited in the MNHN, Paris, France, under accession numbers MNHN-IN-107YT for the holotype female of P. copsychi n. sp. and MNHN-IN-108YT for the allotype male. The paratypes are deposited in the Institute of Biological Sciences, Universiti Malaya, Malaysia, under accession numbers Pf8-1–7 and Pm8-9–11.