Abstract
The flagellated pathogen Giardia duodenalis is one of the leading causes of parasitic gastrointestinal illness worldwide. In many higher income countries, such as the United Kingdom, the disease is often perceived as being travel-related, likely leading to the under-reporting of sporadic cases and outbreaks. A summary of the literature describing outbreaks and risk factors in higher income countries is necessary to improve our understanding of this pathogen and identify existing knowledge gaps. Initial literature searches were carried out in September 2016 and updated at regular intervals until November 2021, using appropriate search terms in Medline, Embase and PubMed databases. A total of 75 papers met the inclusion criteria, revealing that the consumption of contaminated water and contact with young children of diaper-wearing age were the most common transmission routes leading to outbreaks of giardiasis. Of the ten studies where food was primarily associated with outbreaks, food handlers accounted for eight of these. Another reported transmission route was direct contact with fecal material, which was reported in six studies as the primary transmission route. Travel-associated giardiasis was considered the sole transmission route in two studies, whereas multiple transmission routes contributed to giardiasis outbreaks in eleven studies. The evidence around zoonotic transmission was less clear and hampered by the lack of robust and regularly applied parasite molecular typing techniques. This literature review summarizes the findings of Giardia outbreak investigations and epidemiological studies in high-income countries. Transmission routes are identified and discussed to highlight the associated risk factors. These data also indicate gaps in our current knowledge that include the need for robust, in-depth molecular studies and have underscored the importance of water as a transmission route for Giardia cysts. These future molecular studies will improve our understanding of Giardia epidemiology and transmission pathways in higher income countries to prevent spread of this significantly under-reported pathogen.
Keywords: Giardia, Outbreaks, Giardiasis, Epidemiology, Zoonoses
Graphical abstract
Highlights
-
•
Historically giardiasis is associated with patient travel history in high-income countries.
-
•
Our analysis indicates waterborne transmission is a major source of outbreaks.
-
•
Waterborne outbreaks are commonly linked to filtration failure or fecal contamination.
-
•
Outbreaks have also been linked to ambulatory children of diaper-wearing age.
1. Background
Giardia duodenalis (synonyms Giardia intestinalis and Giardia lamblia) is one of the leading causes of parasitic gastrointestinal disease, potentially leading to over 180 million annual cases worldwide (Torgerson et al., 2015). This flagellated protozoan parasite causes the disease giardiasis, with symptoms including diarrhea, nausea, vomiting, abdominal pain, and excessive gas production. The illness can be effectively treated with nitroheterocycles, in particular metronidazole, although there are emerging reports of metronidazole resistance (Ansell et al., 2015; Leitsch, 2015; Muller et al., 2018). Infection can result in long-term complications including irritable bowel syndrome (IBS) and chronic fatigue (Hanevik et al., 2014; Dormond et al., 2016; Litleskare et al., 2018). The parasite is ingested in its cystic form and remains contained until it reaches the stomach. Once exposed to stomach acid, the cyst releases vegetative trophozoites that attach to the small intestine, causing clinical signs as they replicate (Adam, 2001; Bernander et al., 2001). After moving through the proximal portion of the gastrointestinal system, some trophozoites re-encyst in the jejunum before being excreted to continue the life-cycle of the parasite and infect new hosts (Adam, 2001). Although trophozoites rapidly degrade once excreted, cysts are highly robust and can last many months in the environment without a host. Transmission of infectious cysts is possible via a variety of different routes, including person-to-person contact, animal-to-human contact, and contaminated water and food sources. Poor quality sanitation and water filtration systems are typically thought to be responsible for transmission of cysts in lower to middle income countries (LMICs), whereas travel and food are more commonly thought to be the transmission route in higher income countries (Leung et al., 2019). Infection then occurs when fecal material containing infective cysts is ingested through one of these routes. The parasite has a wide host range, and a variety of subtypes exist, known as assemblages A-F. These assemblages infect many mammals and are largely host-specific, with assemblages A and B demonstrating the capacity to be zoonotic (Ryan & Caccio, 2013).
Giardiasis is supposedly less prevalent in high income countries than LMICs, ranging from 2–7% for the former to 20–30% for the latter (Leung et al., 2019). The condition is diagnosed in higher income countries using a range of methods, with conventional identification involving microscopy to directly identify trophozoites and cysts excreted in feces. However, microscopy is being replaced by more sensitive methods, including molecular techniques such as polymerase chain reaction (PCR) and enzyme immunoassays (EIA) that detect parasite-specific antigens. These permit the efficient and rapid screening of large numbers of samples for Giardia and other gastrointestinal pathogens simultaneously. In the past, diagnostic testing of patients in many higher income countries has largely been confined to testing symptomatic individuals with a history of travel to specific, perceived Giardia-risk countries, resulting in significant under-reporting of this pathogen (Alexander et al., 2017). With this increasing awareness of endemic disease in higher income countries, a greater number of samples from symptomatic cases are now being tested using more sensitive tools. As this includes patients without a history of travel, it is likely that a greater number of sporadic cases, clusters, and outbreaks will become evident. This will lead to more accurate assessment of the potential risk factors and transmission routes in higher income countries.
Improving our understanding of Giardia transmission and raising awareness of giardiasis are essential to ensure cases receive appropriate treatment and are important for public health authorities to identify points at which interventions can be made. This is of particular concern as the parasite is easily spread between humans and has the potential to cause long-term complications (Hanevik et al., 2014; Litleskare et al., 2018). There is also evidence from LMICs that infection in young children can impact growth and development, impacting such biological processes as iron absorption, retinal morphology, and hepatic and pancreatic functionality (United States Environmental Protection Agency, 1999; Rogawski et al., 2017, 2018; Lehto et al., 2019). With the increased recognition that there is significant under-reporting of Giardia in higher income countries, we hypothesize that there are underappreciated endemic sources of infection that may impact public health. Additionally, previous studies have primarily focused on one transmission route. These routes have been included and expanded upon (Karanis et al., 2007; Baldursson & Karanis, 2011; Efstratiou et al., 2017). The aim of this work was, therefore, to undertake a systematic review of the literature to identify sources and transmission routes associated with human giardiasis outbreaks in higher income countries and establish the accuracy of the hitherto accepted assertion that giardiasis is primarily a sporadic travel-associated illness.
2. Search approach
2.1. Literature search
The original search was performed between September 2016 up to and including November 2021. Searches of titles and abstracts were undertaken on Medline, Embase, and PubMed databases using the following query: ((Giardia OR Giardiasis) AND Outbreak) OR (((Giardia OR Giardiasis) AND Outbreak) AND (Risk Factor OR Travel OR Pets OR Water OR Swimming Pools OR Food OR Cat OR Dog OR “companion animal”)). The searches yielded a total of 254 articles.
2.2. Inclusion and exclusion criteria
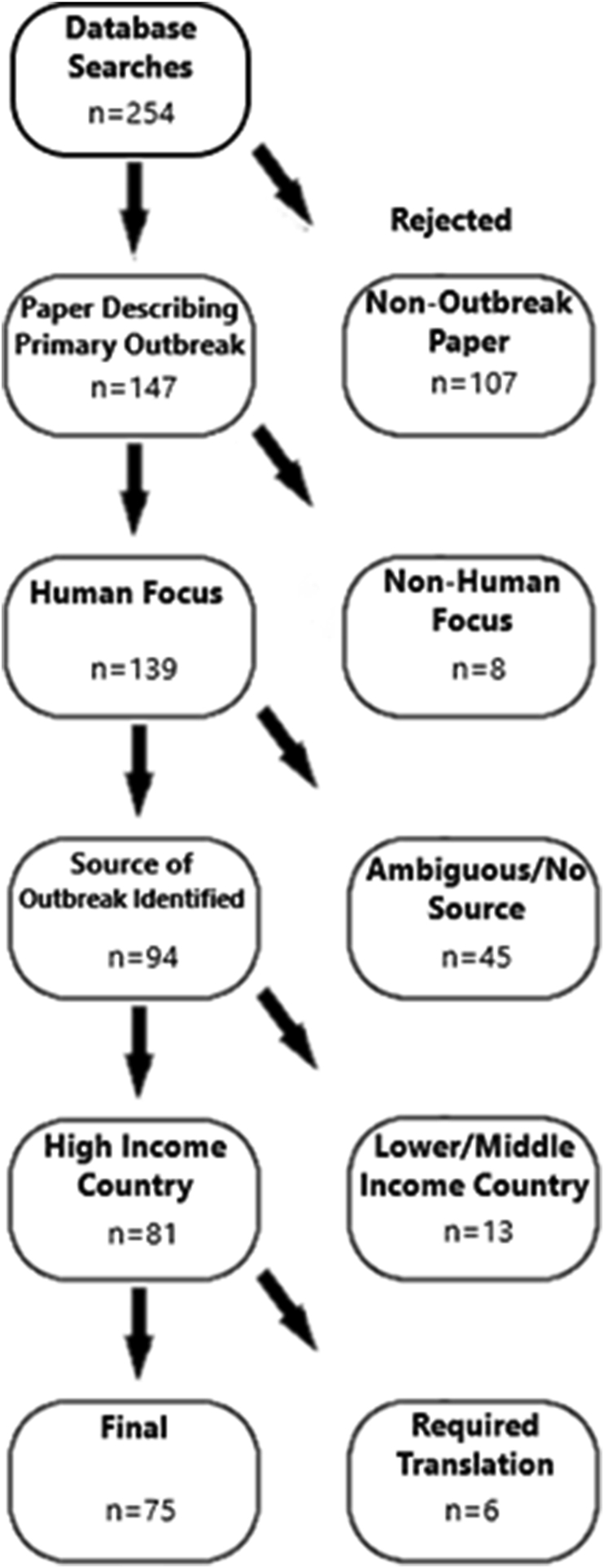
Manuscripts were initially screened based on titles and abstracts to exclude irrelevant studies, such as those that primarily examined animal outbreaks over human outbreaks or were primarily reporting data for another pathogen but mentioned Giardia as a comparator. Manuscripts were also excluded if Giardia was not suspected as the primary pathogen of interest or if they were not originally written in English to avoid issues with translation accuracy. The full text versions of all manuscripts from the initial screen were obtained using a combination of library services and online repositories. Two infectious disease researchers then independently examined the full text of these manuscripts using the following inclusion criteria: (i) that the manuscript reported primary outbreak data and was not a case report describing an individual patient; (ii) that the outbreak primarily focused on human cases; (iii) that the source and causative agent of an outbreak were unambiguously identified; (iv) the reported outbreak occurred in a country on the Organisation for Economic Co-Operation and Development’s (OECD) list of upper-middle-income countries and territories (Fig. 1). All study designs that met these criteria were included, including case-control and observational studies, as the primary aim of the review was to identify potential sources and transmission routes associated with Giardia outbreak rather than estimate the size of these risks. The identified sources and transmission routes for each outbreak were determined by each researcher independently and classed as being associated with travel, water contamination, food contamination, animal contact, person-to-person contact, or exposure to raw sewage. More refined distinctions were made within each class to provide further details (Supplementary Table S1). All articles could be classified into these six categories, which were generated during assessment of the literature. Due to the observational nature of the data, the MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies were applied (Stroup et al., 2000). Identified transmission routes were encoded in a shared data table for ease of access, including additional metadata, such as authors, number of cases, PMID, date of study, date of publication, and country. After a final discussion between the researchers to resolve disparities, a total of 75 papers were included in the review (Fig. 2).
Fig. 1.
Flowchart of paper inclusion process.
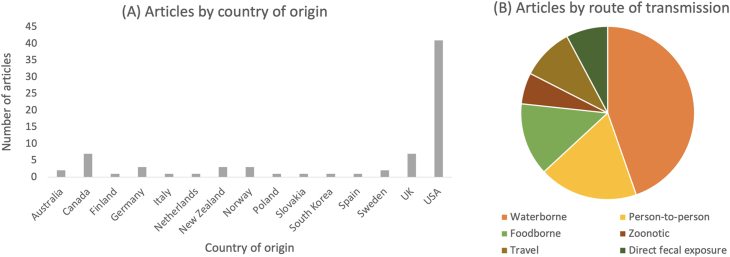
Fig. 2.
Country distribution and transmission routes cited by study (n = 75).
2.3. Sources of bias and heterogeneity
It was noted that very few of the manuscripts were case-control studies or presented an estimate of risk, with the majority reporting a description of a Giardia outbreak in a higher income country with an identified source of infection. This is likely an example of publication bias in which outbreaks without an identifiable source are not deemed sufficiently interesting to merit publication. In addition, there was significant heterogeneity in the study type, methodology, and detection tools used across the 75 papers with almost no study being directly analogous to any other. Both the apparent bias and high study heterogeneity prevent a formal meta-analysis of the data. However, as the aim of this review was to establish a list of potential outbreak sources and transmission routes in higher income countries rather than estimate risks, the systematic analysis of the data serves to highlight areas in which further analysis and formal case-control experiments are required in the future.
3. Waterborne transmission
Waterborne transmission is one of the most important Giardia transmission routes in LMICs (Fakhri et al., 2021) and was found to account for the majority of outbreaks in this systematic analysis of higher income countries (Fig. 3). This includes outbreaks in the USA, Canada, UK, Europe, the Nordics, and Southeast Asia, demonstrating that a range of water treatment approaches across a spectrum of higher income countries can be vulnerable to failure, leading to water contamination. Previously, a review of waterborne parasites in higher income countries found that Giardia was the second most frequently cited protozoan agent after Cryptosporidium, responsible for 37% of waterborne outbreaks (Efstratiou et al., 2017). Similarly, a review of waterborne outbreaks in Nordic countries indicated that parasites accounted for the largest outbreaks of gastrointestinal upset during the time period studied, even when compared to bacterial or viral causes (Guzman-Herrador et al., 2015). Of particular note with respect to waterborne outbreaks is the large number of cases per outbreak, with some having several hundred or more (Dykes et al., 1980; Lopez et al., 1980; Weniger et al., 1983; Navin et al., 1985; Nygard et al., 2006). Waterborne transmission of Giardia cysts occurs via a variety of routes, including contaminated drinking water, swimming pools, rainwater tanks, and recreational lakes. These transmission routes increased the risk of obtaining giardiasis, demonstrated both through case-control studies and outbreak investigations.
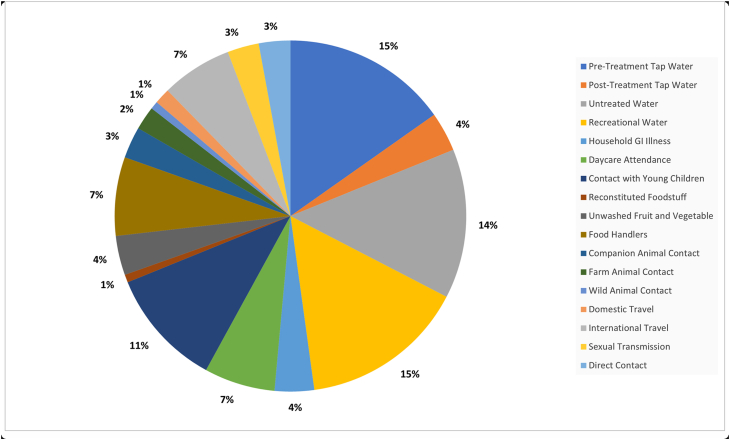
Fig. 3.
Transmission routes for giardiasis cited in studies (n = 75).
3.1. Drinking water
Of the 46 outbreaks described as having a water transmission route or water involvement, approximately 33 involved contaminated drinking water and led to at least 8045 laboratory-confirmed cases of giardiasis in higher income countries from 1974 to 2016 (Table 1; several studies did not attribute outbreaks to case numbers and were therefore not included in the case count). Contamination of treated water by raw, untreated water or sewage was stated as a factor in at least five outbreaks. Other contributing factors cited included structural defects in water distribution systems, insufficient chlorination or poor to no filtration system, and the presence of Giardia-positive North American beavers (Castor canadensis) in the water catchment area (Dykes et al., 1980; Istre et al., 1984; Navin et al., 1985). This has been further explored in work by Tsui et al. (2018), who used whole genome sequencing to suggest the presence of beavers in water catchment areas and along riverbeds was a possible source of human infection via contaminated water, but acknowledged that this was only one factor in a complex cycle of zoonotic spread. Direct contact with raw sewage following a system failure in a private residence caused at least one outbreak in Bratislava, Slovakia (Totkova et al., 2004). Rainwater run-off from sewer systems after severe natural events (including volcanic eruption) also led to contamination of surface water (Weniger et al., 1983). One German study investigated two separate sewer systems that tended to overflow when rainwater contributed to their volume, which would contaminate nearby natural bodies of water. Giardia was found in 12/38 (31.6%) water samples from sewer run-off, which emptied into a nearby catchment area where the local population frequently walked with their companion animals (Schreiber et al., 2019). This highlights how rivers and other water sources contaminated with Giardia from slaughterhouses and sewage run-off can pose a contamination risk for humans and animals in the area (Ma et al., 2019).
Table 1.
Giardiasis outbreaks due to waterborne transmission
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | Tap water |
Untreated water | Recreational water (swimming, etc.) | Beaver involvement | |
|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | |||||||
| Australia | Dale et al. (2010) | 2001–2007 | 12 (3) | × | ||||
| Canada | Isaac-Renton et al. (1999) | 1996 | 590 (590) | × | ||||
| Canada | Isaac-Renton et al., 1993, Isaac-Renton et al., 1994 | 1991–1992 | 124 (124) | × | × | |||
| Canada | Greensmith et al. (1988) | 1986 | 59 (30) | × | ||||
| Finland | Rimhanen-Finne et al. (2010) | 2007–2008 | 37 (37) | × | ||||
| Italy | Resi et al. (2021) | 2018–2019 | 228 (228) | × | ||||
| Netherlands | Pijnacker et al. (2016) | 2010–2013 | 219 (219) | × | ||||
| New Zealand | Wilson et al. (2008) | 2006 | 1214 (1214) | × | × | |||
| Norway | Nygard et al. (2006) | 2004–2005 | 2500 (1268) | × | ||||
| South Korea | Cheun et al. (2013) | 2010 | 9 (7) | × | ||||
| Sweden | Neringer et al. (1987) | 1982 | 56 (56) | × | ||||
| UK | Jephcott et al. (1986) | 1985 | 108 (108) | × | ||||
| UK | Gray et al. (1994) | 1992–1993 | 74 (74) | × | × | |||
| UK | Hall et al. (2017) | 2012 | 4 (4) | × | ||||
| USA | Levine et al. (1990) | 1986–1988 | 4 unique outbreaksa | × | × | |||
| USA | Kramer et al. (1996) | 1993–1994 | 9 unique outbreaksa | × | × | × | × | |
| USA | Moore et al. (1993) | 1991–1992 | 8 unique outbreaksa | × | × | × | × | |
| USA | Herwaldt et al. (1991) | 1989–1990 | 7 unique outbreaksa | × | × | |||
| USA | Birkhead & Vogt (1989) | 1983–1986 | 1211 (1211) | × | × | |||
| USA | Birkhead et al. (1989) | 1986 | 37 (23) | × | × | |||
| USA | Navin et al. (1985) | 1982 | 324 (324) | × | × | |||
| USA | Kent et al. (1988) | 1985–1986 | 703 (703) | × | ×(and muskrat) | |||
| USA | Lopez et al. (1980) | 1977 | 213 (213) | × | ×, C. can | |||
| USA | Dykes et al. (1980) | 1976 | 128 (128) | × | ×, C. can | |||
| USA | Istre et al. (1984) | 1981 | 20 (8) | × | ||||
| USA | Weniger et al. (1983) | 1980 | Estimated 781 (49) | × | ||||
| USA | Karon et al. (2011) | 2007 | 46 (26) | × | ||||
| USA | Shaw et al. (1977) | 1974–1975 | 350 (350) | × | ||||
| USA | Levy et al. (1998) | 1995–1996 | 3 unique outbreaksa | × | × | × | ||
| USA | Reses et al. (2018) | 2003–2004 | 52 (52) | × | × | |||
| USA | Bedard et al. (2016) | 2009 | 36 (36) | × | ||||
| USA | Daly et al. (2010) | 2007 | 31 (17) | × | ||||
| USA | Hopkins & Juranek (1991) | 1983 | 31 (31) | × | ||||
| USA | Porter et al. (1988) | 1985 | 9 (8) | × | ||||
| USA | Katz et al. (2006) | 2003 | 149 (97) | × | ||||
| USA | Harter et al. (1984) | 1982 | 70 (70) | × | ||||
| USA | Eisenstein et al. (2008) | 2006 | 38 (35) | × | ||||
Abbreviation: C. can, Castor canadensis (North American beaver).
Number of individual cases undetermined as some studies referenced in these papers overlap with those included individually this table; cases and specific outbreak studies are not linked to one another in the original study.
Communal water supplies are normally treated to prevent contamination with Cryptosporidium and Giardia, but this requires carefully controlled conditions and proper maintenance of treatment systems. Failure in any aspect of these systems can result in outbreaks due to inadequate removal of infective cysts. Outbreaks may also arise from post-treatment contamination due to pipe system damage or wastewater leakage. Welch (2000) conducted a meta-analysis to test the hypothesis that consumption of water in rural regions of North America posed a statistically significant risk for the acquisition of giardiasis. The authors note that published reports demonstrate a higher incidence of giardiasis among people engaging in outdoor recreational activities, but there is minimal evidence for an association between this and giardiasis. The study also states that although greater emphasis is given to water purification when in the rural outdoors, the reason for increased giardiasis incidence may be due to relaxed hygiene practices on camping trips rather than raw water consumption. The use of private water supplies may also contribute to increased risk of giardiasis as they are more common in rural areas and are not subject to the same stringent water quality testing or regulations as public water supplies (Welch, 2000; Reeve et al., 2018). Masina et al. (2019) and Ma et al. (2019) suggest that the use of indicator bacteria such as Escherichia coli and coliforms to test the quality of tap water for ingestion may not be adequate for all pathogens present, as the absence of indicator bacteria does not necessarily indicate the absence of waterborne parasites such as Giardia spp. Parasites are notably more difficult to identify in water samples as they cannot be readily cultured and are found at lower concentrations in the environment.
3.2. Recreational water
Swimming pool or recreational water was identified as the sole transmission route in 7 outbreaks in higher income countries (Table 1), resulting in at least 463 laboratory-confirmed cases of giardiasis. Swimming in pools or natural water has previously been found to be a risk factor in several case-control studies (Dennis et al., 1993; Hoque et al., 2002; Xiao et al., 2017; Reses et al., 2018). Fecal contamination of pool water was stated as a source for four outbreaks in Canada and the USA with an increased incidence of giardiasis upon diving into the pool, due to the potential for accidental water ingestion (Harter et al., 1984; Greensmith et al., 1988; Porter et al., 1988; Katz et al., 2006). Swimming pools with additional features such as splash pads, water slides, or classes with young children in attendance accounted for several outbreaks (Harter et al., 1984; Greensmith et al., 1988; Eisenstein et al., 2008). While actively flowing water makes infectious agent identification difficult, Giardia was one of a range of pathogens identified among cases of gastrointestinal illness associated with an open swimming event in the River Thames, London (Hall et al., 2017).
4. Person-to-person transmission
Direct or indirect person-to-person transmission was the basis for 12 outbreaks, with a laboratory confirmation of 2195 human cases (Table 2). Giardiasis outbreaks associated with person-to-person contact have been linked to households with young children or in childcare settings where young children are in close contact with each other, likely due to handling diapers (Hoque et al., 2001, 2002, 2003; Minetti et al., 2015b; Reses et al., 2018). Such associations highlight the importance of good personal hygiene in reducing transmission via regular handwashing by those caring for infants. Six outbreaks involved day-care facilities (Table 2), five of which involved children below five years of age. Transmission in childcare facilities was greatest when children were ambulatory but had not yet been toilet-trained. Person-to-person transmission both within and out with households was identified in several studies (Table 2), with a consistent factor being contact with young children and/or involvement in the changing of infants’ diapers. Of particular interest, one study reported a high percentage of asymptomatic cases (37 individuals out of 41 positive cases, with children aged 0–9 years most heavily affected) (Waldram et al., 2017) that were only detected as a result of the household screening undertaken as part of the study. This also underlines the importance of good personal hygiene measures in the prevention of transmission.
Table 2.
Giardiasis outbreaks due to person-to-person transmission
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | Household GI illness | Day-care attendance | Young children |
|---|---|---|---|---|---|---|
| Canada | Keystone et al. (1978) | 1976–1977 | 116 (116) | × | × | |
| New Zealand | Wilson et al. (2008) | 2006 | 1214 (1214) | × | ||
| New Zealand | Hoque et al. (2001) | 1998–1999 | 183 (183) | × | ||
| UK | Waldram et al. (2017) | 2014–2015 | 143 (132) | × | × | |
| UK | Ang (2000) | 1999 | 11 (10), 3 asymptomatic | × | × | |
| UK | Rauch et al. (1990) | 1986–1987 | 27 (27), also asymptomatic outbreaks within the same population | × | ||
| USA | Polis et al. (1986) | 1982 | 39 (39) | × | × | |
| USA | Bartlett et al. (1985) | 1982–1983 | 187 (187), 105 asymptomatic | × | × | |
| USA | Black et al. (1977) | 1975 | 38 (38) | × | × | |
| USA | Katz et al. (2006) | 2003 | 149 (97), 105 via person-to-person | × | ||
| USA | Reses et al. (2018) | 2003–2004 | 80 (80) | × | ||
| USA | White et al. (1989) | 1986 | 88 (72) | × |
Abbreviation: GI, gastrointestinal.
Demonstrating the power of modern molecular genotyping approaches, Wang et al. (2019) investigated the genetic diversity of G. duodenalis in cases in Spain between 2012 and 2018, comparing the distribution and clinical presentation of assemblages A and B between children and adults. They showed a significant difference in the distribution of assemblages between children and adults (P = 0.001) and that children under 12 years of age were more likely to have been infected by assemblage B (44/53, 83%) than assemblage A (9/53, 17%). Conversely, adults in this sample had comparable distributions of assemblages A and B (20/42, 47.6% and 22/42, 52.4% respectively). There was no significant difference in the distribution of assemblages by gender. Cases with assemblage A (4/29, 13.8%) were more likely to have asymptomatic infection than cases with assemblage B (1/66, 1.5%), with OR = 10.4 (95% CI: 1.108–97.625). The genotyping and subtyping results also suggest that anthroponotic transmission, such as within childcare facilities, is an important area of study for giardiasis outbreaks.
Although uncommon, sexual transmission has been identified as a potential route for some communities (Table 3) (Meyers et al., 1977; Reses et al., 2018). This transmission mechanism is recognised for a number of gastrointestinal pathogens including Cryptosporidium (Hellard et al., 2003), Shigella (Borg et al., 2012), and hepatitis A (Ndumbi et al., 2018). A study in England (Mook et al., 2018) used gender distributions in routine surveillance data stratified by age and region to show an excess of Giardia cases among males that the authors posit is linked to transmission in men who have sex with men (MSM). This has also been suggested from studies in the USA (Phillips et al., 1981; Escobedo et al., 2014; Muller et al., 2018; Reses et al., 2018). One multi-centre study testing samples from patients with acute gastroenteritis in Seattle, USA reported that enteric pathogens were detected in 56.3% of MSM cases tested. This was substantially higher than the 33.5% seen in the general population. Of these pathogens, Giardia was found in 20.5% of diarrheic MSM samples compared to 1.9% in the general population (Newman et al., 2020). Both studies used PCR multiplex panels for parasite detection.
Table 3.
Giardiasis outbreaks due to transmission via direct fecal exposure
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | Sexual transmission | Direct fecal contact |
|---|---|---|---|---|---|
| Netherlands | Pijnacker et al. (2016) | 2010–2013 | 219 (219) | × | |
| New Zealand | Wilson et al. (2008) | 2006 | 1214 (1214) | × | |
| Slovakia | Totkova et al. (2004) | 1998 | 7 (7) | × | |
| USA | Newman et al. (2020) | 2017–2018 | 31 (31) | × | |
| USA | Reses et al. (2018) | 2003–2004 | 17 (17) | × | |
| USA | Meyers et al. (1977) | 1975 | 6 (5) | × |
5. Foodborne transmission
Where foodborne transmission has been identified, 1401 laboratory-confirmed human cases were identified and contamination by an infected food handler has been a key feature in these outbreaks. Few reports suggest the possibility of food items being intrinsically infected (Rose & Slifko, 1999; Slifko et al., 2000; Dawson, 2005; Dixon et al., 2013). Ten foodborne outbreaks of giardiasis were identified in this analysis across higher income countries (Table 4), with asymptomatic food handlers or people asymptomatic at the time of food preparation who later developed giardiasis contributing to eight of these. This included an outbreak at a private party (Porter et al., 1988); those who consumed fruit salad were seven times more likely to have been ill than those who did not. It was noted that the household had a child in diapers and a pet rabbit present in the kitchen where the fruit salad was prepared, both of whom were positive for Giardia. Therefore, it was likely that the food preparer became infected by the child and/or rabbit and then contaminated food due to poor hand hygiene. The contaminated food became the primary transmission route for the outbreak. In another outbreak, no individual food item was identified; the assemblage and subtype from one of the asymptomatic food handlers matched the two outbreak cases for which genotyping was available (Figgatt et al., 2017). An outbreak among UK tourists residing at a hotel in Greece was linked to a number of risk factors, including the consumption of raw vegetables (Hardie et al., 1999). Salads were identified as a risk factor in two case-control studies of sporadic giardiasis (Stuart et al., 2003; Espelage et al., 2010), while another study identified inadequate washing of raw fruits and vegetables as a risk factor (de Lucio et al., 2017). This route of transmission is further supported by the identification of Giardia in 10 of 19 salad products tested in a study from Spain (Amoros et al., 2010); however, rates of positivity were lower (10 of 475 samples) in a Norwegian study (Robertson & Gjerde, 2001). Conversely, two studies of sporadic cases in the USA (Reses et al., 2018) and UK (Minetti et al., 2015b) found eating raw fruit and vegetables was inversely associated with giardiasis. Reses et al. (2018) suggested repeated exposure via contaminated raw produce could provide protective immunity and that this inverse association could reflect increased healthy behaviors among controls compared to cases. Individuals who frequently consume fruit and vegetables might possess better general health habits than those who do not and could be less likely to contract giardiasis or develop a systemic infection.
Table 4.
Giardiasis outbreaks due to foodborne transmission
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | Unwashed fruit and vegetables | Food handlers |
|---|---|---|---|---|---|
| Germany | Espelage et al. (2010) | 2007–2008 | 24 (24) | × | |
| New Zealand | Wilson et al. (2008) | 2006 | 1214 (1214) | × | |
| Spain | de Lucio et al. (2017) | 2014 | 6 (6), also 16 (16) dogs 2 (2) cats | × | |
| USA | Porter et al. (1990) | 1986 | 10 (8) | × | |
| USA | Figgatt et al. (2017) | 2015 | 20 (20) | × | |
| USA | Quick et al. (1992) | 1990 | 27 (11) | × | |
| USA | White et al. (1989) | 1986 | 88 (72) | × | |
| USA | Petersen et al. (1988) | 1985 | 13 (11) | × | |
| USA | Mintz et al. (1993) | 1990 | 27 (18) | × | |
| USA | Osterholm et al. (1981) | 1979 | 31 (17) | × |
6. Zoonotic transmission
The search results yielded two studies each for farm animal and companion animal contact transmission routes for giardiasis, affecting 408 people and the elderly at a rate of 5193/10,000 people (Table 5) (Jagai et al., 2010; Wojcik-Fatla et al., 2018; Brunn et al., 2019; Rehbein et al., 2019). Until relatively recently, the lack of robust molecular genotyping for Giardia has hampered work to fully understand zoonotic transmission. One study conducted in the USA (Jagai et al., 2010) on the impact of cattle density on rates of Cryptosporidium and Giardia concluded that higher annual rates of giardiasis were recorded in rural areas with low population density, and these populations were likely to be at greater risk of protozoan infections regardless of cattle density (Jagai et al., 2010). It did, however, find strong seasonal patterns, with areas with a large cattle-to-human population ratio showing a peak in Cryptosporidium and Giardia infections during late October. Conversely, a lack of association with cattle was reported from a study of children and cattle in Spain (Cardona et al., 2011) despite another study in the same area detecting Giardia in 18.8% of cattle fecal samples (Cardona et al., 2015). Brunn et al. (2019) investigated the associations between livestock reservoirs and sporadic cases of giardiasis in Ontario, Canada. Livestock reservoirs were investigated by testing either dairy, beef, or swine farms every month. Case crossover analysis found that livestock reservoirs were associated with an increased risk of human giardiasis with a one-week lag period (OR: 1.65, 95% CI: 1.23–2.22, P = 0.001). Assemblage typing data confirmed that zoonotic assemblages A and B were present in the livestock reservoir, which further supports the likelihood of zoonotic transmission (Brunn et al., 2019). This study is supported by another project undertaken in Scotland that demonstrated the presence of human assemblages in both beef and dairy cattle (Bartley et al., 2019). A separate study among veterinarians in Poland suggested the risk of transmission between animals and humans was low (Wojcik-Fatla et al., 2018), but an Australian study (Zajaczkowski et al., 2018) found contact with domestic, farm animals, or wildlife to be a risk factor. One study investigated shedding of Giardia cysts from pet owners (3/69; 4%) who had either cats or dogs; one household pair of human and dog samples had similar, although not identical, assemblage B genetic sequences, suggesting possible transmission. In this study, more dog than cat fecal samples were found to be Giardia positive (39% vs 14% respectively) (Rehbein et al., 2019) (Table 5). Likewise, a study conducted in northern Spain comprising 63 households with domestic cats and dogs found no evidence that they were a significant reservoir for human infection (de Lucio et al., 2017), nor were domestic or farm animals in a study in Germany (Espelage et al., 2010). A review of Giardia in eastern Europe suggested assemblages A and B were common among domestic animals (Plutzer et al., 2018). Assemblage A is thought to be more likely zoonotically transmitted to humans (Horton et al., 2019) than assemblage B. This concept is supported by a multivariate analysis from England (Minetti et al., 2015b) that found dog ownership was a significant risk factor for developing giardiasis, although this effect was limited to contracting assemblage A infections. In summary, it appears that the involvement of animals in the transmission of Giardia is variable and depends on local factors that require further investigation with accurate genotyping tools.
Table 5.
Giardiasis outbreaks due to zoonotic transmission
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | Animal contact |
|
|---|---|---|---|---|---|
| Companion | Farm | ||||
| Canada | Brunn et al. (2019) | 2006–2013 | 403 | × | |
| Germany | Rehbein et al. (2019) | 2019 | 3 (3) | × | |
| Poland | Wojcik-Fatla et al. (2018) | 2018 | 2 (2) | Occupational exposure in veterinarians | |
| USA | Jagai et al. (2010) | 1991–2004 | 5193 (5193) per 10,000 elderly | × | |
It is also important to note that of the previously described outbreaks in which drinking water was involved, six involved C. canadensis beavers that were positive for Giardia, which may have contributed to multiple cases of human giardiasis (Dykes et al., 1980; Lopez et al., 1980; Navin et al., 1985; Kent et al., 1988; Birkhead et al., 1989; Isaac-Renton et al., 1993, 1994) (Table 1). Contaminated water in these cases was found to be insufficiently filtered and/or treated, suggesting the impact of wild animals in the transmission can be alleviated with proper system maintenance. In outbreaks in which beavers were involved, assemblage typing was not always available but, notably, when beavers were removed from the vicinity of the water supply, there were no further cases of giardiasis (Navin et al., 1985; Isaac-Renton et al., 1993, 1994). While the evidence does not support zoonotic transmission as a major risk for human infections when compared with other transmission routes, it is a route that should be considered, especially when positioning reservoirs and designing water distribution networks.
7. Travel association
International travel was associated with outbreaks in a small number of studies, which affected 1288 people as a primary transmission route (Table 6) (Gray et al., 1994; Wilson et al., 2008) and was suggested as a risk factor in some analyses, although this was not universal. In one of two Australian studies of giardiasis risk factors, international travel was only significant in univariate analysis and not in multivariable analysis (Zajaczkowski et al., 2018). International travel was not considered a risk factor in a study from Spain (de Lucio et al., 2017). The risk identified with both international and domestic travel may be related to activities undertaken in the destination and the resulting water or environmental exposures. Some of the studies included in this review were case-control studies or outbreak investigations that excluded any cases of giardiasis with a travel history from the study cohorts, so this was unable to be explored as a risk factor.
Table 6.
Giardiasis outbreaks due to travel-associated transmission
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | International travel |
|---|---|---|---|---|
| New Zealand | Wilson et al. (2008) | 2006 | 1214 (1214) | × |
| UK | Gray et al. (1994) | 1992–1993 | 74 (74) | × |
A study in England showed assemblage B to be the type most frequently isolated from human samples where companion animals were not involved, accounting for 64% of cases compared to 33% for assemblage A. Cases of mixed assemblages were rare (Minetti et al., 2015a), which is consistent with studies in several other countries. A study in Spain also found assemblage B was more common than assemblage A (66/95, 69.5% and 29/95, 30.5% respectively) (Wang et al., 2019). The opposite was found to be true in a Scottish study of 30 Giardia-positive cases, where assemblage A was isolated most frequently (21/30, 72%). This was followed by assemblage B and mixed infections of assemblages A and B (4/30, 14% and 3/30, 10% respectively) (Alexander et al., 2014). This difference in predominant assemblage by country may also contribute to travel-associated giardiasis, due to traveller exposure to novel assemblages as they move to different regions. Another factor which is not mentioned is a potential selection bias that affects who receives Giardia screening tests, which until recently was predominantly those with a history of travel.
8. Multiple transmission routes
Multiple transmission routes for Giardia were described in 11 studies, which included 1308 laboratory confirmed human cases (Table 7). Among these, international travel was the single most important factor identified in seven studies. However, it should be noted that many countries in which these studies were based require a history of foreign travel before testing for Giardia, adding an element of bias into these multivariate analyses. Other risk factors reflect those described above for person-to-person transmission, contaminated water, and animal and environmental exposures. One study also identified taking antibiotics and having a chronic gastrointestinal condition (Reses et al., 2018) while another showed primary immunodeficiencies such as that of immunoglobulin A (IgA) (Agarwal & Mayer, 2013) as risk factors for giardiasis acquisition.
Table 7.
Giardiasis outbreaks due to multiple modes of transmission
| Location | Reference | Year | No. of cases or samples (Lab-confirmed) | Travel-associated |
Waterborne |
Foodborne |
Person-to-person |
Fecal exposure |
Animal contact/other |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | I | Tap Water PrT | UT | RW | RF | UFV | FH | HH | DC | YC | ST | DFC | CA | FA | WA | Other | ||||
| Australia | Zajaczkowski et al. (2018) | 2016 | 68 (68) | × | × | × | × | × | × | |||||||||||
| UK | Hardie et al. (1999) | 1997 | 58 (58) | × | × | × | × | |||||||||||||
| UK | Stuart et al. (2003) | 1998–1999 | 192 (192) | × | × | × | ||||||||||||||
| UK | Minetti et al. (2015b)a | 2012–2013 | 236 (150) | × | × | |||||||||||||||
| × | × | × | × | Reporting IBS symptoms, taking indigestion medication | ||||||||||||||||
| USA | Reses et al. (2018) | 2003–2004 | 213 (213) | × | × | × | × | × | × | × | Taking antibiotics/having a chronic GI condition | |||||||||
| New Zealand | Hoque et al. (2002) | 1998–1999 | 183 (183) | × | × | × | × | × | × | |||||||||||
| New Zealand | Hoque et al. (2003) | 1999–2000 | 69 (69) children under 5 | × | × | × | ||||||||||||||
| Sweden | Andersson et al. (1972) | 1971 | 30 (30) | × | × | |||||||||||||||
| USA | Lopez et al. (1978) | 1976 | 27 (27) | × | × | × | × | |||||||||||||
| USA | Dennis et al. (1993) | 1984–1985 | 273 (273) | × | × | × | × | |||||||||||||
| USA | Novotny et al. (1990) | 1983 | 45 (45) | × | ×b | |||||||||||||||
Abbreviations: Travel-associated (I, international; D, domestic); Waterborne (PrT, pre-treatment; UT, untreated; RW, recreational water); Foodborne (RF, reconstituted foodstuff; UFV, unwashed fruit/vegetables; FH, food handler); Person-to-person (HH, household GI illness; DC, day-care attendance; YC, young children); Fecal exposure (ST, sexual transmission; DFC, direct fecal contact); Animal contact/other (CA, companion animal; FA, farm animal; WA, wild animal; IBS, irritable bowel syndrome); GI, gastrointestinal.
Study included two separate multivariate analyses, both with (first line) and without (second line) international travel as a risk factor.
Found to be a factor with a family size ≥ 4 people.
9. Conclusions
This review challenges the hypothesis that Giardia outbreaks in higher income countries are primarily associated with foreign travel and shows that transmission can occur through a wide range of local routes. This likely reflects endemic populations of Giardia that have been overlooked due to an insistence of a history of foreign travel before testing in several higher income countries. Of these routes, contaminated water was the most frequently identified route of Giardia transmission in the literature, primarily due to insufficient treatment or post-treatment contamination due to poor maintenance or practices. Water-linked outbreaks are also common to LMICs, but the situations are not directly comparable as poverty and a lack of proper sanitation are the major causes of high giardiasis prevalence in LMICs rather than a disruption in water quality. This suggests that continued investment in water distribution networks in higher income countries is essential to control the disease and there is a need to avoid complacency. This systematic study also highlights a lack of robust case-control studies for assessing the risk of Giardia in higher income countries. Without such analyses, it was not possible to perform a detailed meta-analysis in this review as has been done for LMICs (Fakhri et al., 2021). This was exacerbated by extremely high heterogeneity in study methods and design. It was also noted very few studies examined a range of possible transmission routes for an outbreak, with most focusing on water supply or travel. It is likely that many researchers similarly limit themselves and if an origin is not one of these two common routes, an outbreak is unlikely to be reported, further adding to the publication bias. This may explain the large number of outbreaks linked to water in the literature. This review therefore highlights the need for more in-depth studies with consistent methodology to improve our understanding of this pathogen in higher income countries. It also underscores the need for full and publicly available epidemiological examinations of Giardia outbreaks to avoid such publication bias. Our understanding of the various transmission pathways is further hampered by the lack of studies that include in-depth molecular data, such as assemblage typing, that could be used to understand zoonotic transmission. Indeed, it was noted that only three studies reported molecular genotyping in their results. Wider employment of molecular genotyping and improved tools to determine specific variants will improve surveillance of sporadic cases and help identify outbreaks and associated risk factors. Despite these caveats, just focusing on reported sources and transmission routes rather than estimating risks, our systematic analysis suggests that there are numerous sources and routes for Giardia outbreaks in higher income countries, particularly due to failures in water treatment and infrastructure.
Funding
This study was funded by an award by the Chief Scientist Office, reference TCS/18/22.
Ethical approval
Not applicable.
CRediT author statement
Sarah Krumrie: validation, formal analysis, investigation, data curation, writing - original draft, writing - review & editing, visualisation. Paul Capewell: methodology, software, validation, formal analysis, investigation, resources, data curation, writing - original draft, writing - review & editing, visualisation, supervision. Alison Smith-Palmer: conceptualisation, methodology, resources, writing - original draft, writing - review & editing. Dominic Mellor: writing - review & editing. Willie Weir: resources, writing - review & editing, visualisation, supervision, project administration, funding acquisition. Claire L Alexander: conceptualisation, resources, writing - review & editing, supervision, project administration, funding acquisition.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2022.100084.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Multimedia component 1
References
- Adam R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin. Gastroenterol. Hepatol. 2013;11:1050–1063. doi: 10.1016/j.cgh.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C.L., Currie S., Pollock K., Smith-Palmer A., Jones B.L. An audit of Cryptosporidium and Giardia detection in Scottish National Health Service Diagnostic Microbiology Laboratories. Epidemiology and Infection. 2017;145:1584–1590. doi: 10.1017/S0950268817000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C., Jones B., Inverarity D., Pollock K.G. Genotyping of Giardia isolates in Scotland: A descriptive epidemiological study. Epidemiol. Infect. 2014;142:1636–1639. doi: 10.1017/S0950268813002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoros I., Alonso J.L., Cuesta G. Cryptosporidium oocysts and Giardia cysts on salad products irrigated with contaminated water. J. Food Prot. 2010;73:1138–1140. doi: 10.4315/0362-028x-73.6.1138. [DOI] [PubMed] [Google Scholar]
- Andersson T., Forssell J., Sterner G. Outbreak of giardiasis: Effect of a new antiflagellate drug, tinidazole. Br. Med. J. 1972;2:449–451. doi: 10.1136/bmj.2.5811.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.H. Outbreak of giardiasis in a daycare nursery. Commun. Dis. Public Health. 2000;3:212–213. [PubMed] [Google Scholar]
- Ansell B.R., McConville M.J., Maayeh S.Y., Dagley M.J., Gasser R.B., Svard S.G., Jex A.R. Drug resistance in Giardia duodenalis. Biotechnol. Adv. 2015;33:888–901. doi: 10.1016/j.biotechadv.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Baldursson S., Karanis P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks - an update 2004–2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Bartlett A.V., Moore M., Gary G.W., Starko K.M., Erben J.J., Meredith B.A. Diarrheal illness among infants and toddlers in day care centers. I. Epidemiology and pathogens. J. Pediatr. 1985;107:495–502. doi: 10.1016/s0022-3476(85)80004-4. [DOI] [PubMed] [Google Scholar]
- Bartley P.M., Roehe B.K., Thomson S., Shaw H.J., Peto F., Innes E.A., Katzer F. Detection of potentially human infectious assemblages of Giardia duodenalis in fecal samples from beef and dairy cattle in Scotland. Parasitology. 2019;146:1123–1130. doi: 10.1017/S0031182018001117. [DOI] [PubMed] [Google Scholar]
- Bedard B.A., Elder R., Phillips L., Wachunas M.F. Giardia outbreak associated with a roadside spring in Rensselaer County, New York. Epidemiol. Infect. 2016;144:3013–3016. doi: 10.1017/S0950268816001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander R., Palm J.E., Svard S.G. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell. Microbiol. 2001;3:55–62. doi: 10.1046/j.1462-5822.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- Birkhead G., Janoff E.N., Vogt R.L., Smith P.D. Elevated levels of immunoglobulin A to Giardia lamblia during a waterborne outbreak of gastroenteritis. J. Clin. Microbiol. 1989;27:1707–1710. doi: 10.1128/jcm.27.8.1707-1710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead G., Vogt R.L. Epidemiologic surveillance for endemic Giardia lamblia infection in Vermont. The roles of waterborne and person-to-person transmission. Am. J. Epidemiol. 1989;129:762–768. doi: 10.1093/oxfordjournals.aje.a115191. [DOI] [PubMed] [Google Scholar]
- Black R.E., Dykes A.C., Sinclair S.P., Wells J.G. Giardiasis in day-care centers: Evidence of person-to-person transmission. Pediatrics. 1977;60:486–491. [PubMed] [Google Scholar]
- Borg M.L., Modi A., Tostmann A., Gobin M., Cartwright J., Quigley C., et al. Ongoing outbreak of Shigella flexneri serotype 3a in men who have sex with men in England and Wales, data from 2009–2011. Euro Surveill. 2012;17 [PubMed] [Google Scholar]
- Brunn A., Fisman D.N., Sargeant J.M., Greer A.L. The influence of climate and livestock reservoirs on human cases of giardiasis. EcoHealth. 2019;16:116–127. doi: 10.1007/s10393-018-1385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona G.A., Carabin H., Goni P., Arriola L., Robinson G., Fernandez-Crespo J.C., et al. Identification and molecular characterization of Cryptosporidium and Giardia in children and cattle populations from the province of alava, north of Spain. Sci. Total Environ. 2011;412–413:101–108. doi: 10.1016/j.scitotenv.2011.09.076. [DOI] [PubMed] [Google Scholar]
- Cardona G.A., de Lucio A., Bailo B., Cano L., de Fuentes I., Carmena D. Unexpected finding of feline-specific Giardia duodenalis assemblage F and Cryptosporidium felis in asymptomatic adult cattle in northern Spain. Vet. Parasitol. 2015;209:258–263. doi: 10.1016/j.vetpar.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Cheun H.I., Kim C.H., Cho S.H., Ma D.W., Goo B.L., Na M.S., et al. The first outbreak of giardiasis with drinking water in Korea. Osong Public Health Res. Perspect. 2013;4:89–92. doi: 10.1016/j.phrp.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale K., Kirk M., Sinclair M., Hall R., Leder K. Reported waterborne outbreaks of gastrointestinal disease in Australia are predominantly associated with recreational exposure. Aust. N. Z. J. Public Health. 2010;34:527–530. doi: 10.1111/j.1753-6405.2010.00602.x. [DOI] [PubMed] [Google Scholar]
- Daly E.R., Roy S.J., Blaney D.D., Manning J.S., Hill V.R., Xiao L., Stull J.W. Outbreak of giardiasis associated with a community drinking-water source. Epidemiol. Infect. 2010;138:491–500. doi: 10.1017/S0950268809990744. [DOI] [PubMed] [Google Scholar]
- Dawson D. Foodborne protozoan parasites. Int. J. Food Microbiol. 2005;103:207–227. doi: 10.1016/j.ijfoodmicro.2004.12.032. [DOI] [PubMed] [Google Scholar]
- de Lucio A., Bailo B., Aguilera M., Cardona G.A., Fernandez-Crespo J.C., Carmena D. No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the Province of Alava, northern Spain. Acta Trop. 2017;170:48–56. doi: 10.1016/j.actatropica.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Dennis D.T., Smith R.P., Welch J.J., Chute C.G., Anderson B., Herndon J.L., von Reyn C.F. Endemic giardiasis in New Hampshire: A case-control study of environmental risks. J. Infect. Dis. 1993;167:1391–1395. doi: 10.1093/infdis/167.6.1391. [DOI] [PubMed] [Google Scholar]
- Dixon B., Parrington L., Cook A., Pollari F., Farber J. Detection of Cyclospora, Cryptosporidium, and Giardia in ready-to-eat packaged leafy greens in Ontario, Canada. J. Food Prot. 2013;76:307–313. doi: 10.4315/0362-028X.JFP-12-282. [DOI] [PubMed] [Google Scholar]
- Dormond M., Gutierrez R.L., Porter C.K. Giardia lamblia infection increases risk of chronic gastrointestinal disorders. Trop. Dis. Travel Med. Vaccines. 2016;2:17. doi: 10.1186/s40794-016-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes A.C., Juranek D.D., Lorenz R.A., Sinclair S., Jakubowski W., Davies R. Municipal waterborne giardiasis: An epidemilogic investigation. Beavers implicated as a possible reservoir. Ann. Intern. Med. 1980;92:165–170. doi: 10.7326/0003-4819-92-2-165. [DOI] [PubMed] [Google Scholar]
- Efstratiou A., Ongerth J.E., Karanis P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks - an update 2011–2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Eisenstein L., Bodager D., Ginzl D. Outbreak of giardiasis and cryptosporidiosis associated with a neighborhood interactive water fountain - Florida, 2006. J Environ. Health. 2008;71:18–22. quiz 49-50. [PubMed] [Google Scholar]
- Escobedo A.A., Almirall P., Alfonso M., Cimerman S., Chacin-Bonilla L. Sexual transmission of giardiasis: A neglected route of spread? Acta Trop. 2014;132:106–111. doi: 10.1016/j.actatropica.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Espelage W., an der Heiden M., Stark K., Alpers K. Characteristics and risk factors for symptomatic Giardia lamblia infections in Germany. BMC Public Health. 2010;10:41. doi: 10.1186/1471-2458-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri Y., Daraei H., Ghaffari H.R., Rezapour-Nasrabad R., Soleimani-Ahmadi M., Khedher K.M., et al. The risk factors for intestinal Giardia spp infection: Global systematic review and meta-analysis and meta-regression. Acta Trop. 2021;220:105968. doi: 10.1016/j.actatropica.2021.105968. [DOI] [PubMed] [Google Scholar]
- Figgatt M., Mergen K., Kimelstein D., Mahoney D.M., Newman A., Nicholas D., et al. Giardiasis outbreak associated with asymptomatic food handlers in New York State, 2015. J. Food Protection. 2017:837–841. doi: 10.4315/0362-028X.JFP-16-415. [DOI] [PubMed] [Google Scholar]
- Gray S.F., Gunnell D.J., Peters T.J. Risk factors for giardiasis: A case-control study in Avon and Somerset. Epidemiol. Infect. 1994;113:95–102. doi: 10.1017/s0950268800051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greensmith C.T., Stanwick R.S., Elliot B.E., Fast M.V. Giardiasis associated with the use of a water slide. Pediatr. Infect. Dis. J. 1988;7:91–94. doi: 10.1097/00006454-198802000-00005. [DOI] [PubMed] [Google Scholar]
- Guzman-Herrador B., Carlander A., Ethelberg S., Freiesleben de Blasio B., Kuusi M., Lund V., et al. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.es2015.20.24.21160. [DOI] [PubMed] [Google Scholar]
- Hall V., Taye A., Walsh B., Maguire H., Dave J., Wright A., et al. A large outbreak of gastrointestinal illness at an open-water swimming event in the River Thames, London. Epidemiol. Infect. 2017;145:1246–1255. doi: 10.1017/S0950268816003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanevik K., Wensaas K.A., Rortveit G., Eide G.E., Morch K., Langeland N. Irritable bowel syndrome and chronic fatigue 6 years after Giardia infection: A controlled prospective cohort study. Clin. Infect. Dis. 2014;59:1394–1400. doi: 10.1093/cid/ciu629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R.M., Wall P.G., Gott P., Bardhan M., Bartlett L.R. Infectious diarrhea in tourists staying in a resort hotel. Emerg. Infect. Dis. 1999;5:168–171. doi: 10.3201/eid0501.990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter L., Frost F., Grunenfelder G., Perkins-Jones K., Libby J. Giardiasis in an infant and toddler swim class. Am. J. Publ. Health. 1984;74:155–156. doi: 10.2105/ajph.74.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellard M., Hocking J., Willis J., Dore G., Fairley C. Risk factors leading to Cryptosporidium infection in men who have sex with men. Sex. Transm. Infect. 2003;79:412–414. doi: 10.1136/sti.79.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwaldt B.L., Craun G.F., Stokes S.L., Juranek D.D. Waterborne-disease outbreaks, 1989–1990. MMWR CDC Surveill. Summ. 1991;40:1–21. [PubMed] [Google Scholar]
- Hopkins R.S., Juranek D.D. Acute giardiasis: An improved clinical case definition for epidemiologic studies. Am. J. Epidemiol. 1991;133:402–407. doi: 10.1093/oxfordjournals.aje.a115894. [DOI] [PubMed] [Google Scholar]
- Hoque M.E., Hope V.T., Scragg R., Kjellstrom T. Children at risk of giardiasis in auckland: A case-control analysis. Epidemiol. Infect. 2003;131:655–662. doi: 10.1017/s0950268803008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M.E., Hope V.T., Scragg R., Kjellstrom T., Lay-Yee R. Nappy handling and risk of giardiasis. Lancet. 2001;357:1017–1018. doi: 10.1016/S0140-6736(00)04251-3. [DOI] [PubMed] [Google Scholar]
- Hoque M.E., Hope V.T., Scragg R., Kjellstrom T., Lay-Yee R. Risk of giardiasis in Aucklanders: A case-control study. Int. J. Infect. Dis. 2002;6:191–197. doi: 10.1016/s1201-9712(02)90110-4. [DOI] [PubMed] [Google Scholar]
- Horton B., Bridle H., Alexander C.L., Katzer F. Giardia duodenalis in the UK: Current knowledge of risk factors and public health implications. Parasitology. 2019;146:413–424. doi: 10.1017/S0031182018001683. [DOI] [PubMed] [Google Scholar]
- Isaac-Renton J., Blatherwick J., Bowie W.R., Fyfe M., Khan M., Li A., et al. Epidemic and endemic seroprevalence of antibodies to Cryptosporidium and Giardia in residents of three communities with different drinking water supplies. Am. J. Trop. Med. Hyg. 1999;60:578–583. doi: 10.4269/ajtmh.1999.60.578. [DOI] [PubMed] [Google Scholar]
- Isaac-Renton J.L., Cordeiro C., Sarafis K., Shahriari H. Characterization of Giardia duodenalis isolates from a waterborne outbreak. J. Infect. Dis. 1993;167:431–440. doi: 10.1093/infdis/167.2.431. [DOI] [PubMed] [Google Scholar]
- Isaac-Renton J.L., Lewis L.F., Ong C.S., Nulsen M.F. A second community outbreak of waterborne giardiasis in Canada and serological investigation of patients. Trans. R. Soc. Trop. Med. Hyg. 1994;88:395–399. doi: 10.1016/0035-9203(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Istre G.R., Dunlop T.S., Gaspard G.B., Hopkins R.S. Waterborne giardiasis at a mountain resort: Evidence for acquired immunity. Am. J. Publ. Health. 1984;74:602–604. doi: 10.2105/ajph.74.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagai J.S., Griffiths J.K., Kirshen P.H., Webb P., Naumova E.N. Patterns of protozoan infections: Spatiotemporal associations with cattle density. EcoHealth. 2010;7:33–46. doi: 10.1007/s10393-010-0286-1. [DOI] [PubMed] [Google Scholar]
- Jephcott A.E., Begg N.T., Baker I.A. Outbreak of giardiasis associated with mains water in the United Kingdom. Lancet. 1986;1:730–732. doi: 10.1016/s0140-6736(86)91114-1. [DOI] [PubMed] [Google Scholar]
- Karanis P., Kourenti C., Smith H. Waterborne transmission of protozoan parasites: A worldwide review of outbreaks and lessons learnt. J. Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- Karon A.E., Hanni K.D., Mohle-Boetani J.C., Beretti R.A., Hill V.R., Arrowood M., et al. Giardiasis outbreak at a camp after installation of a slow-sand filtration water-treatment system. Epidemiol. Infect. 2011;139:713–717. doi: 10.1017/S0950268810001573. [DOI] [PubMed] [Google Scholar]
- Katz D.E., Heisey-Grove D., Beach M., Dicker R.C., Matyas B.T. Prolonged outbreak of giardiasis with two modes of transmission. Epidemiol. Infect. 2006;134:935–941. doi: 10.1017/S0950268805005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent G.P., Greenspan J.R., Herndon J.L., Mofenson L.M., Harris J.A., Eng T.R., Waskin H.A. Epidemic giardiasis caused by a contaminated public water supply. Am. J. Publ. Health. 1988;78:139–143. doi: 10.2105/ajph.78.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone J.S., Krajden S., Warren M.R. Person-to-person transmission of Giardia lamblia in day-care nurseries. Can. Med. Assoc. J. 1978;119:241–242. 247-248. [PMC free article] [PubMed] [Google Scholar]
- Kramer M.H., Herwaldt B.L., Craun G.F., Calderon R.L., Juranek D.D. Surveillance for waterborne-disease outbreaks - United States, 1993–1994. MMWR CDC Surveill. Summ. 1996;45:1–33. [PubMed] [Google Scholar]
- Lehto K.M., Fan Y.M., Oikarinen S., Nurminen N., Hallamaa L., Juuti R., et al. Presence of Giardia lamblia in stools of six- to 18-month old asymptomatic Malawians is associated with childrens growth failure. Acta Paediatr. 2019;108:1833–1840. doi: 10.1111/apa.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D. Drug resistance in the microaerophilic parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015;2:128–135. doi: 10.1007/s40475-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K.C., Leung A.A.M., Wong A.H.C., Sergi C.M., Kam J.K.M. Giardiasis: An overview. Inflamm. Allergy Drug Disc. 2019;13:134–143. doi: 10.2174/1872213X13666190618124901. [DOI] [PubMed] [Google Scholar]
- Levine W.C., Stephenson W.T., Craun G.F. Waterborne disease outbreaks, 1986–1988. MMWR CDC Surveill. Summ. 1990;39:1–13. [PubMed] [Google Scholar]
- Levy D.A., Bens M.S., Craun G.F., Calderon R.L., Herwaldt B.L. Surveillance for waterborne-disease outbreaks - United States, 1995–1996. MMWR CDC Survill. Summ. 1998;47:1–34. [PubMed] [Google Scholar]
- Litleskare S., Rortveit G., Eide G.E., Hanevik K., Langeland N., Wensaas K.A. Prevalence of irritable bowel syndrome and chronic fatigue 10 years after Giardia infection. Clin. Gastroenterol. Hepatol. 2018;16 doi: 10.1016/j.cgh.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Lopez C.E., Dykes A.C., Juranek D.D., Sinclair S.P., Conn J.M., Christie R.W., et al. Waterborne giardiasis: A communitywide outbreak of disease and a high rate of asymptomatic infection. Am. J. Epidemiol. 1980;112:495–507. doi: 10.1093/oxfordjournals.aje.a113019. [DOI] [PubMed] [Google Scholar]
- Lopez C.E., Juranek D.D., Sinclair S.P., Schultz M.G. Giardiasis in American travelers to Madeira Island, Portugal. Am. J. Trop. Med. Hyg. 1978;27:1128–1132. doi: 10.4269/ajtmh.1978.27.1128. [DOI] [PubMed] [Google Scholar]
- Masina S., Shirley J., Allen J., Sargeant J.M., Guy R.A., Wallis P.M., et al. Weather, environmental conditions, and waterborne Giardia and Cryptosporidium in Iqaluit. Nunavut. J. Water Health. 2019;17:84–97. doi: 10.2166/wh.2018.323. [DOI] [PubMed] [Google Scholar]
- Ma L., Zhang X., Jian Y., Li X., Wang G., Hu Y., Karanis P. Detection of Cryptosporidium and Giardia in the slaughterhouse, sewage and river waters of the Qinghai Tibetan Plateau area (QTPA), China. Parasitol. Res. 2019;118:2041–2051. doi: 10.1007/s00436-019-06330-w. [DOI] [PubMed] [Google Scholar]
- Meyers J.D., Kuharic H.A., Holmes K.K. Giardia lamblia infection in homosexual men. Br. J. Vener. Dis. 1977;53:54–55. doi: 10.1136/sti.53.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C., Lamden K., Durband C., Cheesbrough J., Fox A., Wastling J.M. Determination of Giardia duodenalis assemblages and multi-locus genotypes in patients with sporadic giardiasis from England. Parasites & Vectors. 2015;8:444. doi: 10.1186/s13071-015-1059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C., Lamden K., Durband C., Cheesbrough J., Platt K., Charlett A., et al. Case-control study of risk factors for sporadic giardiasis and parasite assemblages in North-West England. J. Clin. Microbiol. 2015;53:3133–3140. doi: 10.1128/JCM.00715-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz E.D., Hudson-Wragg M., Mshar P., Cartter M.L., Hadler J.L. Foodborne giardiasis in a corporate office setting. J. Infect. Dis. 1993;167:250–253. doi: 10.1093/infdis/167.1.250. [DOI] [PubMed] [Google Scholar]
- Mook P., Gardiner D., Kanagarajah S., Kerac M., Hughes G., Field N., et al. Use of gender distribution in routine surveillance data to detect potential transmission of gastrointestinal infections among men who have sex with men in England. Epidemiol. Infect. 2018;146:1468–1477. doi: 10.1017/S0950268818001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.C., Herwaldt B.L., Craun G.F., Calderon R.L., Highsmith A.K., Juranek D.D. Surveillance for waterborne disease outbreaks - United States, 1991–1992. MMWR CDC Surveill. Summ. 1993;42:1–22. [PubMed] [Google Scholar]
- Muller J., Hemphill A., Muller N. Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2018;8:271–277. doi: 10.1016/j.ijpddr.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin T.R., Juranek D.D., Ford M., Minedew D.J., Lippy E.C., Pollard R.A. Case-control study of waterborne giardiasis in Reno, Nevada. Am. J. Epidemiol. 1985;122:269–275. doi: 10.1093/oxfordjournals.aje.a114098. [DOI] [PubMed] [Google Scholar]
- Ndumbi P., Freidl G.S., Williams C.J., Mardh O., Varela C., Avellon A., et al. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.33.1700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neringer R., Andersson Y., Eitrem R. A water-borne outbreak of giardiasis in Sweden. Scand. J. Infect. Dis. 1987;19:85–90. doi: 10.3109/00365548709032382. [DOI] [PubMed] [Google Scholar]
- Newman K.L., Newman G.S., Cybulski R.J., Fang F.C. Gastroenteritis in men who have sex with men in Seattle, Washington, 2017–2018. Clin. Infect. Dis. 2020;71:109–115. doi: 10.1093/cid/ciz783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny T.E., Hopkins R.S., Shillam P., Janoff E.N. Prevalence of Giardia lamblia and risk factors for infection among children attending day-care facilities in Denver. Publ. Health Rep. 1990;105:72–75. [PMC free article] [PubMed] [Google Scholar]
- Nygard K., Schimmer B., Sobstad O., Walde A., Tveit I., Langeland N., et al. A large community outbreak of waterborne giardiasis-delayed detection in a non-endemic urban area. BMC Public Health. 2006;6:141. doi: 10.1186/1471-2458-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm M.T., Forfang J.C., Ristinen T.L., Dean A.G., Washburn J.W., Godes J.R., et al. An outbreak of foodborne giardiasis. N. Engl. J. Med. 1981;304:24–28. doi: 10.1056/NEJM198101013040106. [DOI] [PubMed] [Google Scholar]
- Petersen L.R., Cartter M.L., Hadler J.L. A food-borne outbreak of Giardia lamblia. J. Infect. Dis. 1988;157:846–848. doi: 10.1093/infdis/157.4.846. [DOI] [PubMed] [Google Scholar]
- Phillips S.C., Mildvan D., William D.C., Gelb A.M., White M.C. Sexual transmission of enteric protozoa and helminths in a venereal-disease-clinic population. N. Engl. J. Med. 1981;305:603–606. doi: 10.1056/NEJM198109103051102. [DOI] [PubMed] [Google Scholar]
- Pijnacker R., Mughini-Gras L., Vennema H., Enserink R., van den Wijngaard C.C., Kortbeek T., van Pelt W. Characteristics of child daycare centres associated with clustering of major enteropathogens. Epidemiol. Infect. 2016;144:2527–2539. doi: 10.1017/S0950268816001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzer J., Lassen B., Jokelainen P., Djurkovic-Djakovic O., Kucsera I., Dorbek-Kolin E., et al. Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.4.16-00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polis M.A., Tuazon C.U., Alling D.W., Talmanis E. Transmission of Giardia lamblia from a day care center to the community. Am. J. Publ. Health. 1986;76:1142–1144. doi: 10.2105/ajph.76.9.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.D., Gaffney C., Heymann D., Parkin W. Food-borne outbreak of Giardia lamblia. Am. J. Publ. Health. 1990;80:1259–1260. doi: 10.2105/ajph.80.10.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.D., Ragazzoni H.P., Buchanon J.D., Waskin H.A., Juranek D.D., Parkin W.E. Giardia transmission in a swimming pool. Am. J. Publ. Health. 1988;78:659–662. doi: 10.2105/ajph.78.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick R., Paugh K., Addiss D., Kobayashi J., Baron R. Restaurant-associated outbreak of giardiasis. J. Infect. Dis. 1992;166:673–676. doi: 10.1093/infdis/166.3.673. [DOI] [PubMed] [Google Scholar]
- Rauch A.M., Van R., Bartlett A.V., Pickering L.K. Longitudinal study of Giardia lamblia infection in a day care center population. Pediatr. Infect. Dis. J. 1990;9:186–189. doi: 10.1097/00006454-199003000-00008. [DOI] [PubMed] [Google Scholar]
- Reeve N.F., Diggle P.J., Lamden K., Keegan T. A spatial analysis of giardiasis and cryptosporidiosis in relation to public water supply distribution in North-West England. Spat. Spatiotemporal Epidemiol. 2018;27:61–70. doi: 10.1016/j.sste.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Rehbein S., Klotz C., Ignatius R., Muller E., Aebischer A., Kohn B. Giardia duodenalis in small animals and their owners in Germany: A pilot study. Zoonoses Public Health. 2019;66:117–124. doi: 10.1111/zph.12541. [DOI] [PubMed] [Google Scholar]
- Reses H.E., Gargano J.W., Liang J.L., Cronquist A., Smith K., Collier S.A., et al. Risk factors for sporadic Giardia infection in the USA: A case-control study in Colorado and Minnesota. Epidemiol. Infect. 2018;146:1071–1078. doi: 10.1017/S0950268818001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resi D., Varani S., Sannella A.R., De Pascali A.M., Ortalli M., Liguori G., et al. A large outbreak of giardiasis in a municipality of the Bologna province, north-eastern Italy, November 2018 to April 2019. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.35.2001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimhanen-Finne R., Hanninen M.L., Vuento R., Laine J., Jokiranta T.S., Snellman M., et al. Contaminated water caused the first outbreak of giardiasis in Finland, 2007: A descriptive study. Scand. J. Infect. Dis. 2010;42:613–619. doi: 10.3109/00365541003774608. [DOI] [PubMed] [Google Scholar]
- Robertson L.J., Gjerde B. Occurrence of parasites on fruits and vegetables in Norway. J. Food Prot. 2001;64:1793–1798. doi: 10.4315/0362-028x-64.11.1793. [DOI] [PubMed] [Google Scholar]
- Rogawski E.T., Bartelt L.A., Platts-Mills J.A., Seidman J.C., Samie A., Havt A., et al. Determinants and impact of Giardia infection in the first 2 years of life in the MAL-ED birth cohort. J. Pediatric Infect. Dis. Soc. 2017;6:153–160. doi: 10.1093/jpids/piw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski E.T., Liu J., Platts-Mills J.A., Kabir F., Lertsethtakarn P., Siguas M., et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: Longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob. Health. 2018;6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.B., Slifko T.R. Giardia, Cryptosporidium, and Cyclospora and their impact on foods: A review. J. Food Prot. 1999;62:1059–1070. doi: 10.4315/0362-028x-62.9.1059. [DOI] [PubMed] [Google Scholar]
- Ryan U., Caccio S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013;43:943–956. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Schreiber C., Heinkel S.B., Zacharias N., Mertens F.M., Christoffels E., Gayer U., et al. Infectious rain? Evaluation of human pathogen concentrations in stormwater in separate sewer systems. Water Sci. Technol. 2019;80:1022–1030. doi: 10.2166/wst.2019.340. [DOI] [PubMed] [Google Scholar]
- Shaw P.K., Brodsky R.E., Lyman D.O., Wood B.T., Hibler C.P., Healy G.R., et al. A communitywide outbreak of giardiasis with evidence of transmission by a municipal water supply. Ann. Intern. Med. 1977;87:426–432. doi: 10.7326/0003-4819-87-4-426. [DOI] [PubMed] [Google Scholar]
- Slifko T.R., Smith H.V., Rose J.B. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 2000;30:1379–1393. doi: 10.1016/s0020-7519(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283 doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Stuart J.M., Orr H.J., Warburton F.G., Jeyakanth S., Pugh C., Morris I., et al. Risk factors for sporadic giardiasis: A case-control study in southwestern England. Emerg. Infect. Dis. 2003;9:229–233. doi: 10.3201/eid0902.010488. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F., et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totkova A., Klobusicky M., Holkova R., Valent M., Stojkovicova H. A sewage disposal failure as a cause of ascariasis and giardiasis epidemic in a family. Bratislavské Lekárske Listy. 2004;105:117–122. [PubMed] [Google Scholar]
- Tsui C.K., Miller R., Uyaguari-Diaz M., Tang P., Chauve C., Hsiao W., et al. Beaver fever: Whole-genome characterization of waterborne outbreak and sporadic isolates to study the zoonotic transmission of giardiasis. mSphere. 2018;3 doi: 10.1128/mSphere.00090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency . National Service for Environmental Publications (NSCEP); 1999. Giardia: Risk for infants and children.https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1002VQD.txt [Google Scholar]
- Waldram A., Vivancos R., Hartley C., Lamden K. Prevalence of Giardia infection in households of Giardia cases and risk factors for household transmission. BMC Infect. Dis. 2017;17:486. doi: 10.1186/s12879-017-2586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gonzalez-Moreno O., Roellig D.M., Oliver L., Huguet J., Guo Y., et al. Epidemiological distribution of genotypes of Giardia duodenalis in humans in Spain. Parasit. Vectors. 2019;12:432. doi: 10.1186/s13071-019-3692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch T.P. Risk of giardiasis from consumption of wilderness water in North America: A systematic review of epidemiologic data. Int. J. Infect. Dis. 2000;4:100–103. doi: 10.1016/s1201-9712(00)90102-4. [DOI] [PubMed] [Google Scholar]
- Weniger B.G., Blaser M.J., Gedrose J., Lippy E.C., Juranek D.D. An outbreak of waterborne giardiasis associated with heavy water runoff due to warm weather and volcanic ashfall. Am. J. Publ. Health. 1983;73:868–872. doi: 10.2105/ajph.73.8.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K.E., Hedberg C.W., Edmonson L.M., Jones D.B., Osterholm M.T., MacDonald K.L. An outbreak of giardiasis in a nursing home with evidence for multiple modes of transmission. J.Infect. Dis. 1989;160:298–304. doi: 10.1093/infdis/160.2.298. [DOI] [PubMed] [Google Scholar]
- Wilson N., Baker M., Edwards R., Simmons G. Case-case analysis of enteric diseases with routine surveillance data: Potential use and example results. Epidemiol. Perspect. Innov. 2008;5:6. doi: 10.1186/1742-5573-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik-Fatla A., Sroka J., Zajac V., Zwolinski J., Dutkiewicz J. Study on Giardia duodenalis and Cryptosporidium spp. infection in veterinarians in Poland. Ann. Argric. Environ. Med. 2018;25:732–733. doi: 10.26444/aaem/101576. [DOI] [PubMed] [Google Scholar]
- Xiao S., Yin P., Zhang Y., Hu S. Occurrence of Cryptosporidium and Giardia and the relationship between Protozoa and water quality indicators in swimming pools. Korean J. Parasitol. 2017;55:129–135. doi: 10.3347/kjp.2017.55.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajaczkowski P., Mazumdar S., Conaty S., Ellis J.T., Fletcher-Lartey S.M. Epidemiology and associated risk factors of giardiasis in a peri-urban setting in New South Wales Australia. Epidemiol Infect. 2018;147:e–15. doi: 10.1017/S0950268818002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1