Abstract
Escherichia coli isolates from patients with bacteriuria of pregnancy were compared by PCR with isolates from patients with community-acquired cystitis for the presence of established virulence determinants. The strains from patients with bacteriuria of pregnancy were less likely to carry genes for P-family, S-family, and F1C adhesins, cytotoxic necrotizing factor 1, and aerobactin, but virtually all of the strains carried the genes for type 1 fimbriae. Standard mannose-sensitive agglutination of yeast cells showed that only 15 of 42 bacteriuria strains (36%) expressed type 1 fimbriae compared with 32 of 42 strains from community-acquired symptomatic infections (76%) (P < 0.01). This difference was confirmed by analysis of all isolates for an allele of the type 1 fimbrial regulatory region (fim switch), which negates type 1 fimbrial expression by preventing the fim switch from being inverted to the on phase. This allele, fimS49, was found in 8 of 47 bacteriuria strains from pregnant women (17.0%) compared with 2 of 60 strains isolated from patients with cystitis (3.3%) (P < 0.05). Determination of the phase switch orientation in vivo by analysis of freshly collected infected urine from patients with bacteriuria showed that the fim switch was detectable in the off orientation in 17 of 23 urine samples analyzed (74%). These data indicate that type 1 fimbriae are not necessary to maintain the majority of E. coli bacteriurias in pregnant women since there appears to be selection against their expression in this particular group. This is in contrast to the considered role of this adhesin in community-acquired symptomatic infections. The lack of type 1 fimbria expression is likely to contribute to the asymptomatic nature of bacteriuria in pregnant women, although approximately one-third of the bacteriuria isolates do possess key virulence determinants. If left untreated, this subset of isolates pose the greatest threat to the health of the mother and unborn child.
Escherichia coli, the most common cause of bacteriuria, is usually able to express a number of adherence factors that promote initial colonization and allow persistence in the face of regular urine flow (4, 31, 32). The most common E. coli adhesin, expressed by over 80% of uropathogenic strains, is the type 1 fimbria. Type 1 fimbriae are long, thin proteinaceous surface organelles with a tip composed of the FimH protein that binds to α-d-mannose-containing receptors (18, 28). Uroplakins appear to be a receptor for type 1 fimbriae within the bladder and associated tissue (33). Expression of type 1 fimbriae has been shown to occur in vivo and to be important for initiation of urinary tract infections (UTIs) (3, 4, 27). The urinary tract has a number of mechanisms to prevent colonization by bacteria, including an immune response to antigenic fimbriae and mucus to act as a barrier to adherence. To evade this immune response and to save the potential metabolic burden of producing fimbriae, E. coli has evolved complex phase-variable mechanisms to regulate expression of most adhesins. For type 1 fimbriae, the fim promoter is located on a 314-bp “switch” region of the chromosome flanked by inverted repeats (1). In a now well-characterized process, the switch region can be inverted from off to on and vice versa by two recombinases (FimB and FimE) so that the promoter is either correctly or incorrectly oriented to produce type 1 fimbriae (7, 17, 24). We have previously analyzed the switch region of E. coli strains isolated from UTIs and shown that functional heterogeneity exists in the regulation of type 1 phase switching. While the majority of strains could produce type 1 fimbriae under favorable conditions, some strains had the switch locked in the off orientation. One allele (fimS49, screened for in the present study) was isolated which had a single-base-pair insertion in one of the recombinase binding sites, practically preventing phase transition to the on state (20). UTIs occur in 2 to 10% of pregnant women. During the first trimester they are often asymptomatic, and approximately one-third of affected women will develop pyelonephritis if left untreated (15, 16, 22). Asymptomatic bacteriuria (ASB) is also associated with low birth weight, prematurity, hypertension, preeclampsia, maternal anemia, amnionitis, and fetal death (2). Treatment of ASB can reduce these risks, and therefore all women attending the antenatal clinic at the Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom, are screened for ASB by urine culture. All positive culture results are followed up in the bacteriuria clinic, where a fresh sample is obtained from the patient to confirm that an infection is present and appropriate antimicrobial therapy is prescribed when culture results become available. ASB in nonpregnant women is usually uncomplicated and self-limiting.
The bacterial factors that may contribute to asymptomatic infection are not clear. Many bacterial surface and secreted components such as fimbriae and toxins can stimulate inflammatory responses, and these are less likely to be produced by E. coli strains causing bacteriuria during pregnancy (29). A recent study of a strain capable of long-term asymptomatic bladder colonization did not express common adhesins (type 1A and 1C fimbriae, pyelonephritis-associated pili) and showed limited epithelial cell adherence (13).
In this study we have characterized E. coli isolates causing bacteriuria in pregnant woman (BU strains) by comparison with isolates from patients with cystitis in the general population (U strains). The study has focused on the requirement for type 1 fimbrial expression in BU strains, including analysis of fim switch orientation in vivo by analysis of fresh urine samples. An assay was developed to screen for the fimS49 allele, and its frequency was determined in both of the strain sets. The results show that the standard virulence determinants are not as common among the strains from pregnant women (predominantly asymptomatic infections) as among the strains causing symptomatic community-acquired infections in the general population. However, approximately one-third of BU strains do carry the majority of virulence determinants tested for and therefore pose a threat if left untreated. The majority of BU strains, in contrast to the strains causing cystitis, could not produce type 1 fimbriae. There appears to be selection against type 1 fimbrial expression during bacteriuria of pregnancy, and the lack of type 1 fimbrial expression may contribute to the asymptomatic nature of the infections caused by these strains.
MATERIALS AND METHODS
Bacterial isolates.
Pregnant women with significant urine cultures were invited to attend the bacteriuria clinic. Isolates from pregnant women were obtained by plating fresh urine samples provided at the time of the visit. Since fresh urine was cultured, isolates were considered significant at greater than 103 CFU of E. coli per ml of urine. The pregnant women were tested on two separate occasions. E. coli isolates from patients with community-acquired cystitis were obtained from specimens submitted by general practitioners and obtained from patients admitted to the Royal Victoria Infirmary and Freeman Hospital. Isolates selected from this group were present at more than 105 CFU per ml of urine. Of these 46 patients with UTI, 85% were female. The average age was 55.9 years, and there was no clustering of cases to single medical practices. E. coli fecal isolates were also collected from anonymous healthy volunteers not undergoing any antimicrobial treatment. All isolates were selected after growth on MacConkey or cystine lactose electrolyte-deficient medium agar and confirmed as E. coli by using traditional methods, including β-glucuronidase production (26). The following E. coli strains were included as controls for PCRs: K-12 MG1655 (type 1 fimbriae), strain 536 (10) (type 1 fimbriae, P-family, S-family, α-hemolysin), J96 (12) (type 1 fimbriae, P-family, S-family, α-hemolysin, F1C, CNF1), and AD110 (type 1 fimbriae, P-family, S-family, F1C, α-hemolysin, CNF1, and aerobactin). All strains were stored by addition of 20% glycerol and freezing at −70°C.
DNA preparation.
DNA was isolated from fresh urine cultures from the bacteriuria clinic as follows: fresh urine (2 ml) was added to 7 ml of 8 M guanidine hydrochloride, gently mixed, and incubated at room temperature (RT) for over 30 min. The released DNA was bound to diatomaceous earth (1 ml of a 10-mg/ml diatomaceous earth [Sigma-Aldrich] in 6 M guanidine hydrochloride) by gentle inversion at RT for 10 min. The DNA was then pelleted and washed in 50% ethanol containing 200 mM sodium chloride, 10 mM EDTA, and 50 mM Tris-HCl (pH 7.4). The diatomaceous earth was pelleted by pulsed-spinning, and the supernatant was discarded and washed as above. The pellet was washed in acetone, recentrifuged, and then dried at 60°C for 2 min. DNA was eluted in 100 to 500 μl of Tris-EDTA (TE) buffer by incubation at 60°C for 5 min and then microcentrifuged. The DNA was stored with the addition of 1 μl of chloroform as preservative.
DNA from isolates was prepared by growing the isolates overnight in defined rich morpholinepropanesulfonic acid (MOPS) medium, and DNA was released and purified using proteinase K and sodium perchlorate as described previously (20).
Assaying of virulence determinants.
Virulence determinant detection was carried out by PCR using primers characterized and tested at the Institute for the Molecular Biology of Infectious Diseases (IMBID), University of Würzburg, Würzburg, Germany. P-family adhesin determinant detection amplifies the papF gene using the primers 5′ gtgcagattaacatcagggg-3′ and 5′-atgctcatactggccgtggt-3′. S-family sequence detection used primers 5′-atgtctgtgcagcgggttct-3′ and 5′-attaccggcctttaccggaa-3′. The primers for α-hemolysin and cytotoxic necrotizing factor (CNF) were as published (5, 19). Type 1 fimbrial expression was assayed by testing for mannose inhibition of yeast agglutination after isolates were repeatedly cultured overnight (for 3 nights) in static Luria-Bertani broth. fim switch orientation was determined by amplifying the switch region with primers 2535 and 3178 using PCR conditions of 94°C for 45s, 57°C for 45s, and 72°C for 90s (30 cycles) and digesting the product with HinfI to give fragments of 416 and 227 bp in the phase-off orientation or 120 and 523 bp in the phase-on orientation (20). The proportion of the population in the phase-off and phase-on states was measured by separating the digested PCR fragments on a 4% polyacrylamide gel and then staining with ethidium bromide. The relative fluorescence (total DNA) in each band was measured using Bio-Rad gel documentation software and hardware and then adjusted by the size (in base pairs) of each fragment. The values for the two off and on fragments were averaged, and the proportion in the phase-on state was calculated. The primers used to detect the presence of the fimS49 allele (20) were IRLT (5′ ATGATATGGACAGTTTTGG 3′) and CS1 (5′ CCTCATATGTTAAGGCATGC 3′). PCR conditions were 94°C (5 min) and then 30 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 60 s, plus a final extension at 72°C for 10 min.
Strain comparison by PFGE and RAPD.
For pulsed-field gel electrophoresis (PFGE) analysis, chromosomal DNA was prepared in agarose plugs and then cleaved with the restriction enzyme XbaI (9). PFGE was carried out with the CHEF DrII system (Bio-Rad, Munich, Germany) at 200 V in 0.5 M Tris-borate-EDTA) (TBE) buffer at 12°C for 24 h with increasing pulse times from 5 to 50 s. Lambda concatamers from Bio-Rad were used as size markers. Assistance with PFGE was provided by G. Blum-Oehler, IMBID, University of Würzburg.
For randomly amplified polymorphic DNA (RAPD) analysis, three 10-mer oligonucleotide primers (5′ → 3′), CCGAATTCCC (OPF 5), CCGATATCCC (OPF 7), and GGGATATCGG (OPF 8) (14), were used to fingerprint the E. coli isolates. RAPD PCR was carried out in 50-μl reaction volumes containing 20 ng of E. coli chromosomal DNA, 1.25 μM each primer (Molecular Biology Unit, University of Newcastle upon Tyne), 0.5 μl of Taq (Thermus aquaticus) polymerase and respective buffer (Boerhinger Mannheim), per 100 μl of reaction mixture, 0.02 mM each dTTP, dGTP, dCTP, and dATP, and autoclaved Millipore-filtered water. Amplification was performed in a Gene Cycler (Bio-Rad Laboratories) programmed for an initial cycle of 94°C for 4 min and then 40 cycles of 30 s at 94°C, 1 min at 36°C, and 1 min 30 at 72°C. An extension step at 72°C for 10 min was included after the 40 cycles. Amplification products were resolved by electrophoresis in a 1.5% agarose gel stained with ethidium bromide.
RESULTS
Comparison of the distribution of virulence determinants among isolates from bacteriuria of pregnancy with isolates from patients with cystitis.
Isolates from the two groups were analyzed by PCR for the presence of genes for α-hemolysin, CNF1, aerobactin, and the fimbrial adhesins type 1, F1C, P-family, and S-family. All control strains were positive for the anticipated determinants (see Materials and Methods). The distribution of these genes between the two isolate groups is shown in Table 1. The distribution of determinants among the cystitis strains was in agreement with previous studies (see, e.g., references 4 and 5), although the frequency for cnf1 was slightly higher than that for hlyA. The carriage of genes for adhesins, CNF1, and aerobactin was lower among the bacteriuria of pregnancy strains than those isolated from cystitis patients. The majority of strains from both sets carry the type 1 fimbrial switch region. Carriage of virulence determinants was linked in both sets of strains, especially genes for P-family adhesins, S-family adhesins, α-hemolysin, and CNF1 (data not shown). Of the bacteriuria of pregnancy strains, 33% (14 of 43) carried at least four of the seven determinants tested, compared with 51% (24 of 47) of the cystitis strains.
TABLE 1.
Analysis of E. coli isolates for possession of virulence determinants by PCR
| Isolate source | No. of isolates with determinant/total no. (%)
|
||||||
|---|---|---|---|---|---|---|---|
| Type 1 fimbriae | P-family adhesins | S-family adhesins | F1C | α- Hemolysin | CNF1 | Aerobactin | |
| Pregnant women | 43/43 (100) | 13/43 (30) | 11/43 (26) | 8/43 (19) | 12/43 (28) | 11/43 (26) | 17/43 (40) |
| Community-acquired cystitis patients | 40/47 (85) | 28/47 (60) | 24/47 (51) | 17/47 (36) | 14/47 (30) | 18/47 (38) | 30/47 (64) |
Expression of type 1 fimbriae.
A major focus for this study was the expression of type 1 fimbriae in the isolates causing infections in the pregnant women group in comparison with that in the strains isolated from nonpregnant individuals with symptomatic infections. A PCR amplification used to amplify the fim switch demonstrated the switch region to be present in >85% of strains in both groups. Phenotypic testing revealed a marked difference, with 32 of 42 cystitis strains (76%) testing positive for mannose-sensitive agglutination but only 15 of 42 bacteriuria isolates from pregnant women (36%) testing positive (P < 0.01). Therefore, while all the E. coli strains isolated from pregnant women with urinary tract infections carried the fimS regulatory region, 64% could not produce type 1 fimbriae in repeated overnight static culture in Luria-Bertani broth, conditions that usually favor expression. This figure is significantly lower than expression by strains isolated from cystitis patients. Interestingly, 18 of 26 fecal E. coli isolates from healthy volunteers (69%) could express type 1 fimbriae in vitro, a proportion equivalent to that of cystitis isolates (76%), indicating that the low level found in bacteriuria of pregnancy isolates is exceptional.
Detection of the fimS49 allele by PCR.
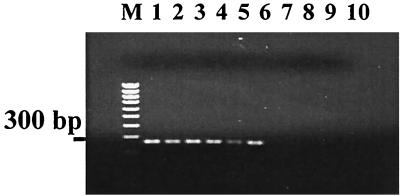
A previous analysis of fim phase variation among urinary tract isolates identified a specific AT base pair insertion in the fim switch region that inhibits FimB activity on the fim switch. The activity of this recombinase is required to turn on the expression of type 1 fimbriae. A bacterium with fimS49 is 500 times less likely to switch on the production of type 1 fimbriae. In essence, this means that such strains do not produce detectable type 1 fimbriae under even favorable laboratory conditions. To determine the frequency of this allele between cystitis and bacteriuria of pregnancy strains, a PCR assay was developed using an allele-specific primer with the AT base pair insertion at the 3′ end (see Materials and Methods). The assay (Fig. 1) was validated by sequencing a selection of positive and negative samples. Of 47 isolates from the prenatal clinic, 8 (17%) possessed the insert, whereas only 2 of 60 isolates from patients with community-acquired UTI (3.3%) possessed the insert (P < 0.05). To rule out the possibility that a single clonal type containing the allele was causing infections among pregnant women being tested at the clinic, the isolates containing the allele were analyzed by RAPD and PFGE. While two of the eight isolates were closely related, the other six were different by both methods (data not shown). In addition, the eight strains differed in their possession of the other virulence determinants screened for in the study. Three carried none of the factors, two carried just aerobactin, two carried just P-family sequences, and one carried just the F1C sequence. The fimS49 allele was therefore found in a number of different strain backgrounds, none of which carried multiple virulence-associated determinants. This allele is capable of eliminating type 1 fimbria production and was more prevalent in the strains isolated from women with bacteriuria of pregnancy than in the cystitis-associated strains isolated from the general population.
FIG. 1.
Detection of the fimS49 allele by PCR. The primers described in Materials and Methods were used to detect the presence of the additional AT base pair in one of the recombinase binding sites within the fim switch (inverted repeat right, on orientation). The sequences of the switch regions shown in the figure were obtained and confirm the presence or absence of the allele as determined by PCR. Lanes: M, molecular weight markers; 1, BU45; 2, BU51; 3, BU67; 4, BU68; 5, BU81; 6, BU140; 7, BU47; 8, BU48; 9, MG1655; 10, blank.
Analysis of fim switch orientation in vivo.
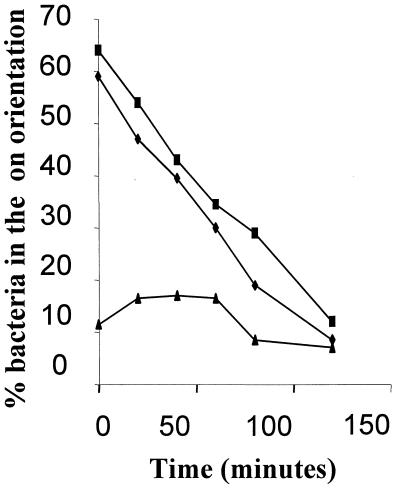
To determine the fim switch orientation in vivo, infected urine from patients visiting the prenatal clinic was collected and the bacterial DNA was prepared immediately as described in Materials and Methods. At a later time, switch orientation was determined by PCR and restriction enzyme analysis as described previously (20). The orientation of the switch under these in vivo conditions was compared with that from in vitro growth of the same isolate in MOPS defined rich medium. The purpose of this study was twofold: (i) to confirm that isolates containing the extra AT allele had the fim switch in the off orientation during infection (bacteria assayed in the urine will either be free or attached to mucus or uroepithelial cells), and (ii) to determine if isolates capable of producing type 1 fimbriae in vitro had expression switched on in vivo and whether regulation differed between the two environments. One possible problem with the in vivo assay is that the orientation of the fim switch is sensitive to environmental conditions, including temperature. Therefore, to show that adding the bacteria to guanidine hydrochloride does preserve the fim switch in the original sample orientation, the following experiment was carried out. The switch orientation from a bacterial population in the process of transition from on to off (controlled by a temperature and medium shift [6]) was determined using three sample preparation methods: (i) immediate boiling of samples and then placing them in 8 M guanidine hydrochloride; (ii) placing them in 8 M guanidine hydrochloride without boiling; and (iii) leaving the samples for 3 h at room temperature. Placing samples into 8 M guanidine hydrochloride was only slightly less effective at preserving the switch state than was immediate boiling of the cells (Fig. 2). As expected, leaving the samples at room temperature allows the population to shift much more to the off phase. Consequently, placing urine samples in 8 M guanidine hydrochloride was selected because this could easily be performed at the clinic.
FIG. 2.
Analysis of the fim switch orientation after treatment of bacteria under different conditions. Samples were taken from a culture of E. coli K-12 undergoing transition from the phase on to the phase off orientation in MOPS rich defined medium (25). The x axis shows the time at which samples were taken following the change in temperature and medium conditions used to bring about a rapid shutdown in type 1 fimbria expression (6). Samples were processed as described in Materials and Methods and left for 3 h at room temperature prior to PCR amplification and restriction digestion with HinfI. The ratio of phase on to phase off bacteria was determined by quantitation of restriction digestion products using Bio-Rad gel documentation hardware and software after polyacrylamide gel electrophoresis (5% TBE) and ethidium bromide staining. Symbols: ■, immediate boiling of the bacteria; ⧫, addition to 8 M guanidine hydrochloride (final concentration, 6M); ▴, left at room temperature.
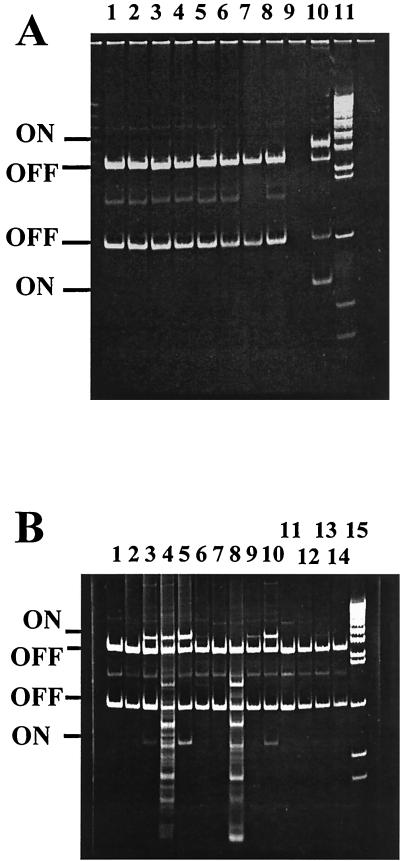
The switch orientation in vivo was determined for 23 infected urine samples in comparison to growth of the appropriate isolate in the laboratory. Of these isolates, 17 were unable to agglutinate yeast cells after static broth culture and 15 of the 17 had the switch in the off orientation after in vitro culture. The same 15 had the switch detectable only in the off orientation in the urine. This analysis included four isolates possessing the fimS49 allele, and these were detectable only in the off orientation both in vivo and in vitro (Fig. 3A). The remaining 6 isolates from the 23 tested could produce type 1 fimbriae in vitro, and 4 of these had the fim switch detectable in the on orientation in vivo (see, e.g., Fig. 3B). Although these six isolates represent a small sample, they indicate that isolates capable of switching on expression in vitro generally do so in vivo, although expression in vivo can be lower than, equal to, or greater than that after growth in vitro (Fig. 3B).
FIG. 3.
Analysis of the fim switch orientation in vivo. Infected urine from pregnant women was treated as described in Materials and Methods, and the orientation of the fim switch was analyzed by PCR and restriction digestion with HinfI. (A) Analysis of strains carrying the fimS49 allele. Switch orientation from infected urine: BU45, BU51, BU67, and BU81 (lanes 2, 4, 6, and 8 respectively). The same strains grown in MOPS rich defined medium are shown in lanes 1, 3, 5, and 7 respectively. Lane 10 contains a sample from a mixed-phase population, and lane 11 contains the DNA molecular weight markers. The switch is detectable only in the phase off orientation in the fimS49 BU strains. (B) The same analysis of the fim switch orientation carried out with other BU strains: BU47, BU48, BU113, BU125, BU128, BU132, and BU139 (lanes 2, 4, 6, 8, 10, 12, and 14, respectively) from infected urine. Lanes 1, 3, 5, 7, 9, 11, and 13 show the fim switch orientation after the respective BU strains were cultured in MOPS rich defined medium. Lane 15 contains the DNA molecular weight markers.
DISCUSSION
It is now 40 years since the first studies delineated the natural history of ASB in pregnancy, and although interventional strategies have been adopted which were based on these pioneering studies, little has been published concerning the bacterial factors important in such patients. Bacteriuria is a significant problem during pregnancy because it can develop into more severe infections that may have repercussions for the health of the mother and unborn child. This research examined the virulence genotypes of E. coli strains isolated from patients with bacteriuria of pregnancy and compared them with the genotypes of E. coli isolates from patients with cystitis in the general population. The bacteriuria of pregnancy strains were less likely to carry genes for S-family, P-family, and FIC adhesins, CNF1, and aerobactin. The women who attended the bacteriuria of pregnancy clinic had been referred to the clinic after being identified by routine screening of urine at prenatal clinics, and they did not all report discomfort normally associated with UTIs. Cytotoxins and adhesins (especially P-related fimbriae) can induce inflammatory responses and should be present less frequently in asymptomatic infections (8, 11, 13, 29). Our data support this, showing that these genotypes occur at a lower frequency in the bacteriuria of pregnancy strains. Previous work by others addressing virulence-associated characteristics of E. coli strains from pregnant women have reached similar conclusions although not for all the determinants analyzed here (29).
A proportion of the isolates (33%) did carry genes for the majority of factors examined and may pose more of a threat to the mother and unborn child if left untreated than the other isolates do. In fact, this number correlates well with the results of early studies looking at the natural history of ASB during pregnancy, in which approximately one-third of women develop pyelonephritis (15, 22, 29). If it is the case that ascending infection is confined to this group of strains, then limiting the use of antimicrobials to the subset of patients with such strains may be valid. Greater understanding of the bacterium-host interaction in ASB will allow more effective targeting of interventional and preventative measures.
A principal aim was to understand the level and importance of type 1 fimbria expression in the two sets of strains. This included an analysis, where possible, of how many isolates from each group (i) possessed the fim switch sequence and the fimS49 allele, (ii) could express type 1 fimbriae in vitro, and (iii) had the fim switch on or off in vivo. Over 85% of isolates from both sets of strains carried genes for type 1 fimbriae. The phenotypic analysis showed that only 36% of the bacteriuria of pregnancy strains could actually express the adhesin under favorable conditions, compared with 76% from the community-acquired symptomatic infections (P < 0.01). This confirmed that the usual adhesins (type 1, P, and S fimbriae) involved in the establishment and persistence of UTIs were not as relevant during bacteriuria of pregnancy, presumably due to the physiological changes in pregnancy that have been reported to put pregnant women at greater risk of developing bacteriuria (23).
The fimS49 allele inhibits wild-type FimB recombinase activity at the fim switch and prevents effective expression of type 1 fimbriae in vitro. This allele occurred at a higher frequency among the bacteriuria of pregnancy-associated strains than among the cystitis-associated strains. Analysis of the fim switch orientation in vivo of strains containing the fimS49 allele also showed the switch to be locked off during infection. This was also the case for the majority of other strains unable to express type 1 fimbriae in vitro. Of six isolates analyzed that were able to express type 1 fimbriae in vitro, four had a proportion of the population with the fim switch in the on orientation during infection. Two studies have analyzed the orientation of the fim switch in vivo during a mouse model of infection (21, 30), and one of these has also analyzed the switch orientation in vivo from a number of patients with urinary tract infection (21). While the majority of bacteria causing symptomatic infections had the fim switch primarily in the off orientation in infected urine and when cultured in vitro, this was not true of bacteria attached to the uroepithelium in the mouse model, in which over 30% had the fim switch in the phase on orientation (21). Our observations support the finding that bacteria able to produce type 1 fimbriae are likely to do so during infection. However, a high proportion of the strains causing bacteriuria during pregnancy cannot express type 1 fimbriae during the infection.
The fimS49 allele will be one of several that prevent the expression of type 1 fimbriae even though the genes are present in the isolates. Of particular interest was the fact that this allele was present in several different E. coli clonal groups (analyzed by PFGE and RAPD). While it remains possible that the fim genes could move horizontally between strains, their presence on a pathogenicity island or on other transmissible elements has never been documented. It is likely, therefore, that the allele has arisen independently in each strain background by selection. The nature of the allele, an AT base insertion within a fim recombinase binding site, would be extremely rare unless the recombination event makes it more likely. However, λ-like site-specific recombination is a well-studied conservative process, with no such mechanism being described. If the allele did arise rarely through recombination, then it appears to have been successfully selected in the bacteriuria of pregnancy strains. Consequently, it appears to be advantageous to these strains not to express this adhesin during the bacteriuria, although a role for type 1 fimbriae in the establishment of the infection remains possible.
As with P fimbriae, type 1 fimbriae are immunogenic and can elicit an inflammatory immune response that causes pyuria. In normal individuals with cystitis and pyelonephritis, this response is a necessary burden associated with the absolute need for such adhesins to initiate and maintain infections. During pregnancy, anatomical and physiological changes mean that infections can arise with afimbriate bacteria, and these are not so easily displaced (for example, if the bladder is not completely voided). Under these conditions, certain E. coli isolates may persist more successfully if they do not initiate inflammatory responses and maintain a low profile. Accordingly, isolates that do initiate a strong response may be removed, selecting for rare variants, such as those containing the fimS49 allele, that do not express key antigens (such as type 1 fimbriae). The advantages of selecting for mutations in this adhesin seem to outweigh the disadvantages in this situation. Whether this “mutation” occurs and can be reversed as a consequence of site-specific recombination at the fim switch remains to be tested. Certainly phase variation has proven to be a common theme for surface components expressed by bacterial pathogens, and the occurrence of fimS49 may represent an example of small-scale evolution in a specific niche that may be reversible.
ACKNOWLEDGMENTS
We are grateful to F. K. Gould, J. Perry, and M. Ford, Microbiology Department, Freeman Hospital, for providing isolates and materials and to G. Blum-Oehler for advice and assistance with PFGE.
The work was supported by a Career Development Research Fellowship to D.L.G. from the British Medical Research Council and a Ph.D. Studentship from the Harker Foundation (University of Newcastle upon Tyne) to S.J.K.
REFERENCES
- 1.Abraham J M, Freitag C S, Clements J R, Eisenstein B I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrington A W, Bint A J. Diagnosis and management of urinary tract infection in pregnancy. Rev Med Microbiol. 1999;10:27–36. [Google Scholar]
- 3.Connell H, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C.: ASM Press; 1996. pp. 135–174. [Google Scholar]
- 5.Foxman B, Zhang L, Palin K, Tallman P, Marrs C F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J Infect Dis. 1995;171:1514–1521. doi: 10.1093/infdis/171.6.1514. [DOI] [PubMed] [Google Scholar]
- 6.Gally D L, Bogan J A, Eisenstein B I, Blomfield I C. Environmental-regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12—effects of temperature and media. J Bacteriol. 1993;175:6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gally D L, Leathart J, Blomfield I C. Interaction of fimB and fimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli k-12. Mol Microbiol. 1996;21:725–738. doi: 10.1046/j.1365-2958.1996.311388.x. [DOI] [PubMed] [Google Scholar]
- 8.Godaly G, Frendeus B, Proudfoot A, Svensson M, Klemm P, Svanborg C. Role of fimbriae-mediated adherence for neutrophil migration across Escherichia coli-infected epithelial cell layers. Mol Microbiol. 1998;30:725–735. doi: 10.1046/j.1365-2958.1998.01104.x. [DOI] [PubMed] [Google Scholar]
- 9.Grothues D, Tummler B. Genome analysis of Pseudomonas aeruginosa by field inversion gel electrophoresis. FEMS Microbiol Lett. 1987;48:419–422. [Google Scholar]
- 10.Hacker J, Knapp S, Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983;154:1145–1154. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedlund M, Wachtler C, Johansson E, Hang L, Somerville J E, Darveau R P, Svanborg C. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses. Mol Microbiol. 1999;33:693–703. doi: 10.1046/j.1365-2958.1999.01513.x. [DOI] [PubMed] [Google Scholar]
- 12.Hull R A, Gill R E, Hsu P, Minshew B H, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981;33:933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hull R A, Rudy D C, Donovan W H, Wieser I E, Stewart C, Darouiche R O. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect Immun. 1999;67:429–432. doi: 10.1128/iai.67.1.429-432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karkkainen U M, Kauppinen J, Ikaheimo R, Katila M L. Random amplified polymorphic DNA (RAPD) analysis of Escherichia coli strains: comparison of urinary and concomitant blood isolates or urosepsis patients. Acta Physiol Microbiol Immunol Scand. 1996;104:437–442. doi: 10.1111/j.1699-0463.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 15.Kass E H. Bacteriuria and pyelonephritis of pregnancy. Arch Intern Med. 1960;105:194–198. doi: 10.1001/archinte.1960.00270140016003. [DOI] [PubMed] [Google Scholar]
- 16.Kincaid-Smith P, Bullen M. Bacteriuria in pregnancy. Lancet. 1965;i:395–399. doi: 10.1016/s0140-6736(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 17.Klemm P. 2 regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986;5:1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogfelt K A, Bergmans H, Klemm P. Direct evidence that the fimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990;58:1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhnert P, Hacker J, Muhldorfer I, Burnens A P, Nicolet J, Frey J. Detection system for Escherichia coli-specific virulence genes: absence of virulence determinants in B and C strains. Appl Environ Microbiol. 1997;63:703–709. doi: 10.1128/aem.63.2.703-709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leathart J B S, Gally D L. Regulation of type 1 fimbriae expression in uropathogenic Escherichia coli: heterogeneity of expression through sequence changes in the fim switch region. Mol Microbiol. 1998;28:371–381. doi: 10.1046/j.1365-2958.1998.00802.x. [DOI] [PubMed] [Google Scholar]
- 21.Lim J K, Gunther N W, Zhao H, Johnson D E, Keay S K, Mobley H L T. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun. 1998;66:3303–3310. doi: 10.1128/iai.66.7.3303-3310.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little P J. The incidence of urinary tract in 5000 pregnant women. Lancet. 1966;ii:925–928. doi: 10.1016/s0140-6736(66)90534-4. [DOI] [PubMed] [Google Scholar]
- 23.Lucas M J, Cunningham F G. Urinary infection in pregnancy. Clin Obstet Gynecol. 1993;36:855–868. doi: 10.1097/00003081-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 24.McClain M S, Blomfield I C, Eisenstein B I. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991;173:5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidhardt F C, Bloch P L, Smith D F. Culture media for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattyn S R, Sion J P, Verhoeven J. Evaluation of the LOGIC system for the rapid identification of members of the family Enterobacteriaceae in the clinical microbiology laboratory. J Clin Microbiol. 1990;28:1449–1450. doi: 10.1128/jcm.28.6.1449-1450.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaeffer A J, Chmiel J S, Duncan J L, Falkowski W S. Mannose-sensitive adherence of Escherichia coli to epithelial cells from women with recurrent urinary-tract infections. J Urol. 1984;131:906–910. doi: 10.1016/s0022-5347(17)50706-5. [DOI] [PubMed] [Google Scholar]
- 28.Sokurenko E V, Courtney H S, Ohman D E, Klemm P, Hasty D L. FimH family of type 1 fimbrial adhesins—functional heterogeneity due to minor sequence variations among fimH genes. J Bacteriol. 1994;176:748–755. doi: 10.1128/jb.176.3.748-755.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenqvist K, Sandberg T, Lidinjanson G, Orskov F, Orskov I, Svanborg-Eden C. Virulence factors of Escherichia coli in urinary isolates from pregnant women. J Infect Dis. 1987;156:870–877. doi: 10.1093/infdis/156.6.870. [DOI] [PubMed] [Google Scholar]
- 30.Struve C, Krogfelt K A. In vivo detection of Escherichia coli type 1 fimbrial expression and phase variation during experimental urinary tract infection. Microbiology. 1999;145:2683–2690. doi: 10.1099/00221287-145-10-2683. [DOI] [PubMed] [Google Scholar]
- 31.Sussman M, Gally D L. The biology of cystitis: host and bacterial factors. Annu Rev Med. 1999;50:149–158. doi: 10.1146/annurev.med.50.1.149. [DOI] [PubMed] [Google Scholar]
- 32.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1997;11:513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 33.Wu X R, Sun T T, Medina J J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins 1a and 1b—relation to urinary-tract infections. Proc Natl Acad Sci USA. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]