Abstract
Background
Antibiotic prescription for uncomplicated upper respiratory tract infection (URTI) in children is not recommended but remains common. The primary objective was to evaluate the relationship between antibiotic prescription for URTI prior to age 2 and antibiotic prescription for URTI after age 2. It was hypothesized that antibiotic prescription for URTI in early childhood may increase the risk of antibiotic use for subsequent URTIs. The secondary objective was to investigate whether this relationship was different for acute otitis media (AOM), for which antibiotics may be indicated.
Methods
A prospective cohort study was conducted between December 2008 and March 2016 at 9 primary care practices in Toronto, Canada. Healthy children aged 0–5 years that met TARGet Kids! cohort eligibility criteria were included if they had at least one sick visit prior to age 2 and least one sick visit after age 2. Generalized Estimating Equation (GEE) models were used to evaluate this relationship while considering within-subject correlation.
Results
Of 2380 participants followed for a mean duration of 4.6 years, children who received an antibiotic prescription for URTI prior to age 2 had higher odds of receiving an antibiotic prescription for URTI in later childhood (adjusted odds ratio: 1.39; 95% confidence interval: 1.19 to 1.63; P < .001). This relationship did not appear to be different for AOM compared to non-AOM URTI.
Conclusion
Antibiotic prescription for URTI before age 2 was associated with antibiotic prescription for URTI in later childhood. Reducing early life antibiotic prescription for URTI may be associated with reduction in antibiotic prescription for subsequent URTIs.
Keywords: acute otitis media, antibiotics, pediatrics, prescription, upper respiratory tract infection
This study found those who received an antibiotic prescription for upper respiratory tract infection (URTI) in early childhood (prior to 2 years of age) had 1.4 times higher odds of receiving an antibiotic prescription for URTI in later childhood (after 2 years of age).
INTRODUCTION
Upper respiratory tract infections (URTI) are among the most common reasons for health service utilization in childhood [1, 2]. In 2008/2009, 23% of Canadian children aged 2–3 years had frequent URTIs and 50% had at least one acute otitis media (AOM; middle ear) infection as reported by their parents [1]. The majority of URTIs are self-limiting viral illnesses that are not complicated by secondary bacterial infections [2–6]. The American Academy of Pediatrics, the National Institute for Health and Clinical Excellence, and the Canadian Paediatric Society recommend judicious antibiotic prescribing practices for respiratory tract infections in children [7–11]. This includes AOM, for which antibiotics are now only recommended for children under the age of 2 years, with more severe symptoms and/or bilateral infection [7]. However, antibiotic prescription for URTIs remains a common practice. In 2014, 38% of all antimicrobial prescriptions in Canada were for URTIs [11].
Antimicrobial resistance is an increasing global concern [11, 12]. Curtailing the use of antibiotics for uncomplicated URTI has been identified as a priority by many groups including Choosing Wisely Canada [13], the US Centers for Disease Control and Prevention [14], the Public Health Agency of Canada [15], and the World Health Organization [16]. Studies that have examined antibiotic prescribing practices for adult respiratory infections suggest that antibiotic prescription for respiratory infections may set expectations for antibiotics for future episodes [17, 18]. We hypothesized that antibiotic prescription for URTI in early childhood (prior to 2 years of age) might increase the risk of antibiotic use for subsequent URTIs. We also hypothesized that this relationship may be less pronounced for children younger than 2 years with AOM where antibiotics may be indicated.
The primary objective of this study was to evaluate the relationship between antibiotic prescription for URTI prior to 2 years of age and antibiotic prescription for URTI in later childhood. The secondary objective was to examine whether antibiotic prescription for AOM versus non-AOM URTI prior to 2 years of age modified the association between antibiotic prescription for URTI prior to 2 years of age and antibiotic prescription for URTI later in childhood.
METHODS
Study Sample and Setting
A prospective cohort study was conducted through TARGet Kids!, a primary care practice-based research network in Toronto, Canada (www.targetkids.ca) [19]. Children were recruited from 9 TARGet Kids! participating primary care pediatric or family medicine clinics during regularly scheduled physician visits between December 2008 and August 2015. Healthy children were included in this study if they had at least one sick visit with their primary care physician prior to 2 years of age and one sick visit after 2 years of age. Participants were followed until they were at least 3 years of age to provide a minimum of 1 year of follow-up. Children were excluded from the TARGet Kids! cohort study if they had conditions affecting growth (eg, cystic fibrosis), acute or chronic conditions (other than asthma and high-functioning autism), and severe developmental delay [19]. All parents provided informed consent to participate in this study and ethics approval was obtained through the Hospital of Sick Children and St. Michael’s Hospital Research Ethics Boards.
Exposure and Outcome Ascertainment
The primary exposure was antibiotic prescription for URTI at a sick visit prior to 2 years of age and was a yes/no binary variable. Antibiotic prescription data and diagnosis at the time of prescription were abstracted from the clinical record using International Classification of Diseases, 10th Revision (ICD-10) coding [20]. Diagnoses of pharyngitis, unspecified viral infection, cold, AOM, and influenza were considered URTIs. ICD-10 categories for relevant diagnoses are listed in Supplementary Table III. Authors N.G., J.O., and J.M. investigated cases of multiple diagnoses at a single visit, and consensus was reached on the primary complaint for each sick visit 100% of the time.
The primary outcome was antibiotic prescription for URTI at a sick visit after 2 years of age which was abstracted from the clinical record and was a yes/no binary variable. Two years of age was selected based on differences in clinical practice guidelines for antibiotic prescription for AOM, which suggest judicious prescribing practices for those under 2 years of age if AOM is uncomplicated and unilateral, and watchful waiting for those over 2 years of age [5, 6].
Covariates that might confound the above relationships were identified a priori through a review of the literature. These covariates included sex [21], age-and-sex standardized body mass index [22], maternal age [23], maternal education achievement [23, 24], number of visits for URTI from birth to 2 years of age [25], median neighborhood household income [26], number of siblings [27], daycare attendance [27], ethnicity [28], and clinic site [29]. Observation time was also included in the model as a continuous variable to account for varying follow-up times among participants. Covariate data were obtained using a standardized parent-completed data collection form adapted from the Canadian Community Health Survey and standardized anthropometric protocols [30].
Data Analysis
Baseline descriptive characteristics of the study sample were summarized using means and standard deviations for continuous variables and proportions for categorical variables. For the primary analysis, unadjusted and adjusted binomial Generalized Estimating Equation (GEE) models with a logit link function and a first-order autoregressive correlation structure (AR1) were used to evaluate the relationship between antibiotic prescription for URTI before and after 2 years of age. Adjusted models included potential confounding variables that were identified a priori. The GEE model was chosen to account for repeated measures from individuals, which is important as children may have had multiple sick visits with their primary care provider. The autoregressive correlation structure allowed for measurements taken closer in time to have higher correlations than measurements taken further apart in time (ie, multiple measurements taken closer in time are likely related to the same illness episode, as opposed to measurements taken further apart in time, which are likely separate episodes of illness), and increased the power and efficiency of the model [31]. For the secondary analysis, an interaction term was added to the primary model to evaluate whether antibiotic prescription for AOM prior to 2 years of age modified the relationship between the primary exposure and outcome.
Multiple imputation by chained equations was used to impute missing covariate data, using 20 datasets that were imputed and results were pooled together [32]. All covariates used in the analysis had less than 10% missing data, and data were assumed to be missing at random. Primary exposure and outcome data were not imputed. Evidence of multicollinearity was evaluated using the variance inflation factor [33]. All statistical analyses were completed using R statistical computing software version 3.4.3 [34, 35].
RESULTS
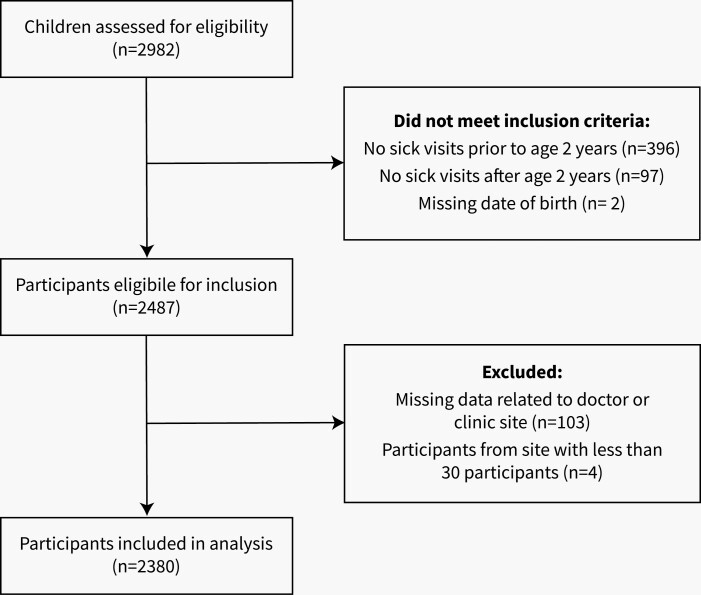
Between December 2008 and August 2015, 2982 children were assessed for eligibility (see Figure 1). Of these, 495 (16.6%) did not meet inclusion criteria (eg, participant had a sick visit before 2 years of age, but not after 2 years of age), 103 (3.5%) were missing data related to practitioner or clinic site, and 4 (<1%) participants were from practice sites with less than 30 participants and were excluded. A total of 2380 children were included in the analysis (see Figure 1). The mean age at the first visit was 37.4 months, and 47% of children were female. The average duration of follow-up from first visit was 4.6 years (Table 1). Those that received antibiotics for URTI prior 2 years were no more likely to be followed-up than those that did not, as there was no statistically significant difference in follow-up time between the two groups.
Figure 1.
Participant flow diagram for participants included in the analysis (n = 2380)..
Table 1.
Characteristics of Participants Included in Analysis (n = 2380)
| Characteristic | Included in the Analysis (n = 2380) |
|---|---|
| Child Gender, n (%) | |
| Female | 1127 (47.4) |
| Male | 1253 (52.6) |
| Child zBMI, mean (SD) | 0.23 (1.1) |
| Missing, n (%) | 23 (1.0) |
| Maternal age at child’s birth (Years), mean (SD) | 33.1 (4.8) |
| Missing, n (%) | 129 (5.4) |
| Maternal education level, n (%) | |
| College/University | 2092 (87.9) |
| High school | 240 (10.0) |
| Public school | 26 (1.1) |
| Missing | 22 (0.9) |
| Received antibiotic for URTI prior to 2 years old, n (%) | |
| Yes | 653 (27.4) |
| No | 1727 (72.6) |
| Received antibiotic for AOM prior to 2 years old, n (%) | |
| Yes | 526 (22.1) |
| No | 1854 (77.9) |
| Number of URTI visits from birth to age 2, mean (SD) | 2.0 (2.6) |
| Number of URTI visits from age 2 to last visit | 2.4 (3.2) |
| Antibiotics for URTI from age 2 to last visit | 0.7 (1.3) |
| Median neighborhood household income ($CAD), mean (SD) | 60,312 (26,984) |
| Missing, n (%) | 168 (7.1) |
| No. of Siblings, n (%) | |
| 0 | 832 (35.0) |
| 1 | 1095 (46.0) |
| 2 | 309 (13.0) |
| 3+ | 78 (3.3) |
| Missing | 66 (3.7) |
| Maternal ethnicity, n (%) | |
| European | 1706 (71.7) |
| East Asian | 271 (11.4) |
| South Asian/ Southeast Asian | 179 (7.5) |
| Other | 98 (4.1) |
| Missing | 126 (5.3) |
| Practice type | |
| Pediatric practice | 2291 (96.2%) |
| Family practice | 89 (3.7%) |
| Child in licensed daycare, n (%) | |
| Yes | 1165 (48.9) |
| No | 1146 (48.1) |
| Missing | 69 (2.9) |
| Child age at first visit (months), mean (SD) | 37.4 (15.7) |
| Observation time (days), mean (SD) | 1684 (761) |
Abbreviations: zBMI, standardized body mass index; GEE, Generalized Estimating Equation; URTI, upper respiratory tract infection; SD, standard deviation; AOM, acute otitis media.
A total of 25 990 sick visits were examined. Of 12 695 sick visits prior to 2 years of age, 1968 (15.5%) of these visits resulted in an antibiotic prescription. Of these 1968 visits, 1001 (50.9%) were for AOM, 299 (15.2%) were for non-AOM URTI, and 668 (33.9%) were for other conditions. Of 13295 sick visits after 2 years of age, 10719 (24.0%) of these visits resulted in an antibiotic prescription. Of the 2576 visits prior to 2 years of age that resulted in an antibiotic prescription, 1065 (41.3%) were for AOM, 719 (27.9%) were for non-AOM URTI, and 792 (30.8%) were for other conditions.
In the primary analysis, both unadjusted and adjusted models revealed that antibiotic prescription for URTI prior to 2 years of age was associated with increased likelihood of antibiotic prescription for URTI after 2 years of age (adjusted odds ratio [OR]: 1.39; 95% confidence interval [CI]: 1.19 to 1.63; P ≤ .001). In the secondary analysis, antibiotic prescription for AOM did not appear to modify the association between antibiotic prescription for URTI prior to 2 years of age and antibiotic prescription for URTI after 2 years of age (interaction P-value = .27). Children who were prescribed antibiotics for AOM prior to age 2 years were also more likely to receive antibiotics for URTI after age 2 (adjusted OR: 1.44; 95% CI: 1.00 to 2.05; P ≤ .05) (Table 2, Supplementary Table III).
Table 2.
Odds of Antibiotic Prescription for URTI per Visit After 2 Years of Age, Given Antibiotics Prescribed for URTI prior to 2 Years of Age
| Unadjusted OR (95% CI) | P | Adjusted OR (95% CI)a | P | |
|---|---|---|---|---|
| Antibiotic prescription for URTI prior to 2years of age | 1.59 (1.38 to 1.83) | <.001*** | 1.39 (1.19 to 1.63) | <.001*** |
| Antibiotic prescription for AOM URTI prior to 2 years of ageb | 1.59 (1.11 to 2.28) | .01** | 1.44 (1.00 to 2.05) | .05* |
Abbreviations: OR, odds ratio; CI, confidence interval; zBMI, standardized body mass index; GEE, Generalized Estimating Equation; URTI, upper respiratory tract infection; AOM, acute otitis media.
aEstimated based on GEE model adjusted for observation time, gender, zBMI, maternal age, maternal education level, number of URTI visits from birth to 2 years of age, median household income based on postal code, number of siblings, whether or not the child was registered in a licensed daycare (Y/N), ethnicity, and site. Covariates with missing data were imputed using the multiple imputation by chained equations (MICE) method.
bInteraction P value for adjusted model = .27.
*P ≤ .05, **P ≤ .01, ***P ≤ .001.
DISCUSSION
In this prospective cohort study of healthy young children, antibiotic prescription for URTI prior to 2 years of age was associated with 39% higher odds of receiving an antibiotic for URTI after 2 years of age. There was no evidence that this relationship was different for antibiotic prescription for AOM prior to 2 years, for which antibiotics may be indicated. URTIs are one of the most common causes of antibiotic prescription [36, 37]. The data suggest that antibiotic prescription for URTI in early life may be an important predictor of antibiotic prescription in later childhood.
Findings from this study are consistent with two previous studies which suggested that children who received an antibiotic for URTI were more likely to seek medical attention for subsequent URTIs. A case-control study by Li et al. [17] found that children aged 0–4 and 5–9 years living in Oregon who received an antibiotic prescription for URTI had 1.39 (95% CI: 1.24 to 1.55) and 1.82 (95% CI: 1.42 to 2.34) higher odds of returning for a subsequent visit, respectively. A retrospective cohort study by Hueston et al. [18] identified that children under the age of 18 years (n = 6060) who received an antibiotic prescription for acute bronchitis had a 1.98 (95% CI: 1.45 to 2.68, P < .001) higher odds of receiving an antibiotic at a subsequent visit. To our knowledge, no previous studies have examined the relationship between early life antibiotic prescription for URTI and antibiotic prescription for URTI in later childhood.
Several studies have examined the reasons for antibiotic prescription for URTIs in children. These include meeting parent expectations [38] and provider concerns about patient satisfaction [39]. Risk factors that may increase the likelihood of antibiotic prescription for URTI include patient household income [26], diagnostic uncertainty [40], and prescriber-related factors including physician age and number of years in practice [29]. Little et al. [41, 42] found that prescribing antibiotics for sore throat in adults may have a “medicalising” effect, increasing the likelihood of seeking medical care for future illness. Prescribing antibiotics at an initial visit may lead patients to believe that antibiotics are effective for future illness [42]. Taken together with previous findings, we speculate that antibiotic prescription early in life may reinforce parental expectations for antibiotics for URTI, making withholding antibiotics for subsequent URTIs more difficult. Our finding that the relationship remained the same regardless of whether antibiotics may have been indicated (ie, for AOM under 2 years of age) is consistent with this hypothesis. Our finding that family practice sites included in the analysis had antibiotic prescribing rates that were significantly lower than pediatric practice sites is also consistent with the idea that prescribing practices may be driven by a combination of clinician- and patient-driven factors.
Additionally, a study by Vernacchio et al. [43] which examined the impact of the 2004 clinical practice guideline on physician behaviors found that although many primary care physicians recognize that watchful waiting is an option, parental reluctance and difficulty of follow-up of children who do not improve were barriers to watchful waiting. It is likely that prescribing practices may be driven by a combination of clinician- and patient-driven factors. Reducing early life antibiotic prescription for URTI may be associated with a reduction in antibiotic prescription for subsequent URTIs.
Strengths of this study include the prospective design, which allowed us to evaluate the temporal relationship between antibiotic prescription for URTI prior to 2 years of age and antibiotic prescription for URTI after 2 years of age. Follow-up for an average of 4 years allowed time to accrue antibiotic prescriptions at subsequent sick visits. The use of multiple trained individuals to abstract antibiotic prescription and sick visit data reduced the likelihood of diagnostic coding errors. Detailed demographic and clinically relevant data allowed for adjustment of multiple potentially confounding factors. The sample size was relatively large, which provided sufficient power to evaluate the observed relationships with relatively narrow confidence intervals. Finally, GEE modeling took advantage of the correlation between multiple URTI visits within the same individual which increased precision in the effect estimates.
Limitations of this study include potential unmeasured confounding. Although multiple variables were accounted for in the statistical analyses, we were unable to adjust for individual physician characteristics such as age and years in practice, which have been found to affect antibiotic prescribing practices [29]. We were also unable to account for individual nonclinical factors such as parent expectations. Future studies may consider the development of a survey component to collect additional parent- and child-level factors that may impact antibiotic prescription. However, we were able to adjust for practice site to account for practice-level factors which may affect antibiotic prescribing practices.
Additionally, due to data limitations, we were unable to include sick visits from outside of the child’s medical home (ie, walk-in clinics or emergency department visits). The use of healthcare system administrative data in future studies may help overcome this limitation. Additionally, with respect to conditions that may stem from viral or bacterial origin (eg, pharyngitis), we were unable to confirm the source, and consequently, whether antibiotic prescription may have been indicated or not. Future cohort studies may overcome this limitation by considering the use of laboratory data to supplement data collection. Another limitation of this study is the minimum follow-up period of 1 year, although observation time was normally distributed with a relatively small number of individuals that had observation times that were short in duration. However, further studies may consider the use of a longer minimum follow-up period to accrue additional sick visits.
Finally, although included children were from an ethnically diverse urban population of children who receive primary healthcare, findings from this population may not be generalizable to all urban children.
CONCLUSION
In this study, antibiotic prescription for URTI for children younger than 2 years of age was associated with higher odds of antibiotic prescription for URTI when children were older than 2 years of age. This relationship appeared unchanged for antibiotic prescription for AOM under 2 years of age for which antibiotics may be indicated. Results from the present study suggest that antibiotic prescription for URTI in early life may establish a pattern for antibiotic prescription for URTI in later childhood. Reducing early life antibiotic prescription for URTI may result in a reduction in antibiotic prescription for URTI in later life.
Supplementary Material
Contributor Information
Bhavna Samtani, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Natasha Gray, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Jessica Omand, Child Health Evaluative Sciences, The Hospital for Sick Children, Toronto, Ontario, Canada.
Charles Keown-Stoneman, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St Michael’s Hospital, Toronto, Ontario, Canada.
Mary Aglipay, Li Ka Shing Knowledge Institute, St Michael’s Hospital, Toronto, Ontario, Canada.
Catherine Birken, Child Health Evaluative Sciences, The Hospital for Sick Children, Toronto, Ontario, Canada.
Jonathon Maguire, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St Michael’s Hospital, Toronto, Ontario, Canada.
Notes
Acknowledgments. We thank all of the participating families for their time and involvement in TARGet Kids! and are grateful to all practitioners who are currently involved in the TARGet Kids! practice-based research network.
Financial support. Funding for this study was provided by Canadian Institutes of Health Research [Grant #333560].
Potential conflicts of interest : J.M. received an unrestricted research grant for a completed investigator-initiated study from the Dairy Farmers of Canada (2011–2012) and Ddrops provided non-financial support (vitamin D supplements) for an investigator-initiated study on vitamin D and respiratory tract infections (2011–2015). C.B. received a research grant from the Centre for Addiction and Mental Health Foundation (CAMH 2017–2020). These agencies had no role in the design, collection, analyses, or interpretation of the results of this study or in the preparation, review, or approval of the manuscript. All other authors declare no conflicts of interest.
Members of the TARGet Kids! Collaboration:
Co-Leads : Catherine S. Birken, MD, and Jonathon L. Maguire, MD.
Advisory Committee : Ronald Cohn, MD; Eddy Lau, MD; Andreas Laupacis, MD; Patricia C. Parkin, MD; Michael Salter, MD; and Shannon Weir-Seeley, MSc.
Science Review and Management Committees : Laura N. Anderson, PhD; Cornelia M. Borkhoff, PhD; Charles Keown-Stoneman, PhD; Christine Kowal, MSc; and Dalah Mason, MPH.
Site Investigators : Murtala Abdurrahman, MD; Kelly Anderson, MD; Gordon Arbess, MD; Jillian Baker, MD; Tony Barozzino, MD; Sylvie Bergeron, MD; Gary Bloch, MD; Joey Bonifacio, MD; Ashna Bowry, MD; Caroline Calpin, MD; Douglas Campbell, MD; Sohail Cheema, MD; Elaine Cheng, MD; Brian Chisamore, MD; Evelyn Constantin, MD; Karoon Danayan, MD; Paul Das, MD; Mary Beth Derocher, MD; Anh Do, MD; Kathleen Doukas, MD; Anne Egger, BScN; Allison Farber, MD; Amy Freedman, MD; Sloane Freeman, MD; Sharon Gazeley, MD; Charlie Guiang, MD; Dan Ha, MD; Curtis Handford, MD; Laura Hanson, MD; Leah Harrington, MD; Sheila Jacobson, MD; Lukasz Jagiello, MD; Gwen Jansz, MD; Paul Kadar, MD; Tara Kiran, MD; Holly Knowles, MD; Bruce Kwok, MD; Sheila Lakhoo, MD; Margarita Lam-Antoniades, MD; Eddy Lau, MD; Denis Leduc, MD; Fok-Han Leung, MD; Alan Li, MD; Patricia Li, MD; Jessica Malach, MD; Roy Male, MD; Aleks Meret, MD; Elise Mok, MD; Rosemary Moodie, MD; Katherine Nash, MD; Sharon Naymark, MD; James Owen, MD; Michael Peer, MD; Marty Perlmutar, MD; Navindra Persaud, MD; Andrew Pinto, MD; Michelle Porepa, MD; Vikky Qi, MD; Noor Ramji, MD; Danyaal Raza, MD; Alana Rosenthal, MD; Katherine Rouleau, MD; Caroline Ruderman, MD; Janet Saunderson, MD; Vanna Schiralli, MD; Michael Sgro, MD; Hafiz Shuja, MD; Susan Shepherd, MD; Barbara Smiltnieks, MD; Cinntha Srikanthan, MD; Carolyn Taylor, MD; Stephen Treherne, MD; Suzanne Turner, MD; Fatima Uddin, MD; Meta van den Heuvel, MD; TheaWeisdorf, MD; PeterWong, MD; John Yaremko, MD; Ethel Ying, MD; Elizabeth Young, MD; and Michael Zajdman, MD.
Research Team : Marivic Bustos, RPN; Pamela Ruth Flores, MD; Mateenah Jaleel, BSc; Tarandeep Malhi, MLT; Ataat Malick, MD; Michelle Mitchell, BA; Martin Ogwuru, MBBS; Frank Ong, MSc; Rejina Rajendran, BE; Sharon Thadani, MLT; Julia Thompson, SSRP; and Laurie Thompson, MLT.
Project Team : Mary Aglipay, MSc; Imaan Bayoumi, MD; Sarah Carsley, PhD; Katherine Cost, PhD; Karen Eny, PhD; Laura Kinlin, MD; Jessica Omand, PhD; Shelley Vanderhout, BASc; and Leigh Vanderloo, PhD.
Applied Health Research Centre : Christopher Allen, BSc; Bryan Boodhoo, MSc; Peter Juni, MD; Gurpreet Lakhanpal, MSc; Gerald Lebovic, PhD and Audra Stitt, MSc.
Mount Sinai Services Laboratory : Rita Kandel, MD, and Michelle Rodrigues, BSc.
REFERENCES
- 1. Thomas EM. Recent trends in upper respiratory infections, ear infections and asthma among young Canadian children. Heal Rep. 2010; 21:47–52. [PubMed] [Google Scholar]
- 2. Dasaraju PV, Liu C.. Infections of the respiratory system. In: Baron S, ed. Infections of the Respiratory System. Galveston, TX: University of Texas Medical Branch; 1996. [PubMed] [Google Scholar]
- 3. Lee GM, Friedman JF, Ross-Degnan D, Hibberd PL, Goldmann DA.. Misconceptions about colds and predictors of health service utilization. Pediatrics 2003; 111:231–6. [DOI] [PubMed] [Google Scholar]
- 4. Schaad UB. Prevention of paediatric respiratory tract infections: emphasis on the role of OM-85. Eur Respir Rev 2005; 14:74–7. [Google Scholar]
- 5. Davis SD, Wedgwood RJ.. Antibiotic prophylaxis in acute viral respiratory diseases. Am J Dis Child 1965; 109:544–53. [DOI] [PubMed] [Google Scholar]
- 6. Soyka LF, Robinson DS, Lachant N, Monaco J.. The misuse of antibiotics for treatment of upper respiratory tract infections in children. Pediatrics 1975; 55:552–6. [PubMed] [Google Scholar]
- 7. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004; 113:1451–65. [DOI] [PubMed] [Google Scholar]
- 8. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics 2013; 131:e964–99. [DOI] [PubMed] [Google Scholar]
- 9. Hersh AL, Jackson MA, Hicks LA.. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics 2013; 132:1146–54. [DOI] [PubMed] [Google Scholar]
- 10. Tan T, Little P, Stokes T.. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. BMJ 2008; 337:a437232–a437. [DOI] [PubMed] [Google Scholar]
- 11. Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System Report 2016; Ottawa ON: Public Health Agency of Canada; 2015. [Google Scholar]
- 12. Le Saux N. Antimicrobial stewardship in daily practice: Managing an important resource. Paediatr Child Heal. 2014; 19:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choosing Wisely Canada. Using Antibiotics Wisely—Communications Toolkit; 2018. [Google Scholar]
- 14. Centers for Disease Control and Prevention (US). Antibiotic Resistance Threats in the United States ; 2013. Accessed August 5, 2019. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- 15. Public Health Agency of Canada; Canadian Food Inspection Agency; Canadian Institutes of Health Research; Health Canada; Agriculture and Agri-Food Canada; Industry Canada; National Research Council Canada. Summary of the Federal Action Plan on Antimicrobial Resistance and Use in Canada. Can Commun Dis Rep. 2015; 41(Suppl 4):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Global Action Plan on Antimicrobial Resistance; 2015. Accessed March 22, 2019. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1. [DOI] [PubMed] [Google Scholar]
- 17. Li J, De A, Ketchum K, Fagnan LJ, Haxby DG, Thomas A.. Antimicrobial prescribing for upper respiratory infections and its effect on return visits. Fam Med 2009; 41:182–7. [PubMed] [Google Scholar]
- 18. Hueston WJ, Jenkins R, Mainous AG.. Does drug treatment of patients with acute bronchitis reduce additional care seeking?: Evidence from the practice partner research network. Arch Fam Med 2000; 9:997–1001. [DOI] [PubMed] [Google Scholar]
- 19. Carsley S, Borkhoff CM, Maguire JL, et al. Cohort profile: the Applied Research Group for Kids (TARGet Kids!). Int J Epidemiol 2015; 44:776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; 1992. Geneva, Switzerland: World Health Organization [Google Scholar]
- 21. Falagas ME, Mourtzoukou EG, Vardakas KZ.. Sex differences in the incidence and severity of respiratory tract infections. Respir Med 2007; 101:1845–63. [DOI] [PubMed] [Google Scholar]
- 22. Campitelli MA, Rosella LC, Kwong JC.. The association between obesity and outpatient visits for acute respiratory infections in Ontario, Canada. Int J Obes 2014; 38:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vinker S, Ron A, Kitai E.. The knowledge and expectations of parents about the role of antibiotic treatment in upper respiratory tract infection - A survey among parents attending the primary physician with their sick child. BMC Fam Pract 2003; 4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zolaly MA, Hanafi MI.. Factors affecting antibiotics’ prescription in general pediatric clinics. J Taibah Univ Med Sci 2011; 6:33–41. [Google Scholar]
- 25. Salah M, Abdel-Aziz M, Al-Farok A, Jebrini A.. Recurrent acute otitis media in infants: Analysis of risk factors. Int J Pediatr Otorhinolaryngol 2013; 77:1665–9. [DOI] [PubMed] [Google Scholar]
- 26. Kozyrskyj AL, Dahl ME, Chateau DG, Mazowita GB, Klassen TP, Law BJ.. Evidence-based prescribing of antibiotics for children: role of socioeconomic status and physician characteristics. CMAJ. 2004; 171:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koopman LP, Smit HA, Heijnen MLA, et al. Respiratory infections in infants: Interaction of parental allergy, child care, and siblings—The PIAMA study. Pediatrics 2001; 108:943–8. [DOI] [PubMed] [Google Scholar]
- 28. Wang KY, Seed P, Schofield P, Ibrahim S, Ashworth M.. Which practices are high antibiotic prescribers? A cross-sectional analysis. Br J Gen Pract 2009; 59:e315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mainous AG, Hueston WJ, Love MM.. Antibiotics for colds in children: who are the high prescribers? Arch Pediatr Adolesc Med 1998; 152:349–52. [PubMed] [Google Scholar]
- 30. Statistics Canada. Canadian Community Health Survey—Annual Component (CCHS); 2018. Accessed March 22, 2019.http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3226. [Google Scholar]
- 31. Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 32. Van Buuren S, Groothuis-Oudshoom K.. Mice: Multivariate imputation by chained equations in R. J Stat. 2011; 45:1–67. [Google Scholar]
- 33. O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant 2007; 41:673–90. [Google Scholar]
- 34. Halekoh U, Højsgaard S, Yan J.. The R package geepack for generalized estimating equations. J Stat Softw 2006; 15:1–11. [Google Scholar]
- 35. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.r-project.org/. [Google Scholar]
- 36. McCaig LF. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA J Am Med Assoc. 1995; 273:214. [PubMed] [Google Scholar]
- 37. Nyquist A-C, Gonzales R, Steiner JF, Sande MA.. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998; 279:875–7. [DOI] [PubMed] [Google Scholar]
- 38. Watson RL, Dowell SF, Jayaraman M, Keyserling H, Kolczak M, Schwartz B.. Antimicrobial use for pediatric upper respiratory infections: reported practice, actual practice, and parent beliefs. Pediatrics. 1999; 104:1251–7. [DOI] [PubMed] [Google Scholar]
- 39. Stearns CR, Gonzales R, Camargo CA Jr, Maselli J, Metlay JP.. Antibiotic prescriptions are associated with increased patient satisfaction with emergency department visits for acute respiratory tract infections. Acad Emerg Med. 2009;16(10):934–941. doi: 10.1111/j.1553-2712.2009.00522.x [DOI] [PubMed] [Google Scholar]
- 40. Grossman Z, Del Torso S, Hadjipanayis A, Van Esso D, Drabik A, Sharland M.. Antibiotic prescribing for upper respiratory infections: European primary paediatricians’ knowledge, attitudes and practice. Acta Paediatr Int J Paediatr. 2012; 101:935–40. [DOI] [PubMed] [Google Scholar]
- 41. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL.. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997; 315:350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL.. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997; 314:722722.–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vernacchio L, Vezina RM, Mitchell AA.. Knowledge and practices relating to the 2004 acute otitis media clinical practice guideline: a survey of practicing physicians. Pediatr Infect Dis J. 2006; 25(5):385–9. doi: 10.1097/01.inf.0000214961.90326.d0. PMID: 16645499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.