Abstract
Osteopenia occurs in a subset of phenylalanine hydroxylase (PAH) deficient phenylketonuria (PKU) patients. While osteopenia is not fully penetrant in patients, the Pahenu2 classical PKU mouse is universally osteopenic, making it an ideal model of the phenotype. Pahenu2 Phe management, with a Phe-fee amino acid defined diet, does not improve bone density as histomorphometry metrics remain indistinguishable from untreated animals. Previously, we demonstrated Pahenu2 mesenchymal stem cells (MSCs) display impaired osteoblast differentiation. Oxidative stress is recognized in PKU patients and PKU animal models. Pahenu2 MSCs experience oxidative stress determined by intracellular superoxide over-representation. The deleterious impact of oxidative stress on mitochondria is recognized. Oximetry applied to Pahenu2 MSCs identified mitochondrial stress by increased basal respiration with concurrently reduced maximal respiration and respiratory reserve. Proton leak secondary to mitochondrial complex 1 dysfunction is a recognized superoxide source. Respirometry applied to Pahenu2 MSCs, in the course of osteoblast differentiation, identified a partial complex 1 deficit. Pahenu2 MSCs treated with the antioxidant resveratrol demonstrated increased mitochondrial mass by MitoTracker green labeling. In hyperphenylalaninemic conditions, resveratrol increased in situ alkaline phosphatase activity suggesting partial recovery of Pahenu2 MSCs osteoblast differentiation. Up-regulation of oxidative energy production is required for osteoblasts differentiation. Our data suggests impaired Pahenu2 MSC developmental competence involves an energy deficit. We posit energy support and oxidative stress reduction will enable Pahenu2 MSC differentiation in the osteoblast lineage to subsequently increase bone density.
Keywords: Pahenu2, Phenylketonuria, Osteopenia, Oxidative stress, Respiratory complex 1
1. Introduction
Phenylalanine hydroxylase (PAH) deficient phenylketonuria (PKU) is prominently defined by neurologic phenotypes. However, a patient subset presents with osteopenia. Feinberg and Fisch initially described osteopenia in PKU affected children [1], confirmed by Murdoch and Holman [2], and subsequently reported many times [3-10]. Pathophysiology of PKU osteopenia remains ambiguous. Originally and without supportive evidence, it was suggested PKU osteopenia was secondary to dietary therapy whereby calcium, phosphorous, and other bone forming material are reduced or rendered biologically unavailable; however, osteopenia is observed in patients that never received diet therapy and young patients after short-term therapy making dietary causation a less tenable hypothesis. Several studies find no correlation between plasma Phe concentration and osteopenia [11-17]; while, others show a negative correlation [18,19]. Biochemical parameters including bone formation markers [18,19], bone resorption markers [20,21], and other bone-related biochemistry [16,18,21] provide no consensus for PKU osteopenia risk.
While osteopenia is not fully penetrant in PKU-affected patients, the Pahenu2 classical PKU mouse is universally osteopenic. Osteoblast differentiation studies in Pahenu2 mesenchymal stem cells (MSCs) demonstrated reduced capacity for osteoblast differentiation and reduced expression of genes critical to osteoblast identify and function [22,23]. In the present study, we determined Phe-restriction with amino acid defined diet did not improve bone histomorphometry metrics. Oxidative stress is described in PKU models [24,25] and patients [26-28], being an imbalance between reactive oxygen species (ROS) and the capacity to buffer reactive species. We demonstrate superoxide over-representation in Pahenu2 MSCs, indicating oxidative stress. Resting and proliferating MSCs derive energy primarily from glycolysis [29]; however, upon stimuli for osteoblasts differentiation, oxidative energy production is upregulated [30,31 ]. Oximetry in Pahenu2 MSCs demonstrates reduced maximal respiration, and respiratory reserve. Respirometry of differentiating Pahenu2 MSCs determined a partial complex 1 respiratory chain deficit contributes to mitochondrial dysfunction. Moreover, proton leak, a common consequence of complex 1 dysfunction, is a recognized source of superoxide, precipitating oxidative stress.
A variety of small molecule antioxidants scavenges ROS. The antioxidant resveratrol (3,5,4′-trihydroxy-trans-stilbene) enhances human MSC osteoblast differentiation [32]. Resveratrol applied to Pahenu2 MSCs within the context of hyperphenylalaninemic conditions, increased mitochondria mass and up-regulated in situ alkaline phosphatase activity suggesting enhanced osteoblast differentiation. PKU osteopenia is without any specific intervention; as systemic Phe management is the singular approach applied to all disease phenotypes. This study suggests oxidative stress management augments Pahenu2 MSC osteoblast differentiation, which may improve bone density.
2. Methods
2.1. Animal management
Pahenu2 and control animal propagation used an approved protocol at Rangos Research Center vivarium at Children's Hospital of Pittsburgh. Experimental cohorts were generated crossing a heterozygous female to a heterozygous male. Genotyping to distinguish affected from unaffected animals was performed as described [33]. A cohort of homozygous Pahenu2 received dietary Phe management at weaning (day 21 of life) as described to generate consistent blood Phe of ~200 μM [22,23]. Control animals and unrestricted Pahenu2 (blood Phe ~2000–2200 μM) received standard laboratory chow. Animals for microcomputed tomography assessment (control, PKU without Phe management, PKU with Phe management) were sacrificed at 4 months of age. Animals for MSC harvest (control, PKU) were sacrificed at 2–3 months.
2.2. Microcomputed tomography
Each experimental (Pahenu2 Phe managed, Pahenu2 without management) and control animal cohort consisted of six animals (three male, three female). Pahenu2 cohorts (Phe restricted, unrestricted) were sacrificed 3 months after weaning. Blood Phe among animals receiving amino acid defined diet was maintained at ~200 μM for 12 weeks. Trabecular bone histomorphomery by microcomputed tomography was performed in a randomized and blinded manner. Analysis was performed as described [22,23]. Briefly, 4% formalin fixed lumbar vertebrae where scanned in 70% ethanol (6 μM resolution) with a Skyscan 1272 microCT. Image reconstruction and analysis used Data-viewer, CTvox, and CTscan software [22,23].
2.3. Mesenchymal stem cell culture and osteoblast differentiation
From Pahenu2 animals receiving a standard diet (blood Phe ~2000–2200 μM) and control animals, epiphysis were removed from the dissected femur and tibia, and bone marrow was flushed with media (RPMI-1640, 10% fetal calf serum) using an insulin syringe. MSCs were cultured as described [22,23]. Briefly, from primary tissue aspirate, erythrocytes were removed with red cell lysis buffer. Cells are plated in T25 flasks for 3–8 h after which non-adherent cells are collected. Short-term plating removes rapidly adhering fibroblast-like cells. Non-adherent cells are re-plated in a T25 flask for 72 h after which remaining non-adherent cells are discarded. Proliferation media (MesenCult Proliferation Kit (mouse), Stemcell Technologies) is provided to adherent cell with subsequent passage at 60% confluence, to avoid unintended differentiation. Osteogenic differentiation applies media supplemented with 35 μg/ml l-ascorbic acid, 10 mM β-glycerolphosphate, 10 pM ACTH, 10 nM 1α,25-dihydroxyvitamin D3, and 0.5 mM CaCl2 [34]. Hyperphenylalaninemic conditions are achieved in some cultures by supplementing Phe to a final concentration of 1200 μM being the minimum Phe concentration defining classical PKU [22,35]. Cultures were provided fresh media at 72-h intervals. A resveratrol stock was created by dissolving dry chemical in ethanol to a concentration of 5 mM. Stock material was diluted in culture media to a final concentration of 5 μM. Resveratrol renewal occurred in the course of culture maintenance as described above. Osteoblast differentiation was assessed by in situ alkaline phosphatase activity. In situ alkaline phosphatase activity used 0.05% napthol AS-MX substrate and visualized as described [22,23]. Stained culture plate scoring was as described [22,23].
2.4. Assessment of superoxide and mitochondrial mass
Cellular superoxide quantification stains cells with MitoSox Red (superoxide staining) and MitoTracker Green (mitochondria mass determination) as described [36-38]. Briefly, cells are harvested, washed in PBS, and resuspended (106 cells/ml) in complete media. MitoSox Red is added to 5 μM while MitoTracker Green is added to 450 nM. Following a 30 min incubation at 37 °C, samples of 104 cell are analyzed in a Becton Dickinson FACSAria II flow cytometer. Experimental and control cohorts each consist of cells from four animals (two male, two female).
2.5. Oximetry
An XF96 Analyzer (Seahorse, Agilent Technologies, Santa Clara, CA) was used to measure oxygen consumption. MSCs were prepared from Pahenu2 or control animal cohorts (four animals/cohort, two male, two female). MSCs were seeded in poly-d-Lysine-coated plates and grown to confluence. Individual plates compared one Pahenu2 and one control animal, where each assessment utilized eight technical replicate wells. Cellular oxygen consumption was assessed as described [37,38]. Prior to assessment, medium was replaced with Seahorse XF DMEM medium, pH 7.4 supplemented with 10 mM glucose, 1 mM pyruvate, 2 mM l-glutamine, and cells were incubated in a non-CO2 incubator at 37 °C for 1 h prior to oximetry. Oxygen consumption rate was determined at baseline, with sequential addition of oligomycin (1.5 μM), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 1 μM), and rotenone + antimycin A (0.5 μM). Finally, cells were lysed and protein content determined as described [37,38]. Data is normalized to protein concentration and oxygen consumption rate is reported in pmol/min/mg protein. ATP production was calculate from oximetry data as described [37,38].
2.6. High resolution respirometry
An Oxygraph 2 K HRR was used (Oroboros Instruments, GMBH, Innsbruck, Austria) for respirometry. Pahenu2 and control MSCs were grown to confluence in T75 flasks. Induction of osteoblast differentiation was as above. Following 14 days of induction, cells are scraped from the culture surface, washed with PBS, and pelleted. While trypsinization is ineffective to release differentiating MSCs monolayers, trypsin was applied secondarily (1 min 37 °C) to scraped cells, followed by vortexing (15–20 s). Subsequently, cells were washed twice with complete media and resuspended. This process leveraged greater surface area access of scraped material, which released individual cells to enable counting with a hemocytometer. One million MSCs were homogenized in Mir05 buffer (110 mM sucrose, 0.5 mM EGTA, 3 mM MgCl2, 60 mM potassium lactobionate, 10 mM KH2PO4, 20 mM HEPES, pH 7.2) and strained to remove debris. Homogenate is added to respiration chambers. Malate (5 mM), ADP (5 mM), pyruvate (5 mM) and glutamate (5 mM) successive addition stimulates Complex I [39]. At steady state, 10 mM succinate is added assessing combined Complex I and II activities. Next, 10 mM cytochrome C is added assessing mitochondrial integrity followed by 0.5 M carbonyl cyanide 3-chlorophenylhydrazone uncoupling the mitochondria membrane inducing maximum respiration. Complex II respiration is defined as respiration after addition of 10 mM succinate (Complexes I, II) minus the rate of respiration on malate/pyruvate/ADP (Complex I). Oxygen consumption was normalized to protein content with scaling of experimental to control data.
2.7. Statistics
Data were compared using Student's t-test. Graphpad software is used for data analysis with differences considered significant when p ≥ 0.05.
3. Results
3.1. Phe management does not improve Pahenu2 bone density
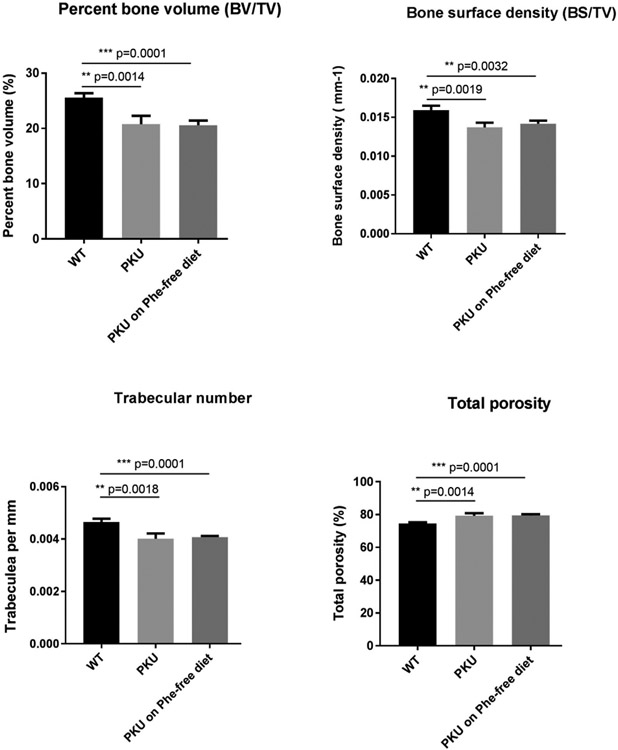
Fig. 1 provides histomorphometry metrics for Pahenu2, Pahenu2 after 3 months of Phe restriction, and control animals. Displayed are bone volume/total volume, bone surface density, trabecular number, and total porosity. While Pahenu2 differs from control in all metrics, no difference is observed between animals with uncontrolled PHE homeostasis (~2000–2200 μM Phe) and animal under PHE management (~200 μM Phe). These data show dietary Phe management with amino acid defined diet is without effect to improve Pahenu2 bone density. Aggregate data from male and female animals is analyzed together. These studies focus on PKU verses managed PKU and no attempt is made to demonstrate a bone density difference related to sex.
Fig. 1.
Bone metrics in control animals, untreated PKU, and PHE restricted PKU. (N = 6 animals/group). PKU animals display reduced bone volume, surface density, and trabecula number compared to control; however, PHE-restriction does not improve these metrics. Untreated and PHE restricted animal have greater porosity. In all metrics assessed, there are no significant differences between untreated Pahenu2 and treated Pahenu2. WT = wild type C57bl/6 mouse, PKU = Pahenu2 mouse. N = 6 animals/cohort, 3 male, 3 female.
3.2. Superoxide Over-representation in Pahenu2 MSCs
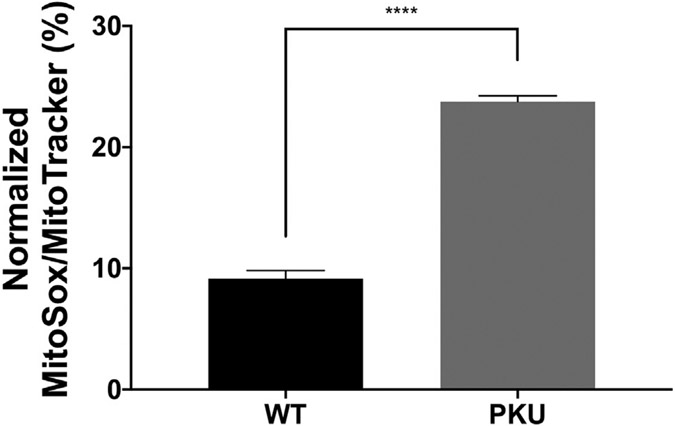
Oxidative stress occurs in PKU models and PKU patients [24-28]. MitoSox Red dye specifically stains superoxide. Fig. 2 demonstrates Pahenu2 MSCs have greater superoxide content than control MSCs. Over-representation of the reactive oxygen species superoxide defines oxidative stress is occurring in Pahenu2 MSCs.
Fig. 2.
Superoxide labeling (MitoSox Red) normalized to mitochondrial mass (MitoTracker Green). The Pahenu2 MSCs have greater superoxide content than control MSCs, N = 4, **** = p < 0.0001.
3.3. Oximetry of Pahenu2 MSCs identifies mitochondrial impairment
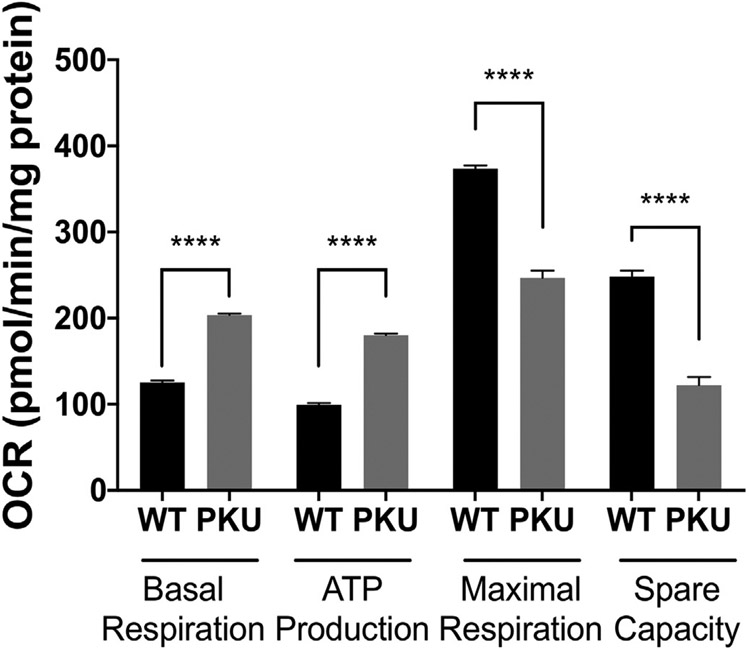
Intra-mitochondria oxidative stress adversely affect mitochondrial function [40-42]. Oximetry measurements utilized MSCs from four control animals (two male, two female) and four Pahenu2 animals (two male, two female). Fig. 3 compares oxygen consumption of Pahenu2 and control MSCs. Compared to control MSCs, Pahenu2 MSCs display increased basal respiration with increased ATP production. Concurrently, there is reduced maximal respiration and respiratory reserve capacity. These data are consistent with mitochondrial stress. In normal human MSCs and mouse MSCs, oximetry studies demonstrated that up-regulation of oxidative metabolism is required for MSC osteoblast differentiation [31,32]. These data show aberrant oxygen consumption in Pahenu2 MSCs suggesting stress, which may impair capacity to up-regulate energy production and support osteoblast differentiation.
Fig. 3.
Seahorse oximetry of Pahenu2 and Control MSCs. Basal respiration and ATP production are higher in Pahenu2 MSC. Maximal respiration and spare respiratory capacity are lower in Pahenu2 MSCs. This combination of phenotypes suggests mitochondrial stress. Y-axis OCR = oxygen consumption rate. * pW.05, *** p:50.001; **** p:50.0001; WT = C57BL/6 MSCs; PKU = Pahenu2 MSCs.
3.4. Respirometry demonstrates complex 1 functional deficit
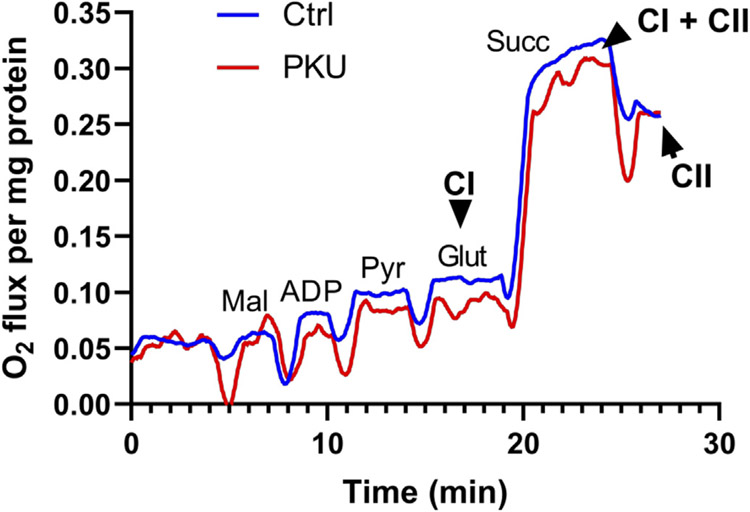
Fig. 2 oxidative stress and Fig. 3 mitochondria oxygen consumption motivated respiratory complex functional assessment. The Oroboros system compares respiratory complex 1 and complex 2 activities in response to substrate activation. Fig. 4 shows attenuated complex 1 stimulation following successive induction by malate, ADP, pyruvate, and glutamate in Pahenu2 MSCs compared to control MSCs. The profile shown in Fig. 4 is representative of three assessments (MSCs from one male and two female animals, both control and Pahenu2) where reduced complex 1 activity is consistently observed. These data observed concurrently with ROS over-representation suggest complex 1 deficit contributes to mitochondria functional impairment as displayed in Fig. 3.
Fig. 4.
Oroboros respirometry of differentiating Pahenu2 and Control MSCs. Fig. 4 provides a representative profile of consistent results from three independent assessments using MSCs from one male and two female animals. Substrate induced respiratory chain complex 1 activity is lower in Pahenu2 MSCs. Ctrl = control MSCs, PKU = Pahenu2 MSCs, Cl = respiratory chain complex 1, Cll = respiratory chain complex 2, Mal = malate, ADP = adenosine diphosphate, Pyr = pyruvate, Glut = glutamate.
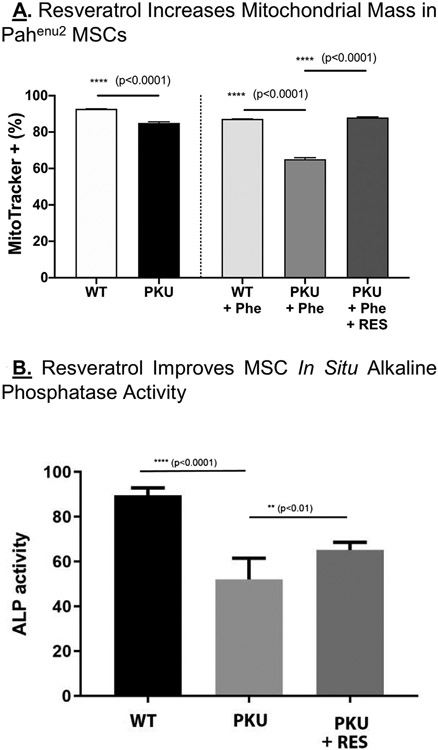
3.5. Resveratrol increases mitochondrial representation and partially rescues osteoblast differentiation
The polyphenol antioxidant resveratrol enhances human MSC osteoblast differentiation [32]. Pahenu2 MSCs (four animals, two male, two female) and control MSCs (four animals, two male, two female) were treated with 5 μM resveratrol for 2 weeks. Fig. 5A shows Mitotracker Green staining demonstrating mitochondrial mass in the context of standard culture conditions, PHE insult, and PHE insult plus resveratrol. Bars on the left (control, Pahenu2), show mitochondria content of Pahenu2 is reduced under standard culture conditions. Bars on the right, show PHE insult further reduces mitochondria content; however, even in the context of hyperphenylalaninemia resveratrol serves to increase mitochondria mass. The far right bar demonstrates in the context of 1200 μM PHE, mitochondria content is significantly increased. Fig. 5B assesses in situ alkaline phosphatase activity among control MSCs, Pahenu2 MSCs, and Pahenu2 MSCs treated with resveratrol (5 μM) following21 days of osteoblast differentiation. In the context of hyperphenylalaninemic conditions (1200 μM PHE), resveratrol treatment partially rescues alkaline phosphatase activity suggesting improved osteoblast differentiation.
Fig. 5. -.
Fig. 5A. Resveratrol Increases mitochondrial mass in PKU MSCs. The left two bars show control and Pahenu2 MSCs cultured in standard media. Mitochondria mass is greater in control cells. The right Bars show control and Pahenu2 MSCs cultured in 1200 μM PHE, where resveratrol leads to increased mitochondrial mass in Pahenu2 cells. Fig. 5B. Resveratrol Improves MSC Osteoblast Differentiation. In situ alkaline phosphatase activity is shown in differentiating control MSCs, Pahenu2 MSCs, and Pahenu2 MSCs treated with resveratrol. A statistically significant increase in alkaline phosphatase activity is seen with resveratrol treatment. N = 4, ** p < 0.01, **** p < 0.0001.
4. Discussion
Legacy data (1969–1975) identified mitochondrial dysfunction in PKU precipitated by Phe and the Phe catabolite phenylpyruvate [43-46]. Contemporary investigations of mitochondria involvement in PKU and characterization of oxidative stress in patients and animal models gives further credence to mitochondrial participation in PKU pathophysiology [47-50]. Essentially the entire PKU osteopenia literature is clinical and descriptive. In the PKU osteopenia space, there is a genuine paucity of pathophysiological investigation. The Pahenu2 mouse is universally osteopenic providing a system to investigate bone pathology. Our prior studies demonstrated Pahenu2 MSCs display compromised osteoblast differentiation. Data presented herein demonstrates Pahenu2 MSCs experience oxidative stress and mitochondria functional deficits that we hypothesize are contributory to impaired osteoblast development.
Oxidative stress in PKU is established. The relationship between oxidative stress and mitochondria dysfunction is equally established. Oximetry studies in normal human MSCs and two stains of mouse MSCs demonstrate osteoblast differentiation requires increased oxidative metabolism [30,31]. It is hypothesized, oxidative metabolism is required to support extracellular matrix production [51]. MitoSox Red dye specifically stains superoxide. Oxidative stress in Pahenu2 MSCs owing to superoxide (Fig. 2 )led us to suspect an energy production defect contributed to impaired osteoblast differentiation. The Seahorse MitoStress test identified deficient mitochondrial functional metrics in Pahenu2 MSCs, the most relevant being reduced maximum respiratory rate and respiratory reserve (Fig. 3). These data coincide with oxidative stress (Fig. 2) to further support energy deficit. Superoxide is a recognized product of proton leak associated with respiratory chain complex 1 dysfunction [52]. Data in Fig. 4, we interpret as a Pahenu2 MSCs complex 1 respiratory deficit as it occurs concurrently with superoxide over-representation (Fig. 2). Similar respirometry data could be generated by a mitochondrial pyruvate transport deficit, pyruvate dehydrogenase deficit, or a Kreb cycle enzyme deficit. Future studies will address alternative means whereby respirometry results may be generated. Regardless, the summation of these data (ROS overrepresentation, oximetry, respirometry) suggests mitochondria functional deficit contributes to Pahenu2 impaired osteoblast development.
PKU medical formula contains antioxidants. However, oxidative stress has been observed in therapy compliant patients suggesting it may be necessary to consider increased antioxidant utilization [27,28]. Figs. 5A-B provide evidence that antioxidant treatment increases mitochondrial mass and MSC competence for osteoblast differentiation. These data provide encouraging evidence that regimens to reduce oxidative stress may increase in vivo MSC differentiation to subsequently increase bone density. This initial observation gives indication for further assessment of antioxidants in MSC developmental (alternative antioxidants, higher dosage, longer dose duration, molecular assessment, oximetry, etc); however, we suggest energy substrates may further support osteoblast development. MSCs have unique energy substrate specificity which includes glutamine, glutamine catabolic products (glutamate, α-ketoglutarate), and unsaturated fatty acids [53-55]. Providing MSC-preferred substrates may enhance energy production leading to increased osteoblast differentiation and improved bone density. A further benefit of glutamate (through direct supplementation or glutamine catabolism) is repleting glutathione to combat oxidative stress. Relating to both antioxidants and preferred energy substrates are studies of bone density and response to glycomacropeptide diet. It is demonstrated in Pahenu2 that glycomacropeptide Phe management increases bone density yet does so in the context of higher Phe homeostasis (~750 ~M Phe in blood) [56]. Glycomacropeptide prebiotic properties may reduce oxidative stress to support the Fig. 5 antioxidant study [57]. Furthermore, plasma amino acid assessment of Pahenu2 and control animals managed with glycomacropeptide presented higher glutamate and glutamine concentrations than cohorts (Pahenu2, Control) provided either amino acid defined diet or casein-based diet [56]. These independently generated data, showing bone density improvement concurrent with increased MSC preferred energy substrates gives indication for further investigation of substrate-based intervention.
In the Pahenu2 mouse, we determined dietary Phe management (Fig. 1) with amino acid defined diet does not restore bone density. Our previous investigation demonstrated Pahenu2 MSCs have a developmental defect in the osteoblast lineage. Presently, we demonstrate Pahenu2 MSCs experience oxidative stress, aberrant mitochondrial oxygen consumption, and a partial complex 1 respiratory chain deficit. These data indicate dysfunctional energy production. Up-regulation of oxidative energy production is required for osteoblast differentiation; therefore, the current study suggests energy deficit contributes to Pahenu2 osteopenia. Resveratrol partially rescued Pahenu2 MSC in situ alkaline phosphatase activity suggesting oxidative stress reduction enhances osteoblast differentiation. Continuing studies are required investigate in vitro MSC cell culture systems for the effect of supplemental energy substrates and additional antioxidant regimens. Transition to Pahenu2 animal studies is underway.
Grant support
Supported in part by Department of Veterans Affairs Grant I01BX002490 and by National Institutes of Health Grant AR076146-01A1.
Footnotes
Disclosure summary
No authors had relationships during the past 2 years with companies that make or supply products or services related to the subject of the paper, or other financial and non-financial interests.
References
- [1].Feinberg SB, Fisch RO, Roentgenologic findings in growing long bones in phenylketonuria, Preliminary study. Radiol 78 (1962) 394–398. [DOI] [PubMed] [Google Scholar]
- [2].Murdoch MM, Holman GH, Roentgenologic bone changes in phenylketonuria. Relationship to dietary phenylalanine and serum alkaline phosphatase, Am J Dis Child. 107 (1964) 523–532. [PubMed] [Google Scholar]
- [3].Porta F, Spada M, Lala R, Mussa A, Phalangeal quantitative ultrasound in children with phenylketonuria: a pilot study, Ultrasound Med. Biol 34 (2008) 1049–1052. [DOI] [PubMed] [Google Scholar]
- [4].Schwahn B, Mokov E, Scheidhauer K, Lettgen B, Schönau E, Decreased trabecular bone mineral density in patients with phenylketonuria measured by peripheral quantitative computed tomography, Acta Paediatr. 87 (1998) 61–63. [DOI] [PubMed] [Google Scholar]
- [5].Zeman J, Bayer M, Stepán J, Bone mineral density in patients with phenylketonuria, Acta Paediatr. 88 (1999) 1348–1351. [DOI] [PubMed] [Google Scholar]
- [6].Allen JR, Humphries IR, Waters DL, Roberts DC, Lipson AH, Howman-Giles RG, Gaskin KJ, Decreased bone mineral density in children with phenylketonuria, Am. J. Clin. Nutr 59 (1994) 419–422. [DOI] [PubMed] [Google Scholar]
- [7].Coakley KE, Douglas TD, Goodman M, Ramakrishnan U, Dobrowolski SF, Singh RH, Modeling correlates of low bone mineral density in patients with phenylalanine hydroxylase deficiency, J. Inherit. Metab. Dis 39 (2016) 363–372. [DOI] [PubMed] [Google Scholar]
- [8].Demirdas S, Coakley KE, Bisschop PH, Hollak CE, Bosch AM, Singh RH, Bone health in phenylketonuria: a systematic review and meta-analysis, Orphanet J Rare Dis. 15 (10) (2015) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stroup BM, Hansen KE, Krueger D, Binkley N, Ney DM, Sex differences in body composition and bone mineral density in phenylketonuria: a cross-sectional study, Mol Genet Metab Rep. 15 (2018) 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hansen KE, Ney D, A systematic review of bone mineral density and fractures in phenylketonuria, J. Inherit. Metab. Dis 37 (2014) 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Greeves LG, Carson DJ, Magee A, Patterson CC, Fractures and phenylketonuria, Acta Paediatr. 86 (1997) 242–244. [DOI] [PubMed] [Google Scholar]
- [12].de Groot MJ, Hoeksma M, van Rijn M, Slart RH, van Spronsen FJ, Relationships between lumbar bone mineral density and biochemical parameters in phenylketonuria patients, Mol. Genet. Metab 105 (2012) 566–570. [DOI] [PubMed] [Google Scholar]
- [13].Lage S, Bueno M, Andrade F, Prieto JA, Delgado C, Legarda M, Sanjurjo P, Aldámiz-Echevarría LJ, Fatty acid profile in patients with phenylketonuria and its relationship with bone mineral density, J. Inherit. Metab. Dis 33 (Suppl. 3) (2010) S363–S371. [DOI] [PubMed] [Google Scholar]
- [14].Zeman J, Bayer M, Stepán J, Bone mineral density in patients with phenylketonuria, Acta Paediatr. 88 (1999) 1348–1351. [DOI] [PubMed] [Google Scholar]
- [15].Allen JR, Humphries IR, Waters DL, Roberts DC, Lipson AH, Howman-Giles RG, Gaskin KJ, Decreased bone mineral density in children with phenylketonuria, Am. J. Clin. Nutr 59 (1994) 419–422. [DOI] [PubMed] [Google Scholar]
- [16].Hillman L, Schlotzhauer C, Lee D, Grasela J, Witter S, Allen S, Hillman R, Decreased bone mineralization in children with phenylketonuria under treatment, Eur.J. Pediatr 155 (Suppl. 1) (1996) S148–S152. [DOI] [PubMed] [Google Scholar]
- [17].Modan-Moses D, Vered I, Schwartz G, Anikster Y, Abraham S, Segev R, Efrati O, Peak bone mass in patients with phenylketonuria, J. Inherit. Metab. Dis 30 (2007) 202–208. [DOI] [PubMed] [Google Scholar]
- [18].Adamczyk P, Morawiec-Knysak A, Płudowski P, Banaszak B, Karpe J, Pluskiewicz W, Bone metabolism and the muscle-bone relationship in children, adolescents and young adults with phenylketonuria, J. Bone Miner. Metab 29 (2011) 236–244. [DOI] [PubMed] [Google Scholar]
- [19].Barat P, Barthe N, Redonnet-Vernhet I, Parrot F, The impact of the control of serum phenylalanine levels on osteopenia in patients with phenylketonuria, Eur. J. Pediatr 161 (2002) 687–688. [DOI] [PubMed] [Google Scholar]
- [20].Nagasaka H, Tsukahara H, Takatani T, Sanayama Y, Takayanagi M, Ohura T, Sakamoto O, Ito T, Wada M, Yoshino M, Ohtake A, Yorifuji T, Hirayama S, Miida T, Fujimoto H, Mochizuki H, Hattori T, Okano Y, Cross-sectional study of bone metabolism with nutrition in adult classical phenylketonuric patients diagnosed by neonatal screening, J. Bone Miner. Metab 29 (2011) 737–743. [DOI] [PubMed] [Google Scholar]
- [21].Porta F, Roato I, Mussa A, Repici M, Gorassini E, Spada M, Ferracini R, Increased spontaneous osteoclastogenesis from peripheral blood mononuclear cells in phenylketonuria, J. Inherit. Metab. Dis 31 (Suppl. 2) (2008) S339–S342. [DOI] [PubMed] [Google Scholar]
- [22].Dobrowolski SF, Tourkova IL, Robinson LJ, Secunda C, Spridik K, Blair HC, A bone mineralization defect in the Pahenu2 model of classical phenylketonuria involves compromised mesenchymal stem cell differentiation, Mol. Genet. Metab 125 (2018) 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dobrowolski SF, Tourkova IL, Blair HC, PKU osteopenia involves a mesenchymal stem cell developmental defect, Mol. Genet. Metab 126 (2019) 296. [Google Scholar]
- [24].Martinez-Cruz F, Pozo D, Osuna C, Espinar A, Marchante C,Guerrero JM, Oxidative stress induced by phenylketonuria in the rat: prevention by melatonin, vitamin E, and vitamin C, J. Neurosci. Res, 69 (2002) 550–558. [DOI] [PubMed] [Google Scholar]
- [25].Ercal N, Aykin-Burns N, Gurer-Orhan H, McDonald JD, Oxidative stress in a phenylketonuria animal model, Free Radic. Biol. Med 32 (2002) 906–911. [DOI] [PubMed] [Google Scholar]
- [26].Colomé C, Artuch R, Vilaseca MA, Sierra C, Brandi N, Lambruschini N, Cambra FJ, Campistol J, Lipophilic antioxidants in patients with phenylketonuria, Am. J. Clin. Nutr 77 (2003) 185–188. [DOI] [PubMed] [Google Scholar]
- [27].Sitta A, Barschak AG, Deon M, Terroso T, Pires R, Giugliani R, Dutra-Filho CS, Wajner M, Vargas CR, Investigation of oxidative stress parameters in treated phenylketonuric patients, Metab. Brain Dis, 21 (2006) 287–296. [DOI] [PubMed] [Google Scholar]
- [28].Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Belló-Klein A, Giugliani R, Deon M, Vargas CR, Oxidative stress in patients with phenylketonuria, Biochim. Biophys. Acta 1740 (2005) 68–73. [DOI] [PubMed] [Google Scholar]
- [29].Nuschke A, Rodriguez M, Wells AW, Sylakowski K, Wells A, Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation, Stem Cell Res Ther 7 (2016) 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shum LC, White NS, Mills BN, Bentley KL, Eliseev RA, Energy Metabolism in mesenchymal stem cells during osteogenic differentiation, Stem Cells Dev. 25 (2016) 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guntur AR, Le PT, Farber CR, Rosen CJ, Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass, Endocrinology. 155 (2014) 1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moon DK, Kim BG, Lee AR, In Choe Y, Khan I, Moon KM, Jeon RH, Byun JH, Hwang SC, Woo DK, Resveratrol can enhance osteogenic differentiation and mitochondrial biogenesis from human periosteum-derived mesenchymal stem cells, J. Orthop. Surg. Res 15 (2020) 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dobrowolski SF, Lyons-Weiler J, Spridik K, Vockley J, Skvorak K, Biery A, DNA methylation in the pathophysiology of hyperphenylalaninemia in the PAHenu2 mouse model of phenylketonuria, Mol. Genet. Metab 119 (1-2) (2016. Sep) 1–7 pii: S1096-7192(16)30001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tourkova IL, Liu L, Sutjarit N, Larrouture QC, Luo J, Robinson LJ, Blair HC, Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes, Lab. Investig 97 (2017) 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, Mitchell J, Smith WE, Thompson BH, Berry SA, American college of medical genetics and genomics therapeutics committee. phenylalanine hydroxylase deficiency: diagnosis and management guideline, Genet Med. 16 (2014) 188–200. [DOI] [PubMed] [Google Scholar]
- [36].Rodenacker K, Bengtsson E, A feature set for cytometry on digitized microscopic images, Anal. Cell. Pathol 25 (2003) 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ghaloul-Gonzalez L, Mohsen AW, Karunanidhi A, Seminotti B, Chong H, Madan-Khetarpal S, Sebastian J, Vockley CW, Reyes-Múgica M, Vander Lugt MT, Vockley J Reticular Dysgenesis, Mitochondriopathy induced by adenylate kinase 2 deficiency with atypical presentation, Sci. Rep 9 (2019), 15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dobrowolski SF, Alodaib A, Karunanidhi A, Basu S, Holecko M, Lichter-Konecki U, Pappan KL, Vockley J, Clinical, biochemical, mitochondrial, and metabolomic aspects of methylmalonate semialdehyde dehydrogenase deficiency: report of a fifth case, Mol. Genet. Metab 129 (4) (2020. Apr) 272–277. [DOI] [PubMed] [Google Scholar]
- [39].Zhang Y, Bharathi SS, Beck ME, Goetzman ES, The fatty acid oxidation enzyme long-chain acyl-CoA dehydrogenase can be a source of mitochondrial hydrogen peroxide, Redox Biol. 26 (2019), 101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cenini G, Lloret A, Cascella R, Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view, Oxidative Med. Cell. Longev 2019 (2019) 2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lin MT, Beal MF, Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases, Nature. 443 (2006) 787–795. [DOI] [PubMed] [Google Scholar]
- [42].Bhatti JS, Bhatti GK, Reddy PH, Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies, Biochim. Biophys. Acta Mol. basis Dis 2017 (1863) 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Halestrap AP, The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors, Biochem. J 148 (1975) 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Patel MS, Grover WD, Auerbach VH, Pyruvate metabolism by homogenates of human brain: effects of phenylpyruvate and implications for the etiology of the mental retardation in phenylketonuria, J. Neurochem 20 (1973) 289–296. [DOI] [PubMed] [Google Scholar]
- [45].Weber G, Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpyruvate: possible relevance to phenylketonuric brain damage, Proc. Natl. Acad. Sci. U. S. A 63 (1969) 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Patel MS, Arinze IJ, Phenylketonuria: metabolic alterations induced by phenylalanine and phenylpyruvate, Am. J. Clin. Nutr 28 (1975) 183–188. [DOI] [PubMed] [Google Scholar]
- [47].Mezzomo NJ, Becker Borin D, Ianiski F, Dotto Fontana B, Diehl de Franceschi I, Bolzan J, Garcez R, Grings M, Parmeggiani B, da Silva Fernandes L, de Almeida Vaucher R, Leipnitz G, Duval Wannmacher CM, Cielo Rech V, Creatine nanoliposome reverts the HPA-induced damage in complex II-III activity of the rats’ cerebral cortex, Mol. Biol. Rep 46 (2019) 5897–5908. [DOI] [PubMed] [Google Scholar]
- [48].Bortoluzzi VT, Brust L, Preissler T, de Franceschi ID, Wannmacher CMD, Creatine plus pyruvate supplementation prevents oxidative stress and phosphotransfer network disturbances in the brain of rats subjected to chemically-induced phenylketonuria, Metab. Brain Dis 34 (2019) 1649–1660. [DOI] [PubMed] [Google Scholar]
- [49].Dimer NW, Ferreira BK, Agostini JF, Gomes ML, Kist LW, Malgarin F, Carvalho-Silva M, Gomes LM, Rebelo J, Frederico MJS, Silva FRMB, Rico EP, Bogo MR, Streck EL, Ferreira GC, Schuck PF, Brain bioenergetics in rats with acute hyperphenylalaninemia, Neurochem. Int 117 (2018) 188–203. [DOI] [PubMed] [Google Scholar]
- [50].Stepien KM, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D, Hargreaves IP, Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders, J. Clin. Med 19 (6) (2017)pii: E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shares BH, Busch M, White N, Shum L, Eliseev RA, Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation, J. Biol. Chem 293 (2018) 16019–16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Murphy MP, How mitochondria produce reactive oxygen species, Biochem. J 417 (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fillmore N, Huqi A, Jaswal JS, Mori J, Paulin R, Haromy A, Onay-Besikci A, Ionescu L, Thébaud B, Michelakis E, Lopaschuk GD, Effect of fatty acids on human bone marrow mesenchymal stem cell energy metabolism and survival, PLoS One 13 (2015), e0120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhou T, Yang Y, Chen Q, Xie L, Is Glutamine Metabolism, Essential for stemness of bone marrow mesenchymal stem cells and bone homeostasis, Stem Cells Int. 12 (2019)2019:8928934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yu Y, Newman H, Shen L, Sharma D, Hu G, Mirando AJ, Zhang H, Knudsen E, Zhang GF, Hilton MJ, Karner CM, Glutamine Metabolism regulates proliferation and lineage allocation in skeletal stem cells, Cell Metab. 2 (2019) 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Solverson P, Murali SG, Litscher SJ, Blank RD, Ney DM, Low bone strength is a manifestation of phenylketonuria in mice and is attenuated by a glycomacropeptide diet, PLoS One 7 (9) (2012), e45165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sawin EA, De Wolfe TJ, Aktas B, Stroup BM, Murali SG, Steele JL, Ney DM, Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice, Am. J. Physiol. Gastrointest. Liver Physiol 309 (2015) G590–G601. [DOI] [PMC free article] [PubMed] [Google Scholar]