Abstract
The interaction of microbes with dendritic cells (DCs) is likely to have an enormous impact on the initiation of the immune response against a pathogen. In this study, we compared the interaction of Mycobacterium tuberculosis with murine bone marrow-derived DCs and macrophages (Mφ) in vitro. M. tuberculosis grew equally well within nonactivated DCs and Mφ. Activation of DCs and Mφ with gamma interferon and lipopolysaccharide inhibited the growth of the intracellular bacteria in a nitric oxide synthase-dependent fashion. However, while this activation enabled Mφ to kill the intracellular bacteria, the M. tuberculosis bacilli within activated DCs were not killed. Thus, DCs could restrict the growth of the intracellular mycobacteria but were less efficient than Mφ at eliminating the infection. These results may have implications for priming immune responses to M. tuberculosis. In addition, they suggest that DCs may serve as a reservoir for M. tuberculosis in tissues, including the lymph nodes and lungs.
Mycobacterium tuberculosis, the causative agent of tuberculosis, is responsible for 1.5 million deaths per year, although only 10% of immunocompetent people infected with M. tuberculosis develop active tuberculosis (13). Most people contain, although probably do not eliminate, the initial infection as a result of cell-mediated immunity. It has been established that T cells provide protection against M. tuberculosis (reviewed in reference 9), and there are studies supporting roles for both CD4 and CD8 T cells (4, 6, 16, 27, 40, 51). The organism resides and multiplies within macrophages (Mφ), which can be activated by gamma interferon (IFN-γ) and other signals to control the infection.
The interactions between dendritic cells (DCs) and pathogens are of prime importance in establishing an appropriate immune response. It has been demonstrated that DCs internalize various microbes (14, 20, 23–25, 32, 36, 37, 39). We reported previously that human peripheral blood-derived DCs phagocytose M. tuberculosis and subsequently display a phenotype consistent with that of a mature DC (24). Recently, it has been reported that a murine DC line also can be infected with M. tuberculosis, which results in a mature phenotype with secretion of inflammatory cytokines (50). The initiation of a protective immune response depends on the interaction of antigen-presenting cells (APCs) and naive T cells, which occurs in lymphoid organs, including lymph nodes. DCs are considered to be the most potent APCs and play a crucial role in the initiation of an adaptive immune response. Following phagocytosis of an antigen (such as a bacterium), mannose and Fc receptors on DCs are downregulated, while adhesion, antigen-presenting, and costimulatory molecules for T cells are upregulated, resulting in a mature DC (42, 54). The murine model of tuberculosis has provided considerable insight into the immune responses to M. tuberculosis. In this study we extend the findings from our human DC studies to the mouse system, indicating that the responses of DCs and Mφ to M. tuberculosis infection differ. In addition, we present data showing that the fate of the organism within these two cell types is different. This may have implications for the immune response against this microbe, as well as for the persistence of bacilli in the host.
MATERIALS AND METHODS
Mice.
Adult female 8- to 10-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were used. Nitric oxide synthase (NOS2)−/− mice on a C57BL/6 background were generated as described by MacMicking et al. (30) and kindly provided by Timothy Billiar (University of Pittsburgh School of Medicine). All mice were maintained in a specific-pathogen-free biosafety level 3 facility.
Culture and purification of DCs and Mφ.
DCs and Mφ were generated from the bone marrow cells of C57BL/6 mice. Briefly, cells were extracted from the femur and tibia bones of mice in Dulbecco modified Eagle medium (DMEM). For the Mφ cultures, the cells were washed twice in DMEM, and 2.5 × 106 cells were plated in LabTek PS petri dishes (Fisher Scientific, Pittsburgh, Pa.) in 25 ml of DMEM supplemented with 10% certified fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine (Life Technologies, Grand Island, N.Y.), and 33% supernatant from L-cell fibroblasts cultured for 5 to 6 days. All reagents were lipopolysaccharide (LPS)-free, and no antibiotics were used. The medium was changed on day 3. On day 5, adherent cells were washed twice with ice-cold phosphate-buffered saline (PBS) (Life Technologies), incubated for 20 min on ice, and harvested by using cell scrapers (Becton Dickinson Labware, Lincoln Park, N.J.). The cell concentration was adjusted to 1.0 × 106 cells/ml, and cells were placed in Teflon jars (1 ml) (Savillex, Minnetonka, Minn.) or aliquoted into a 96-well plate (200 μl/well) for infection.
For DC cultures, bone marrow cells were centrifuged at 1,200 rpm for 7 min, and red blood cells were lysed with NH4Cl-Tris solution. T cells (CD4+ and CD8+) were removed using Low-Tox–M rabbit complement (Accurate Chemical and Scientific Corporation, Westbury, N.Y.) after incubation with anti-CD4 antibody (GK1.5, 10 μg/107 cells) and anti-CD8 antibody hybridoma supernatant (anti-CD8α, clone 83-15-5). The cell concentration was adjusted to 106 cells/ml, and adherent cells were depleted by overnight culture in DC medium containing DMEM, 2 mM l-glutamine, and heat-inactivated 5% mouse serum (Sigma, St. Louis, Mo.). The nonadherent cells were cultured at 0.25 × 106 cells/ml in 24-well plates (Costar, Cambridge, Mass.) in DC medium containing 1,000 U of rm-granulocyte-macrophage colony-stimulating factor (rm-GM-CSF) and rm-interleukin-4 (IL-4; Schering-Plough, Kenilworth, N.H., and kindly provided by Walter Storkus). At day 5, nonadherent cells were harvested, adjusted to 1.0 × 106 cells/ml in DC medium containing rm-GM-CSF (1,000 U/ml), and either dispersed into a 24-well plate (1 ml/well) or aliquoted into a 96-well plate (200 μl/well) for infection.
Bacteria.
M. tuberculosis strain Erdman (obtained from the Trudeau Institute, Saranac Lake, N.Y.) was passed through mice, grown once in liquid (7H9 Middlebrook; Difco) medium, and frozen in aliquots at −80°C. Aliquots were used to start cultures at a concentration of 2.5 × 106 /ml in 7H9 medium; bacteria were grown in 5% CO2 at 37°C. Cultures were used at day 6 or 7 to infect cells. The bacteria were washed and resuspended in DC or Mφ medium, sonicated 10 s in a cup-horn sonicator, and then added to the cell cultures after estimation of bacterial numbers based on previous experience. Enumeration of viable bacteria to confirm the multiplicity of infection (MOI) was done by plating for viable CFU on 7H10 Middlebrook medium and incubated for 18 days at 37°C with 5% humified CO2.
Infection of DCs and Mφ.
After culture for 5 days, DCs (at 106/ml in DC medium plus rm-GM-CSF but without IL-4) were infected in 24- or 96-well plates with M. tuberculosis at an estimated MOI of 3 to 5. After 12 h, unincorporated bacteria were removed by pelleting the DCs at low speed (<1,000 rpm) and reculturing them with fresh media. In some experiments, Mφ were cultured and infected in 96-well plates; monolayers were then washed to remove extracellular bacteria, and fresh medium was added. In experiments involving quantitative cultures of intracellular bacterial growth, stasis, or killing, the MOI was reduced to 1, and extracellular bacteria were removed after 4 h. To estimate the percentage of infected cells for each experiment, DCs and Mφ were either air dried on poly-l-lysine-coated slides or grown in parallel in glass culture well slides (Nalgene) and fixed in 1% paraformaldehyde at each time point. Slides were stained by the Kinyoun method for acid-fast bacteria. For phenotypic assays, DCs and Mφ were cultured for an additional 48 h.
Transmission electron microscopy.
Uninfected and M. tuberculosis-infected DCs were cultured as described above for 72 h postinfection. Cells (4 × 105) were gently pelleted at 2,000 rpm for 2 min in microcentrifuge tubes, washed twice in PBS, and then fixed in 1.5% paraformaldehyde and 1.0% glutaraldehyde in PBS. Cells were postfixed in 1% osmium tetroxide in PBS, dehydrated through a graded series of alcohols, and embedded in Epon (Energy Beam Sciences, Agawam, Mass.). Thin (60-nm) sections were cut using a Reichert Ultracut S (Leica, Deerborn, Mich.), mounted on 200-mesh copper grids, counterstained with 2% aqueous uranyl acetate for 7 min and 1% aqueous lead nitrate for 2 min, and observed using a JEOL 1210 transmission electron microscope (Peabody, Mass.).
Flow cytometry analysis of cell surface markers.
DCs and Mφ were obtained and infected as described above. Approximately (2 to 5) × 105 were aliquoted into tubes and stained for surface markers using antibodies against major histocompatibility complex (MHC) class I (phycoerythrin [PE]-conjugated anti-mouse H2Db clone KH95 with control BALB/c immunoglobulin G2b [IgG2b]), MHC class II [fluorescein isothiocyanate (FITC) anti-mouse IAb clone AF6-120.1 with control mouse IgG2a (κ)], intercellular adhesion molecule-1 (ICAM-1) (FITC anti-mouse CD54 clone 3E2 with control hamster IgG), B7.1 (FITC-conjugated anti-mouse CD80 clone 16-10A1 with control hamster IgG), B7.2 (FITC-conjugated anti-mouse CD86 clone GL-1 with control rat IgG2a), and CD14 (PE-conjugated anti-mouse clone rm-C5-3 with control PE-conjugated rat IgG1) in PBS containing 20% mouse serum, 0.1% bovine serum albumin, and 0.1% sodium azide (fluorescence-activated cell sorter [FACS] buffer) for 30 min at 4°C. All antibodies were used at 0.2 μg/106 cells and were obtained from Pharmingen (San Diego, Calif.). Cells were fixed in 2% paraformaldehyde for 4 to 15 h and analyzed by flow cytometry (FACSCaliber) using Lysis II (acquisition) and CellQuest (analysis) software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Phagocytosis assay.
Infected or control bone marrow-derived DCs and Mφ were harvested from culture and resuspended at 4 × 105/ml in DMEM and kept on ice. Then, 8 μl of FITC-latex conjugated beads 3 μm in diameter (Polysciences, Warrington, Pa.) was added to the cells and mixed well. The cells were incubated with the beads for 2 h at 37 or 4°C. The cells were washed five times after incubation with ice-cold FACS medium and then fixed for 1 h with 1% formaldehyde before analysis by flow cytometry (FACScan; Becton Dickinson).
Cytokine production.
Supernatants from control and M. tuberculosis-infected DCs and Mφ cultures were harvested postinfection, filtered (0.2-μm-pore-size filters), and stored at −80°C. Enzyme-linked immunosorbent assay (ELISA) antibody pairs were used to detect IL-10 (JES5-SXC1 and JES5-2A5), IL-12p70 heterodimer (C15.6 and C17.15) (Biosource International, Camarillo, Calif.), and tumor necrosis factor alpha (TNF-α; MP6-XT22 and MP6-XT3) (Pharmingen) in the supernatants. Recombinant cytokines (Pharmingen and The Genetics Institute, Cambridge, Mass.) were used to generate standard curves. The ELISAs were performed according to Pharmingen's protocol.
RPA.
Determination of the levels of mRNA for the genes of interest at various time intervals after the infection was performed using a multiprobe RNase assay system (Pharmingen). Total RNA was extracted from DCs and Mφ uninfected or infected with M. tuberculosis as detailed above, using Trizol reagent (Life Technology). The extracted RNA was subjected to an RNase protection assay (RPA) according to the manufacturer's instructions. Protected [32P]UTP-labeled probes were resolved on a 6% polyacrylamide gel and analyzed by autoradiography. Cytokine analysis was performed using custom-made probe sets specific for NOS2, IL-12p40, IL-1α, IL-1β, IFN-γ, and IL-10 (mCK3) and for IL-4, IL-2p40, TNF-α, IFN-γ, IL-1α, and IL-1β (mCK2B). The expression of specific genes was quantified densitometrically (ImageQuant; Molecular Dynamics, Sunnyvale, Calif.) relative to the abundance of the housekeeping genes for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or L32.
T-cell proliferation and cytokine production assays.
DCs and Mφ uninfected or infected (MOI = 4) as described above for 24 h and then plated in triplicate in 96-well U-bottom plates (Corning Incorporated) at various concentrations to achieve 1:20 to 1:100 APC/T-cell ratio in RPMI 1640 medium (Life Technologies) containing 10% certified fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 25 mM HEPES (Life Technologies), and 50 μM 2-mercaptoethanol (Sigma). As a source of sensitized T cells, spleens were obtained from C57BL/6 mice infected for 4 to 5 weeks, and single-cell suspensions were obtained by crushing the spleens in cell strainers (Becton Dickinson Labware). Red blood cells were lysed with NH4Cl-Tris solution, and cells were washed twice. Mφ were depleted by adherence on plastic petri dishes for 2 h at 37°C. In some experiments, B cells were depleted by adherence on anti-IgG–anti-IgM antibody-coated plates (Zymed Laboratories, Inc., San Francisco, Calif.). Lymphocyte-enriched splenocytes were added at (2 to 4) × 105 cells/well and cultured with different APCs for 3 days. The proliferation of T cells in medium alone served as a baseline. As a positive control, cells were stimulated with concanavalin A (ConA; Boehringer Mannheim Corp., Indianapolis, Ind.) at 5 μg/ml. Cells were pulsed for the final 12 to 18 h of culture with 1 μCi of [3H]thymidine (Amersham Life Sciences, Inc., Arlington Heights, Ill.) per well, and the incorporation of radioactivity was measured by counting cell lysates on filters in a scintillation counter. The stimulation index (SI) was determined as follows: SI = (cpm of T cells plus infected APCs)/(cpm of T cells plus uninfected APCs). Culture supernatants were harvested after 3 days of culture, and IFN-γ production was measured by sandwich ELISA using the antibodies R4-A62 and XMG1.2 (biotinylated) (Pharmingen) according to the manufacturer's protocol. Recombinant murine IFN-γ used to generate a standard curve was a gift from Genentech (San Francisco, Calif.).
Antimycobacterial activity of Mφ and DCs.
The antimycobacterial activity of DCs and Mφ was assessed by metabolic labeling of intracellular M. tuberculosis with [3H]uracil as previously described (8, 43). DCs and Mφ (2 × 105 cells/well, duplicate or triplicate wells for each condition) were primed with IFN-γ (100 U/ml; Genentech, Inc., San Francisco, Calif.) for 12 to 24 h, and then LPS (1 μg/ml) (Sigma, St. Louis, Mo.) was added. Activated and resting cells were infected with M. tuberculosis (sonicated in a cup-horn sonicator for 20 s to reduce clumping) at an MOI of 3 to 5. Aminoguanidine (AG), an NOS2 enzyme inhibitor, was added to some conditions of the IFN-γ-treated and untreated cells 4 h prior to LPS addition and infection to inhibit NOS2 activity. At 24 h postinfection, the cells were pulsed with 2.5 μCi of [3H]uracil, which is incorporated predominantly by the bacteria and not by Mφ or DCs. The supernatants were removed 8 to 16 h later, the cell pellets were lysed with 1% saponin and trichloroacetic acid (TCA) precipitated onto GF/C glass fiber filters (Fisher Scientific, Pittsburgh, Pa.), and radioactive incorporation was measured by using a β-scintillation counter. The percent inhibition was calculated as follows: 100 − [(cpm of activated cells/cpm of resting cells) × 100].
For the determination of actual intracellular CFU, DCs and Mφ cultures were prepared as described above, and cell lysates at each time point were cultured on 7H10 plates (10-fold dilutions in PBS plus 0.05% Tween). The number of extracellular bacteria was determined by plating the undiluted sonicated supernatant obtained at each time point. The number of initial intracellular bacteria was determined at 4 h postinfection, and the reduction of input was based on that number. CFU were counted after incubation of the plates at 37°C for 18 days.
Determination of nitrite accumulation.
Nitrite (NO2−) accumulation in the supernatant of cultured cells was measured as an indicator of NO production by a Griess assay, with a sodium nitrite standard, as previously described (21). Supernatants from 2 × 105 cells (100 μl) of each condition were assayed in duplicate or triplicate, and the absorbancy was measured at 570 nm using an Emax precision microplate reader (Molecular Dynamics).
Statistics.
For statistical analysis of samples, paired and unpaired Student t tests were used (Instat, v.2.03; GraphPad Software, San Diego, Calif., and Stat View, Abacus Concepts, Berkeley, Calif.). P values of <0.05 were considered significant.
RESULTS
Infection of murine DCs with M. tuberculosis.
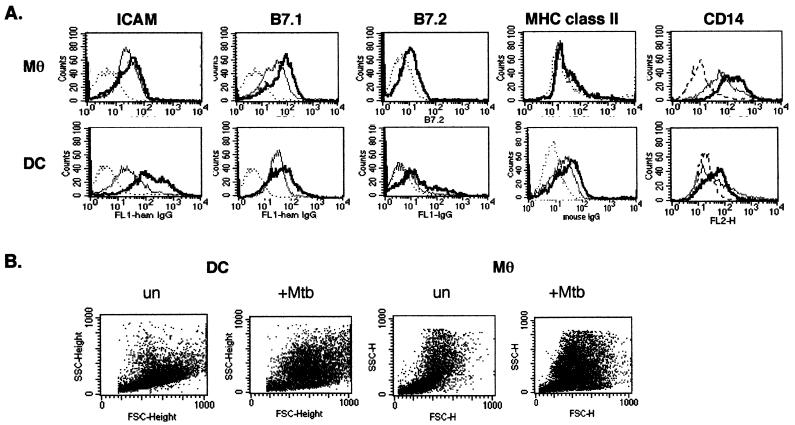
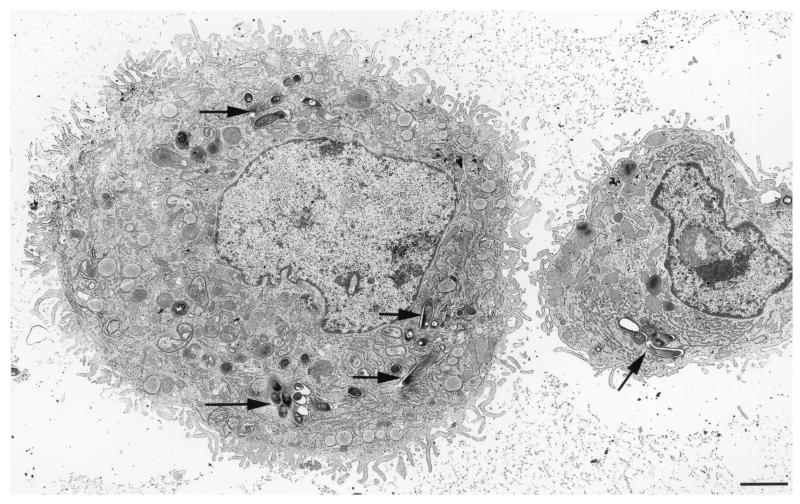
Immature DCs are highly phagocytic and readily take up various microbes (46, 48). We generated immature murine DCs by culture of bone marrow cells for 5 days in LPS-free media supplemented with 5% mouse serum, rm-GM-CSF, and rm-IL-4. These DCs have a very immature phenotype, as judged by low cell surface expression of MHC class II, B7.1, and B7.2, as well as moderate expression of ICAM (Fig. 1), morphology, and a high phagocytic ability, as determined by the uptake of FITC-labeled beads (Fig. 2). Mφ were also derived from bone marrow precursors. The DCs and Mφ were infected with M. tuberculosis at an estimated MOI of 3 to 5 for 12 h, which resulted in 50 to 70% of the cells infected, as judged by staining for acid-fast bacilli. The use of a higher MOI (5 to 10) resulted in a higher percentage of infected cells or more bacteria per cell but was associated with a loss of cell viability. To confirm that the mycobacteria were internalized, infected DCs were examined using transmission electron microscopy (Fig. 3). M. tuberculosis bacilli were observed within vacuoles of the DCs, and multiple bacilli were often present within the cell. Bacteria free in the cytoplasm were not observed. After infection with M. tuberculosis, both DCs and Mφ undergo a change in cell density and size (Fig. 1B).
FIG. 1.
Cell surface phenotype of DCs infected with M. tuberculosis. Purified bone marrow-derived DCs and Mφ were infected with M. tuberculosis or uninfected and harvested 48 h postinfection. (A) The cells were stained with antibodies as follows: MHC class II (IAb)-FITC conjugated and an isotype control mouse IgG2a; ICAM (CD54)-FITC and B7.1 (CD80)-FITC and an isotype control hamster IgG; B7.2 (CD86)-FITC and an isotype control rat IgG2a; and CD-14 (PE conjugated) and an isotype control rat IgG1(κ). The cells were then fixed and analyzed by FACS analysis. Top row, Mφ; bottom row, DCs; dotted line, IgG control; thin gray line, uninfected cells; thick black line, M. tuberculosis-infected cells. (B) The forward versus side-scatter plots show that both the DCs and the Mφ undergo morphologic changes after infection. A representative experiment of 10 independent experiments is shown.
FIG. 2.
Phagocytic capability is decreased in M. tuberculosis-infected DCs but not macrophages. Purified DCs and Mφ were cultured without (top panel) or with M. tuberculosis (bottom panel). After 72 h, cells were incubated with FITC-labeled latex beads (3 μm) for 2 h at 4 or 37°C as indicated and examined by flow cytometry. Dead cells and free beads were excluded from the gate, and 104 cells were collected within the gate for each sample. The MFI is indicated in each histogram. The bar above the histograph represents the gate. Percent gated: DCs, uninfected, 20% at 4°C and 76% at 37°C; DCs plus M. tuberculosis, 5% at 4°C and 37% at 37°C; Mφ, uninfected, 33% at 4°C and 93% at 37°C; Mφ plus M. tuberculosis, 14% at 4°C and 83% at 37°C. The experiment was repeated once.
FIG. 3.
Immature murine DCs internalize live M. tuberculosis. DCs infected with M. tuberculosis were fixed, embedded, sectioned, and examined by transmission electron microscopy. The arrows indicate M. tuberculosis. The micrograph shown is representative of 25 individual cells observed. Bar = 1 μm.
Murine DCs and Mφ respond differently to M. tuberculosis infection.
We previously reported that M. tuberculosis infection of human peripheral blood mononuclear cell (PBMC)-derived DCs resulted in the phenotypic maturation of the cells. To confirm a similar effect on murine DCs, we examined cell surface marker expression following M. tuberculosis infection of murine bone marrow-derived DCs and Mφ by flow cytometry. The DCs generated from bone marrow were cultured in mouse serum and have a very immature phenotype. DCs infected with M. tuberculosis showed a consistent upregulation in expression of the cell surface molecules ICAM, B7.2, and B7.1 compared to uninfected cells (Fig. 1A, Table 1), suggesting a shift to a more mature phenotype. Although the Mφ express MHC classes I and II, B7.1, B7.2, and ICAM-1, these were not generally upregulated following infection with M. tuberculosis. The Mφ express high levels of CD14, whereas the DCs express low-level CD14 which is slightly upregulated upon infection, as described by others (31) (Fig. 1A, Table 1). The morphology of both populations of cells is modified postinfection (Fig. 1B).
TABLE 1.
Cell surface phenotypes of DCs infected with M. tuberculosisa
| Antibody | Mφ
|
DCs
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected
|
Infected
|
Fold increaseb | Uninfected
|
Infected
|
Fold increaseb | |||||
| MFI | % Positive | MFI | % Positive | MFI | % Positive | MFI | % Positive | |||
| MHC molecules | ||||||||||
| CD14-PE | 209.69 | 68.0 | 272.80 | 93.0 | 1.3 | 129.3 | 28.0 | 138.9 | 38.0 | 1.1 |
| IAb-FITC (class II) | 110.0 | 12.6 | 126.0 | 14.0 | 1.1 | 49.0 | 18.0 | 210.2 | 44.0 | 4.0 |
| Rat IgG-PE | 10.52 | 2.5 | 20.6 | 2.3 | ||||||
| Murine IgG-FITC | 11.5 | 1.8 | 8.96 | 1.4 | ||||||
| Costimulatory molecules | ||||||||||
| CD-80 (B7.1) | 84.0 | 55.0 | 101.0 | 77.0 | 1.2 | 67.0 | 58.0 | 113.4 | 68.0 | 1.7 |
| CD-86 (B7.2) | 103.1 | 16.5 | 107.9 | 16.0 | 0.9 | 50.0 | 4.19 | 118.0 | 27.8 | 2.4 |
| Hamster IgG-FITC | 6.09 | 1.23 | 4.4 | 2.3 | ||||||
| Rat IgG2a-FITC | 6.24 | 0.2 | 4.9 | 0.58 | ||||||
| Adhesion molecules | ||||||||||
| CD54 (ICAM-1) | 128.0 | 36.0 | 129.0 | 38.0 | 1.0 | 62.0 | 45.0 | 245.0 | 93.0 | 3.9 |
| Hamster IgG-FITC | 6.51 | 0.7 | 4.4 | 1.9 | ||||||
Purified bone marrow-derived DCs and Mφ were infected with M. tuberculosis or uninfected and then harvested 4 h postinfection. The cells were stained for H2D-PE (MHC class I), IAb FITC (MHC class II), CD54-FITC (ICAM), CD80-FITC (B7.1), CD86-FITC (B7.2), and CD14-PE antibodies with the appropriate isotype controls and then fixed and analyzed by FACS analysis. Cells were gated on size (forward angle scatter) and density (side scatter) to exclude cellular debris and dead cells. The table shows the results of a representative FACS experiment out of a total of 10 independent experiments.
MFI compared to the MFI of uninfected cells.
Immature DCs are quite phagocytic, but this is reduced upon maturation (41, 45). We compared the effect of M. tuberculosis infection on the phagocytic potential of murine DCs and Mφ using FITC-labeled latex beads (3 μm; Fig. 2). Upon infection, DCs showed reduced phagocytic ability at 37°C compared to uninfected DCs (lower mean fluorescence intensity [MFI] and percent gated). In contrast, no change in the phagocytic potential of Mφ was observed following infection (Fig. 2). These data suggest that M. tuberculosis infection results in the maturation of DCs, confirming the results obtained with human DCs (24).
Inflammatory cytokines induced by M. tuberculosis infection of DCs.
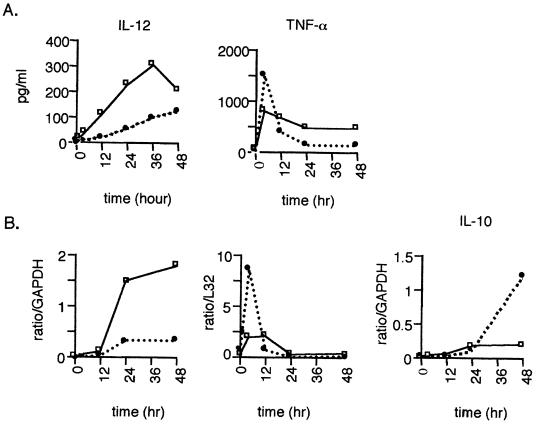
Mφ infected with M. tuberculosis secrete inflammatory cytokines, which can influence cytokine production by T cells (3). The production of cytokines by DCs during the priming of T cells in the lymph node is likely to affect the initiation of an immune response against an infection. TNF-α, IL-12, and IL-10 secretion and gene expression as measured by ELISA and RPA, respectively, were assessed in M. tuberculosis-infected DC and Mφ cultures. TNF-α was produced by M. tuberculosis-infected DCs and Mφ by 4 h postinfection, with decreased production at later time points (Fig. 4A). Infected DCs consistently produced more TNF-α early postinfection compared with infected Mφ, but TNF-α production by infected Mφ increased over time. This pattern was also observed at the RNA level (Fig. 4B). Uninfected DCs and Mφ produced negligible amounts of TNF-α (Fig. 4, time zero). IL-12 protein was detectable by 12 h in M. tuberculosis-infected DCs and by as early as 4 h in infected Mφ and increased over the course of infection (Fig. 4A). Infected Mφ produced two- to fivefold more IL-12 than infected DCs. Uninfected DCs and Mφ produce negligible amounts of IL-12 (Fig. 4, time zero). IL-12 gene expression was barely detectable in M. tuberculosis-infected DCs and Mφ until 24 h; no gene expression was observed in uninfected cells. IL-10 was not detected in either uninfected or M. tuberculosis-infected cells at any of the time points examined either by ELISA or by intracellular cytokine staining (data not shown). However, a low level of IL-10 mRNA was detected in M. tuberculosis-infected DCs and Mφ, and this level increased over time (Fig. 4B). In addition, IL-1α and IL-1β gene expression increased with infection in both cell types (not shown).
FIG. 4.
Cytokine secretion in response to M. tuberculosis-infected DCs and Mφ. (A) Supernatants from M. tuberculosis-infected unactivated DCs (●) and Mφ (□) were assayed in duplicate for production of IL-12 (ELISA for IL-12p70) or TNF. (B) Time zero represents cytokine production from the cells prior to infection. Recombinant murine cytokines were used as standards in each assay. Representative experiments of four and two total experiments are shown for IL-12 and TNF-α, respectively. (B) Analysis of gene expression by RNase protection assay. A total of 6 μg of RNA from either uninfected or M. tuberculosis-infected DC (•) and Mφ (□) at various time points was used in the assay, and specific genes of interest were standardized to GAPDH by densitometerization of the autoradiograph. A representative experiment (of two experiments) is shown.
Stimulation of T-cell proliferation and cytokine production by APCs.
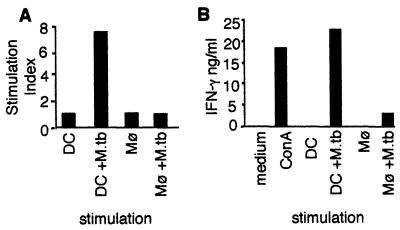
The differential effect of mycobacterial infection on expression of costimulatory molecules by DCs and Mφ suggested that they might differ in mycobacterial antigen-presenting functions. To assess the ability of infected DCs and Mφ to stimulate T-cell effector functions, T cells from M. tuberculosis-infected mice were cultured with either DCs or Mφ as APCs. Both uninfected and live M. tuberculosis-infected APCs were used. M. tuberculosis-infected DCs induced substantial proliferation of T cells from infected mice (Fig. 5A). In contrast, uninfected DCs and Mφ and, surprisingly, even infected Mφ, were very poor stimulators of T cells from infected mice (Fig. 5A). T cells, DCs, or Mφ alone did not proliferate to a significant extent (data not shown). A correlation was observed between the stimulation of T-cell proliferation and IFN-γ production. M. tuberculosis-infected DCs readily stimulated secretion of IFN-γ by T cells from infected mice, at levels comparable to that induced by ConA (Fig. 5B). However, little IFN-γ secretion was observed when either infected Mφ or uninfected DCs and Mφ were used as APCs (Fig. 5B).
FIG. 5.
Stimulation of T-cell proliferation and cytokine production by APCs. T-cell-enriched splenocytes from C57BL/6 mice were cultured for 3 days with bone marrow-derived DCs or Mφ either infected with M. tuberculosis (MOI = 4) or left uninfected at a 1:50 APC/T-cell ratio. (A) Proliferation was measured by [3H]thymidine incorporation and is represented as the SI. Stimulation with ConA resulted in ∼18,000 cpm, and stimulation with infected DCs resulted in ∼16,000 cpm. DCs, Mφ, or T cells alone incorporated fewer than 2,000 cpm. (B) Supernatants of the cultures described above were collected at the end of the culture period, and the concentration of IFN-γ was measured by sandwich ELISA. A representative experiment (of three experiments) is shown.
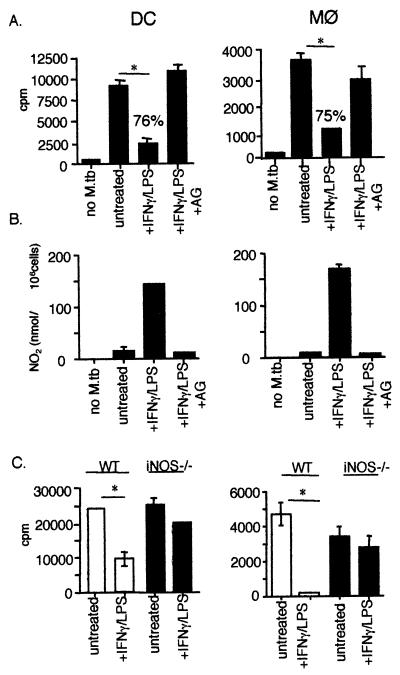
Activated DCs inhibited the proliferation of M. tuberculosis in vitro.
M. tuberculosis can grow within unactivated macrophages, and this is a major site for bacterial replication in vivo. However, murine Mφ activated by IFN-γ and either TNF-α or LPS inhibit intracellular growth of M. tuberculosis and kill 50 to 70% of the bacteria via NOS2-dependent reactive nitrogen intermediate (RNI) production (10). It has been reported that murine DCs can produce NO in response to LPS and/or IFN-γ (5). We examined the ability of DCs, either unactivated or activated with IFN-γ and LPS, to support or inhibit the growth of intracellular M. tuberculosis. Intracellular mycobacterial proliferation was assessed by measuring [3H]uracil incorporation into M. tuberculosis, which indicates bacterial growth but does not determine whether the bacteria are killed (10, 44). M. tuberculosis in unactivated DCs or Mφ incorporated [3H]uracil, indicating the replication of intracellular bacteria (Fig. 6A). DCs and Mφ activated with IFN-γ and LPS inhibited the growth of intracellular M. tuberculosis compared to unactivated cells, and the inhibition was comparable (76% inhibition for DCs compared to 75% inhibition for Mφ). The inhibition correlated with RNI production by activated DCs or Mφ (Fig. 6B). Addition of an NOS2 inhibitor, aminoguanidine, to both DCs and Mφ prior to infection abrogated the effect on intracellular M. tuberculosis (Fig. 6A). Confirmation of the importance of RNI production in the antimycobacterial activity of DCs was obtained using DCs and Mφ generated from NOS2−/− mice. IFN-γ- and LPS-activated NOS2−/− DCs failed to inhibit M. tuberculosis proliferation (Fig. 6C), similar to the result obtained with activated NOS2−/− Mφ (Fig. 6C).
FIG. 6.
Activated DCs and Mφ are comparable in the inhibition of M. tuberculosis growth via RNI production. (A) Incorporation of [3H]uracil into M. tuberculosis within DCs and Mφ was assessed in untreated and IFN-γ–LPS-activated DCs and Mφ. The lysates of [3H]uracil-labeled uninfected, M. tuberculosis-infected, or IFN-γ–LPS-activated M. tuberculosis-infected wild-type DCs and Mφ were TCA precipitated, collected on glass filters, and counted in a β-scintillation counter. An inhibitor of NO production, aminoguanidine (AG), was added to the cells prior to infection as indicated. (B) Griess assays were performed with supernatants of M. tuberculosis-infected DCs and Mφ and read at 570 nm. The standard curve was generated using NaNO2. (C) Lysates of [3H]uracil-labeled uninfected, M. tuberculosis-infected, or IFN-γ–LPS-activated M. tuberculosis-infected NOS2−/− DCs and Mφ were TCA precipitated and counted using liquid scintillation. Samples were tested in triplicate, and a representative experiment from three experiments is shown. For statistical analysis for all panels, IFN-γ–LPS or IFN-γ–LPS plus AG samples were compared to untreated cells. ∗, P < 0.02.
There was consistently a higher incorporation of [3H]uracil into M. tuberculosis within DCs compared to Mφ (Fig. 6), although similar infection levels were obtained, as judged by acid-fast bacillus staining and the intracellular CFU determination (see below) for each culture. To determine whether there was a differential uptake of [3H]uracil by DCs compared to Mφ, both cell types (uninfected) were pulsed with [3H]uracil, washed, centrifuged, and quantitated by liquid scintillation. There was a twofold increase in [3H]uracil uptake in DCs compared to Mφ (data not shown). DCs have a high fluid phase uptake, and the additional [3H]uracil inside the cell may have resulted in increased incorporation into the bacteria. Varying the concentration of [3H]uracil in a cell-free M. tuberculosis proliferation assay indicated that the incorporated counts per minute (cpm) declined linearly with the dilutions of [3H]uracil. These results confirmed that availability of [3H]uracil affects incorporation into the bacteria. Thus, the observed differences in the total cpm values between M. tuberculosis in DCs and Mφ probably reflect differences in the availability of [3H]uracil rather than any differences in bacterial growth within the cells.
Mycobacterial growth and killing within DCs and Mφ.
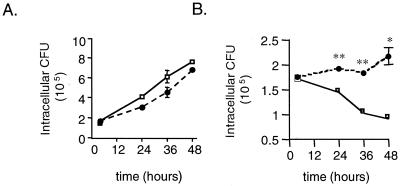
DCs encountering M. tuberculosis in the lung might not be activated initially, and thus the intracellular bacteria would be able to multiply and perhaps use this migrating cell to gain access to the lymph node. The ability of DCs to produce RNI and so inhibit bacterial replication suggests a potential role for DCs as an effector cell. Therefore, the antimicrobial effects of activated DCs were studied further. IFN-γ–LPS-activated DCs clearly inhibited the growth of intracellular M. tuberculosis, as described above. However, the ability of DCs to actually kill the organism was unknown. To address this, intracellular CFU levels at various times postinfection were determined by plating lysates from an equal number of unactivated and activated infected DCs and Mφ (Fig. 7). Initial (4 h postinfection) intracellular CFU levels were similar between DCs and Mφ (P = 0.63). M. tuberculosis grew equally well within unactivated DCs and Mφ over 60 h (Fig. 7A, Table 2), confirming the ability of DCs to support the growth of intracellular M. tuberculosis. In activated Mφ (treated with IFN-γ and LPS), the number of viable bacteria was reduced by ∼50% by 48 h postinfection compared to 4 h postinfection (P = 0.02) (Fig. 7B, Table 2). The number of viable bacteria in activated DCs did not increase over time, confirming that these cells can inhibit mycobacterial replication. However, in contrast to activated Mφ, the numbers of intracellular M. tuberculosis in activated DCs were not reduced over the course of the infection in six independent experiments (4 h versus 48 h, P = 0.21), demonstrating a lack of killing of intracellular bacteria (Fig. 7B, Table 2). Examination of the viability of the DC and Mφ cultures over the course of infection was performed by staining parallel cultures of infected cells in chamber slides and staining them with trypan blue and for acid-fast bacilli. At 48 h, there was no difference in the viabilities of the DCs and Mφ, although at later time points (72 to 90 h) there was a marked deterioration of both cell types. For this reason, we compared intracellular killing only for up to 48 h postinfection. In addition, the culture supernatants at each time point were plated to determine the number of bacteria that had escaped the cells, perhaps due to cell death. In all cases up to 48 h, very few bacteria (<1% of the total bacterial numbers) were present in the supernatants, and there was no difference between DC and Mφ cultures. Thus, the discrepancy in killing of intracellular organisms between DCs and Mφ cannot be attributed simply to differences in the cell viability. The activated DCs and Mφ both produced RNI (Table 2), which has been shown to be essential in killing intracellular M. tuberculosis (10). There is not a higher level of NOS2 at either the protein or the RNA level in activated Mφ compared to DCs (data not shown).
FIG. 7.
DCs inhibit the growth of but do not kill intracellular M. tuberculosis. Cell pellet lysates of M. tuberculosis-infected DCs or Mφ (MOI = 1), unactivated or activated with IFN-γ–LPS, were serially diluted in PBS plus 0.05% Tween 80 and plated on 7H10 plates, which were incubated for 18 days at 37°C and 5% CO2. Three wells per condition were assessed at each time point, and the mean intracellular CFU values are reported at various time points after infection. (A) Intracellular CFU in resting DCs (●) and resting Mφ (□). (B) Intracellular CFU in activated DCs (●) and activated macrophages (□). At each time point, <1% of the total CFU was found in the supernatant. In panel A, CFU levels within unactivated DCs and Mφ at each time point revealed no statistical differences (P > 0.5). In panel B, bacterial numbers within DC were not significantly reduced (P = 0.2) compared to intracellular CFU at 4 h (input). Comparison of CFU within Mφ at each time point to the CFU level at 4 h revealed statistically significant reductions over time (∗∗, P < 0.01; ∗, P < 0.02). Error bars show the standard error. A representative experiment (of six experiments) is shown.
TABLE 2.
Activated Mφ kill M. tuberculosis, whereas activated DCs inhibit the growth of M. tuberculosisa
| Cell type | Time (h) | Mean CFU (10−5) ± SDb
|
Fold change (CFU)c
|
NO2−d (nmol)
|
|||
|---|---|---|---|---|---|---|---|
| Untreated | IFN-γ–LPS treated | Untreated | IFN-γ–LPS treated | Untreated | IFN-γ–LPS treated | ||
| Mφ | 4 | 1.73 ± 0.05 | 1.70 ± 0.13 | 10.97 | 105.50 | ||
| 24 | 4.18 ± 0.06 | 1.43 ± 0.10 | +2.4 | −1.1 | 23.80 | 76.80 | |
| 36 | 6.20 ± 0.64 | 1.01 ± 0.07 | +3.6 | −1.6 | 50.50 | 142.70 | |
| 48 | 7.65 ± 0.07 | 0.89 ± 0.15 | +4.5 | −1.9 | 43.5 | 130.5 | |
| DCs | 4 | 1.75 ± 0.05 | 1.74 ± 0.06 | 0 | 37.5 | ||
| 24 | 3.14 ± 0.26 | 1.91 ± 0.11 | +1.8 | +1.1 | 0.14 | 90.9 | |
| 36 | 4.64 ± 0.50 | 1.83 ± 0.30 | +2.6 | +1.1 | 2.31 | 150.6 | |
| 48 | 6.90 ± 0.07 | 2.15 ± 0.16 | +4.3 | +1.2 | 1.74 | 135.3 | |
These data are graphically represented in Fig. 7.
The mean of the triplicate intracellular CFU level of the untreated (resting) and IFN-γ–LPS-treated (activated) cells.
The fold change of intracellular CFU level at each time point compared to the intracellular CFU level at 4 h (input).
Nitrite level in the supernatant of these samples as determined by a Griess assay. Shown is a representative experiment of six performed.
DISCUSSION
The interaction of DCs with infectious agents plays a vital role in the initiation of the immune response against a microbe. These potent APCs encounter antigen at the site of infection, traffic to the lymph node, and prime T cells to respond to the microbial antigen (2). The effector T cells migrate back to the site of infection, produce cytokines, activate macrophages, and lyse target cells in an effort to eliminate the microbial agent. In the studies presented here, we demonstrate that DCs interact with live M. tuberculosis bacilli in a manner different from that of Mφ. Mφ are the cells in which M. tuberculosis is believed to live and multiply within the host. However, M. tuberculosis grew equally well within DCs and Mφ, and activated DCs and Mφ were equivalent in their ability to inhibit replication of M. tuberculosis in an NOS2-dependent manner. Our data indicate that, whereas activated Mφ could kill intracellular M. tuberculosis, activated DCs were not mycobacteriocidal and did not eliminate intracellular bacilli.
DCs readily internalized M. tuberculosis bacilli and subsequently displayed phenotypic and functional changes, including the upregulation of various cell surface molecules important in initiating immune responses and the downregulation of phagocytic ability, in addition to producing inflammatory cytokines. These DCs were superior to macrophages in stimulating proliferation and IFN-γ production of mycobacterium-specific T cells (47, 50; this study). Mouse bone marrow-derived DCs infected with M. tuberculosis for 48 h demonstrated increased cell surface expression of ICAM-1, B7.1, and B7.2 molecules (both as percent positive cells and the MFI) while decreasing their phagocytic ability, and this differed from the M. tuberculosis infection of macrophages. These data are consistent with our previous work with M. tuberculosis infection of human PBMC-derived DCs, although the upregulation of cell surface molecules was less profound in the murine system, and with the results reported by Tascon et al. using the tsDC cell line (50). Although the levels of B7 expression were similar between our primary DCs and the tsDC line, we also observed an increase in the level of ICAM-1 after infection, which was not observed using the tsDC cell line (50). A consistent up- or downregulation of MHC class I or II was not observed in our studies. It may be that MHC levels are susceptible to variables such as MOI, the percentage of infected cells, and the number of live (or dead) bacilli per cell. We also did not observe downregulation of MHC class II on macrophages following infection, in either this study or in our study with human DCs and Mφ (24), in contrast to published reports (17, 22, 38).
Inflammatory cytokine production by DCs would be expected to influence the T-cell phenotype primed in the lymph node (29). M. tuberculosis-infected DCs produced low to moderate amounts of IL-12, which would skew the primed T-cell response toward a type 1 (IFN-γ-producing) response. Production of this cytokine was lower in DCs than in infected Mφ. IL-12 production by DCs following infection with microbial pathogens has been reported, although the downregulation of constitutive IL-12 production by DCs following infection with certain pathogens has also been reported (1, 53). DCs infected with Toxoplasma gondii have a refractory period where IL-12 production is low but, upon restimulation, IL-12 production can increase (7). TNF-α production following M. tuberculosis infection was initially higher in DCs compared to Mφ, but the Mφ did not decrease TNF-α production as quickly. This is similar to the pattern seen with M. tuberculosis infection of human DCs (24). IL-10 production was not observed in infected DCs or Mφ for up to 60 h postinfection. Low levels of mRNA were detected and increased by 24 h (Fig. 4C). This downregulatory cytokine may be produced later in infection or may be produced primarily by T cells during this infection.
The fate of a microbe within a DC may affect the presentation of antigen by the DC to naive T cells. The bactericidal capabilities of DCs might be expected to correspond to those of Mφ, given the similarity in the progenitor cells, but studies examining the fate of microorganisms within DCs are rare. DCs encountering a pathogen in tissue, for example, the lung, early in infection are not likely to also encounter specific T-cell-secreting cytokines, such as IFN-γ, to activate the DCs. M. tuberculosis bacilli replicated equally well within unactivated DCs and Mφ. Thus, maturation of the DCs in response to M. tuberculosis infection had little effect on the intracellular survival and growth of the microbe. Although it has been reported that M. tuberculosis H37Rv did not grow within the tsDC line, we consistently observed that untreated murine bone marrow-derived DCs were as permissive for M. tuberculosis growth as bone marrow-derived Mφ (Fig. 7). The discrepancy may be due to differences between primary DCs and an immortalized line. A mature DC would be expected to traffic to the lymph nodes, and our data suggest that such a DC would be carrying live M. tuberculosis bacilli. This may be a mechanism by which M. tuberculosis gains access to the lymph node. Studies in the Leishmania murine model suggest that DCs persist in the lymph node even when infected (36). It has been hypothesized that the host's ability to control an infection is a complex balance between a low number of persistent organisms and the specific effector cells in the immune system (19, 33, 36). Therefore, a continuous supply of antigen from a living bacterium may be advantageous for the priming and maintenance of an effective immune response. In two recent studies, mice vaccinated with BCG- or M. tuberculosis-infected DCs were shown to generate a protective immune response and to reduce the bacterial burden after challenge with M. tuberculosis (11, 50). These studies suggest that targeting DCs in vivo will be an important consideration for vaccine development and design.
In an environment in which T cells or other cells are producing IFN-γ, the DCs would be expected to be activated in a manner similar to the Mφ. Activated Mφ have been shown by various groups to inhibit the growth of intracellular M. tuberculosis bacilli (10, 12, 15) and, more importantly, to kill at least 50% of the intracellular mycobacteria (10) via NOS2-dependent mechanisms. In our studies, activation of Mφ with IFN-γ and LPS also resulted in a 50% reduction in intracellular M. tuberculosis by 48 h postinfection, as determined by CFU in cell lysates. DCs treated with IFN-γ and LPS were capable of restricting the growth of intracellular M. tuberculosis, and this was dependent on NOS2 activity. However, in contrast to Mφ, activated DCs were unable to reduce the intracellular bacterial numbers over time, despite the essentially similar levels of RNI production in DCs and Mφ. In some experiments, there were differences in RNI production at 4 h postinfection (Table 2). Initially, we speculated that a higher amount of RNI early in the infection produced by activated Mφ compared to activated DCs could be responsible for the differential ability of the cells to kill M. tuberculosis. However, analysis of data from a series of experiments indicated that, regardless of the RNI production by DCs at 4 h, including experiments in which the RNI levels were identical to those of the Mφ, the DCs did not reduce the number of input bacteria over time. It is possible that M. tuberculosis bacilli within DCs avoid the killing effects of RNI by persisting within special vacuoles of DCs. Other possibilities for the lack of mycobacterial killing include differences in the phagosome pH, lysosomal enzymes, and reactive oxygen production in DCs compared to Mφ. These possibilities are under investigation.
There have been relatively few studies on the effect of DCs, particularly activated DCs, on intracellular bacteria. DCs have been demonstrated to take up various microbes, including Salmonella spp. (32, 49), Escherichia coli (49), Listeria monocytogenes (21), Borrelia burgdorferi (14, 35), Bordetella bronchiseptica (20), M. bovis BCG (23, 26), Leishmania spp. (18, 36, 37), and Chlamydia spp. (25, 28, 39, 55). Salmonella was reported to survive and replicate within unactivated murine DCs (32), while Chlamydia was killed via phagolysosome fusion (39). Leishmania parasites have been shown to persist within DCs in lymph nodes, suggesting that DCs are impaired in their ability to kill Leishmania (36, 37). Recently, studies indicate that certain parasite infections, such as Trypanosoma cruzi and Plasmodium falciparum, downregulate inflammatory cytokine production and prevent maturation of the DCs (52, 53). These parasites apparently manipulate DCs as an immune evasion strategy.
We hypothesize that the ability of M. tuberculosis to survive, although perhaps not replicate, within activated DCs may be beneficial in priming a T-cell response by mature DCs in the lymph node. Live bacteria are capable of secreting antigens, which are believed to be important in the protective T-cell response against tuberculosis. In addition, it has been suggested that M. tuberculosis forms a pore within the phagosomal membrane which allows access to the cytoplasm for mycobacterial peptides; presumably, priming of MHC class I-restricted CD8 T-cell responses would be more efficient in DCs harboring live, rather than dead, tubercle bacilli (34). An alternative hypothesis is that M. tuberculosis has evolved a strategy to evade killing by the DCs and uses this cell as a vehicle for dissemination from the lung to the lymph nodes and other organs.
ACKNOWLEDGMENTS
This work was supported by NIH RO1 AI37859 (J.L.F.).
We are grateful to Simon Watkins for the electron microscopy images and for assistance in the imaging facility. We thank John Chan and Joel Ernst for invaluable advice, discussion, and critical reading of the manuscript; Heather Joseph for technical assistance; and Joseph Ahearn for use of his flow cytometer. In addition, we thank the members of the Flynn lab for helpful discussion.
REFERENCES
- 1.Ahuja S S, Mummidi S, Malech H L, Ahuja S K. Human dendritic cell (DC)-based anti-infective therapy: engineering DCs to secrete functional IFN-gamma and IL-12. J Immunol. 1998;161:868–876. [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P F, Modlin R L, Ellner J J. T-cell responses and cytokines. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. p. 417. [Google Scholar]
- 4.Behar S M, Dascher C C, Grusby M J, Wang C R, Brenner M B. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonham C A, Lu L, Hoffman R A, Simmons R L, Thomson A W. Generation of nitric oxide by mouse dendritic cells and its implications for immune response regulation. Adv Exp Med Biol. 1997;417:283–290. doi: 10.1007/978-1-4757-9966-8_46. [DOI] [PubMed] [Google Scholar]
- 6.Caruso A M, Serbina N, Klein E, Triebold K, Bloom B R, Flynn J L. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 7.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T-cell stimulatory capacity: T-T help via APC activation J. Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J, Tanaka K, Carroll D, Flynn J L, Bloom B R. Effect of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Kaufmann S H E. Immune mechanisms of protection. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 389–415. [Google Scholar]
- 10.Chan J, Xing Y, Magliozzo R, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demangel C, Bean A G D, Marin E, Feng C G, Kamath A T, Britton W J. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis bacillus Calmette Guerin-infected dendritic cells. Eur J Immunol. 1999;29:1972–1979. doi: 10.1002/(SICI)1521-4141(199906)29:06<1972::AID-IMMU1972>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 13.Dolin P J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull W H O. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 14.Filgueira L, Nestle F O, Rittig M, Joller H I, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 15.Flesh I, Kaufmann S H E. Mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gercken J, Pryjma J, Ernst M, Flad H D. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorak P M, Engwerda C R, Kaye P M. Dendritic cells but not macrophages produce IL-12 immediately following Leishmania donovani. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman C, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman C A, Domann E, Rohde M, Bruder D, Darjii A, Weiss S, Wehland J, Charraborty T, Timmis K N. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamma Z, Gabathuler R, Jefferies W A, de Jong G, Reiner N E. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J Immunol. 1998;161:4882–4893. [PubMed] [Google Scholar]
- 23.Havenith C E G, Breedijk A J, Hoefsmit E C M. Effect of bacillus Calmette-Guerin inoculation on numbers of dendritic cells in bronchoalveolar lavages of rats. Immunobiology. 1992;184:336–347. doi: 10.1016/S0171-2985(11)80591-7. [DOI] [PubMed] [Google Scholar]
- 24.Henderson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 25.Igietseme J U, Perry L L, Ananaba A G, Uriri I M, Ojior O O, Kumar S N, Caldweld H D. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect Immun. 1998;66:1282–1286. doi: 10.1128/iai.66.4.1282-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus-Calmette Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leveton C, Barnass S, Champion B, Lucas S, De Souza B, Nicol M, Banerjee D, Rook G. T-cell mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. 1989;57:390–395. doi: 10.1128/iai.57.2.390-395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu H, Zhong G. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect Immun. 1999;67:1763–1769. doi: 10.1128/iai.67.4.1763-1769.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz M, Girolomoni G, Ricciardi-Castagnoli P. The role of cytokines in functional regulation and differentiation of dendritic cells. Immunbiology. 1996;195:431–455. doi: 10.1016/S0171-2985(96)80014-3. [DOI] [PubMed] [Google Scholar]
- 30.MacMicking J D, Nathan C, Hom G, Chartrain N, Trumbauer M, Stevens K, Xie Q-W, Sokol K, Fletcher D S, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 31.Mahnke K, Becher E, Ricciardi-Castagnoli P, Luger T A, Schwarz T, Grabbe S. CD14 is expressed by subsets of murine dendritic cells and upregulated by lipopolysaccharide. Adv Exp Med Biol. 1997;417:145–159. doi: 10.1007/978-1-4757-9966-8_25. [DOI] [PubMed] [Google Scholar]
- 32.Marriott I, Hammond T G, Thomas E K, Bost K L. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokines. Eur J Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Matzinger P. Memories are made of this? Nature. 1994;369:605–606. doi: 10.1038/369605a0. [DOI] [PubMed] [Google Scholar]
- 34.Mazzaccaro R J, Gedde M, Jensen E R, van Santem H M, Ploegh H L, Rock K L, Bloom B R. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1996;93:11786–11791. doi: 10.1073/pnas.93.21.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbow M L, Zeidner N, Panella N, Titus R G, Piesman J. Borrelia burdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect Immun. 1997;65:3386–3390. doi: 10.1128/iai.65.8.3386-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moll H, Flohe S, Rollinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol. 1995;25:693–699. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 37.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 38.Noss E H, Harding C V, Boom W H. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201:63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 39.Ojcius D M, Bravo de Alba Y, Kanellopoulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of chlamydia by dendritic cells and stimulation of chlamydia-specific T cells J. Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 40.Orme I, Collins F. Adoptive protection of the Mycobacteria tuberculosis-infected lung. Cell Immunol. 1984;84:113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- 41.Reise-Sousa C, Stahl P D, Austyn J M. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–518. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rescigno M, Winzler C, Delia D, Mutini C, Lutz M, Ricciardi-Castagnoli P. Dendritic cell maturation is required for initiation of the immune response. J Leukoc Biol. 1997;61:415–421. [PubMed] [Google Scholar]
- 43.Rook G A, Champion B R, Steele J, Varey A M, Stanford J L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985;59:414–420. [PMC free article] [PubMed] [Google Scholar]
- 44.Rook G A W, Rainbow S. An isotope incorporation assay for the antimycobacterial effects of human monocytes. Ann Immunol. 1981;132D:281–289. [Google Scholar]
- 45.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus IL-4 and downregulated by tumor necrosis factor-α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serbina N V, Flynn J L. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67:3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman R, Swanson J. The endocytic activity of dendritic cells. J Exp Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Svensson M, Stockinger B, Wick M J. Bone-marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 50.Tascon R E, Soares C S, Ragno S, Stavropoulos E, Hirst E M A, Colston M J. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99:473–480. doi: 10.1046/j.1365-2567.2000.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Protection against Mycobacterium tuberculosis infection by CD8 T cells requires production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urban B C, Perfuson D J P, Pain A, Willcox N, Plebanski M, Austyn J M, Roberts D J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 53.van Overtvelt L, Vanderheyde N, Verhasselt V, Ismaili J, de Vos L, Goldman M, Willems F, Vray B. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect Immun. 1999;67:4033–4040. doi: 10.1128/iai.67.8.4033-4040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia W, Pinto C, Kradin R. The antigen-presenting activities of Ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med. 1995;181:1275–1283. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang D, Yang X, Lu H, Zhong G, Brunham R C. Immunity to Chlamydia trachomatis mouse pneumonitis induced by vaccination with live organisms correlates with early granulocyte-macrophage colony-stimulating factor and interleukin-12 production and with dendritic cell-like maturation. Infect Immun. 1999;67:1606–1613. doi: 10.1128/iai.67.4.1606-1613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]