Abstract
Richter Transformation (RT) is the phenomenon of progression from Chronic Lymphocytic Leukemia (CLL) into an aggressive Large-Cell Lymphoma and is typically characterized by diffuse lymphadenopathy combined with the classical “B symptoms”. While rare, other causes of sudden onset diffuse lymphadenopathy in patients with CLL can occur; one of the rarest being necrotic herpes simplex lymphadenitis. We report a case that presented similarly to Richter Transformation including PET-CT scan findings consistent with RT but was histologically proven to be necrotic herpes simplex lymphadenitis. We identified less than 20 reported cases of this phenomenon in English language literature. Our patient was successfully treated with appropriate antiviral therapy due to timely recognition of the correct disease process. Our case reinforces the importance of maintaining diagnostic suspicion when approaching sudden onset lymphadenitis in this patient population.
Keywords: Herpes simplex virus, Necrosis, Richter transformation, Leukemia, Lymphadenopathy
Highlights
-
•
Richter's transformation (RT) represents transformation into aggressive lymphoma.
-
•
HSV can rarely present as necrotic lymphadenitis.
-
•
Cases of necrotic HSV lymphadenitis have been mistaken for RT.
Introduction
Richter Transformation is characterized by the development of aggressive Large-Cell Lymphoma in a patient with underlying CLL or Small Lymphocytic Lymphoma (SLL). This clinical transformation is rare, occurring in around 5% of CLL/SLL cases [1], [2], [3]. Evidence of Richter Transformation is usually denoted by rapid clinical deterioration including sudden increased lymphadenopathy with “B Symptoms” (fevers, night sweats, weight loss), combined with histological evidence of aggressive Diffuse Large B-Cell Lymphoma (DLBCL) [4], [5]. Richter Transformation has typically been associated with a poor prognosis, exhibiting a median survival of 5–8 months [6], [7]. Treatment of Richter Transformation depends on the histological features but usually consists of aggressive chemotherapeutic regimens with the possibility of Hematopoietic Cell Transplantation (HCT) [8], [9]. Lymphadenitis secondary to Herpes Simplex Viral (HSV) infection is an exceedingly rare condition, with less than 20 cases in CLL/SLL patients reported in the literature to date [10], [11], [12], [13], [14], [15]. The clinical presentation of HSV lymphadenitis is characterized by sudden widespread or local lymphadenopathy combined with fevers and sweating, often causing suspicion for Richter Transformation. Here we report a case of biopsy proven necrotic HSV lymphadenitis disguised as Richter Transformation in a patient with CLL who had not received treatment for the preceding 5 years.
Case report
An 86-year-old male with a past medical history of Hypertension, Chronic Kidney Disease (CKD) and CLL presented to the emergency department with a one-week history of fevers, progressive weakness, intermittent night sweats and decreased appetite. Upon presentation the patient's partner also reported that the patient's mental status had been deteriorating to the point that he could only answer yes/no questions, a major regression from his baseline. A week prior the patient visited his primary care provider who noted that the patient's creatinine was more than double his baseline, prompting the physician to order a computed tomography (CT) scan of the abdomen which showed extensive retroperitoneal lymphadenopathy. The patient had previously been diagnosed with Waldenstrom’s macroglobulinemia which was treated Rituximab, and was subsequently diagnosed with CLL. Given clinical stability of his CLL, the patient had not yet undergone treatment. The patient was admitted for altered mental status and likely uremic encephalopathy.
The physical exam was remarkable for firm lymphadenopathy in the bilateral axilla, supraclavicular and inguinal regions. Labs on admission were significant for a wbc of 9.58 K /uL (normal range 4–11 K/uL) with a neutrophilic predominance, Hb of 10.3 g/dL (normal range 13.5–18 g/dL), Platelets of 91 k/uL (normal range 150–400 K /uL), Cr of 9.23 mg/dL (baseline ∼2, normal range 0.6–1.5 mg/dL), BUN of 145 mg/dL (normal range 8–20 mg/dL) with a protein gap of 5.3 g/dL (normal is >4 g/dL). The initial LDH level was 313 U/L (normal range 50–242 U/L) and Immunoglobulin levels were as follows; IgG of 2117 mg/dL (normal range 610–1660 mg/dL), IgA of 1864 mg/dL (normal range 84–499 mg/dL) and IgM of 83 mg/dL (normal range of 35–242 mg/dL) with a normal kappa/lambda free light chain ratio of 1.56 (normal range 0.26–1.65). CT Scan of the chest, abdomen and pelvis showed diffuse lymphadenopathy of the mediastinal, subclavian, axillary, retroperitoneal and pelvic lymph nodes. The Hematology/Oncology team was consulted in the setting of his CLL and concern for Richter Transformation.
Serum and Urine Protein Electrophoresis (SPEP and UPEP) with Immunofixation identified IgG kappa and weak IgA lambda bands, consistent with underlying CLL/SLL. Positron-emission tomography-computed tomography (PET-CT) scan exhibited the previously identified diffuse lymphadenopathy with high levels of fludeoxyglucose (FDG) avidity, consistent with metabolically active disease and Richter Transformation (Fig. 1, Fig. 2). Bone marrow biopsy exhibited a cell population consistent with CLL/SLL with fluorescence in-situ hybridization (FISH) studies showing TP53 mutations, favoring a poor prognosis.
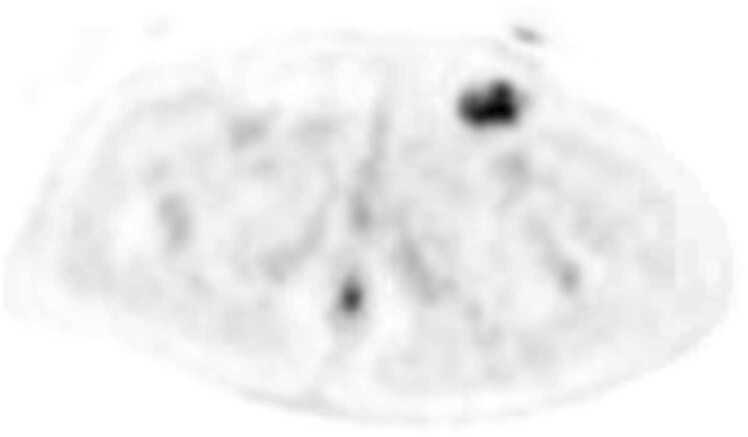
Fig. 1.
This is a PET-CT, transverse view, of the pelvis at the level of the pubic symphysis exhibiting FDG-Avidity of the left inguinal lymph node, from which the consequential diagnostic biopsy was taken.
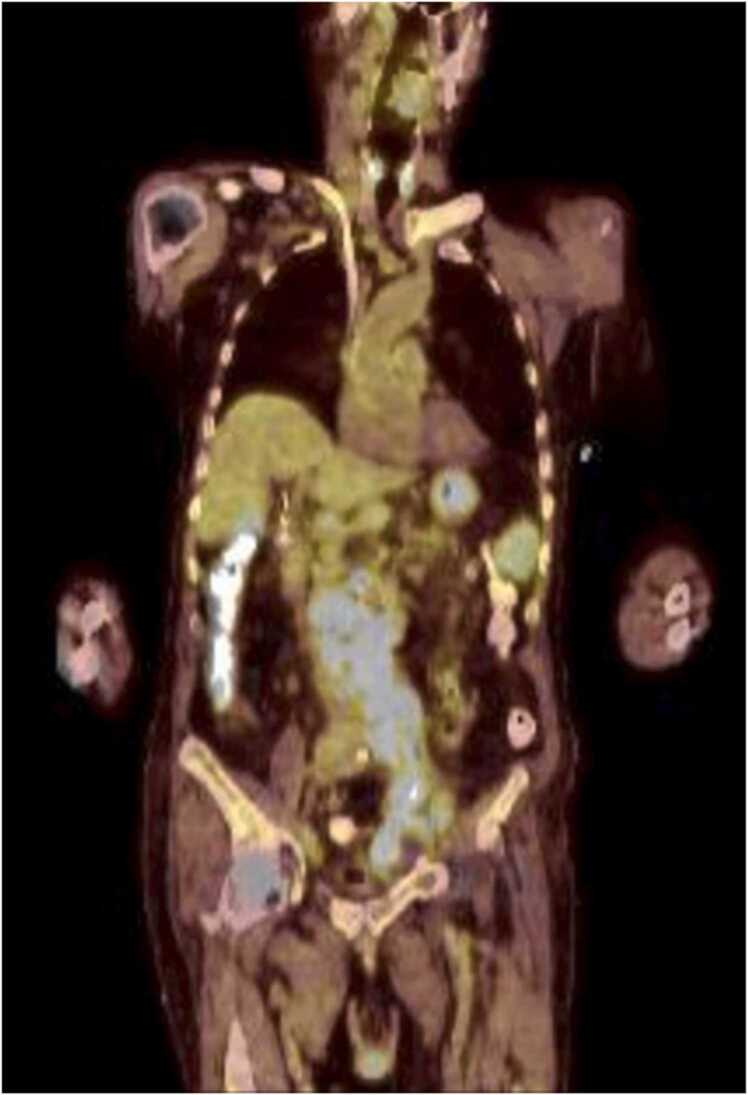
Fig. 2.
This is a PET-CT, coronal view, exhibiting increased FDG-Avid (light blue regions) lymph nodes in the retroperitoneum and right inguinal region.
The final investigatory measure was a biopsy of the left inguinal FDG-avid lymph node. The pathology report of the biopsied lymph node reported necrotizing acute inflammation, with negative staining for fungal or bacterial organisms. This initial biopsy showed no evidence of large cell transformation, prompting another lymph node biopsy for further investigation. Subsequent stains of the new biopsied node exhibited HSV-1 and HSV-2 positivity, confirming the diagnosis of necrotic HSV lymphadenitis (Fig. 3).
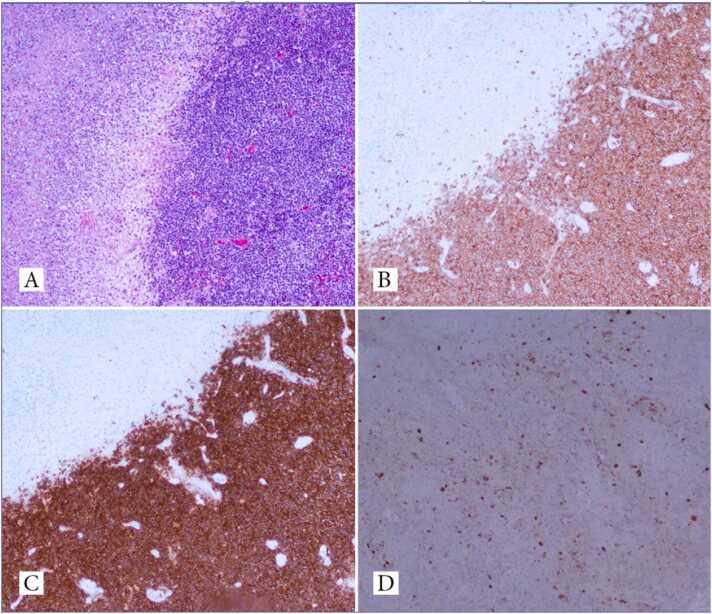
Fig. 3.
Lymph node biopsy tissue showing diffuse small lymphocytes with large necrotic areas (A). Positivity for CD5 (B) and CD20 (C) and HSV-1 and HSV-2 (D) on immunohistochemical studies.
The patient was initiated on Acyclovir (400 mg daily, intravenously) for 4 days and discharged to a sub-acute rehab on Valacyclovir (500 mg orally, daily) for an additional 17 days to complete a 21 day total course of antiviral treatment. Upon discharge, the patient's lymphadenopathy and clinical status were already improving. Treatment of the patients CLL was deferred until after the necrotic HSV lymphadenitis had been adequately treated, with plans to treat the CLL with a bruton’s tyrosine kinase (BTK) inhibitor in the form of acalabrutinib.
Discussion
We have described a case of suddenly progressive lymphadenopathy combined with fevers and night sweats sparking clinical and radiographic suspicion for Richter Transformation. The diagnosis was subsequently histologically proven to be necrotic HSV lymphadenitis. Cases demonstrating PET-CT findings consistent with Richter Transformation turning out to be necrotic HSV lymphadenitis are exceedingly rare.
While generalized lymphadenopathy in CLL patients is not the rarest of occurrences, the differential diagnosis is broad and treatment of the many possibilities differs greatly. One large scale study investigated the etiology of rapidly progressive lymphadenopathy in greater than 250 CLL/SLL patients, including the collection of over 3000 lymph node biopsies. Only 3 of these biopsies contained active HSV, outlining the rarity of this infection [16]. Other differential diagnoses of rapidly progressive lymphadenopathy in this patient population include progression of the CLL/SLL (including Richter Transformation) versus infection with fungal, bacterial or parasitic pathogens. The most common of the differentials is progression of the underlying disease, but as proven by our case it is important to keep other possibilities in mind regardless of how clear the clinical picture may seem.
Though CLL/SLL patients commonly encounter opportunistic infections, the predisposition to these infections is usually immunosuppression in the setting of chemotherapy for the underlying disease. Our case is significant due to the fact that the patient was not on any immunosuppressive treatment, and was receiving no CLL therapy in any form. The proposed mechanism by which HSV infects CLL cells is facilitation of viral entry by CLL cell surface receptors. In non-diseased cells, two cell surface proteins named HveA and HveC facilitate viral entry into the cell [17]. CLL cells have been found to express heightened proportions of HveA on the cell surface, facilitating the entry of HSV into the cells [18]. This could explain the occurrence, though rare, of this phenomenon in patients with CLL.
Our patient was treated with the appropriate antiviral therapy following their diagnosis, with improvement of the generalized lymphadenopathy and infectious symptoms. Given the rarity of necrotic HSV lymphadenitis, the first lymph node biopsy tissue was not stained for HSV-1 and 2, but was instead stained for more common fungal and bacterial pathogens. Only after final pathology of the first biopsy tissue did not indicate any definitive cause, including lack of CLL progression or Richter Transformation, was a second biopsy performed and stained for HSV 1 and 2. Our case underscores the importance of maintaining clinical suspicion for rare causes of lymphadenopathy in this patient population, especially in the setting of the known avidity of HSV entry into CLL cells. Prompt recognition of HSV lymphadenitis confers very favorable treatment outcomes.
Funding
The authors have no sources of funding to disclose.
CRediT authorship contribution statement
Smith, Thakker, Levine and Kondo contributed to the conceptualization, literature review and writing of the case report. Marabti, Freid and Hosseini contributed to the editing, literature review and the figures of the case report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Tsimberidou A.M., Keating M.J. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103(2):216–228. doi: 10.1002/cncr.20773. [DOI] [PubMed] [Google Scholar]
- 2.Robertson L.E., Pugh W., O'Brien S., Kantarjian H., Hirsch-Ginsberg C., Cork A., et al. Richter's syndrome: a report on 39 patients. J Clin Oncol. 1993;11(10):1985–1989. doi: 10.1200/JCO.1993.11.10.1985. [DOI] [PubMed] [Google Scholar]
- 3.Rossi D., Cerri M., Capello D., Deambrogi C., Rossi F.M., Zucchetto A., et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol. 2008;142(2):202–215. doi: 10.1111/j.1365-2141.2008.07166.x. [DOI] [PubMed] [Google Scholar]
- 4.Harousseau J.L., Flandrin G., Tricot G., Brouet J.C., Seligmann M., Bernard J. Malignant lymphoma supervening in chronic lymphocytic leukemia and related disorders. Richter's syndrome: a study of 25 cases. Cancer. 1981;48(6):1302–1308. doi: 10.1002/1097-0142(19810915)48:6<1302::aid-cncr2820480609>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Tsimberidou A.M., O'Brien S., Khouri I., Giles F.J., Kantarjian H.M., Champlin R., et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24(15):2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- 6.Abrisqueta P., Delgado J., Alcoceba M., Oliveira A.C., Loscertales J., Hernández-Rivas J.A., et al. Clinical outcome and prognostic factors of patients with Richter syndrome: real-world study of the Spanish Chronic Lymphocytic Leukemia Study Group (GELLC) Br J Haematol. 2020;190(6):854–863. doi: 10.1111/bjh.16748. [DOI] [PubMed] [Google Scholar]
- 7.Bento L., Díaz-López A., Barranco G., Martín-Moreno A.M., Baile M., Martín A., et al. New prognosis score including absolute lymphocyte/monocyte ratio, red blood cell distribution width and beta-2 microglobulin in patients with diffuse large B-cell lymphoma treated with R-CHOP: Spanish Lymphoma Group Experience (GELTAMO) Br J Haematol. 2020;188(6):888–897. doi: 10.1111/bjh.16263. [DOI] [PubMed] [Google Scholar]
- 8.Eyre T.A., Riches J.C., Patten P.E.M., Walewska R., Marr H., Follows G., et al. Richter transformation of chronic lymphocytic leukaemia: a British Society for Haematology Good Practice Paper. Br J Haematol. 2022;196(4):864–870. doi: 10.1111/bjh.17882. [DOI] [PubMed] [Google Scholar]
- 9.Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol., 32(1); 2021. pp. 23–33. 〈DOI: 10.1016/j.annonc.2020.09.019〉. Epub 2020 Oct 19. PMID: 33091559. [DOI] [PubMed]
- 10.Hodgson Y.A., Jones S.G., Knight H., Sovani V., Fox C.P. Herpes simplex necrotic lymphadenitis masquerading as Richter's transformation in treatment-naive patients with chronic lymphocytic leukemia. J Hematol. 2019;8(2):79–82. doi: 10.14740/jh517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klepfish A., Vaknine H., Schattner A. Never say never: unexpected herpes lymphadenitis. Lancet. 2014;384(9954):1640. doi: 10.1016/S0140-6736(14)61466-5. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J.P., Warnke R.A. Herpes lymphadenitis in association with chronic lymphocytic leukemia. Cancer. 1999;86(7):1210–1215. [PubMed] [Google Scholar]
- 13.Salem A., Loghavi S., Khoury J.D., Agbay R.L., Jorgensen J.L., Medeiros L.J. Herpes simplex infection simulating Richter transformation: a series of four cases and review of the literature. Histopathology. 2017;70(5):821–831. doi: 10.1111/his.13137. [DOI] [PubMed] [Google Scholar]
- 14.Parmar V., Bayya M., Kak V. Herpes simplex virus causing necrotizing granulomatous lymphadenitis. Cureus. 2022;14(3) doi: 10.7759/cureus.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierre C., Jaubert D., Carloz E., Duval P., Boudon A. Massive necrotizing adenitis complicating a disseminated herpes simplex virus 2 infection in chronic lymphoid leukemia. Ann Pathol. 1991;11(1):31–35. [PubMed] [Google Scholar]
- 16.Bowen D.A., Rabe K.G., Schwager S.M., Slager S.L., Call T.G., Viswanatha D.S., et al. Infectious lymphadenitis in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma: a rare, but important, complication. Leuk Lymphoma. 2015;56(2):311–314. doi: 10.3109/10428194.2014.914202. [DOI] [PubMed] [Google Scholar]
- 17.Krummenacher C., Rux A.H., Whitbeck J.C., Ponce-de-Leon M., Lou H., Baribaud I., et al. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol. 1999;73(10):8127–8137. doi: 10.1128/JVI.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eling D.J., Johnson P.A., Sharma S., Tufaro F., Kipps T.J. Chronic lymphocytic leukemia B cells are highly sensitive to infection by herpes simplex virus-1 via herpesvirus-entry-mediator A. Gene Ther. 2000;7(14):1210–1216. doi: 10.1038/sj.gt.3301241. [DOI] [PubMed] [Google Scholar]