Abstract
Background
Guidelines for oligometastatic breast cancer (OMBC) propagate multimodality treatment including polychemotherapy and local ablative treatment (LAT) of all lesions. The aim of this approach is prolonged disease remission, or even cure. Long-term outcomes in OMBC and factors associated with prognosis are largely unknown, due to the rarity of this condition. We report overall survival (OS), event-free survival (EFS), and prognostic factors in a large real-world cohort of patients with OMBC.
Methods
Patients with breast cancer and 1–3 distant metastatic lesions, treated in the Netherlands Cancer Institute between 1997 and 2020, were identified via text mining of medical files. We collected patient, tumor and treatment characteristics. The Kaplan-Meier method was used to calculate OS and EFS estimates, and Cox regression analyses to assess prognostic factors.
Results
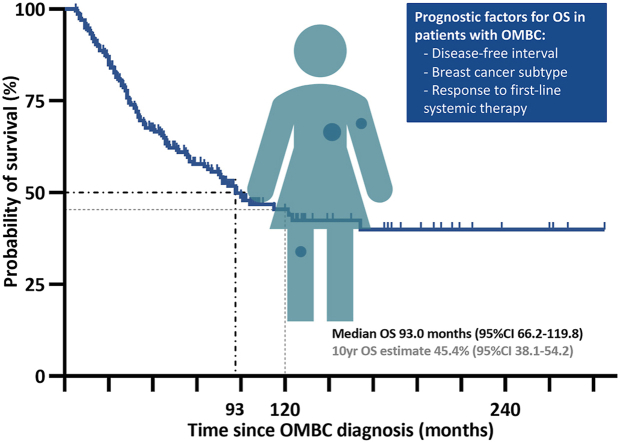
The cohort included 239 patients, of whom 54% had ERpos/HER2neg, 20% HER2pos and 20% triple negative disease. Median follow-up was 88.0 months (95% confidence interval (CI) 82.9–93.1) during which 107 patients died and 139 developed disease progression/recurrence; median OS was 93.0 months (95%CI 66.2–119.8). Factors associated with OS in multivariable analysis were subtype, disease-free interval and radiologic response to first-line systemic therapy; LAT was associated with EFS, but not OS.
Conclusions
In this large real-world cohort of patients with OMBC, OS and EFS compare favorably to survival in the general MBC population. Radiologic complete response to first-line systemic therapy was associated with favorable OS and EFS, indicating the importance of early optimal systemic therapy. The value of LAT in OMBC requires further study.
Keywords: Oligometastasis, Breast cancer, Chemotherapy, Local ablative therapy, Multimodality treatment
Graphical abstract
Highlights
-
•
Patients with OMBC have favorable prognosis compared to the general MBC population.
-
•
Complete response to first-line systemic therapy is associated with better OS and EFS.
-
•
Optimizing systemic therapy for patients with OMBC should be a research priority.
-
•
The value of local ablative therapy in OMBC is unclear and requires further study.
Abbreviations
- (O)MBC
(Oligo)metastatic breast cancer
- ASCO
American Society of Clinical Oncology
- BC
Breast cancer
- CAP
College of American Pathologists
- CI
Confidence interval
- DFI
Disease-free interval
- EFS
Event-free survival
- EORTC
European Organisation for Research and Treatment of Cancer
- ER
Endocrine receptor
- ESTRO
European Society for Radiotherapy and Oncology
- FDG-PET
Fluorodeoxyglucose-positron emission tomography
- Gy
Gray
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- IQR
Interquartile range
- LAT
Local ablative therapy
- MARI
Marking axillary lymph nodes with radioactive iodine seeds
- MWA
Microwave ablation
- NED
No evidence of disease
- NKI-AVL
Netherlands Cancer Institute
- OS
Overall survival
- PFS
Progression-free survival
- rCR
Radiological complete response
- RCT
Randomized controlled trial
- RFA
Radiofrequency ablation
- rPD
Radiological progressive disease
- SABR
Stereotactic ablative radiotherapy
- SN
Sentinel node
- TN
Triple negative
- WHO
World Health Organization
1. Introduction
Metastatic breast cancer (MBC) is almost invariably an incurable disease for which patients may receive therapy with a palliative intent. Median overall survival (OS) varies per breast cancer subtype from one to four years, but a subset of MBC patients survives long-term (>10 years) [[1], [2], [3]]. Limited disease burden, commonly referred to as oligometastatic breast cancer (OMBC), is an independent prognostic factor for long-term survival [[4], [5], [6]]. No uniform definition to classify OMBC exists, but a prevailing characterization is a maximum of 3–5 metastatic lesions, all amenable for local ablative therapy (LAT) [7].
Given the limited spread of oligometastatic disease, there is a potential for cure if all metastases receive LAT and, as such, further spread of the disease is halted [8]. For breast cancer, treatment guidelines recommend a so-called multimodality approach that includes LAT and systemic therapy, tailored to the individual patient, to treat both macroscopic and microscopic disease [9,10].
The potential benefit of LAT originated in retrospective, observational studies [8]. SABR-COMET was the first randomized study on the subject. The study included 99 patients with different tumor types, of whom 18 breast cancer, and demonstrated a clear OS benefit of the addition of stereotactic ablative radiotherapy (SABR) to standard of care systemic therapy [11]. The more recent NRG-BR002 study included 129 patients with OMBC and found no benefit of metastasis-directed therapy in terms of progression-free survival (PFS) or OS [12]. These results suggest a differential effect of LAT for different tumor types; the debate on its value in OMBC is ongoing.
Systemic therapy is a key component of OMBC treatment, but it is unclear which systemic regimen is optimal for the individual patient with OMBC. Maximal systemic therapy, as proposed by the current guidelines, resembles a (neo-)adjuvant approach rather than a palliative approach and may include combination chemotherapy, targeted therapy and endocrine therapy, depending on breast cancer subtype and previously received treatments. This approach clearly leads to added toxicity, but might be worthwhile with the prospect of significantly prolonged survival or even cure.
In summary, the optimal treatment (both local and systemic) for patients with OMBC has yet to be determined and long-term outcomes for this patient group are largely unknown. In this real-world cohort study, we aim to explore treatment patterns and prognostic factors relevant in OMBC. We further aim to estimate long-term OS and event-free survival (EFS) in this population.
2. Material and methods
2.1. Patient selection
In this single center study at the Netherlands Cancer Institute, we performed automated text mining on all available electronic medical records up until July 31, 2020. Text mining consisted of a simple text search, using Microsoft SQL Server Management Studio. Breast cancer patients whose record contained the word ‘oligo’ were selected for eligibility screening. Patients with a maximum of three distant metastases (according to the 8th edition of the American Joint Committee on cancer staging system) at first diagnosis of MBC, were included in the cohort. Lymph nodes within a single field suitable for high dose radiotherapy (e.g., 3 nodes within head and neck level IV), were considered one lesion; all other lesions were counted separately based on available imaging in the medical file (without formal review of imaging). The Institutional Review Board of the Netherlands Cancer Institute approved the study and waived informed consent.
2.2. Data collection
We collected patient, tumor and treatment characteristics as well as recurrence and survival dates from the medical files and through the municipality registry. Patients were classified as having either synchronous (distant metastases ≤6 months of primary diagnosis) or metachronous (distant metastases >6 months after primary diagnosis) OMBC [6]. For patients with metachronous OMBC, disease-free interval (DFI) was defined as the interval between diagnosis of the primary tumor or last locoregional recurrence and diagnosis of distant metastases. If either the estrogen or progesterone receptor were scored positive according to Dutch guidelines (positive stain in ≥10% tumor cells), the tumor was classified as endocrine receptor positive (ERpos) [13]. Human epidermal growth factor receptor 2 (HER2) status was scored according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [14]. We recorded initial systemic therapy for OMBC, which included adjuvant therapy after local treatment. Radiologic response to initial systemic therapy was defined as complete response with disappearance of all visible lesions (rCR), any response (not complete) or progressive disease (rPD) based on the radiologic report. If a patient also underwent local therapy for metastatic disease, the radiologic response prior to local therapy was noted. For distant metastases, we classified local therapy as ablative (LAT) if the patient underwent radical surgery or radiofrequency and/or microwave ablation. For radiotherapy, an equivalent dose of 2 Gy (Gy) per fraction of >50Gyα/β = 10 was classified as ablative.
2.3. Endpoint definitions
Overall survival was defined as time from OMBC diagnosis until death by any cause. Event-free survival was defined as time from OMBC diagnosis until recurrence of disease (in patients who were rendered no evidence of disease (NED) by initial OMBC-therapy) or until disease progression (in patients who were not rendered NED) based on the medical file.
2.4. Statistical analysis
Data cut-off for the current analyses was May 2022. Descriptive statistics (median, interquartile range (IQR)) were used to describe baseline characteristics. Overall and event-free survival estimates were calculated with the Kaplan-Meier method. The effect of different prognostic factors was assessed with Cox regression analysis. Variables of interest included age at OMBC diagnosis, T-stage and N-stage at primary diagnosis, year of OMBC diagnosis (1997–2012 versus 2013–2020), subtype (ERpos/HER2neg versus HER2 positive (HER2pos) and triple negative (TN)), DFI (synchronous metastases versus short (≤24 months) and long (>24 months) DFI), number of lesions (1 versus 2–3), bone-only disease, presence of visceral disease, node-only disease, proof of metastatic disease (tissue biopsy or cytology), chemotherapy regimen (anthracycline or taxane-based regimens versus capecitabin-vinorelbin and non-anthracycline/non-taxane-based regimens), radiologic response to first-line systemic therapy (any response versus rCR and rPD) and LAT for all metastatic disease. Potential prognostic factors were first tested in univariable analysis. If the p-value was ≤0.10, that factor was entered in the multivariable model. We performed analyses in the whole cohort, and in two subgroups: patients with metachronous disease (primarily to investigate the effect of locoregional recurrence at time of OMBC diagnosis) and patients diagnosed from 2013 onwards (to evaluate potential immortal time bias and the effect of changing therapies over time).
3. Results
3.1. Patients
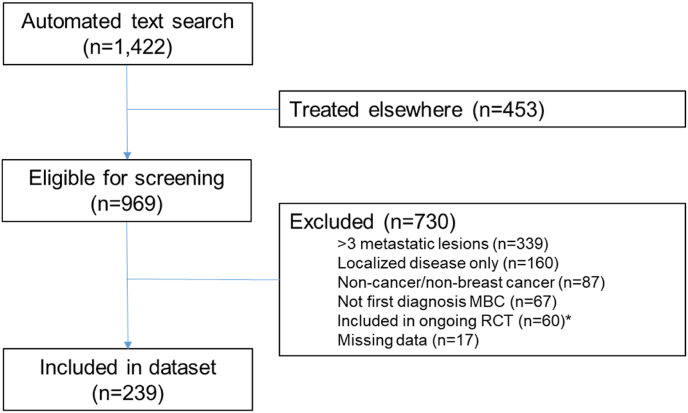
The automated text search yielded 1422 cases, of which 969 were treated at the NKI-AVL. Following chart review, 730 cases were excluded, mainly due to too extensive metastatic disease (n = 339) or the absence of distant metastases (n = 160). The final cohort consisted of 239 patients (Fig. 1).
Fig. 1.
Flow chart of patient selection.
Median age at OMBC diagnosis was 49.0 years (IQR 42.0–58.0). Year of OMBC diagnosis varied from 1997 to 2020, 81% of patients were diagnosed after 2012. Fifty-four percent of patients had ERpos/HER2neg disease, 20% HER2pos and 20% TN. Subtype was available for both primary tumor as well as metastatic lesion in 106 patients, and in 14 of these patients, there was a discrepancy between subtype of the primary tumor and that of the metastasis (Supplementary Table S1). OMBC was metachronous in 126 patients (53%), and of these, 60 patients had concurrent breast and/or locoregional recurrence. There was proof of metastatic disease in 64% of patients (Table 1).
Table 1.
Patient and tumor characteristics.
| N | % | ||
|---|---|---|---|
| TOTAL | 239 | 100 | |
| Female sex |
237 |
99.2 |
|
| Characteristics at first BC diagnosisa | |||
| Age at diagnosis in years, median (IQR) | 45,0 (39,0–54,0) | ||
| Year of diagnosis | 1990–2004 | 48 | 20.1 |
| 2005–2012 | 81 | 33.9 | |
| 2013–2020 | 110 | 46.0 | |
| T-stageb | Tx | 3 | 1.3 |
| T1 | 53 | 22.2 | |
| T2 | 97 | 40.6 | |
| T3 | 44 | 18.4 | |
| T4 | 32 | 13.4 | |
| Unknown | 10 | 4.2 | |
| N-stage | N0 | 62 | 25.9 |
| N1 | 70 | 29.3 | |
| N2 | 36 | 15.1 | |
| N3 | 61 | 25.5 | |
| Unknown | 10 | 4.2 | |
| Grade | 1 | 11 | 4.6 |
| 2 | 94 | 39.3 | |
| 3 | 92 | 38.5 | |
| Unknown | 42 | 17.6 | |
| Subtype (primary tumor) | ERpos/HER2neg | 123 | 51.5 |
| HER2pos | 48 | 20.1 | |
| Triple negative | 46 | 19.2 | |
|
Unknownc |
22 |
9.2 |
|
| Characteristics at OMBC diagnosis | |||
| Age at diagnosis in years, median (IQR) | 49,0 (42,0–58,0) | ||
| Year of diagnosis | 1997–2012 | 80 | 33.5 |
| 2013–2020 | 159 | 66.5 | |
| WHO performance score | 0 | 152 | 63.6 |
| 1 | 38 | 15.9 | |
| Unknown | 49 | 20.5 | |
| Synchronous disease | 113 | 47.3 | |
| Metachronous disease | Short DFI (metastases ≤24 months of primary | 37 | 15.5 |
| Long DFI (metastases > 24 months of primary) | 89 | 37.2 | |
| DFI in months, median (IQR) (in case of metachronous disease, n = 126)d | 45,0 (23,8–85,0) | ||
| Simultaneous breast and/or locoregional recurrence (in case of metachronous disease, n = 126) | 60 | 25.1 | |
| Number of metastases | 1 | 148 | 61.9 |
| 2 | 60 | 25.1 | |
| 3 | 31 | 13.0 | |
| FDG-PET for staging | 194 | 81.2 | |
| Proof of distant metastatic diseasee | 154 | 64.4 | |
| Subtype (metastatic lesion) | ERpos/HER2neg | 75 | 31.4 |
| HER2pos | 18 | 7.5 | |
| Triple negative | 19 | 7.9 | |
| Unknown | 127 | 53.1 | |
| Subtype - summary (used for analysis: metastatic lesion if available, otherwise primary tumor) | ERpos/HER2neg | 129 | 54.0 |
| HER2pos | 47 | 19.7 | |
| Triple negative | 47 | 19.7 | |
| Unknown | 16 | 6.7 | |
| Visceral disease | 59 | 24.7 | |
| Bone-only disease | 109 | 45.6 | |
| Node-only disease | 55 | 23.0 | |
BC, breast cancer; DFI, disease-free interval; ER, estrogen receptor; FDG-PET, fluorodeoxyglucose-positron emission tomography; HER2, human epidermal growth factorreceptor 2; IQR, interquartile range; OMBC, oligometastatic breast cancer; WHO, World Health Organization.
Moment of first BC diagnosis equals moment of OMBC diagnosis for patients with synchronous disease.
In case of neo-adjuvant systemic therapy: c- or yp-stage (whichever was highest); in case of no neo-adjuvant systemic therapy: p-stage.
1/22 unknowns was diagnosed after 1-1-2005, the moment HER2-testing became routinely available in the Netherlands.
Defined as the interval between primary tumor and diagnosis of distant metastases; in case of intercurrent locoregional recurrence without distant metastases: interval between locoregional recurrence and distant metastases.
By histology (tissue biopsy or cytology).
MBC, metastatic breast cancer; RCT, randomized controlled trial.
-
∗
ClinicalTrials.gov identifier NCT01646034, ongoing RCT on the added effect of high-dose chemotherapy for patients with oligometastatic breast cancer harboring homologous recombination deficiency
3.2. Treatment
Two-hundred patients received any local therapy for all metastases; in 111 patients, local therapy was ablative according to our criteria. In earlier years, SABR was not available and high dose radiotherapy was delivered with conventional fractionated radiotherapy in the range of 30 × 2Gy to 17 × 3Gy. With the introduction of SABR, more hypofractionated schemes were used and doses in the range of 1 × 24Gy to 8 × 7.5Gy could safely be achieved. Nearly all patients (n = 236, 98.7%) received some form of systemic therapy, of whom the majority chemotherapy (n = 195, 81.6%) and/or endocrine therapy (n = 166, 69.5%). Of the patients who received no chemotherapy, the majority had ERpos/HER2neg disease (32/44 patients). The majority of chemotherapy regimens contained anthracyclines and/or taxanes (n = 157, 65.7%), although patients with HER2-positive disease often received an anthracycline-free regimen (Supplementary Table S2). Radiologic response to first-line systemic treatment was evaluable in 185 patients, of whom 40 achieved an rCR and 21 had rPD (Table 2).
Table 2.
Treatment characteristics.
| N | % | ||
|---|---|---|---|
|
TOTAL |
239 |
100 |
|
| BC therapy prior to OMBC diagnosis (metachronous population only, n = 126) | |||
| Surgical treatment breast | Breast-conserving | 52 | 21.8 |
| Ablative | 71 | 29.7 | |
| Nonea | 3 | 1.3 | |
| Surgical treatment locoregional disease | SN | 40 | 16.7 |
| MARI | 6 | 2.5 | |
| ALND | 67 | 28.0 | |
| None | 13 | 5.4 | |
| Locoregional radiotherapy | Chest only or nodes only | 38 | 15.9 |
| Chest + axilla and/or other nodes | 45 | 18.8 | |
| None | 43 | 18.0 | |
| Systemic treatment | Any | 94 | 39.3 |
| Chemotherapy | 88 | 36.8 | |
| Endocrine therapy | 65 | 27.2 | |
|
HER2-targeted therapy |
12 |
5.0 |
|
| At OMBC diagnosis | |||
| Surgical treatment breast | Breast-conserving | 60 | 25.1 |
| Ablative | 34 | 14.2 | |
| Re-surgery | 17 | 7.1 | |
| Surgical treatment locoregional disease | SN | 21 | 8.8 |
| MARI | 28 | 11.7 | |
| ALND | 57 | 23.8 | |
| Surgical debulking otherwise | 2 | 0.8 | |
| Locoregional radiotherapy | Chest only or nodes only | 21 | 8.8 |
| Chest + axilla and/or other nodes | 82 | 34.3 | |
| Re-irradiation ( ± hyperthermia) | 26 | 10.9 | |
| Unknown | 2 | 0.8 | |
| Local treatment of all metastatic lesionsb | 200 | 83.7 | |
| Local treatment modality metastases | Radiotherapy only | 155 | 64.9 |
| Surgery only | 29 | 12.1 | |
| RFA/MWA | 6 | 2.5 | |
| Two or more modalitiesc | 13 | 5.4 | |
| Ablative local treatment metastasesd | 111 | 46.4 | |
| Systemic treatment (first-line)e | Any | 236 | 98.7 |
| Chemotherapy | 195 | 81.6 | |
| Endocrine therapyf | 166 | 69.5 | |
| HER2-targeted therapy | 52 | 21.8 | |
| Chemotherapy regimen | Anthracyclins + cyclophosphamide | 13 | 5.4 |
| Anthracyclins + cyclophosphamide + taxanes±platinum | 70 | 29.3 | |
| Taxanes±platinum | 37 | 15.5 | |
| Capecitabin + vinorelbin | 17 | 7.1 | |
| Other, with anthracyclins and/or taxanesg | 37 | 15.5 | |
| Other, no anthrayclines or taxanes | 21 | 8.8 | |
| Radiologic response to first-line systemic therapy | Complete response | 40 | 16.7 |
| Any response, not complete | 124 | 51.9 | |
| Progression | 21 | 8.8 | |
| Not evaluable | 54 | 22.6 | |
ALND, axillary lymph node dissection; BC, breast cancer; HER2, human epidermal growth factorreceptor 2; MARI, marking axillary lymph nodes with radioactive iodine seeds; MWA, microwave ablation; OMBC, oligometastatic breast cancer; RFA, radiofrequency ablation; SN, sentinel node.
2 patients refused primary surgery, 1 patient received radiotherapy.
Local treatment of all metastases within 1 year of OMBC diagnosis.
For either the same or different lesions.
Defined as surgery, RFA/MWA, or ablative radiotherapy (an equivalent dose of 2Gy per fraction of >50Gyα/β = 10).
First systemic therapy for OMBC; adjuvant therapy for patients with no evidence of disease (NED) after local treatment was also considered part of the first systemic therapy.
Includes 3 patients who received a CDK4/6 inhibitor, no patient received everolimus in first-line.
Includes 10 patients who underwent high-dose chemotherapy with carboplatin, thiothepa and cyclophosphamide.
3.3. Survival
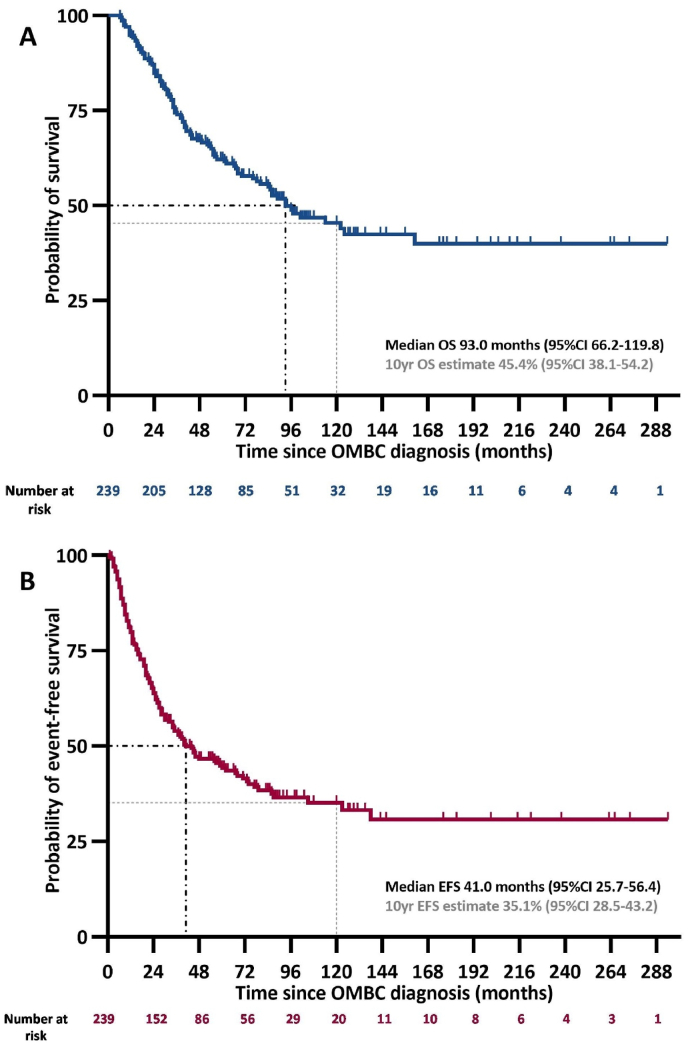
Median follow-up for OS was 88.0 months (95%CI 82.9–93.1); 107 patients died, five without prior documented disease recurrence/progression. Median OS estimate in the whole cohort was 93.0 months (95%CI 66.2–119.8) (Fig. 2A). In the subgroup of patients diagnosed in or after 2013, median OS was also 93.0 months (95%CI not reached) (Supplementary Fig. S1). OS did not significantly differ between patients with or without proof of metastatic disease (Supplementary Fig. S1).
Fig. 2.
Overall (A) and event-free (B) survival in the whole cohort.
CI, confidence interval; EFS, event-free survival; OMBC, oligometastatic breast cancer; yr, year.
After a median follow-up of 84.0 months (95%CI 72.7–95.3), we observed 139 recurrence/progression events with a medium EFS estimate of 41.0 months (95%CI 25.7–56.4) (Fig. 2B). The majority (n = 113, 81.2%) of EFS-events involved new lesions (with or without progression of known disease), whereas 18 EFS-events were based on progression of previously known lesions(s) only; for the remaining eight events, the site of recurrence/progression was unknown.
At data cut-off, 95 patients were alive without documented disease recurrence or progression.
3.4. Prognostic factors
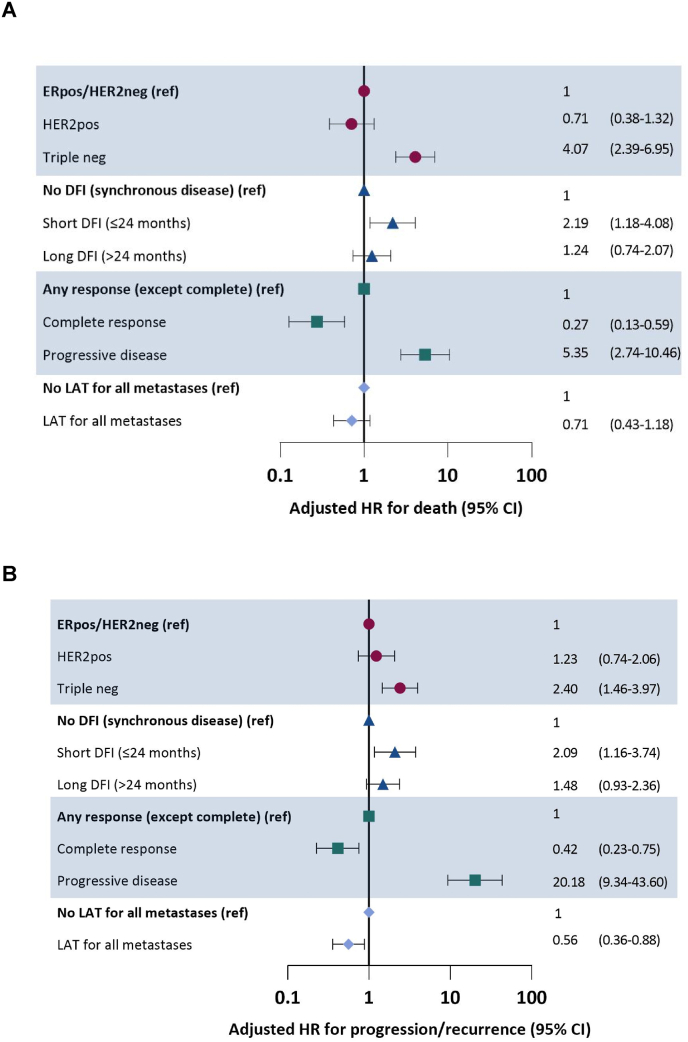
In univariable analyses breast cancer subtype, DFI, chemotherapy regimen, radiologic response to first systemic therapy and LAT for all metastases were statistically significantly associated with OS (Supplementary Table S3). The multivariable model included all these factors except chemotherapy, which we excluded because it strongly correlated with DFI (chi squared p < 0.001). TN subtype was associated with worse OS (hazard ratio (HR) for death: 4.07, 95%CI 2.39–6.95), as was short DFI (HR 2.19, 95%CI 1.18–4.08) and rPD to first-line systemic therapy (HR 5.35, 95%CI 2.74–10.46). rCR to first-line systemic therapy (HR 0.27, 95%CI 0.13–0.59) was associated with better OS. LAT was not significantly associated with OS (Fig. 3A, Supplementary Fig. S2).
Fig. 3.
Factors associated with overall (A) and event-free survival (B) in multivariable analysis.
Subtype according to metastatic lesion if available, otherwise primary tumor.
ER, endocrine receptor; DFI, disease-free interval; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; LAT, local ablative therapy; neg, negative; pos, positive; ref, reference category.
Similar to OS, breast cancer subtype, DFI, chemotherapy regimen, radiologic response to first systemic therapy and LAT of all metastases were statistically significantly associated with EFS in univariable analyses (Supplementary Table S3). The multivariable model included all variables except chemotherapy regimen (because of strong correlation with DFI). TN subtype was associated with worse EFS (HR 2.40, 95%CI 1.46–3.97), as was short DFI (HR 2.09, 95%CI 1.16–3.74) and rPD to first-line systemic therapy (HR 20.18, 95%CI 9.34–43.60). LAT was associated with favorable EFS (HR 0.56, 95%CI 0.36–0.88) (Fig. 3B).
In the subgroup of patients with metachronous OMBC, we identified no association between the presence of locoregional recurrence at time of OMBC diagnosis and OS or EFS (Supplementary Tables S4 and S5).
4. Discussion
We describe a large real-world cohort of 239 patients with OMBC, with a median follow-up of 88.0 months. Median OS in the cohort was 93.0 months, which is notably longer than the reported one to four years for the overall MBC population [[1], [2], [3]]. This finding supports the notion that OMBC is a distinct clinical entity with favorable prognosis [1,2]. We identified breast cancer subtype, DFI and radiologic response to first-line systemic therapy as independent prognostic factors for OS.
Breast cancer subtype is perhaps the most widely acknowledged prognostic factor for MBC; our cohort validates this observation in OMBC [15]. The HER2pos subtype was associated with comparable OS and EFS as the ERpos/HER2neg subtype in our cohort. Due to the introduction of new anti-HER2 targeting agents such as trastuzumab-emtansine and trastuzumab-deruxtecan in the last couple of years, survival in the HER2pos subgroup will further improve [16,17]. The TN subtype had unfavorable OS compared to the ERpos/HER2neg subtype, but in this small subgroup long-term survivorship also occurred. Recent promising therapies for TN breast cancer such as immune checkpoint inhibitors and sacituzumab-govitecan have expanded systemic options for this subgroup [[18], [19], [20]]. The effect of these new therapies in patients with TN OMBC is eagerly awaited. Subtype discrepancy between primary and metastatic tumor has been recognized as a prognostic factor for survival [21]. In our cohort, the discrepancy rate was 6%, which is lower than in the literature, but availability of both subtypes was limited in our cohort, which hinders further interpretation of this phenomenon [22,23].
Short DFI was independently associated with worse OS, in line with data in the overall population with MBC [[24], [25], [26], [27]]. In our previously published systematic review, we also identified DFI as an independent prognostic factor for OMBC [28]. These results underline the heterogeneity within the OMBC population, and plea for a more detailed categorization of patients with OMBC, such as suggested by the European Society for Radiotherapy and Oncology (ESTRO) and European Organisation for Research and Treatment of Cancer (EORTC) consensus statement [7]. DFI should be incorporated in the design of new trials in OMBC, but also in the clinical decision making process for individual patients.
A third independent prognostic factor in our cohort was radiologic response to first-line systemic therapy. Patients with rCR had favorable survival compared to patients with any response while those with rPD clearly fared worse. Due to lack of standardized radiologic evaluations in our study, we could not discern patients with stable disease from partial responders. This led to a relatively heterogeneous control group of patients with ‘any response’. Nevertheless, the association between radiologic response and survival remained statistically significant. Our results are in line with other MBC studies, in which radiologic response to systemic therapy was associated with OS although some other studies questioned the association between objective response and OS [[29], [30], [31], [32]]. These findings support the hypothesis that early maximal systemic therapy in OMBC is important. A ‘curative’ regimen, comparable to the regimens used in early breast cancer (doublet or triplet chemotherapy), provides the best chances of favorable response and may therefore be preferable [33]. The fact that the majority of patients in our cohort relapsed at new disease sites further supports the idea that optimal systemic therapy is key in achieving long-term remission. We would like to stress that there is very limited data on what constitutes optimal systemic therapy in OMBC. Patients and physicians need to be aware of this knowledge gap and carefully weigh the benefits of a curative regimen (higher chances of tumor response) against the costs (toxicity). Future prospective studies are necessary to provide more clarity on this topic, as retrospective, non-randomized studies such as ours have limited value in determining the value of specific therapies.
LAT of all metastases was not significantly associated with OS, but it was independently associated with EFS. Our OS-results are in line with the results of the NRG-BR002 study, the only randomized study that investigated the value of LAT in OMBC [12]. The OS-benefit of LAT in OMBC might be limited due to the availability of multiple systemic therapies with reasonable chances of durable response, again making the case for increasing knowledge on optimal systemic therapy in OMBC [34]. Whilst our study, and other retrospective studies, suggest an EFS-benefit from LAT, the NRG-BR002 showed no benefit in PFS [6,35,36]. This discrepancy might be the result of confounding by indication in observational studies, but it might also be the result of different study populations or differences in systemic therapy. Since the full publication of NRG-BR002 is not out yet, information on certain relevant prognostic factors (such as the number of patients with visceral disease or the median DFI) lacks. In terms of systemic therapy, only 27% of the patients in NRG-BR002 received chemotherapy, compared to 82% in our cohort. Given the sample size (n = 129) of NRG-BR002 and the relative overrepresentation of patients with an ER+/HER2neg tumor (80% of participants), additional data are highly desirable to establish the value of LAT in OMBC, or the lack thereof. Results from the OligoCare project and the TAORMINA study are therefore eagerly awaited [7,37].
Although not one uniform definition to classify OMBC exists, most definitions leave room for patients with up to five distant metastases [7,38]. For this project, we chose a more conservative limit of three distant metastases, because this has been the cut-off for OMBC in our institute in the past decade. A recent nationwide cohort study from our group reinforced this cut-off [6].
Important strengths of our study are the size of the cohort and the duration of follow-up. To our knowledge, this is the largest OMBC cohort to date with the longest follow-up. Second, we selected patients based on disease characteristics only, regardless of therapy or outcome. Our results are therefore generalizable to the whole OMBC population and are less likely affected by selection bias in favor of fit patients than previous studies that focused on patients who underwent a specific type of LAT [39,40]. Third, we had detailed data on potential relevant prognostic factors.
Some limitations of our study require consideration. The NKI-AVL is a tertiary referral hospital, and many patients only visit for a second opinion or for radiotherapy; all these patients were excluded from this cohort. The fact that patients in our cohort were notably younger than in the average MBC population is probably also the result of the tertiary referral function of our hospital (mainly young and healthy patients are referred to the NKI-AVL). Our selection method may have caused selection bias. The term ‘oligo’ might be used in particular to describe fit patients, who are eligible for ‘oligo’-directed treatment. Our search, however, also identified a large sample of non-oligo patients, indicating that ‘oligo’ is a common term in our hospital. It is possible that our strategy failed to identify patients with OMBC who were diagnosed long ago, because the term ‘oligo’ was not yet common at that time. On the one hand, this indeed poses a risk of selection bias. On the other hand, it is highly debatable if patients who were diagnosed with limited metastatic disease over 20 years ago are representative of the patients with OMBC we see in the clinic today. Novel imaging techniques such as FDG-PET and MRI have changed which patients classify as ‘oligo’ and both systemic as well as local treatment options have improved. Availability of an electronic medical record was a prerequisite for inclusion in the cohort and, as such, patients who were diagnosed with OMBC prior to introduction of the electronic medical record in our hospital (February 2013) could only be included in the cohort if they were still alive at that time which may have caused immortal time bias. The fact that the sensitivity analysis based on year of diagnosis demonstrated similar OS in patients diagnosed prior to and in/after 2013 indicates that this effect has had very limited impact on the results. A final limitation of our study is that the data were not primarily registered for research purposes. For many parameters (e.g., age at diagnosis) this need not be problematic, but for some parameters this may have caused unwanted heterogeneity (e.g., there was no standardized radiology protocol to evaluate disease status).
5. Conclusions
This real-world cohort confirms a favorable long-term outcome in patients with OMBC, with a 10-year overall survival estimate of 45%. Breast cancer subtype, DFI and radiologic response to first-line systemic therapy are independent prognostic factors for OS. These factors can aid in designing future prospective OMBC studies, which should focus on optimizing systemic therapy and clarifying the role of LAT.
Author contributions according to CRediT
Annemiek van Ommen-Nijhof: Conceptualization, Investigation, Data curation, Formal analysis, Methodology, Visualization, Writing - original draft, Writing - review & editing. Tessa G. Steenbruggen: Conceptualization, Investigation, Data curation, Methodology, Writing - review & editing. Laura Capel: Investigation, Writing - review & editing Michel Vergouwen: Investigation, Data curation, Software, Writing - review & editing. Marie-Jeanne T. Vrancken Peeters: Writing - review & editing. Terry G Wiersma: Supervision, Writing - review & editing. Gabe S. Sonke: Conceptualization, Methodology, Supervision, Writing - review & editing.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: GS reports the following competing interests: Consulting or Advisory Role - Biovica (Inst); Novartis (Inst); Seattle Genetics (Inst).
Research Funding - Agendia (Inst); AstraZeneca/Merck (Inst); Merck Sharp & Dohme (Inst); Novartis (Inst); Roche (Inst).
AvON, TS, LC, MV, MVP, TW report no competing interests.
Acknowledgements
The authors would like to thank Vincent van der Noort for his help with the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.12.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Surveillance, epidemiology, and end results program. https://seer.cancer.gov/explorer/ (SEER): SEER*Explorer 2022 [Available from:
- 2.Netherlands Cancer Registry (Ncr) 2022. www.iknl.nl/nkr-cijfers Available from:
- 3.Grinda T., Antoine A., Jacot W., Blaye C., Cottu P.H., Diéras V., et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open. 2021;6(3) doi: 10.1016/j.esmoop.2021.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen D.H., Truong P.T., Walter C.V., Hayashi E., Christie J.L., Alexander C. Limited M1 disease: a significant prognostic factor for stage IV breast cancer. Ann Surg Oncol. 2012;19(9):3028–3034. doi: 10.1245/s10434-012-2333-3. [DOI] [PubMed] [Google Scholar]
- 5.Dalenc F., Lusque A., De La Motte Rouge T., Pistilli B., Brain E., Pasquier D., et al. Impact of lobular versus ductal histology on overall survival in metastatic breast cancer: a French retrospective multicentre cohort study. Eur J Cancer. 2022;164:70–79. doi: 10.1016/j.ejca.2021.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Steenbruggen T.G., Schaapveld M., Horlings H.M., Sanders J., Hogewoning S.J., Lips E.H., et al. Characterization of oligometastatic disease in a real-world nationwide cohort of 3447 patients with de Novo metastatic breast cancer. JNCI Cancer Spectr. 2021;5(3) doi: 10.1093/jncics/pkab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., deSouza N.M., et al. Characterisation and classification of oligometastatic disease: a European society for radiotherapy and Oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18–e28. doi: 10.1016/s1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 8.Palma D.A., Salama J.K., Lo S.S., Senan S., Treasure T., Govindan R., et al. The oligometastatic state - separating truth from wishful thinking. Nat Rev Clin Oncol. 2014;11(9):549–557. doi: 10.1038/nrclinonc.2014.96. [DOI] [PubMed] [Google Scholar]
- 9.Pagani O., Senkus E., Wood W., Colleoni M., Cufer T., Kyriakides S., et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102(7):456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gennari A., André F., Barrios C.H., Cortés J., de Azambuja E., DeMichele A., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020 doi: 10.1200/jco.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmura S.J., Winter K.A., Woodward W.A., Borges V.F., Salama J.K., Al-Hallaq H.A., et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer ( NCT02364557) J Clin Oncol. 2022;40(16_suppl):1007. doi: 10.1200/JCO.2022.40.16_suppl.1007. [DOI] [Google Scholar]
- 13.https://richtlijnendatabase.nl/richtlijn/borstkanker/pathologie/receptorbepaling.html Richtlijnendatabase (Dutch breast cancer guideline) [Internet]. [cited August 1, 2022]. Available from:
- 14.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/jco.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 15.Kennecke H., Yerushalmi R., Woods R., Cheang M.C., Voduc D., Speers C.H., et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/jco.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 16.Cortés J., Kim S.B., Chung W.P., Im S.A., Park Y.H., Hegg R., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 17.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardia A., Hurvitz S.A., Tolaney S.M., Loirat D., Punie K., Oliveira M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 19.Schmid P., Rugo H.S., Adams S., Schneeweiss A., Barrios C.H., Iwata H., et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/s1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 20.Voorwerk L., Slagter M., Horlings H.M., Sikorska K., van de Vijver K.K., de Maaker M., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 21.Grinda T., Joyon N., Lusque A., Lefèvre S., Arnould L., Penault-Llorca F., et al. Phenotypic discordance between primary and metastatic breast cancer in the large-scale real-life multicenter French ESME cohort. npj Breast Cancer. 2021;7(1):41. doi: 10.1038/s41523-021-00252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrijver W.A.M.E., Suijkerbuijk K.P.M., van Gils C.H., van der Wall E., Moelans C.B., van Diest P.J. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. JNCI: J Natl Cancer Inst. 2018;110(6):568–580. doi: 10.1093/jnci/djx273. [DOI] [PubMed] [Google Scholar]
- 23.Callens C., Driouch K., Boulai A., Tariq Z., Comte A., Berger F., et al. Molecular features of untreated breast cancer and initial metastatic event inform clinical decision-making and predict outcome: long-term results of ESOPE, a single-arm prospective multicenter study. Genome Med. 2021;13(1):44. doi: 10.1186/s13073-021-00862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang E., Mougalian S.S., Adelson K.B., Young M.R., Yu J.B. Association between prolonged metastatic free interval and recurrent metastatic breast cancer survival: findings from the SEER database. Breast Cancer Res Treat. 2019;173(1):209–216. doi: 10.1007/s10549-018-4968-7. [DOI] [PubMed] [Google Scholar]
- 25.Lobbezoo D.J., van Kampen R.J., Voogd A.C., Dercksen M.W., van den Berkmortel F., Smilde T.J., et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 26.Malmgren J.A., Mayer M., Atwood M.K., Kaplan H.G. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res Treat. 2018;167(2):579–590. doi: 10.1007/s10549-017-4529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Rahman O. Outcomes of metastatic breast cancer patients in relationship to disease-free interval following primary treatment of localized disease; a pooled analysis of two clinical trials. Breast J. 2019;25(5):823–828. doi: 10.1111/tbj.13346. [DOI] [PubMed] [Google Scholar]
- 28.van Ommen-Nijhof A., Steenbruggen T.G., Schats W., Wiersma T., Horlings H.M., Mann R., et al. Prognostic factors in patients with oligometastatic breast cancer - a systematic review. Cancer Treat Rev. 2020;91 doi: 10.1016/j.ctrv.2020.102114. [DOI] [PubMed] [Google Scholar]
- 29.Bruzzi P., Mastro L.D., Sormani M.P., Bastholt L., Danova M., Focan C., et al. Objective response to chemotherapy as a potential surrogate end. Point Surv Metastatic Breast Cancer Patient. 2005;23(22):5117–5125. doi: 10.1200/jco.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 30.Hackshaw A., Knight A., Barrett-Lee P., Leonard R. Surrogate markers and survival in women receiving first-line combination anthracycline chemotherapy for advanced breast cancer. Br J Cancer. 2005;93(11):1215–1221. doi: 10.1038/sj.bjc.6602858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrelli F., Barni S. Surrogate endpoints in metastatic breast cancer treated with targeted therapies: an analysis of the first-line phase III trials. Med Oncol. 2013;31(1):776. doi: 10.1007/s12032-013-0776-4. [DOI] [PubMed] [Google Scholar]
- 32.Burzykowski T., Buyse M., Piccart-Gebhart M.J., Sledge G., Carmichael J., Lück H.-J., et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26(12):1987–1992. doi: 10.1200/jco.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 33.Butters D.J., Ghersi D., Wilcken N., Kirk S.J., Mallon P.T. Addition of drug/s to a chemotherapy regimen for metastatic breast cancer. Cochrane Database Syst Rev. 2010;2010(11) doi: 10.1002/14651858.CD003368.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/s0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 35.Milano M.T., Katz A.W., Zhang H., Huggins C.F., Aujla K.S., Okunieff P. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: some patients survive longer than a decade. Radiother Oncol. 2019;131:45–51. doi: 10.1016/j.radonc.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Scorsetti M., Franceschini D., De Rose F., Comito T., Villa E., Iftode C., et al. Stereotactic body radiation therapy: a promising chance for oligometastatic breast cancer. Breast. 2016;26:11–17. doi: 10.1016/j.breast.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 37.https://www.clinicaltrials.gov/ct2/show/NCT05377047?term=taormina&cond=breast+cancer&draw=2&rank=1 TAORMINA study - ClinicalTrials.gov [Internet]. Available from:
- 38.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott D.E., Brouquet A., Mittendorf E.A., Andreou A., Meric-Bernstam F., Valero V., et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery. 2012;151(5):710–716. doi: 10.1016/j.surg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akyurek S., Chang E.L., Mahajan A., Hassenbusch S.J., Allen P.K., Mathews L.A., et al. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol. 2007;30(3):310–314. doi: 10.1097/01.coc.0000258365.50975.f6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.