Highlights
-
•
Sustained interference is observed on a test of cognitive flexibility in PTSD.
-
•
Higher theta band activity in DLPFC reflects greater cognitive control need in PTSD.
-
•
Low suppression of beta band activity impairs cognitive flexibility in PTSD.
Keywords: Post-traumatic stress disorder, Cognitive flexibility, Task-switching, Magnetoencephalography
Abstract
Post-traumatic stress disorder (PTSD) is associated with deficits in cognitive flexibility, with evidence suggesting that these deficits may be a risk factor for the development of core PTSD symptoms. Understanding the neurophysiological substrate of this association could aid the development of effective therapies for PTSD. In this study, we investigated the relationship between post-traumatic stress severity (PTSS) in service members with combat exposure and the modulation of cortical oscillatory activity during a test of cognitive flexibility. Participants were assigned to three groups based on PTSS scores: low (well below a threshold consistent with a diagnosis of PTSD, n = 30), moderate (n = 32), and high (n = 29) symptom severity. Magnetoencephalography data were recorded while participants performed a cued rule-switching task in which two matching rules were repeated or switched across consecutive trials. Participants with high PTSS had longer reaction times for both switch and repeat trials, and showed evidence of sustained residual interference during repeat trials. During the cue-stimulus interval, participants with moderate and high PTSS showed higher relative theta power in switch trials over left dorsolateral prefrontal cortex (DLPFC). After test-stimulus onset, participants with high PTSS showed less suppression of beta band activity, which was present over multiple prefrontal, parietal, and temporal regions in switch trials, but it was confined to ventromedial prefrontal cortex in repeat trials. Higher theta band activity is a marker of effortful voluntary shifting of attention, while lower suppression of beta band activity reflects difficulties with inhibition of competing perceptual information and courses of action. These findings are consistent with a role for altered suppression of beta band activity, which can be due to less effective top-down bias signals exerted by DLPFC, in the etiology of cognitive flexibility deficits in PTSD.
1. Introduction
Exposure to psychological trauma may result in a spectrum of symptoms including intrusive memories and flashbacks, emotional numbing, negative thoughts and mood, and difficulty sleeping and concentrating. Most people show a remarkable capacity to recover with time following the traumatic event. However, for a significant proportion of individuals (Kessler et al., 1995) such symptoms may persist over long periods of time, leading to post-traumatic stress disorder (PTSD) (Diagnostic and Statistical Manual of Mental Disorders DSM-5, American Psychiatric Association, 2013). Military combat, for example, is one of the common causes of PTSD among men, with combat-related PTSD being reported in 14 % to 19 % of war veterans (Dohrenwend et al., 2006, Schell and Marshall, 2008). PTSD is associated with poor physical health (Pacella et al., 2013), comorbid psychiatric conditions (Kessler et al., 1995), and increased risk of suicide (Sareen et al., 2007). PTSD is also accompanied by a spectrum of cognitive difficulties related to attention, memory and executive function (e.g. Vasterling et al., 1998, Bremner et al., 2004, Yehuda et al., 2005, Aupperle et al., 2012, Scott et al., 2015). These cognitive impairments attract significant research interest because they may share a common neurobiological substrate with core emotional and arousal symptoms of PTSD (Aupperle et al., 2012). If the presence of cognitive impairments facilitates the development or maintenance of some of the core symptoms of PTSD, then it becomes paramount to understand the neurophysiological basis of such impairments in order to develop more effective mechanistically based therapies to treat or prevent PTSD.
One of the deficits in executive function seen in PTSD is related to cognitive flexibility (Beckham et al., 1998, Jenkins et al., 2000, Gilbertson et al., 2006, Koso and Hansen, 2006, Pang et al., 2014, Qureshi et al., 2011 for a review), which refers to the ability to rapidly shift attention and inhibit pre-existing action plans, and it is typically tested using task-switching paradigms (Monsell, 2003). A meta-analytic study (Polak et al., 2012) has reported that differences in performance on tests of cognitive flexibility are more pronounced when patients with PTSD are compared to trauma-exposed participants without PTSD rather than to healthy individuals who were not exposed to traumatic events. This finding suggests that people who were exposed to traumatic events without having developed PTSD might have better cognitive flexibility associated with more efficient coping strategies and resilience to PTSD. This hypothesis received support from a study in a large sample of trauma exposed participants, which has found that lower cognitive flexibility at one month after trauma predicted PTSD severity 14 months later (Ben-Zion et al., 2018), suggesting that impaired cognitive flexibility is a risk factor for the development of PTSD. These findings align with the prevailing theories that posit a mutual reinforcement between deficits in executive function and core symptoms of PTSD (Aupperle et al., 2012), and underscore the importance of a greater understanding of the neurobiological substrate of individual differences in susceptibility to the disorder. Given the established role of oscillatory cortical activity in cognitive function, including activation, maintenance or suppression of neuronal cell assemblies representing information (e.g. Uhlhaas and Singer, 2006, Düzel et al., 2010, Hanslmayr et al., 2012, Buschman et al., 2012), we focused our current study on characterizing the dynamic activity in specific frequency bands in relation to cognitive flexibility in PTSD.
Task-switching paradigms (also referred to as rule-switching), in which participants are instructed to evaluate stimuli and respond in accordance to a rule that is either repeated or switched on a trial-by-trial basis, have been extensively used by research studies in healthy individuals to characterize the ability to re-orient attention and inhibit pre-existing action plans (Monsell, 2003, for a review). One of the main findings from these studies is that performance speed and accuracy are typically lower on switch compared to repeat trials. This difference has been ascribed to effortful processes required by what has been often referred to as the task-set reconfiguration, such as shifting attention to new stimulus attributes and activating corresponding neuronal representations of stimulus–response contingencies, while inhibiting the neuronal representations for cognitive operations involved in the execution of the prior task. The difference in accuracy or reaction time between switch and repeat trials is known as switch cost (switch costs may also be conceptualized instead in terms of repetition benefits). When cues are used to provide advanced knowledge about the upcoming rule in each trial and enough time is allowed to prepare for it, the switch cost is usually reduced but not completely eliminated.
Evidence from electrophysiological studies in healthy individuals indicates that cognitive processes invoked by cued task-switching paradigms are associated with modulations of oscillatory activity in specific frequency bands, which suggests that alterations in such modulatory processes may contribute to the executive dysfunction in PTSD. The instruction to repeat or switch a rule on a trial-by-trial basis is typically followed by an increase in theta band activity, and a suppression of alpha and beta band activity (Cunillera et al., 2012, Foxe et al., 2014, Cooper et al., 2017). During the preparatory (cue-test stimuli) interval, these patterns of modulation of cortical oscillations are more elevated for switch compared to repeat trials. Similar results have also been reported in a task using simultaneous cues and test stimuli (Capizzi et al., 2020). The higher increase in theta band activity following the cue onset in switch trials is believed to reflect neuronal activity associated with proactive goal-updating processes (Cooper et al., 2017) and increased cognitive control (Cavanagh and Frank, 2014) required by shifts of attention. The suppression of alpha activity over unimodal sensory areas has been considered to reflect top-down attentional effects that facilitate processing of relevant sensory features and are stronger when tasks need to be switched (Foxe et al., 2014). This interpretation is based on observations that anticipatory allocation of attention is linked to suppression of alpha band activity in sensory areas that process the attended sensory inputs, while enhancement of alpha oscillations in other sensory areas signals the disengagement of other sensory systems (Worden et al., 2000, Gould et al., 2011, Haegens et al., 2011). Finally, the suppression of beta band activity has been linked to interference control (suppression) that is manifested in multiple brain regions and predominantly in regions involved in motor response selection (Proskovec et al., 2019, Capizzi et al., 2020). This interpretation receives support from findings of stronger suppression of beta band activity for incongruent compared to congruent trials in Stroop tasks (Zhao et al., 2015, Tafuro et al., 2019) particularly in the presence of interference that affects the process of motor response selection (Zhao et al., 2015).
A series of electrophysiological studies in PTSD have reported alterations in brain oscillatory activity during rest (e.g. Kolassa et al., 2007, Huang et al., 2014, Mišić et al., 2016, Popescu et al., 2016) as well as during performance of cognitive tasks (Dunkley et al., 2015, Khanna et al., 2017, Waldhauser et al., 2018, Popescu et al., 2019, Popescu et al., 2020). It is therefore plausible that a distinct dynamic modulation of oscillatory activity may be present in PTSD during task-switching as well. If so, this may help to understand the proactive or reactive processes elicited by the task which are associated with impaired performance for individuals with PTSD. Potential alterations in oscillatory activity could be manifested as distinct modulations of theta, alpha, or beta band oscillations, given their established role in cognitive operations involved in set-shifting. We had specific hypotheses regarding the modulations of cortical oscillations in each of these frequency bands. One hypothesis was that modulation of theta power in prefrontal regions implicated in cognitive control would be lower in PTSD, suggesting a primary role of an alteration in theta oscillatory activity in the etiology of the cognitive flexibility deficit. An alternative hypothesis was that if impaired modulation of theta power does not represent a primary pathophysiological process, then an increase in theta power in PTSD might emerge as a manifestation of a compensatory mechanism in the presence of impaired cognitive flexibility caused by dysregulation of other neurophysiological mechanisms. Among them, we hypothesized that an altered modulation of alpha band oscillations in sensory areas could reflect general difficulties to engage/disengage sensory processing systems, whereas a lower suppression of beta band oscillations in areas involved in cognitive control, sensory processing and/or response selection may reflect difficulties with inhibition of neuronal representations for competing perceptual information or courses of action, respectively, that impairs the ability to shift sets. To test these hypotheses, we used a cued task-switching paradigm and examined the relationship between the modulation of oscillatory activity, behavioral performance, and the severity of PTSD symptoms in service members with combat exposure. In line with the executive dysfunction theory of PTSD (Aupperle et al., 2012), we hypothesized that PTSD is associated with impaired behavioral performance reflected in measures of accuracy, reaction time or switch costs. Using neuromagnetic recordings and source estimation methods, we sought to characterize the potential alterations in the modulation of cortical oscillatory activity to gain physiological insights into the cognitive processes and associated brain networks that may be affected in PTSD.
2. Methods
2.1. Participants
Study participants (n = 99) were service members enrolled in an outpatient program for patients with post-concussive and post-traumatic psychological health symptoms at the National Intrepid Center of Excellence (NICoE), Walter Reed National Military Medical Center. Patients were not included in this study if they had a history of moderate or severe traumatic brain injury or other neurological, developmental or psychiatric disorders such as stroke, epilepsy, bipolar disorder, etc. The study was approved by the Institutional Review Board of the Walter Reed National Military Medical Center in compliance with all applicable federal regulations governing the protection of human subjects. Informed consent was obtained from each participant before participation in the study.
All participants completed the PTSD Check List-version 5 (PCL-5), which is a 20-item self-report scale that is used to screen individuals for post-traumatic stress severity (PTSS) (Bovin et al., 2015, Blevins et al., 2015). PCL-5 items ask about the presence and severity of re-experiencing, avoidance, emotional numbing and hyperarousal symptoms elicited by stressful experiences and are rated on a scale from 0 to 4 (the total score ranges from 0 to 80, with higher values indicating higher symptom severity). Participants completed a modified version of the Combat Exposure Scale (Hoge et al., 2008), which characterizes the level of exposure to combat experiences, such as receiving incoming artillery, rocket, or mortar fire, seeing seriously injured bodies, etc. The exposure to combat-related stressful experiences generally occurred over an extended period of time, during which the participants have also experienced one or more mild traumatic brain injury (defined according to standard criteria established by the American Congress of Rehabilitation Medicine, 1993). Participants also completed the Patient Health Questionnaire PHQ-9 that measures the severity of depression symptoms (Spitzer et al., 1999, Kroenke et al., 2001), and the AUDIT-C alcohol consumption screening test (Bush et al., 1998).
Eight participants had to be excluded from the analysis for the following reasons: four participants experienced significant drowsiness/sleepiness during the recording, two participants had excessive noise in the data, one participant had a co-registration error, and one participant had very low accuracy in performing the task (between 23 % and 33 % correct responses in the different conditions of the experiment) indicating that he did not follow instructions. The remaining participants (n = 91, all males) were assigned to three groups based on the PCL-5 scores: Group 1 included participants with low PTSS (n = 30, PCL-5 score ≤ 15), Group 2 included participants with moderate PTSS (n = 32, 16 ≤ PCL-5 score ≤ 33), and Group 3 included participants with high PTSS (n = 29, PCL-5 score ≥ 35). Participants in Group 1 had very low PCL-5 scores, well below a threshold that is consistent with a diagnosis of PTSD; thus, Group 1 served in our study as a control group of participants with trauma exposure. Participants with moderate scores who were assigned to Group 2 had more elevated PCL-5 scores that were still lower than the cut-off score recommended to screen veterans with combat exposure for probable PTSD (the optimal cut-off score was estimated to be 34, Murphy et al., 2017). With respect to criteria that can warrant inclusion in a diagnostic category, this intermediate group was inhomogeneous, as it included participants who met various criteria used to define subthreshold (subclinical) PTSD (McLaughlin et al., 2015), as well as participants who did not meet the criteria for inclusion in this diagnostic category. The assignment of these participants into a separate intermediate group aligns with observations that PTSD can be characterized as the upper end of a stress-response continuum rather than a discrete pathological syndrome (Ruscio et al., 2002). All participants in Group 3 had PCL-5 scores higher than the cut-off score used to screen for probable PTSD (Murphy et al., 2017).
No significant group differences were present for age, education, combat exposure, and AUDIT-3 scores (Table 1). The proportions of participants with a history of mild traumatic brain injuries with loss of consciousness (37 % in Group 1, 41 % in Group 2, and 38 % in Group 3) was not significantly different between groups (p = 0.96 using the Fisher’s exact test of proportions). Four participants (all of them assigned to Group 3) had PHQ-9 scores in the range of severe depression (PHQ-9 scores greater or equal to 20). The severity of depressive symptoms was not used as an exclusion criterion given that PTSD and depression share a series of symptoms (anhedonia, sleep disturbances, difficulties concentrating) that are probed by both PCL-5 and PHQ-9 scales. It is well documented that a significant proportion of participants with PTSD also experience depressive symptoms (Rytwinski et al., 2013), and the frequent co-occurrence of PTSD and major depressive disorder (MDD) may in fact reflect a trauma-related phenotype that may be distinct from MDD in the absence of trauma exposure (Flory and Yehuda, 2015). Our current study aimed to investigate the neurobiological mechanisms involved in the potential association between cognitive flexibility difficulties and PTSD symptoms without seeking to determine if co-occurrence of MDD does or does not represent a distinct phenotype. No participants in the study, who were all active-duty service members, had a history of use or abuse of recreational drugs.
Table 1.
Demographic and neuropsychological data: descriptive statistics (mean and standard deviation) and results of statistical tests for significance of group differences (ANOVA or Kruskal-Wallis tests were used as appropriate).
| Group 1 (n = 30) |
Group 2 (n = 32) |
Group 3 (n = 29) |
Test statistics | p | |
|---|---|---|---|---|---|
| PCL-5 | 10.5 ± 3.8 | 23.1 ± 5.9 | 48.8 ± 9.9 | ||
| Age (years) | 43.2 ± 5.7 | 40.8 ± 6.8 | 41.8 ± 5.4 | F = 1.24a | 0.29 |
| Education (years) | 15.4 ± 2.0 | 14.9 ± 2.2 | 15.4 ± 2.0 | F = 0.72a | 0.49 |
| Combat Exposure § | 81.2 ± 21.1 | 84.6 ± 19.3 | 91.1 ± 22.2 | H = 3.4b | 0.18 |
| AUDIT-C | 2.8 ± 1.7 | 2.9 ± 1.9 | 2.8 ± 1.7 | H = 0.06b | 0.97 |
ANOVA test of group differences.
Kruskal-Wallis test of group differences.
combat exposure information was available for 95% of the participants (it was not available for 3 participants from Group1, 1 participant from Group 2, and 1 participant from Group 3).
Participants were not excluded from the study based on medication use. Three participants in Group 1, ten participants in Group 2, and sixteen participants in Group 3 were taking antidepressant medication. Additionally, eight participants in each group were taking Gabapentin for headache prophylaxis. Three participants in Group 1, three participants in Group 2, and nine participants in Group 3 were taking Prazosin as a treatment for nightmares. Some participants were taking multiple medications from the categories described above; the total number of medicated participants was 12 (Group1), 15 (Group 2), and 22 (Group3).
2.2. Experimental paradigm
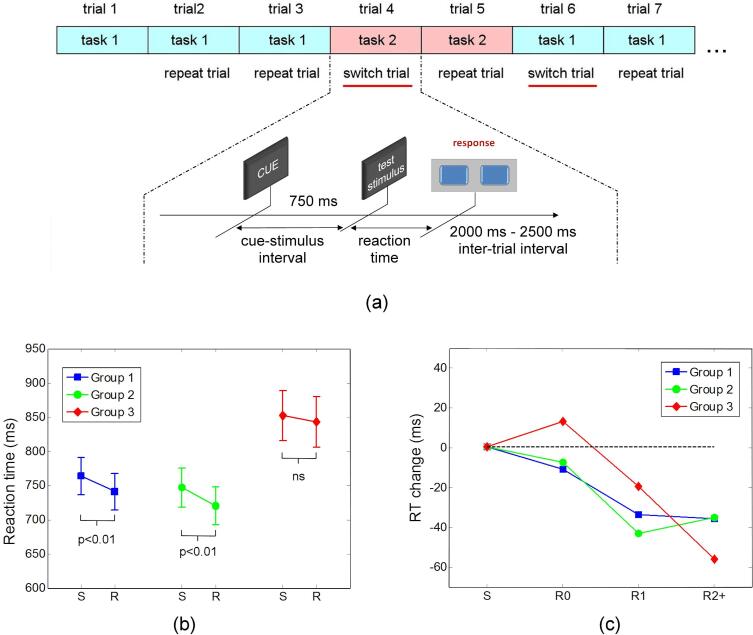
MEG data were recorded while participants performed a rule-switching task (illustrated schematically in Fig. 1a), in which they were asked if two geometric figures displayed simultaneously on a screen match with respect to either their color (color rule) or their shape (shape rule). Each trial started with a cue (the word ‘color’ or ‘shape’) displayed in the center of a screen positioned at 95 cm in front of the subjects. Cues indicated whether the color or shape rule needs to be used in the current trial. The cue word was displayed for 750 ms and was replaced by two colored geometric figures (test stimuli) shown to the left and right of the screen center. Participants had to respond with the right hand by pressing one of two buttons to indicate as quickly and as accurately as possible if the two figures match or do not match according to the current rule. The correct response in each trial therefore depends on both the stimulus identity and the current rule in effect. Stimulus-response associations corresponding to the two rules were incongruent in each trial, such that a correct response always indicated that the participant followed the current rule. The current trial ended with a button press response or if a response was not given within a maximum allowed time interval of 4750 ms after presentation of the figures. Each response (or trial-end) was followed by an inter-trial interval with randomized duration in the range of 2000 ms to 2500 ms, during which a fixation cross was shown at the screen center. Combinations between a set of four shapes and four colors were used to create 16 figures. Stimuli were selected from a pool of 48 pairs of same shape-different color figures and 48 pairs of same color-different shape figures. A total of 192 trials (intermixed with respect to the cues) were presented in a randomized sequence for each participant, such that the rule to be applied in each trial was unpredictable. Positive and negative responses (indicating that the two geometric figures match or do not match, respectively, according with the current rule) were required with equal probability across trials. The recording session was split into four blocks, with short breaks between blocks. The sequence of stimuli was presented using the Presentation software (Neurobehavioral Systems Inc.).
Fig. 1.
(a) Schematic illustration of the task-switching paradigm. Each trial started with a cue (the word ‘color’ or ‘shape’) informing the participants about the matching rule that needs to be used in the current trial. The cue word was displayed for 750 ms and was replaced by two colored geometric figures (test stimuli). Participants had to respond by pressing one of two buttons to indicate as quickly and as accurately as possible if the two figures match or do not match according to the current rule. (b) Mean reaction times for switch and repeat trials are shown separately for each group (vertical bars show the standard error of mean). (c) Mean values of the changes in reaction time (RT) for repeat trials are shown for each group as a function of serial position in trains of repeat trials (serial position is identified by R0, R1, and R2+, as explained in the text). For each group, the changes in reaction time are shown relative to the corresponding group mean reaction time from all switch trials (denoted by S).
For the analyses of behavioral performance and MEG data, only epochs with correct responses were included. The first trials in each block were discarded from the analyses. Trials that followed immediately after a trial with an incorrect or missing response were also discarded from the analysis of reaction time and MEG data. For analyses of both behavioral and MEG data, two separate trial sets were created for repeat and switch trials, respectively, by pooling together corresponding trials irrespective of rule (match by color and match by shape) or response type (figures match or do not match).
2.3. MEG data acquisition and pre-processing
MEG recordings were performed inside a magnetically shielded room using the Elekta VectorView™ whole-head MEG system (Elekta-Neuromag Oy, Helsinki, Finland) with 102 triplet-sensors (each made of one magnetometer and two orthogonal planar gradiometers) with the participant in a seated position. The location of three fiduciary points (nasion, and left and right auricular points) defining the head-frame coordinate system, as well as the location of four localization coils placed on the head and of a set of head surface points were digitized using a 3D Fastrak digitizer (Polhemus, Colchester, VT, USA) to allow co-registration of the MEG data with T1-weighted MRI data acquired in a separate session with a 3T MRI scanner (General Electric, Milwaukee, WI). The head position relative to the sensor array was registered at the beginning of the recording using the localization coils. Data were acquired with 1 kHz sampling rate.
Datasets were band-pass filtered off-line between 1 Hz and 100 Hz, with a powerline filter at 60 Hz, and then down sampled at 500 Hz. Filtering was done using frequency domain, zero-phase and zero-delay finite impulse response (FIR) filters implemented in the MNE software (Gramfort et al., 2014). Datasets were concatenated with empty room noise recordings of 1 min duration that were acquired immediately before the MEG recordings and were band-pass filtered and down sampled in the same way. Independent Component Analysis using an Infomax algorithm (EEGLAB, Delorme and Makeig, 2004) was subsequently used on the concatenated datasets to segregate the activity of different signal generators on separate independent components (ICs). ICs corresponding to cardiac and eye movement interferences, as well as other sources of external artifacts (if any) were removed (concatenation facilitated the identification and removal of the same ICs corresponding to external artifacts from both empty room noise recordings and MEG recordings). The ICA-filtered data were divided into epochs from − 1600 ms to 2000 ms relative to the onset of the cue marking the beginning of each trial. The signals were band-pass filtered in theta (4–7 Hz), alpha (7.5–13 Hz), beta (14–30 Hz) and gamma (35–100 Hz) bands. Filtering was done at this stage using frequency domain, zero-phase and zero-delay finite impulse response (FIR) filters implemented in the Brainstorm software (Tadel et al., 2011). The source reconstruction was performed separately for signals filtered in each frequency band.
2.4. Source reconstruction
The cortical surface was determined for each participant using the FreeSurfer image analysis software (Fischl, 2012, https://surfer.nmr.mgh.harvard.edu) from individual T1-weighted MR images. The source reconstruction was done using the Brainstorm software (Tadel et al., 2011). Cortical sources were estimated at 10,000 locations using a minimum norm estimator (Hämäläinen and Ilmoniemi, 1994) and a multiple overlapping sphere model of the volume conductor that fits a sphere to the local curvature of the skull for each sensor (Leahy et al., 1998, Huang et al., 1999). The inverse projection operator incorporated a diagonal noise-covariance matrix derived from the empty room noise recordings. For the analysis in each frequency-band, the noise-covariance matrix was computed after filtering the empty-room noise recordings in the corresponding frequency band. Cortical currents with unconstrained orientation were estimated using a depth weighting parameter of 0.5 and were subsequently projected on the averaged FreeSurfer template brain. The power of the reconstructed currents was spatially integrated in each of the 84 cortical regions of a modified Desikan-Killiany anatomical atlas (Desikan et al., 2006). The original Desikan-Killiany atlas with 68 regions was refined by dividing several regions of relatively large area into smaller, functionally more specific sub-regions. The regional power was averaged separately for repeat and switch trials, respectively, in each frequency band. Subsequently, the mean regional power in each frequency band was transformed into percent change relative to a baseline interval from −800 ms to –300 ms with respect to the cue onset.
2.5. Statistical analysis
Behavioral performance (mean reaction time and response accuracy) was compared between the three groups and two trial types (switch trials and repeat trials) using analysis of variance (ANOVA). Prior to applying the parametric tests, the accuracy data were transformed using the arcsin-square root transformation (which is appropriate to normalize the distribution of proportion data), and reaction times were log-transformed to normalize their right-skewed distributions. After data transformation, Shapiro-Wilk tests were used to confirm the validity of the normality assumption. Levene’s tests were used to confirm the validity of the assumption of equal variances for each ANOVA.
Although an assumption implicit in most studies is that the task-set reconfiguration can be completed within a single switch trial, there is some evidence that repeat trials (which are used as baseline in the estimation of switch costs) may also be affected by residual interference from the competing task (Wylie and Allport, 2000). We hypothesized that the unpredictable nature of the rule in any given trial in our task would increase the potential for residual set related interference compared with set-switching task designs in which the current set is always reinforced with several consecutive repeat trials before the occurrence of a single switch trial. Thus, we conducted additional analyses to characterize the dependence of the reaction time on the serial position in trains of repeat trials and to investigate if such a relationship may be different between groups. The repeat trials were grouped into three categories, denoted by R0, R1, and R2+, respectively, depending on how many consecutive repeat trials preceded a current trial: R0 denotes repeat trials that are not immediately preceded by other repeat trials (i.e. they immediately follow a switch trial), R1 denotes repeat trials that are immediately preceded by one and only one previous repeat trial, and R2+ denotes repeat trials that are immediately preceded by two or more consecutive repeat trials. Similarly, switch trials were also split into three categories denoted by S0, S1, and S2+, respectively: S0 denotes switch trials that are not immediately preceded by repeat trials (i.e. they immediately follow another switch trial), S1 denotes switch trials that are immediately preceded by one and only one repeat trial, and S2+ denotes switch trials that are immediately preceded by two or more consecutive repeat trials. Separate 3x3 ANOVAs with factors group and trial category were conducted for switch and repeat trials, respectively, to investigate if the reaction time is different for the different categories of trials and if such a potential relationship is different among groups. The Greenhouse-Geisser correction was used if the sphericity assumption was violated (Mauchly’s test of sphericity, p < 0.05).
In preliminary MEG data analyses, we investigated with a relatively high temporal resolution the regional temporal course of the relative change in signal power during a temporal interval spanning both the interval between cue and test stimuli, as well a subsequent temporal interval following the onset of test stimuli. The goal of these preliminary MEG data analyses was twofold: first, we sought to understand if the modulation of the brain activity in each frequency band is qualitatively similar with that reported by previous EEG studies using different versions of task switching paradigms, and second, we sought to assess if the modulation of the brain activity can be considered approximately stationary during the whole duration of the cue-stimulus and early post-stimulus intervals (which would allow to use average power values estimated from the whole duration of these intervals in subsequent group analyses). For each frequency band, the relative change in regional power was determined on a series of seven temporal intervals of 300 ms duration with 50 % overlap (half of the duration of the temporal interval used for power integration). These intervals were centered at latencies starting from 150 ms and ending at 1050 ms with respect to the cue onset. For each of these temporal intervals, we tested if the relative change in power was significantly different than zero using one-sample t-tests on data from all participants. In a second analysis, we used paired t-tests on data from all participants to compare the relative change in signal power between repeat and switch trials. For each of these preliminary analyses, significance thresholds were adjusted to control the false discovery rate (FDR) at q = 0.1, to account for the multiple comparisons performed across 84 brain regions and 7 temporal intervals. Since differences in the modulation of brain activity between the two conditions could be due to differences in baseline power, we also conducted paired t-tests to compare the log-transformed baseline power between the two conditions. For these analyses, significance thresholds were adjusted to control the false discovery rate at q = 0.1 to account for the multiple comparisons performed across 84 brain regions.
For the main MEG data analyses, we adopted an approach that was guided by findings of the behavioral data analysis (presented in section 3.1). The specific objective was to investigate group differences in brain oscillatory activity for each of the two trial types, i.e. repeat and switch trials, respectively, while ensuring consistency in the control for multiple comparisons for tests conducted in multiple brain regions, frequency bands, and temporal intervals. To do this, we used separate one-way ANOVAs with factor group on the relative change of regional power for repeat and switch trials, respectively. The one-way ANOVA applied to switch trials was used to investigate group differences in brain activity associated with set-shifting, while the one-way ANOVA applied to repeat trials was used to investigate group differences in brain activity associated with a sustained interference that increases the reaction time in repeat trials for Group 3 (as described in section 3.1). To characterize the association between PTSD and proactive processes, the analyses were performed on the early (0–450 ms) and late (300–750 ms) parts of the cue-test stimulus interval. The selection of these intervals was based on information gained from the preliminary analyses, which showed different patterns of modulation across frequency bands during these two intervals (notably, the first part of the cue-test stimulus interval includes also the time-locked early evoked response components). To investigate the association between PTSD and the modulation of oscillatory brain activity during the post-stimulus interval, analyses were performed on a 450 ms long temporal interval starting from the onset of the test-stimuli (from 750 ms to 1200 ms following the cue onset). For these analyses, the FDR was controlled at q = 0.1 using the data driven Benjamini-Hochberg procedure for multiple hypotheses with group structure (Hu et al., 2010), with groups determined by frequency bands and temporal intervals. Results that were significant when using a less conservative approach that controls the FDR at q = 0.1 for each frequency band and temporal interval will also be reported.
As mentioned before, our main analyses sought to characterize the group differences in the modulation of brain activity, which has been defined as the relative change in power with respect to a baseline interval preceding the cue onset. Since group differences in the modulation of brain activity could be due, at least in part, to differences in the baseline power, we performed additional analyses to ascertain if the baseline power was significantly different between groups. These analyses were performed for each frequency band and condition (repeat and switch trials) using one-way ANOVAs with factor group on the corresponding log-transformed absolute power values determined on the baseline interval. For each of these additional analyses, the results are also reported using significance thresholds adjusted to control the FDR at q = 0.1.
3. Results
3.1. Behavioral performance
Descriptive statistics for response accuracy (percentage of correct responses) and reaction time data are presented in Table 2, Table 3, and in Fig. 1b. Mixed two-factor ANOVA with factors group and trial type (repeat vs switch trials) on response accuracy data showed no main effect of group (F = 0.9, p = 0.41), a main effect of trial type (F = 27.7, p < 0.0001) indicating that the percentage of correct responses was higher in repeat relative to switch trials, and no group × trial type interaction (F = 0.1, p = 0.90).
Table 2.
Performance accuracy: descriptive statistics (mean and standard deviation) for each condition of the experiment.
| Accuracy (%) |
||||
|---|---|---|---|---|
| Rule | Trial type | Group 1 (n = 30) |
Group 2 (n = 32) |
Group 3 (n = 29) |
| Match by shape or color | repeat | 96.2 ± 3.2 | 94.4 ± 5.4 | 95.5 ± 5.7 |
| switch | 94.2 ± 4.3 | 92.4 ± 6.0 | 93.7 ± 6.6 | |
Table 3.
Reaction times: descriptive statistics (mean and standard deviation) and results of pairwise tests of group differences for repeat and switch trials, respectively.
| Reaction Time (ms) |
||||
|---|---|---|---|---|
| Rule | Trial type | Group 1 (n = 30) |
Group 2 (n = 32) |
Group 3 (n = 29) |
| Match by shape or color | repeat | 741 ± 147 | 720 ± 156 | 843 ± 199 |
| switch | 764 ± 148 | 746 ± 162 | 852 ± 198 | |
Repeat trials: post-hoc t-tests: Group 3 vs Group 1: t = 2.22, p = 0.03; Group 3 vs Group 2: t = 2.73, p = 0.008; Group 2 vs Group 1: t = −0.6, p = 0.49;
Switch trials: post-hoc t-tests: Group 3 vs Group 1: t = 1.9, p = 0.06; Group 3 vs Group 2: t = 2.33, p = 0.023; Group 2 vs Group 1: t = −0.59, p = 0.55.
Reaction times were also analyzed using mixed ANOVA with factors group and trial type (repeat vs switch trials). This analysis showed a main effect of group (F = 4.0, p = 0.022) indicating a general increase in reaction time for participants with high PTSS (Group 3), a main effect of trial type (F = 20.2, p < 0.0001) indicating faster responses in repeat relative to switch trials, and no group × trial type interaction (F = 1.4, p = 0.25). The results of pair wise tests of group differences are summarized in the note of Table 3.
Across participants, 43.5 ± 4.4 of repeat trials were in the R0 category, 20.9 ± 3.7 of repeat trials were in the R1 category, and 21.2 ± 7.0 of repeat trials were in the R2+ category. For switch trials, 42.6 ± 8.0 of trials were in the S0 category, 22.4 ± 4.4 of trials were in the S1 category, and 20.5 ± 3.5 of trials were in the S2+ category. The number of trials in these categories reflects the fact that the frequency of occurrence for trains of n consecutive repeat trials decreases with n for randomized sequences. The number of trials in each category was not significantly different between groups. The descriptive statistics for the reaction time data for each category of trials is summarized in Supplementary Table 1 (included in Supplementary Material).
For switch trials, the mixed 3x3 ANOVA with factors group and switch trial category (S0, S1, and S2+) showed no main effect of trial category (F = 1.3, p = 0.28), a main effect of group (F = 3.3, p = 0.04), and no group × trial category interaction (F = 0.6, p = 0.62). These results indicate longer reaction times for Group 3 for all switch trial categories, and no significant differences in reaction time across the three switch trial categories.
The mixed 3x3 ANOVA conducted for repeat trials showed a main effect of trial category (F = 15.2, p < 0.001), a main effect of group (F = 4.0, p = 0.021), and no significant group × trial category interaction (F = 2.0, p = 0.11). These results indicate a decrease in reaction time with serial position in the train of repeat trials and longer reaction times for Group 3 across all three categories of repeat trials. The main effect of repeat trial category shows that interference from neuronal representations of the alternative set is not completely suppressed by the end of a switch trial but rather it may continue to exert a behavioral effect during repeat trials.
The mean decrement in reaction time with the serial position in a train of repeat trials is shown for each of the three groups in Fig. 1c. Participants in Groups 1 and 2 show a mean decrease in reaction time from R0 to R1 followed by no further decrement from R1 to R2+, suggesting that residual interference is primarily present only during the first two repeat trials in a train of repeat trials. For participants in Group 3, however, the mean decrease in reaction time from repeat trials R0 to R1 is followed by another noticeable decrement from R1 to R2+, suggesting that residual interference amenable to suppression is still present after the first two repeat trials in a train of repeat trials. Orthogonal contrasts validated the observations from visual inspection: Group 3 showed significantly steeper decrements in reaction time from R1 to R2+ compared to Groups 1 and Group 2 considered together (t = 2.04, p = 0.045), while Group 1 and Group 2 did not differ from each other (t = 0.47, p = 0.64).
In summary, the supplementary analyses led to several notable findings. First, the results indicate that for the set-up of our paradigm, a residual interference from neuronal representations of the previous task continues to be present during repeat trials. This finding is consistent with observations of previous studies (Wylie and Allport, 2000), which helped to refine task-switching models for which a complete “task reconfiguration” (or suppression of the neuronal representations associated with the alternative task) is already achieved by the end of a switch trial (Rogers and Monsell, 1995). Second, Group 3 shows evidence of stronger residual interference that continues to be present while being progressively suppressed over multiple consecutive repeat trials. This sustained interference increases the reaction time in repeat trials and decreases the switch cost for Group 3.
3.2. Modulation of the cortical oscillatory activity
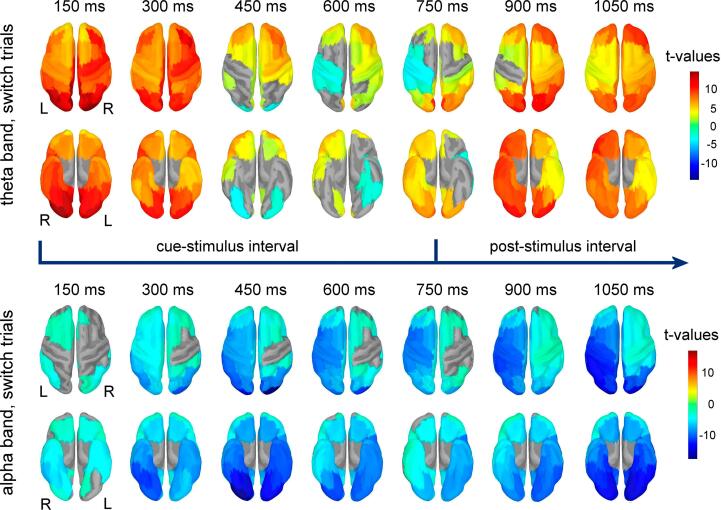
Results of the t-tests comparing the relative change in power against baseline are exemplified in Fig. 2 for theta and alpha bands (switch trials) and in Fig. 3 for beta band (switch trials). Additional results obtained for repeat trials are shown for each of these frequency bands in Supplementary Fig. 1 (included in Supplementary Material). For the theta band, significant increases in power were present in all brain regions immediately after the cue onset. In multiple prefrontal regions, the increase in power persisted throughout the late part of the temporal interval between the cue and test stimuli, as well as on the temporal interval following the onset of the test stimuli. Bilateral regions of the occipital and temporal lobes, as well as regions of the left posterior parietal, somatomotor, premotor and insular cortex showed a relative decrease in theta power during the late part of the cue-test stimulus interval. Following the onset of the test stimuli, a relative increase in theta power was observed again in the majority of these regions. The alpha and beta band power showed significant decrease compared to the baseline over extended cortical areas from occipital, parietal, temporal and frontal lobes. This decrease in power started immediately after the cue onset and persisted throughout the late cue-test stimulus interval, as well as on the interval following the onset of the test-stimuli. A similar decrease relative to baseline was observed for gamma power (Supplementary Fig. 2) in multiple brain regions, with the exception of occipital regions that showed significant bilateral increases in gamma power starting immediately after the cue onset and persisting throughout the cue-test stimulus interval and on the interval following the test-stimuli onset.
Fig. 2.
Results of t-tests contrasting the regional theta power (upper two rows) and alpha power (lower two rows) against the corresponding baseline power for the switch condition. Power was estimated on 300 ms long intervals, with 50 % overlap between consecutive intervals. Latencies correspond to the center of each interval and are relative to the cue onset. Results show regions with significant power change in top (upper rows) and bottom views (lower rows) for each frequency band. Test stimuli were presented at 750 ms after the cue onset.
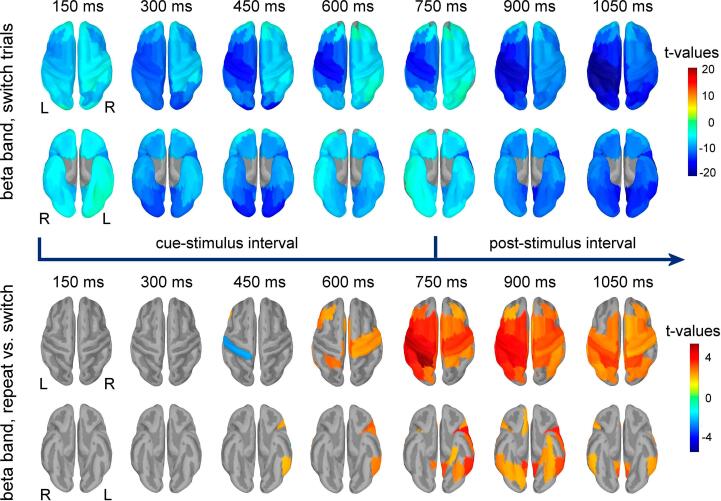
Fig. 3.
Results of the t-tests contrasting the regional beta power against baseline power for the switch condition are shown in the upper two rows. Power was estimated on 300 ms long intervals, with 50 % overlap between consecutive intervals. Latencies correspond to the center of each interval and are relative to the cue onset. For each temporal interval, regions with significant power change are shown in top (upper rows) and bottom (lower rows) views of the brain. Test stimuli were presented at 750 ms after the cue onset. The results of paired t-tests contrasting the regional relative change in beta power in repeat versus switch trials are shown for each temporal interval in the lower two rows in top and bottom views of the brain, respectively.
Results of the paired t-tests comparing the relative change in power in repeat versus switch trials are exemplified in Fig. 3 for the beta band, and in Supplementary Fig. 3 (Supplementary Material) for the theta and alpha bands. During the interval between cue and test-stimuli, significant differences between these two conditions were present only in alpha and beta bands. These differences generally reflected lower power suppression in the repeat compared to switch trials on the last part of this temporal interval (from 450 ms after cue onset). Specifically, less suppression of alpha power was seen in repeat trials over the left temporal lobe (inferior and lateral temporal regions) and left frontal lobe (lateral orbitofrontal and pars opercularis) and left insular cortex. Similarly, less suppression of beta power was present in repeat trials over regions of the left posterior temporal lobe (inferior and lateral temporal regions), left frontal lobe (pars triangularis, pars opercularis, rostral middle frontal), left insular cortex, left superior parietal cortex, bilateral anterior cingulate and paracentral area, and right posterior cingulate and somatomotor cortex. During the peristimulus interval (centered at 750 ms), these differences in alpha and beta band power were present in more cortical areas, including areas that are adjacent to the ones mentioned before; in beta frequency band, differences were also seen over more areas of the right hemisphere.
Following the onset of the test-stimuli, we observed a similar pattern indicative of lower suppression of alpha and beta band activity in the repeat compared to switch trials. In the alpha frequency band (Supplementary Fig. 3), this difference was seen over frontal, temporal and parietal regions of the left hemisphere and over the bilateral anterior cingulate. For beta band (Fig. 3), this difference was present in bilaterally distributed regions including the dorsolateral and ventrolateral prefrontal cortex, the posterior part of the superior frontal gyrus, parietal cortex, posterior cingulate and high order visual processing regions of the inferior temporal and fusiform gyri, right anterior cingulate and right orbitofrontal cortex. Following the onset of the test stimuli, differences were observed also in theta band (Supplementary Fig. 3), with higher theta band activity in repeat compared to switch trials in left supramarginal, left superior parietal and bilateral paracentral cortical regions.
No significant differences between repeat versus switch trials were observed for the relative power in gamma band on any temporal interval (either preceding or following the test stimuli).
No significant differences in baseline power between the two conditions (repeat versus switch trials) were observed in any frequency band.
3.3. Relationship between the modulation of cortical oscillatory activity and PTSD symptom severity
3.3.1. Modulation of oscillatory brain activity during pro-active processes
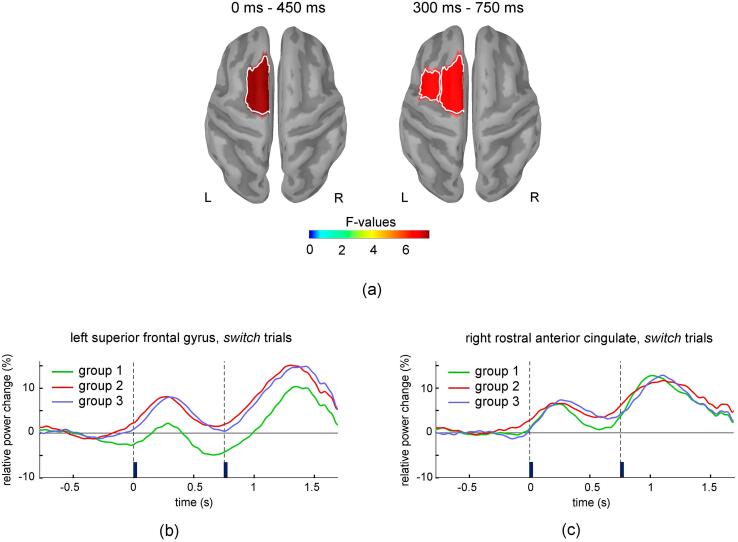
Significant group differences were found in the modulation of theta band activity corresponding to pro-active processes (Fig. 4). These differences were observed only in switch trials during the late part of the cue-test stimulus interval in left caudal middle frontal cortex and posterior part of the left superior frontal gyrus. Using the less conservative approach that controlled the FDR separately for each frequency band and temporal interval, only one more significant group difference was also observed in left superior frontal gyrus during the early part of the cue-test stimulus interval. In these regions, theta activity was higher for Groups 2 and 3 relative to Group 1 (Fig. 4b). Notably, the negative values of the relative change in theta power that are predominant on the late part of the cue-stimulus interval for Group 1 in Fig. 4b indicate that the relatively low theta power is accompanied by a blending effect of the power suppression that is typically present at higher frequencies (alpha band) during this time interval. Furthermore, a slight suppression of theta power appears to emerge for Group 1 shortly before the cue onset, possibly reflecting some preparatory processes in anticipation of the cue. For comparison, the time course of the theta activity is also exemplified in Fig. 4c for the rostral anterior cingulate, which does not show significant group differences and exhibits transient increases in theta band activity following the onsets of cue and test-stimuli in all three groups.
Fig. 4.
Regions with significant group differences for the relative change in theta band power in switch trials are shown in (a) for the early and late part of cue-test stimulus interval. Panel (b) and (c) exemplify the time courses of the mean relative change in theta band power in the switch condition for the left superior frontal gyrus (a region that showed significant group differences on the cue-test stimulus interval), and right rostral anterior cingulate (a region that did not show significant group differences). Vertical lines mark the onset of the cue and test stimulus. To assess its temporal variation, theta band power was averaged on 300 ms long intervals centered at each time sample.
For alpha, beta and gamma bands, no significant group differences were detected during the cue-test stimulus interval for the switch or repeat trials.
3.3.2. Modulation of oscillatory brain activity during reactive processes
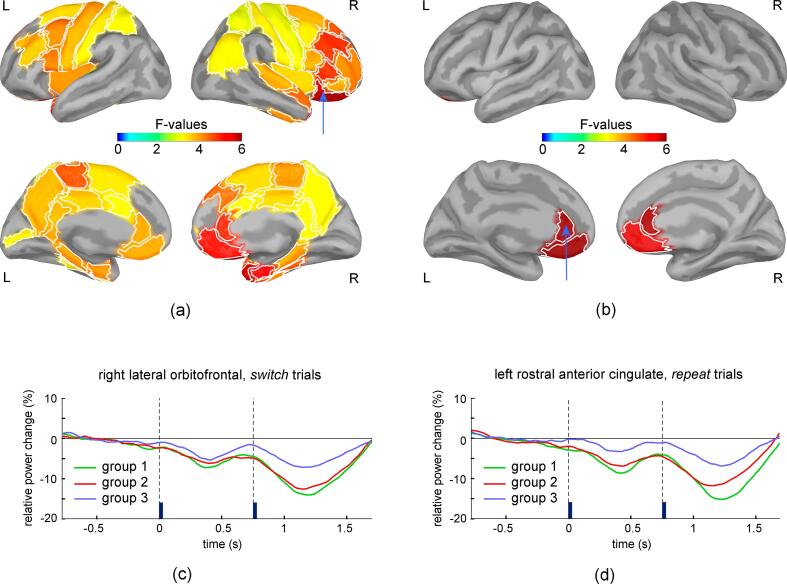
Significant group differences in the modulation of beta band activity were present during the post-stimulus interval (Fig. 5). For switch trials, these differences were present bilaterally over multiple prefrontal, temporal and parietal cortical regions. Particularly strong effects were present in the right lateral and medial orbitofrontal cortex, entorhinal cortex and temporal pole. Group differences were also present for repeat trials, but they were restricted to the bilateral rostral anterior cingulate and medial orbitofrontal cortex. In all these regions, Group 3 showed less suppression of beta band activity compared to Group1 and Group 2 (Fig. 5 c, d). For theta, alpha, and gamma bands, no significant group differences were detected during the post-stimulus interval for the switch or repeat trials. These results indicate that the stronger residual interference that is present for Group 3 during repeat trials, as indicated by the analysis of reaction time data, is associated with less suppression of beta band activity confined to regions of the bilateral rostral anterior cingulate and medial orbitofrontal cortex.
Fig. 5.
Results of one-way ANOVA with factor group for the relative change in beta band power in switch (a) and repeat (b) trials for the temporal interval following the stimulus onset (750 ms to 1200 ms). Regions with significant group differences after correcting for multiple comparisons are shown in lateral views (upper row) and medial views (lower row) of the two hemispheres. Panels (c) and (d) exemplify the time courses of the mean relative change in beta band power for the right lateral orbitofrontal and left rostral anterior cingulate (regions indicated by arrows in (a) and (b)), in the switch and repeat conditions, respectively. Vertical lines mark the onset of the cue and test stimulus. To assess its temporal variation, beta band power was averaged on 300 ms long intervals centered at each time sample.
3.3.3. Assessment of group differences in baseline power
No significant group differences in baseline power were present in any of the frequency bands and conditions (switch or repeat trials). These statistical results were also scrutinized for detection of any potential trends that might have been present for those brain regions, frequency bands and conditions that showed significant group differences for the modulation of brain activity in the main analyses of our study. No such trends were present for the theta band in switch trials or for the beta band in the repeat or switch condition (all uncorrected p-values were higher than 0.05). Based on these results, we conclude that the observed group differences in the modulation of the brain activity cannot be accounted for by group differences in baseline power.
3.4. Assessment of antidepressant medication effects on behavioral performance and cortical oscillatory activity
Some participants in our study were taking medications with central nervous system effects, specifically selective serotonin reuptake inhibitors (SSRI) antidepressants, Prazosin and Gabapentin. Notably, several studies that investigated the effect of antidepressant medication on brain activity have reported changes in the resting-state EEG spectrum that included an increase in beta band power (e.g. Veltmeyer et al., 2006, Hyun et al., 2011). To investigate the potential effect of antidepressant medication on the brain activity recorded during task-switching, we conducted follow-up analyses that compared the behavioral performance and the modulation of oscillatory brain activity between the subgroup of participants with high PTSD symptom severity who were taking antidepressant medication (n = 16) versus the subgroup of participants with high PTSD symptom severity who were not taking antidepressant medication (n = 13). Four participants in each subgroup were taking Gabapentin, whereas six participants in the subgroup who were taking antidepressants were taking Prazosin versus three participants in the subgroup who were not taking antidepressants. The PCL-5 scores did not differ between these two subgroups (Wilcoxon-Mann-Whitney test: uA = 88.5, p = 0.51). Furthermore, t-tests for independent samples showed no significant differences in behavioral performance (accuracy or reaction time) between the two subgroups (all ps > 0.1). Similarly, we used t-tests for independent samples to compare the oscillatory brain activity in theta and beta frequency bands and on the corresponding temporal intervals and conditions (switch and repeat trials) for which significant differences in oscillatory brain activity were identified for participants in Group 3, i.e. during the cue-stimulus interval in the switch condition for the theta band, and during the post-stimulus interval in both switch and repeat conditions for the beta band. As for the main analyses, these comparisons were done over all brain regions and significance thresholds were corrected to control the FDR at q = 0.1 for each test. The analyses did not show any significant differences between the two subgroups of participants. Thus, these follow-up analyses do not provide evidence for an effect of antidepressant medication on the behavioral performance or on the modulation of oscillatory brain activity during task-switching, but we acknowledge that additional studies using a larger number of medicated and unmedicated participants may be necessary to reach a definitive conclusion.
4. Discussion
Our study characterized the association between the severity of PTSD symptoms, behavioral measures of cognitive flexibility, and frequency-specific modulation of cortical oscillatory activity during performance of a test of cognitive flexibility in service members with combat exposure. We used a cued task-switching paradigm that elicits neuronal activity associated with pro-active voluntary attention shifting, and with reactive interference-control mechanisms needed for the execution of motor responses. Participants with high PTSS (Group 3) showed (1) response accuracy that was not different than the other two groups, (2) an increase in reaction time for both switch and repeat trials, which reflects an increased difficulty in performing the task, and (3) a more sustained residual interference during repeat trials, leading to longer reaction time in repeat trials and attenuation of the switch cost. The analysis of MEG data showed higher relative theta power in switch trials during the cue-stimulus interval for participants with moderate (Group 2) and high (Group 3) PTSS over the left dorsal prefrontal cortex. Participants with high PTSS (Group 3) also showed lower suppression of beta band activity in switch trials after stimulus onset compared to the other two groups. This lower suppression was present bilaterally over multiple prefrontal, temporal and parietal cortical regions, but it was confined to the bilateral rostral anterior cingulate and medial orbitofrontal cortex in repeat trials. No group differences were observed for the modulation of alpha and gamma band oscillations. In the following, we will discuss the potential mechanisms underlying the distinct modulation of brain activity in theta and beta bands for participants with high PTSD symptom severity, and how these findings may be related to core symptoms of PTSD.
We had two competing hypotheses regarding potential alterations in theta band oscillatory activity with increasing PTSS. First, if an alteration in theta band power is a primary neurophysiological alteration contributing to impaired cognitive flexibility in PTSD, then patients with higher PTSS would demonstrate lower theta power in cortical regions implicated in cognitive control. Alternatively, if impaired modulation of theta band power is not a primary pathophysiological process, then these patients would demonstrate an increase in theta power as a compensatory mechanism in the presence of impaired cognitive flexibility caused by dysregulation of other neurophysiological mechanisms. Participants with moderate and high PTSS showed higher relative theta power over the left dorsolateral prefrontal cortex (DLPFC) compared to participants with low PTSS, consistent with a compensatory process. The two regions that showed group differences in the modulation of theta band oscillations, i.e. caudal middle frontal cortex and superior frontal gyrus, include pre-motor, supplementary and pre-supplementary motor areas (SMA, pre-SMA), which have been shown to be active in fMRI studies of cognitive control involving attention shifting (Wager et al., 2004) and to exhibit switch-specific preparatory activity during the cue-test stimulus interval (Rushworth et al., 2002, Slagter et al., 2006). Notably, the lateralization of these regions may be related to the fact that participants used the right hand for motor responses and/or to the linguistic nature of the cues used in our study (visual words).
One of the potential mechanisms underlying the emergence of theta band oscillatory activity in cortical regions is the competitive interaction between neighboring neuronal ensembles that are located in each other’s area of significant surround inhibition (Kienitz et al., 2018). Activity of neuronal ensembles from dorsolateral prefrontal cortex regions could reflect, for example, the control of bias inputs to other regions involved in processing of relevant visual stimulus features indicated by the cues or in response selection (MacDonald et al., 2000, Banich et al., 2000, Banich, 2009). The need for increased cognitive control purportedly signaled by the presence of theta oscillations (Cavanagh and Frank, 2014) may be determined by such competitive interactions between neighboring neuronal ensembles; in this case, prefrontal theta activity may also be regarded as a manifestation of increased cognitive control. The lower modulation of theta band activity for participants in Group 1 may indicate that the competition between neuronal ensembles involved in the execution of the alternative tasks is relatively low and/or quickly resolved after the cue presentation, reflecting the effectiveness of attention control. This would be analogous with the drop in activity seen with fMRI in DLPFC with increased practice on a task (Milham et al., 2003). On the other hand, the higher relative theta power observed for Groups 2 and 3 would reflect more effortful shifting and maintenance of attention, associated with higher perceived task difficulty. Notably, more effortful shifting and maintenance of attention can lead to different behavioral outcomes (faster reaction times for Group 2 compared to Group 3) determined by the effectiveness of attention control in suppressing competing neuronal representations; that is, the high theta band activity was effectively compensatory for the group with moderate PTSS but was less so for those with severe symptoms. It is also conceivable that the effectiveness of effortful cognitive control may lead to slightly faster mean reaction times for participants in Group 2 compared to Group 1 (Table 3), although this group difference in reaction times was not statistically significant.
A second working hypothesis was that we would observe a lower suppression of beta power with higher PTSS. Indeed, participants with high PTSS (Group 3) demonstrated lower suppression of beta band activity following the stimulus onset compared to participants with low and moderate PTSD symptom severity (Groups 1 and 2). After the presentation of the test-stimulus, the relevant stimulus features must be processed and integrated across the visual hemifields and a motor response selection must be performed according to the current rule. There is evidence that beta band oscillations may represent the sustained activation or re-activation of neuronal ensembles that perform specific functions (Spitzer and Haegens, 2017), and in some circumstances may reflect the maintenance of a cognitive, emotional or sensorimotor state or status quo (Engel and Fries, 2010). In particular, beta oscillations are normally suppressed during periods of motor preparation and execution (e.g. Doyle et al., 2005, Engel and Fries, 2010, Heinrichs-Graham et al., 2014, Heinrichs-Graham et al., 2018, Wilson et al., 2014). Thus, we will first discuss how the lower beta band suppression for participants in Group 3 may reflect mechanisms related to motor response selection and execution; then, we will address how similar mechanisms may be also expressed in brain areas that are primarily involved in processing of perceptual information.
The task used in our study requires the execution of a motor response according to a stimulus–response contingency that depends on the attended stimulus feature, while inhibiting the neuronal representation of the conflicting stimulus–response contingency used in previous trials. The stronger suppression of beta band activity in switch versus repeat trials observed in our study and others (Cunillera et al., 2012, Foxe et al., 2014, Capizzi et al., 2020) may be explained based on two premises. First, beta band oscillations in prefrontal regions may reflect activity in competitive neuronal ensembles encoding the stimulus–response contingencies corresponding to alternative rules (Buschman et al., 2012). Second, the amplitude of beta band oscillations in the rule appropriate neuronal ensemble may decay with time after the execution of the corresponding motor response, with a lifetime in the range of trial duration. This is supported by evidence that with the cue-test stimulus interval held constant, a longer delay after the last performance of the previous task improves performance on switch trials (Meiran et al., 2000), suggesting a passive dissipation of the neuronal representations of the alternate (interfering) stimulus–response contingency. Based on these premises, relatively strong beta oscillations will be present in a switch trial in neuronal ensembles that encode the stimulus–response mapping corresponding to the irrelevant/conflicting rule used in the immediately preceding trial due to its recency. Planning and execution of the motor response in a switch trial requires the disengagement/inhibition of the activity in these competitive neuronal ensembles exhibiting strong beta band activity, resulting in strong suppression of the regional beta band activity. During a repeat trial, comparatively weaker beta oscillations will be present in neuronal ensembles that encode the irrelevant/conflicting stimulus–response mapping since this rule was used in the more distant past, before the immediately preceding trial. The planning and execution of the response in a repeat trial is thus associated with disengagement/inhibition of neuronal ensembles with comparatively weaker beta band activity, which results in less suppression of the regional beta band activity compared to a switch trial.
It is conceivable that the time needed to disengage (de-synchronize) the relatively strong activity in neuronal assemblies representing the conflicting stimulus–response mapping in a switch trial is longer compared to a repeat trial, contributing to the well documented switch costs. The lower suppression of beta band activity and slower reaction time for participants with high PTSD symptom severity may reflect in part a degree of difficulty disengaging the activity of neuronal ensembles involved in the encoding of the alternate (conflicting) stimulus–response contingency during selection of motor responses, despite effortful attention shifts exerted by dorsal prefrontal cortex as reflected by the higher modulation of theta oscillations. It is also plausible that the execution of a response has a suppressive effect on the neuronal representation of the conflicting stimulus–response contingency. In the presence of a small suppressive effect (e.g. for participants in Group 3), the temporal decay of the neuronal representation of the conflicting stimulus–response contingency may be characterized by longer lifetimes, leading to high activity in competitive neuronal ensembles even during repeat trials. This may explain the sustained residual interference for participants with high PTSS and suggests that the low switch cost for these participants can be more intuitively thought of as a lack of repetition benefit.
In switch trials, the participants with high PTSS show lower suppression of beta band activity after stimulus onset bilaterally over multiple prefrontal, temporal and parietal cortical regions. Since information regarding the relevant stimulus features must be processed and integrated across visual hemifields prior to response selection, the lower suppression of beta band activity in high-order visual processing regions of the temporal lobe, as well as in some prefrontal and parietal regions to which they are connected, likely reflects persistent activity in local neuronal ensembles that were involved in processing and integration of the alternative stimulus feature in the immediately preceding trial. A lower suppression of beta band activity was also present for participants from Group 3 in repeat trials, but it was confined to bilateral rostral anterior cingulate and medial orbitofrontal cortex, suggesting a longer lasting representation of conflicting neuronal representations in these regions that was manifested as sustained residual interference during repeat trials. The longer reaction times for participants in Group 3 could also stem in part from a voluntary adjustment of behavior, i.e. a compensatory speed-accuracy tradeoff indicative of a more cautious approach after experiencing difficulty with response selection in preceding trials. In particular, since the orbitofrontal cortex is thought to play an important role in voluntary inhibition of action (Balasubramani et al., 2020), the lower suppression of beta band activity in this region may reflect activity in neuronal pools that control the delay of motor responses based on adaptive strategies, such as the choice of a cautious approach.
The different patterns of interplay observed across groups between modulations of theta band activity in left DLPFC during the cue-test stimulus interval and of beta band activity in other brain regions after test-stimulus onset can be interpreted within the framework of the cascade model of cognitive control described in (Banich, 2009). This model posits that DLPFC regions are involved in shifting and maintaining attention to task-relevant information (Banich et al., 2000, MacDonald et al., 2000), providing bias signals that confer a competitive advantage for processing of relevant stimulus features in other brain regions and for conflict resolution and selection of appropriate motor responses in anterior cingulate cortex (ACC). When bias signals from DLPFC are less effective, this will ultimately affect (e.g. slow down) the selection of motor responses that takes place at later stages in ACC. Notably, as control by DLPFC becomes more effective with increased practice, the activity measured with fMRI diminishes in DLPFC and ACC (Milham et al., 2003). In our study, Group 1 showed lower theta band activity in left DLPFC in switch trials compared to the other two groups, accompanied by strong suppression of beta-band activity in other brain regions including ACC. Since these findings are associated with relatively fast reaction times, they are analogous to the lower activity observed with fMRI in DLPFC and ACC with increased practice on a task (Milham et al., 2003), being likely indicative of low perceived task difficulty. Group 2 showed a marker of more effortful cognitive control (high theta band activity in left DLPFC) when compared to Group 1, accompanied by similarly strong suppression of beta-band activity in other brain regions including ACC. Since this pattern is also associated with relatively fast reaction times, it suggests that the effortful cognitive control exerted by the left DLPFC is effective at suppressing competitive neuronal representations in other cortical regions. Lastly, Group 3 showed markers of effortful cognitive control (similar to Group 2) but low suppression of beta-band activity in multiple brain regions including ACC. This pattern suggests that the bias signals from left DLPFC are less effective at suppressing the representations of competitive information in other brain regions in general and in ACC in particular. As a consequence, this leads to longer reaction time in switch trials, as well as to sustained interference and high beta band activity in ventromedial prefrontal cortex during repeat trials for participants in this group.
A general difficulty with inhibition of neuronal representations in anterior cingulate and medial orbitofrontal cortex, which is due in part to ineffective bias signals exerted by DLPFC, may represent a shared neurobiological substrate for difficulties with set-shifting and core PTSD symptoms. Evidence has shown that the orbitofrontal cortex (Rolls and Grabenhorst, 2008, Rolls, 2013) and the anterior cingulate, particularly its rostral region (Etkin et al., 2006, Etkin et al., 2011), belong to a brain network involved in the representation of emotions. Emotions can be conceptualized as intermediary states elicited by rewards and punishers and are intrinsically associated with activation of the neuronal representations for specific goals that guide selection of appropriate behavioral actions (Rolls, 2013). Top-down cognitive control can influence these neuronal representations through biased competition mechanisms in a way that is analogous to attention control (Rolls and Grabenhorst, 2008, Rolls, 2013, page 40), suggesting a modulatory route of cognition on emotion representation. Hence, general difficulties with inhibition of neuronal representations in these ventromedial prefrontal regions may undermine the ability to inhibit emotional reactions (or regulate emotions), a symptom known to be associated with PTSD (Jovanovic and Ressler, 2010). Difficulties with disengaging and re-orienting attention away from or with inhibition of automatic responses to trauma related memories with strong emotional components may contribute in turn to the development and maintenance of a whole spectrum of characteristic PTSD symptoms such as hyperarousal, irritability, or difficulties concentrating, and may lead to the adoption of alternative coping strategies reflected in avoidance behaviors (Aupperle et al., 2012).
As we have noted, deficits in cognitive flexibility in the aftermath of a psychologically traumatic event are predictive of the subsequent development of PTSD, with the severity of the cognitive flexibility impairment correlating with the severity of PTSD symptoms; additionally, successful remediation of cognitive flexibility deficits with cognitive flexibility training has been shown to reduce the incidence of subsequent PTSD (Ben-Zion et al., 2018). What has not been established is whether or not such deficits in cognitive flexibility are present prior to the traumatic event, whether the experience of the traumatic event induces deficits in cognitive flexibility de novo or whether the traumatic event produces a worsening of a milder pre-existing deficit in cognitive flexibility. To the degree that such deficits are premorbid, they could be assessed in certain high risk populations, such as active duty military personnel, to predict relative vulnerability to the development of PTSD in association with traumatic stress. If so, strategies such as cognitive therapy and/or neurofeedback therapy to reduce beta band activity during rule switching tasks could be utilized therapeutically in a preventative manner. To the degree that such deficits may be acquired in the aftermath of an exposure to a traumatic stressor during the experience of acute stress or the development of post-traumatic stress syndrome, such neurofeedback therapy could be initiated as an early treatment strategy to minimize acute stress symptoms and potentially prevent the ultimate development of PTSD. Animal studies have demonstrated that exposure to prolonged stress could in fact result in newly acquired deficits in cognitive flexibility in association with PTSD-like symptoms (George et al., 2015), suggesting that in at least a subgroup of individuals with PTSD and cognitive flexibility impairments, the latter phenomenon was a result of the traumatic stressor rather than a premorbid deficit. Furthermore, alleviation of conditioned fear extinction deficits in a PTSD animal model with the administration of brain derived growth factor in infralimbic cortex (the rodent analogue of ventromedial prefrontal/medial orbitofrontal cortex in humans) secondarily resulted in an improvement in a deficit in set shifting (Paredes et al., 2021). This implies that fear extinction learning (an impairment of which has been proposed to be a primary process underlying PTSD core symptoms), and set shifting share a common neurobiological substrate and that effective treatment of one will have secondary beneficial effects on the other, which may explain the beneficial effect of cognitive flexibility training in the aftermath of a traumatic event. Whether or not fear extinction deficits and set shifting deficits also share patterns of altered cortical oscillatory activity (such as impaired beta activity modulation in a ventromedial prefrontal cortex circuit during extinction trials correlating with impaired extinction) remains to be elucidated.
Our sample of participants was limited to adult males with a history of combat exposure, which necessitates a discussion about the generalizability of the results to other populations and types of trauma. It is noteworthy that lower cognitive flexibility is a risk factor for the development of PTSD irrespective of gender and trauma type, and interventions aiming to improve cognitive flexibility were successful at reducing PTSD symptom severity over time for both males and females (Ben-Zion et al., 2018). This suggests that the neurobiological substrate linking cognitive flexibility to core PTSD symptoms is not dependent of gender or trauma type. Nevertheless, since other studies have found that male gender, higher age, and war trauma are factors related to poorer executive functioning in PTSD (Polak et al., 2012), we conclude that future studies are needed to understand how our results generalize to other populations and types of trauma.
CRediT authorship contribution statement
Mihai Popescu: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft. Elena-Anda Popescu: Methodology, Software, Formal analysis, Writing – review & editing. Thomas J. DeGraba: Conceptualization, Writing – review & editing. John D. Hughes: Conceptualization, Methodology, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Authors would like to thank the participants who volunteered for this study. They also want to thank Jacqueline Dyer, Isabella Salmon and Rebecca Sandlain for study coordination, David Fernandez-Fidalgo and Andrew Bryant for MEG data acquisition, and Adam Cliffton, Joe Hindinger and the neuroimaging team led by Dr. Grant Bonavia for technical and logistical support with MRI acquisition.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclaimers
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of Defense or the U.S. Government.
The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the authors, DoD, or any component agency.
The study protocol was approved by the Walter Reed National Military Medical Center in compliance with all applicable Federal regulations governing the protection of human subjects.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103297.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data may be made available on request contingent on institutional approval.
References
- American Congress of Rehabilitation Medicine Definition of mild traumatic brain injury. J. Head Trauma Rehab. 1993;8(3):86–87. [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. Arlington, VA, American Psychiatric Association 2013, p. 271-276.
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani P.P., Pesce M.C., Hayden B.Y. Activity in orbitofrontal neuronal ensembles reflects inhibitory control. Eur. J. Neurosci. 2020;51(10):2033–2051. doi: 10.1111/ejn.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich M.T. Executive function: the search for an integrated account. Curr. Dir. Psychol. Sci. 2009;18:89. [Google Scholar]
- Banich M.T., Milham M.P., Atchley R.A., Cohen N.J., Webb A., Wszalek T., Kramer A.F., Liang Z., Barad V., Gullett D., Shah C., Brown C. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cogn. Brain Res. 2000;10(1–2):1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Beckham J.C., Crawford A.L., Feldman M.E. Trail making test performance in Vietnam combat veterans with and without posttraumatic stress disorder. J. Trauma. Stress. 1998;11(4):811–819. doi: 10.1023/A:1024409903617. [DOI] [PubMed] [Google Scholar]
- Ben-Zion Z., Fine N.B., Keynan N.J., Admon R., Green N., Halevi M., Fonzo G.A., Achituv M., Merin O., Sharon H., Halpern P., Liberzon I., Etkin A., Hendler T., Shalev A.Y. Cognitive flexibility predicts PTSD symptoms: observational and interventional studies. Front. Psychiatry. 2018;9:477. doi: 10.3389/fpsyt.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma. Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Bovin M.J., Marx B.P., Weathers F.W., Gallagher M.W., Rodriguez P., Schnurr P.P., Keane T.M. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in Veterans. Psychol. Assess. 2015;28:1379–1391. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E., Afzal N., Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2004;192(10):643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- Buschman T.J., Denovellis E.L., Diogo C., Bullock D., Miller E.K. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76(4):838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Capizzi M., Ambrosini E., Arbula S., Vallesi A. Brain oscillatory activity associated with switch and mixing costs during reactive control. Psychophysiology. 2020;57:e13642. doi: 10.1111/psyp.13642. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.S., Wong A.S.W., McKewen M., Michie P.T., Karayanidis F. Frontoparietal theta oscillations during proactive control are associated with goal-updating and reduced behavioral variability. Biol. Psychol. 2017;129:253–264. doi: 10.1016/j.biopsycho.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Cunillera T., Fuentemilla L., Periañez J., Marco-Pallarès J., Krämer U.M., Càmara E., Rodríguez-Fornells A. Brain oscillatory activity associated with task switching and feedback processing. Cogn. Affect. Behav. Neurosci. 2012;12(1):16–33. doi: 10.3758/s13415-011-0075-5. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into Gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dohrenwend B.P., Turner J.B., Turse N.A., Adams B.G., Koenen K.C., Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L.M., Yarrow K., Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin. Neurophysiol. 2005;116(8):1879–1888. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dunkley B.T., Sedge P.A., Doesburg S.M., Grodecki R.J., Jetly R., Shek P.N., Taylor M.J., Pang E.W. Theta, mental flexibility, and post-traumatic stress disorder: connecting in the parietal cortex. PLoS One. 2015;10(4):e0123541. doi: 10.1371/journal.pone.0123541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzel E., Penny W.D., Burgess N. Brain oscillations and memory. Curr. Opin. Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations–signalling the status quo? Curr. Opin. Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Etkin A., et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory J.D., Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 2015;17(2):141–150. doi: 10.31887/DCNS.2015.17.2/jflory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe J.J., Murphy J.W., De Sanctis P. Throwing out the rules: Anticipatory alpha-band oscillatory attention mechanisms during task-set reconfigurations. Eur. J. Neurosci. 2014;39(11):1960–1972. doi: 10.1111/ejn.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S.A., Rodriguez-Santiago M., Riley J., Abelson J.L., Floresco S.B., Liberzon I. Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav. Brain Res. 2015;286:256–264. doi: 10.1016/j.bbr.2015.02.051. [DOI] [PubMed] [Google Scholar]
- Gilbertson M.W., Paulus L.A., Williston S.K., et al. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J. Abnorm. Psychol. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- Gould I.C., Rushworth M.F., Nobre A.C. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J. Neurophysiol. 2011;105:1318–1326. doi: 10.1152/jn.00653.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D., Strohmeier D., Brodbeck C., Parkkonen L., Hämäläinen M.S. MNE software for processing MEG and EEG data. Neuroimage. 2014;86:446–460. doi: 10.1016/j.neuroimage.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S., Händel B.F., Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J. Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M.S., Ilmoniemi R.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Compu. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Staudigl T., Fellner M.C. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci. 2012;6:74. doi: 10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W., Santamaria P.M., Heithoff S.K., Torres-Russotto D., Hutter-Saunders J.A., Estes K.A., Meza J.L., Mosley R.L. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb. Cortex. 2014;24:2669–2678. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., McDermott T.J., Mills M.S., Wiesman A.I., Wang Y.P., Stephen J.M., Calhoun V.D., Wilson T.W. The lifespan trajectory of neural oscillatory activity in the motor system. Dev. Cogn. Neurosci. 2018;30:159–168. doi: 10.1016/j.dcn.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hu J.X., Zhao H., Zhou H.H. False discovery rate control with groups. J. Am. Stat. Assoc. 2010;105(491):1215–1227. doi: 10.1198/jasa.2010.tm09329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X., Mosher J.C., Leahy R.M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999;44:423–440. doi: 10.1088/0031-9155/44/2/010. [DOI] [PubMed] [Google Scholar]
- Huang M., Yurgil K.A., Robb A., Angeles A., Diwakar M., Risbrough V.B., Nichols S.L., McLay R., Theilmann R.J., Song T., Huang C.W., Lee R.R., Baker D.G. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. Neuroimage Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J., Baik M.J., Kang U.G. Effects of psychotropic drugs on quantitative EEG among patients with Schizophrenia-spectrum disorders. Clin. Psychopharmacol. Neurosci. 2011;9(2):78–85. doi: 10.9758/cpn.2011.9.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.A., Langlais P.J., Delis D.A. Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. Clin. Neuropsychol. 2000;14:7–12. doi: 10.1076/1385-4046(200002)14:1;1-8;FT007. [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Ressler K.J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry. 2010;167(6):648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Khanna M.M., Badura-Brack A.S., McDermott T.J., Embury C.M., Wiesman A.I., Shepherd A., Ryan T.J., Heinrichs-Graham E., Wilson T.W. Veterans with post-traumatic stress disorder exhibit altered emotional processing and attentional control during an emotional Stroop task. Psychol. Med. 2017;47(11):2017–2027. doi: 10.1017/S0033291717000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz R., Schmiedt J.T., Shapcott K.A., Kouroupaki K., Saunders R.C., Schmid M.C. Theta rhythmic neuronal activity and reaction times arising from cortical receptive field interactions during distributed attention. Curr. Biol. 2018;28(15):2377–2387. doi: 10.1016/j.cub.2018.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I.T., Wienbruch C., Neuner F., Schauer M., Ruf M., Odenwald M., Elbert T. Altered oscillatory brain dynamics after repeated traumatic stress. BMC Psychiatry. 2007;7:56. doi: 10.1186/1471-244X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koso M., Hansen S. Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. Eur. Psychiatry. 2006;21:167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy R.M., Mosher J.C., Spencer M.E., Huang M.X., Lewine J.D. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr. Clin. Neurophysiol. 1998;107(2):159–173. doi: 10.1016/s0013-4694(98)00057-1. [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., 3rd, Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Koenen K.C., Friedman M.J., Ruscio A.M., Karam E.G., Shahly V., Stein D.J., Hill E.D., Petukhova M., Alonso J., Andrade L.H., Angermeyer M.C., Borges G., de Girolamo G., de Graaf R., Demyttenaere K., Florescu S.E., Mladenova M., Posada-Villa J., Scott K.M., Takeshima T., Kessler R.C. Subthreshold posttraumatic stress disorder in the world health organization world mental health surveys. Biol. Psychiatry. 2015;77(4):375–384. doi: 10.1016/j.biopsych.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N., et al. Component processes in task switching. Cogn. Psychol. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Milham M.P., Banich M.T., Claus E., Cohen N. Practice related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Mišić B., Dunkley B.T., Sedge P.A., Da Costa L., Fatima Z., Berman M.G., Doesburg S.M., McIntosh A.R., Grodecki R., Jetly R., Pang E.W., Taylor M.J. Post-traumatic stress constrains the dynamic repertoire of neural activity. J. Neurosci. 2016;36(2):419–431. doi: 10.1523/JNEUROSCI.1506-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn. Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Murphy D., Ross J., Ashwick R., Armour C., Busuttil W. Exploring optimum cut-off scores to screen for probable posttraumatic stress disorder within a sample of UK treatment-seeking veterans. Eur. J. Psychotraumatol. 2017;8(1):1398001. doi: 10.1080/20008198.2017.1398001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella M., Hruska B., Delahanty D. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J. Anxiety Disord. 2013;27(1):33–46. doi: 10.1016/j.janxdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Pang E.W., Sedge P., Grodecki R., Robertson A., MacDonald M.J., Jetly R., Shek P.N., Taylor M.J. Color or shape: examination of neural processes underlying mental flexibility in posttraumatic stress disorder. Transl. Psychiatry. 2014 doi: 10.1038/tp.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes D., Knippenberg A.R., Morilak D.A. Infralimbic BDNF signaling is necessary for the beneficial effects of extinction on set shifting in stressed rats. Neuropsychopharmacology. 2021;47(2):1–9. doi: 10.1038/s41386-021-01171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A.R., Witteveen A.B., Reitsma J.B., Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J. Affect. Disord. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Popescu M., Hughes J.D., Popescu E.A., Reidy G., DeGraba T.J. Reduced prefrontal MEG alpha-band power in mild traumatic brain injury with associated posttraumatic stress disorder symptoms. Clin. Neurophysiol. 2016;127(9):3075–3085. doi: 10.1016/j.clinph.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Popescu M., Popescu E.A., DeGraba T.J., Fernandez-Fidalgo D., Riedy G., Hughes J.D. Post-traumatic stress disorder is associated with altered modulation of prefrontal alpha band oscillations during working memory. Clin. Neurophysiol. 2019;130:1869–1881. doi: 10.1016/j.clinph.2019.06.227. [DOI] [PubMed] [Google Scholar]
- Popescu M., Popescu E.A., DeGraba T.J., Hughes J.D. Altered modulation of beta band oscillations during memory encoding is predictive of lower subsequent recognition performance in post-traumatic stress disorder. NeuroImage: Clinical. 2020;25 doi: 10.1016/j.nicl.2019.102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec A.L., Wiesman A.I., Wilson T.W. The strength of alpha and gamma oscillations predicts behavioral switch costs. Neuroimage. 2019;188:274–281. doi: 10.1016/j.neuroimage.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi S.U., Long M.E., Bradshaw M.R., Pyne J.M., Magruder K.M., Kimbrell T., Hudson T.J., Jawaid A., Schulz P.E., Kunik M.E. Does PTSD impair cognition beyond the effect of trauma? J. Neuropsychiatry Clin. Neurosci. 2011;23(1):16–28. doi: 10.1176/jnp.23.1.jnp16. [DOI] [PubMed] [Google Scholar]
- Rogers R.D., Monsell S. Costs of a predictable switch between simple cognitive tasks. J. Exp. Psychol. Gen. 1995;124:207–231. [Google Scholar]
- Rolls E.T. Oxford University Press; Oxford: 2013. Emotion and Decision Making Explained. [Google Scholar]
- Rolls E.T., Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog. Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Ruscio A.M., Ruscio J., Keane T.M. The latent structure of posttraumatic stress disorder: a taxometric investigation of reactions to extreme stress. J. Abnorm. Psychol. 2002;111:290–301. [PubMed] [Google Scholar]
- Rushworth M.F.S., Hadland K.A., Paus T., Sipila P.K. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J. Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rytwinski N.K., Scur M.D., Feeny N.C., Youngstrom E.A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J. Trauma. Stress. 2013;26(3):299–309. doi: 10.1002/jts.21814. [DOI] [PubMed] [Google Scholar]
- Sareen J., Cox B.J., Stein M.B., Afifi T.O., Fleet C., Asmundson G.J. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom. Med. 2007;69(3):242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- Schell T.L., Marshall G.N. In: Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Tanielian T., Jaycox L.H., editors. RAND Corporation; Santa Monica, CA: 2008. Survey of individuals previously deployed for OEF/OIF; pp. 87–117. [Google Scholar]
- Scott J.C., Matt G.E., Wrocklage K.M., Crnich C., Jordan J., Southwick S.M., Krystal J.H., Schweinsburg B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015;141(1):105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter H.A., Weissman D.H., Giesbrecht B., Kenemans J.L., Mangun G.R., Kok A., Woldorff M.G. Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn. Affect. Behav. Neurosci. 2006;6:175–189. doi: 10.3758/cabn.6.3.175. [DOI] [PubMed] [Google Scholar]
- Spitzer, B., Haegens, S. (2017). Beyond the status quo: a role for beta oscillations in endogenous content (re)activation. eNeuro 4 (4) ENEURO.0170-17.2017. [DOI] [PMC free article] [PubMed]
- Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of the PRIME-MD: the PHQ primary care study. J. Am. Med. Assoc. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Tadel F., Baillet S., Mosher J.C., Pantazis D., Leahy R.M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011;2011:1–13. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuro A., Ambrosini E., Puccioni O., Vallesi A. Brain oscillations in cognitive control: a cross-sectional study with a spatial Stroop task. Neuropsychologia. 2019;133:107190. doi: 10.1016/j.neuropsychologia.2019.107190. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125e133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Veltmeyer M.D., McFarlane A.C., Bryant R.A., Mayo T., Gordon E., Clark C.R. Integrative assessment of brain function in PTSD: brain stability and working memory. J. Integr. Neurosci. 2006;5(1):123–138. doi: 10.1142/s0219635206001057. [DOI] [PubMed] [Google Scholar]
- Wager T., Jonides J., Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Waldhauser G.T., Dahl M.J., Ruf-Leuschner M., Müller-Bamouh V., Schauer M., Axmacher N., Elbert T., Hanslmayr S. The neural dynamics of deficient memory control in heavily traumatized refugees. Sci. Rep. 2018;8(1):13132. doi: 10.1038/s41598-018-31400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Heinrichs-Graham E., Becker K.M. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 2014;102:531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden M.S., Foxe J.J., Wang N., Simpson G.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific-band electroencephalography increases over occipital cortex. J. Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G., Allport A. Task switching and the measurement of “switch costs”. Psychol. Res. 2000;63:212–233. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Golier J.A., Tischler L., Stavitsky K., Harvey P.D. Learning and memory in aging combat veterans with PTSD. J. Clin. Exp. Neuropsychol. 2005;27(4):504–515. doi: 10.1080/138033990520223. [DOI] [PubMed] [Google Scholar]
- Zhao J., Liang W.K., Juan C.H., Wang L., Wang S., Zhu Z. Dissociated stimulus and response conflict effect in the Stroop task: evidence from evoked brain potentials and brain oscillations. Biol. Psychol. 2015;104:130–138. doi: 10.1016/j.biopsycho.2014.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be made available on request contingent on institutional approval.