Abstract

The synthesis and investigation of the physicochemical properties of a novel one-dimensional (1D) hybrid organic–inorganic perovskitoid templated by the 1,1,1-trimethylhydrazinium (Me3Hy+) cation are reported. (Me3Hy)[PbI3] crystallizes in the hexagonal P63/m symmetry and undergoes two phase transitions (PTs) during heating (cooling) at 322 (320) and 207 (202) K. X-ray diffraction data and temperature-dependent vibrational studies show that the second-order PT to the high-temperature hexagonal P63/mmc phase is associated with a weak change in entropy and is related to weak structural changes and different confinement of cations in the available space. The second PT to the low-temperature orthorhombic Pbca phase that corresponds to the high change in entropy and dielectric switching is associated with an ordering of the trimethylhydrazinium cations, re-arrangement and strengthening of hydrogen bonds, and slightly shifted lead-iodide octahedral chains. The high-pressure Raman data revealed two additional PTs, one between 2.8 and 3.2 GPa, related to the symmetry decrease, ordering of the cations, and inorganic chain distortion, and the other in the 6.4–6.8 GPa range related to the partial and reversible amorphization. Optical studies revealed that (Me3Hy)[PbI3] has a wide band gap (3.20 eV) and emits reddish-orange excitonic emission at low temperatures with an activation energy of 65 meV.
Short abstract
We present the synthesis and detailed physicochemical evaluation of a novel perovskitoid, 1,1,1-trimethylhydrazinium lead iodide. The compound undergoes two phase transformations, one of which is an order−disorder transformation associated with dielectric switching. At low temperatures, the material emits a reddish-orange luminescence, with temperature having a significant impact on intensity.
Introduction
Lead halides are one of the most well-known and researched groups of hybrid organic–inorganic perovskites, particularly for their photovoltaic applications.1,2 They may exhibit interesting ferroelectric,3−5 ferroelastic,6,7 magnetic,8 second harmonic generation,9 switchable dielectric,10 or luminescence8,10,11 properties. While typical hybrid perovskites with a tolerance factor (TF) of 0.8 to 1 adopt a three-dimensional (3D) corner-sharing structure, compounds with higher TF are more likely to form one- or two-dimensional structures. Some of them have recently been called “perovskitoids.”
Stoumpos et al. introduced this new term in materials science to describe compounds having ABX3 stoichiometry and exclusively face-sharing connectivity between BX6 octahedra.12 The physicochemical properties of those hybrids (in oxide nomenclature termed 2H polytype) significantly differ from corner-sharing archetypical perovskites (3C polytype).12 Later, the term “perovskitoid” was extended to include structures similar to perovskite but with connectivity other than corner-sharing, such as face- or edge-sharing.13
Similar to perovskites, perovskitoids also have interesting physicochemical properties that are important for optoelectronics.12−14 One of them is the improvement of the application properties of perovskite solar cells (PSCs).15−17 Despite many advantages of the commonly used MAPbI3 and FAPbI3 (MA+ = methylammonium cation; FA+ = formamidinium cation), such as low-production costs and high-power conversion efficiency, PSCs have relatively low intrinsic stability. One of the suggested ways to boost photovoltaic properties of PSCs is the passivation of the surface with other 1D perovskitoids templated by bulky ammonium cations, like diethyl-(2-chloroethyl)ammonium,16 cyclohexylmethylammonium,18 or methyl-1,3-propanediammonium.15
In recent years, hybrids templated by the hydrazinium cation19 and its derivatives, such as methylhydrazinium8−11,20 or 1,1-dimethylhydrazinium,21,22 have received attention. Both cations were successfully employed to form 3D or two-dimensional (2D) perovskites in halide,9−11 hypophosphite,8 or formate frameworks.21−23
Despite the suggestions that partially substituting the MA+ in MAPbI3 with the 1,1,1-trimethylhydrazinium cation (Me3Hy+) would improve the photovoltaic properties and increase the intrinsic stability,17 there is no published structure and detailed physicochemical characterization of (Me3Hy)[PbI3]. As a result, we have decided to undertake methodological research on this perovskitoid in order to define the observed phase transitions (PTs), clarify their mechanisms in detail, and to characterize the optical and phonon properties of each phase. We have also performed high-pressure Raman measurements of the low-wavenumber mode region, which is believed to be particularly sensitive even to very subtle structural changes, in order to assess the stability and flexibility of (Me3Hy)[PbI3].
Experimental Section
Materials and Synthesis
Commercially available lead(II) iodide (99%, Sigma-Aldrich, US), 1,1,1-trimethylhydrazinium iodide (97%, Sigma-Aldrich, US), N,N-dimethylformamide (DMF) (99.8%, Sigma-Aldrich, US), dimethylsulfoxide (DMSO) (99.9%, Merck), and methyl acetate (99.5%, Sigma-Aldrich, US) were employed without additional purification.
The antisolvent method was used to synthesize (Me3Hy)[PbI3]. Lead(II) iodide and 1,1,1-trimethylhydrazinium iodide were dissolved in a mixture of DMF and DMSO (5:1). This solution was placed in a glass vial with a loose top, which was then placed in a bigger vial containing methyl acetate (antisolvent). After 5 days, yellow crystals were extracted, filtered from the mother solution, and dried at room temperature in air. The phase purity of crystals was validated by the good agreement of the powder X-ray diffraction (PXRD) pattern with the simulated one based on the single-crystal data (Figure S1).
X-ray Diffraction
All of the data for (Me3Hy)[PbI3] were collected using an XtaLAB Synergy-S (Rigaku—Oxford Diffraction) four circle diffractometer with monochromatic and microfocus MoKα (λ = 0.71073 Å) radiation. Experiments were carried out for a single yellow crystal with dimensions 0.139 × 0.112 × 0.083 mm, at 215, 230, 260, 290, 310, 330, 350, and 375 K temperatures. The complete data sets were collected at 230 and 375 K. For other temperatures, the strategy implemented in CrysAlis was applied. The data sets were obtained and processed with CrysAlisPro software (CrysAlisPRO (ver. 41_64.104a), Oxford Diffraction/Agilent Technologies UK Ltd, Yarnton, England). Absorption correction was also taken into account in CrysAlisPro software. The structures were solved using SHELX2014 program packages by direct methods and refined with the full-matrix least-squares method on F2.24 Nonhydrogen atoms in all phases were refined anisotropically, and the positions of hydrogen atoms were idealized with geometry and constrained during refinement with an implemented model in SHELX. The final refinement cycles were performed with the EXTI command due to the very high absorption coefficient reaching ca. 20 mm–1. In the final model of the structure at 375 K, two hydrogen atoms from theNH2 group are missing. The figures of (Me3Hy)[PbI3] were prepared using Mercury 4.0 software.25
The powder data for low-temperature (LT) phase were collected using a Rigaku XtaLAB Synergy-S system and CuKα radiation (λ = 1.54056 Å). The data were reduced first in CrysAlisPro and then in EXPO2014. The cell parameters were found using the McMaille routine, and then the space group was determined. The structure was solved by direct methods followed by a resolution bias modification procedure, and the chain was identified. Fourier recycling resulted in the location of the organic cation. Hydrogen atoms were assigned to calculated positions. To keep the reasonable geometry of the Me3Hy+ cation, several restraints for bond lengths and valence angles were applied. In the final model, two nitrogen atoms from the NH2 group were missing.
The structural data have been deposited at the Cambridge Crystallographic Data Centre [2159778 for the room-temperature (RT) phase and 2159781 for the high-temperature (HT) phase].
Thermal Properties
Heat capacity was measured using a Mettler Toledo DSC-1 calorimeter (DSC, differential scanning calorimetry) with a high resolution of 0.4 μW. Nitrogen was used as a purging gas, and the heating and cooling rate was 5 K min–1. The mass of the measured sample was 16.46 mg. The excess heat capacity associated with the PT was calculated by subtracting from the data a baseline representing the system variation in the absence of PTs.
Thermogravimetric analysis (TGA) was performed in the temperature range of 303–973 K using a PerkinElmer TGA 4000. The sample weighed 28.80 mg, and a 5 K min–1 heating rate was used. The atmosphere was pure nitrogen gas.
Dielectric Properties
Dielectric measurements of the examined samples were carried out using a broad-band impedance Novocontrol Alpha analyzer. Since the obtained single crystals were not large enough to perform single-crystal dielectric measurements, a pellet was placed between two copper, flat electrodes of the capacitor with a gap of 0.5 mm. A sinusoidal voltage with an amplitude of 1 V and a frequency in the range of 1 Hz to 1 MHz was applied across the sample. The measurements were taken every 1 K in the temperature range of 140–360 K. The temperature stability of the samples was better than 0.1 K. The temperature was stabilized by means of nitrogen gas using the Novocontrol Quattro system.
Vibrational Properties
The Raman spectra (3500–75 cm–1 range) of a polycrystalline sample were recorded at RT using a Bruker FT 100/S spectrometer and YAG:Nd laser excitation at 1064 nm. Temperature-dependent (80–400 K) Raman spectra of randomly oriented single crystals in the 3500–50 cm–1 range were measured using a Renishaw inVia Raman spectrometer with a confocal DM2500 Leica optical microscope, a thermoelectrically cooled CCD detector, and an Ar+ ion laser operating at 488 nm. A Linkam THMS600 stage was used to regulate the temperature.
A Labram Evolution spectrometer from Horiba equipped with a microscope was used to record the high-pressure Raman spectra. A solid-state 514.5 nm laser line was used for excitation. The spectral resolution was set to 2 cm–1. A diamond anvil cell Diacell μScopeDAC-RT(G) from Almax easyLab with a diamond of 0.4 mm culets was used to achieve high pressures. The sample was put into a 100 μm hole drilled in a stainless-steel gasket with a thickness of 200 μm using an electric discharge machine from Almax easyLab. The pressure transmitting medium was Nujol (mineral oil). The values of pressure were calculated using the shifts of the ruby R1 and R2 fluorescence lines.
Mid-infrared (IR) (4000–400 cm–1) and far-IR (500–50 cm–1) transmittance spectra were measured in the KBr disc and Nujol mull in a polyethylene plate, respectively, using a Nicolet iS50 FT-IR spectrometer with a resolution of 2 cm–1. The temperature-dependent IR spectra in the 4000–600 cm–1 range were collected using a Nicolet iN10 FTIR microscope and a Linkam THMS600 cryostat cell equipped with ZnSe windows.
Optical Properties
The absorption measurement was performed in the back-scattering mode using an Agilent Cary 5000 spectrophotometer. Temperature-dependent emission spectra were measured with the Hamamatsu photonic multichannel analyzer PMA-12 equipped with a BT-CCD linear image sensor. The laser diode operating at 405 nm was applied as an excitation source. The temperature of the samples during emission measurements was controlled by a Linkam THMS600 heating/freezing stage. The luminescence decay profiles were recorded using a femtosecond laser (Coherent Model Libra).
Results and Discussion
Thermal Properties
The PT behavior of (Me3Hy)[PbI3] was investigated before performing detailed crystal structure characterizations. The DSC curve demonstrates that (Me3Hy)[PbI3] undergoes two PTs during heating (cooling) at T1 = 322 (320) K and T2 = 207 (202) K (Figure S2). The ΔCp peaks associated with the PT at T1 are asymmetric and do not show temperature hysteresis between heating and cooling cycles (Figure 1). The associated change in entropy (ΔS) varies continuously with temperature, indicating the second-order nature of PT at T1. The heat capacity peaks associated with PT at T2, on the other hand, are symmetrical, sharp, and with thermal hysteresis (5 K), indicating a first-order PT. This is further supported by the associated discontinuous change in ΔS (see Figure 1). The entropy changes were experimentally determined to be approximately 1.8 J mol–1 K–1 at T1 and 28.5 J mol–1 K–1 at T2.
Figure 1.

Temperature dependence of the excess heat capacity (ΔCp) obtained for heating (red) and cooling (blue); the inset shows the corresponding change of entropy (ΔS).
According to X-ray diffraction (XRD) data (see further sections of this paper), the Me3Hy+ cations exhibit a sixfold disorder above T2. Assuming that the LT phase III is completely ordered, the PT at T2, separating the RT (II) and LT phases, according to the Boltzmann equation, would have a ratio of NRT/NLT = 6, where Ni is the number of accessible microstates in the ith phase and R is the gas constant. As a result, the predicted entropy change at T2 would be around 15 J mol–1 K–1. Nearly twice as low as the experimental value may imply that this PT is more complicated than a simple order–disorder model would predict.
The estimate of the ΔS is higher than expected, suggesting that the disorder may be caused by both the movement of the cations and the disturbance of the entire skeleton. A further increase in entropy after going from RT to the HT phase is associated with subtle changes in unit cell parameters (see further sections). Furthermore, the total change of ΔS in (Me3Hy)[PbI3] is higher than recently reported for other known lead iodides, such as (methylhydrazinium)2[PbI4] (2D) (∼2.88 J mol–1 K–1), (N-methyldabconium)[PbI3] (1D) (∼4.3 J mol–1 K–1), and (R-2-methylpiperidinium)[PbI3] (1D) (∼29.5 J mol–1 K–1) analogues.26−28
Thermal stability has been used to evaluate the thermal stability of (Me3Hy)[PbI3]. The compound is stable up to roughly 573 K, according to the registered TGA curve shown in Figure S3. The (Me3Hy)[PbI3] decomposes in three stages, with weight losses of around 4.7, 19.2, and 44.8% at inflection points at 595, 650, and 830 K, respectively. According to the literature data, the first step of trimethylhydriazine decomposition leads to N-methylmetanimine and the release of methylamine,29 which is in good agreement with the registered weight loss. The residual N-methylmetaniminium lead iodide decomposes into N-methylmetanimine, PbI2, and HI in the subsequent stage at 650 K.30 The third stage involves the formation of HCN, CH4, and the I2 molecules, as well as residual of pure lead.30
Dielectric Properties
In order to characterize the dielectric response of (Me3Hy)[PbI3], the complex dielectric permittivity as a function of frequency and temperature was investigated. Parts a and b of Figure 2 show the temperature dependences of the real ε′ and imaginary ε″ parts of the complex dielectric permittivity ε* = ε′ – iε″. During heating, after the PT at T2, ε′ first shows a step-like rise from 26.5 to 30.4. Such a sharp increase in the dielectric response related to a first-order PT is a common example of the phenomenon called dielectric switching.21,28,31,32 During continued heating, a high-frequency dispersion becomes dominant in the RT phase, which is most likely associated with ionic/electrical conductivity processes. Some hybrid perovskites, such as (FA)MII[H2PO2]3 (MII = Cd, Mn), (MHy)2[PbI4], (MHy)[PbBr3] (MHy+ = methylhydrazinium cation), or [(CH3)4N]4Pb3Cl10, already demonstrated similar behaviors.10,11,33,34
Figure 2.

Temperature dependence of the dielectric permittivity (a) and dielectric loss (b) as a function of temperature measured for the pelletized (Me3Hy)[PbI3] during heating. The dashed lines represent the PT temperatures determined by DSC measurements.
To better visualize the relaxation dynamics and exclude the contribution of electrode polarization, the frequency-dependent electric modulus (M* = 1/ε*) for several isotherms was analyzed for selected ones (Figure S4). The occurrence of the ionic/electrical conductivity process at HTs was revealed by isothermal complex electric modulus spectra with the characteristic bell (M″) and step (M′) shapes. Additionally, the dielectric permittivity was measured as a function of temperature and time to confirm the phase stability during many switches between on/off states. The material was left at the specified temperatures for 20 min following the switching cycles, which involved temperature changes between 180 and 210 K at a rate of 2 K min–1 (Figure 3). These results indicate that the examined material, when subjected to repeated temperature changes, is reversible and stable.
Figure 3.

Several cycles of the temperature-induced dielectric switching of (Me3Hy)[PbI3] at a frequency of 0.05 MHz.
Single-Crystal XRD
Depending on the temperature (Me3Hy)[PbI3] crystal exists in three phases, namely HT phase I, RT phase II, and LT phase III. The crystal data and refinement details for I and II phases are gathered in Table S1. Tables S2 and S3 list the geometric parameters for phases I and II, respectively. Crystals in the phase I adopt hexagonal P63/mmc symmetry with unit cell volume VHT = 658.44 Å3. The PT to the phase II is associated with a symmetry lowering to P63/m and a reduction in the unit cell volume to VRT = 635.57 Å3. The disappearance of c planes during PT at T1 (P63/mmc → P63/m) was confirmed by systematic absence analysis. Eventually, each structure-solving attempt with higher symmetry in phase II failed. In phase III, (Me3Hy)[PbI3] crystal alters symmetry to the orthorhombic system with the Pbca space group.
It should be highlighted that structural and symmetry modifications justify DSC data showing a strong peak at T2 and a weak one at T1. In the former case, this strong peak, with a corresponding high change in entropy, represents considerable symmetry and unit cell volume change. In the latter case, both parameters change slightly, and the crystal structure is very similar. Furthermore, repeated cycle diffraction experiments in the 215–375 K range showed that crystal-to-crystal PT at T1 is totally reversible and the crystal integrity is fully preserved. In contrast, the PT at T2 leads to permanent crystal disintegration and multidomain diffraction pattern (see powder experiments).
In phases I and II, the inorganic part of the structure was built by two Pb2+ cations and six I– ions. The face-sharing octahedral chain is created by [PbI6]4– ions with three iodine atoms shared between every two octahedra (Figure 4).
Figure 4.

Packing of (Me3Hy)[PbI3] at 375 K (I) (a) and at 230 K (II) (b) (with the thermal ellipsoids plotted at 30% probability) and at 190 K (III) (c) (structure solved from powder measurement); all structures are plotted along the [100] direction; hydrogen atoms are omitted for clarity.
The polymer chains are oriented along with the [001] direction, and the distance between the Pb atoms in the chain is c/2. The distance between adjacent lead atoms in the directions [100] and [010] defines the parameters a and b of the unit cell and is at 230 and 375 K, respectively, 9.620 and 9.774 Å. The organic part of the unit cell contains Me3Hy+, located in the voids of the framework. They fill the whole available volume, assuring dense packing. In I and II, cations show significant disorder due to rotation around sixfold inversion axes (N1 nitrogen atom is located at this axis and N2 in the m plane). The positions of the organic part are stabilized by a system of N–H···I and C–H···I hydrogen bonds. Due to the high symmetry, the intermolecular interactions are multiplied by symmetry operations, and the robust hydrogen bond network is created. Selected examples of contacts for II were presented in Table S4. For phase I, only C–H···I interactions were found (Table S5). No N–H···I interactions were found for the structure model in the HT phase, which is related to the high measurement temperature resulting in missing hydrogens in the amino group. The hydrogens from the terminal NH2 group cannot be observed from electron density because of higher thermal distortion parameters related to the elevated experiment temperature.
The PT at T1 is connected with distortions in the organic part. The chain structure is composed of slightly distorted octahedra. It is rigid and remains unchanged with temperature and symmetry increase (Figure S5). The values of the I–Pb–I angles are practically identical for both polymorphs, being ca. 86 and 94°. Similarly, Pb–I distances are nearly identical—3.2361 and 3.2279 Å, in I and II, respectively. It is justified by subtle changes in cell parameters and cell volume. The c parameter shows some fluctuations (Figure 5), whereas for a, we observed a steady increase. The most linear increase was found for the cell volume, which showed an expansion of ca. 3.6% with a temperature increase from 215 to 375 K.
Figure 5.

Superposition of (Me3Hy)[PbI3] structures at 375 K (blue, phase I) and at 230 K (gray, phase II) with the thermal ellipsoids plotted at 30% probability (along the [001] direction); hydrogen atoms are omitted for clarity.
The single crystal studies for phase III (below 205 K) showed that single crystals crack into several domains related by rotation around the c axis (Figure S6). The treatment of them as domains of the twinned crystal failed. These experiments provided cell parameters, but the space group was incorrectly assigned and SHELXT24 did not show a reasonable solution in the correct space group. Hence, the powder experiments were carried out (obtained data is in Table S6).
The cell parameters of the LT phase III showed that the volume expanded by approximately four times, and the c parameter remained retained. This parameter corresponds to the chain direction, forming the most robust part of the structure. Hence, we can assume that this topology might be preserved. The structure determination from powder data indicated a centrosymmetric Pbca space group. The structure clearly confirms that the chain composed of face-sharing PbI6 octahedra is robust and did not undergo disruption despite the single crystal integrity being lost. The Pb–I distances range from 3.18 to 3.28 Å (dav = 3.244 Å), which is comparable to the phases I and II (dav being 3.228 and 3.236 Å for phases II and I, respectively). We conclude that there are subtle changes related to lowered symmetry of the [PbI6]4– octahedron (Figure 4 and Table S7). It should be noted that adjacent chains are slightly shifted. It can be assessed by lead atom position—in I and II it is positioned in the (001) plane, whereas in III it is displaced by ca. 0.06 (in fractional coordinates) from this plane. The positions of (Me3Hy)+ cations in the unit cell show that they were slightly repositioned in the crystal framework (Figure S7), which should affect hydrogen bonds network. Moreover, PT results in reorientation of the cations in phase III, causing that the N–N bonds to be approximately parallel to the chain direction. It is conceivable that this change affects the hydrogen bond network. This effect cannot be fully discussed because in the final model, two hydrogen atoms from the NH2 group are missing. Nevertheless, the organic cation is trapped in the crystal network by a robust network of C–H···I hydrogen bonds (Table S8). Summarizing, we can hypothesize that crystal cracking below T2 and PT are related to the mutual shift of adjacent chains, the relocation of the cation and its reorientation in terms of the N–N bond position according to the chain propagation direction. All these factors can affect the N–H···I and C–H···I hydrogen bond network. These structural changes are much more severe than those between I and II phases and justify the big entropy change as well as distinct features observed in IR and Raman spectra (see Temperature-Dependent Raman and IR Studies).
Vibrational Properties
Molecular Vibrations of the (Me3Hy)+ Cation
The free Me3Hy+ cation has 42 vibrational degrees of freedom, including 14 stretching (ν) and 28 deformational modes. The stretching modes can be described as νsNH2 (symmetric), νasNH2 (antisymmetric), 3× νsCH3, 6× νasCH3, νNN, νsCNC, and νasCNC, whereas the bending as δNH2 (bending), ρNH2 (rocking), τNH2 (twisting), ωNH2 (wagging), 3× δsCH3, 6× δasCH3, 6× ρCH3, 3× τCH3, 3× δCNN, and 3× δCNC.
Assignment of Bands
RT Raman and IR spectra are presented in Figure S8. The proposed assignment, presented in Table S9, was based on comparative analysis with literature sources, including papers describing IR and Raman spectra of 1,1,1-methylhydrazinium iodide,35 coordination nickel(II) compound with 1,1,1-trimethylhydrazinium as ligand36 and perovskite-like compounds templated by 1,1-dimethylhydrazinium22 and methylhydrazinium37,38 cations. Bands corresponding to internal vibrations of Me3Hy+ are observed in a characteristic ranges as reported in literature.
The strongest Raman band, which has a maximum at 105 cm–1 and an IR counterpart at 101 cm–1, was assigned to Pb–I stretching modes, while the weaker Raman band, which has a maximum at 60 cm–1, was attributed to bending I–Pb–I vibrations as well as librational motions of the entire PbI6 units.
Temperature-Dependent Raman and IR Studies
In order to understand the mechanisms of PTs, IR and Raman spectra were measured upon heating as a function of temperature in the range of 80–400 K (see Figures S9–S13). As may be seen, IR and Raman spectra evolve with noticeable changes at T2 and very minor changes at T1. This is consistent with the XRD measurements, which demonstrate an increase in symmetry from orthorhombic to hexagonal at T2 and maintain hexagonal symmetry at T1. Furthermore, it can be noticed that PT at T2 causes strong splitting of the IR and Raman bands, which are due to symmetry change and the expected increased number of inequivalent Me3Hy+ cations in phase III. Furthermore, all the IR and Raman bands are very narrow in phase III, suggesting that the organic cations become fully ordered. The abrupt nature of the changes seen at T2 demonstrates that organic cations completely order shortly below T2 rather than gradually freeze upon cooling.
The Me3Hy+ cation is distinct among other hydrazine derivatives since only one amino group may create hydrogen bonding with the PbI6 octahedra. The positions of bands corresponding to stretching vibrations of the NH2 terminal group are a determinant of the strength of these interactions. Lorentz curves were used to fit spectra in order to identify shifts in band maxima and variations in full-width-at-half-maxima (fwhm) with temperature. Figures 6 and 7 show results obtained for stretching vibrations of the NH2 terminal group. The positions of bands and fwhms hardly change during PT at T1. In contrast, at T2, a majority of bands are downshifted in phase III. The most pronounced shifts, by 16.4 and 15.8 cm–1, were noticed for the Raman band at 3313 cm–1 and the IR band at 3314 cm–1, respectively (see Figure 6). Additionally, while changes in fwhms at T1 are undetectable, shifts at T2 are accompanied by a considerable strong narrowing of bands, decreased most by 17.7 cm–1 for the Raman band at 3313 cm–1 (Figure 7). These findings suggest that the HB network is irrelevant to the mechanism of PT at T1, while it is reorganized and strengthened during PT at T2, which is driven by the ordering of the Me3Hy+ cations. This implies that phase III is stabilized by stronger HBs, which prevent further disorder of the cations and cause their lock-in. The same results are achieved by observing other characteristic vibrations related to the amino group (Figure S14). In addition, the δNH2 and ρNH2 vibrations are more sensitive to PT, exhibiting modest inflections at T1.
Figure 6.

Thermal evolution of positions of the Raman (blue) and IR (red) bands corresponding to stretching vibrations of the amino group; vertical lines correspond to temperatures of PTs determined from DSC.
Figure 7.

Thermal evolution of fwhms of the Raman bands corresponding to stretching vibrations of the amino group; vertical lines correspond to temperatures of PTs determined from DSC.
The thermal behavior of vibrational modes corresponding to the skeleton of Me3Hy+ cations (without H atoms) is depicted in Figure S15. Changes at T1 are, as predicted, within the fitting error, but they are apparent at T2. However, the changes at T2 are minor, implying that the geometry of the skeleton is only marginally changed during this PT.
The positions of bands corresponding to stretching and bending vibrations of methyl groups shift during PT at T2 (see Figure S16), which supports the dynamic character of this PT. The variations at T1 are less pronounced because they are likely related to confinement changes induced by changes in cell volume.
The significant splitting and narrowing of low-wavenumber Raman modes below T2 demonstrate that the symmetry is reduced to orthorhombic (Figure S17). The splitting of the Raman band near 100 cm–1 into three narrow components also demonstrates the presence of unequal Pb–I bonds in the LT phase. This kind of splitting needs to be connected with distortion of the lead-iodide chains. Moreover, the abrupt evolution of Raman spectra at T2 confirmed the discontinuous nature of the transformation.
High-Pressure Raman Studies
In order to better understand the stability of (Me3Hy)[PbI3], the low-wavenumber Raman spectra were measured as a function of pressure (Figure 8a). Figure 8b presents the pressure-dependent evolution for the observed modes. The values of pressure coefficients (α = dω/dP) and wavenumber intercepts at zero pressure (ω0) derived by fitting the experimental data with a linear function ω(P) = ω0 + αP are shown in Table S10.
Figure 8.

(a) Low-wavenumber Raman bands in the 0–10.0 GPa range during compression compared to a spectrum at 2.0 GPa measured during decompression (D, dashed line) and Raman spectrum obtained at ambient pressure at 80 K; (b) pressure evolution of Raman bands; the vertical dashed lines in (b) indicate the high-pressure PTs.
At first, the increase in pressure causes only minor changes. At 2.0 GPa, the components of the strongest band at 100 cm–1 start to experience intensity changes. At 3.2 GPa, the spectrum changes qualitatively, including the appearance of new bands, indicating a high-pressure PT (1st high-pressure phase, HPI) occurring between 2.8 and 3.2 GPa. The fact that there are more bands suggests that the HPI phase has lower symmetry than ambient-pressure (AP) phase. It is also possible that the symmetry of the HPI phase is orthorhombic based on the comparable number of bands observed at ambient pressure at 80 K (see Figure 8a). Strong narrowing of low-wavenumber modes also implies that the HPI phase may be ordered.
Furthermore, α coefficients change values, which confirms the PT between 2.8 and 3.2 GPa. Bands shift strongly toward higher wavenumber with increasing pressure in both the AP and HPI phases, indicating significant compression of the Pb–I bonds, similar to MHyPbCl3.39 A few Raman bands disappear when the pressure rises to 6.4–6.8 GPa, and the remaining bands broaden. This may simply be another PT to 2nd HP (HPII) or start of amorphization. The quality of the crystal remains unchanged up to 10 GPa and after decompression (see Figure S18). However, without more investigation, this is difficult to address. The process is entirely reversible, whether or not there is partial amorphization (see Figure 8a).
Optical Properties
The diffuse reflectance spectrum
of (Me3Hy)[PbI3] crystals recorded at RT is
presented in Figure S19a. It consists of
a broad band in the 200–500 nm range. On its edge (at 400 nm)
one can observe an excitonic band characteristic for halide perovskites.10,40 The recalculation of the data obtained from the absorption measurements
made it possible to determine the energy band gap of the analyzed
compound using the Kubelka–Munk function 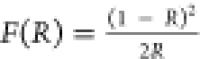 , where R stands for reflectance.41 The band gap energy (Eg) of the (Me3Hy)[PbI3] crystals was
calculated to be 3.20 eV (Figure S19b).
The obtained value is similar to the size of the energy band gap of
other 1D iodide hybrid perovskites (2.58–3.18 eV),42 but higher compared to organic or inorganic
3D analogues such as MAPbI3 (1.57 eV), FAPbI3 (1.48 eV), or CsPbI3 (1.73 eV).43
, where R stands for reflectance.41 The band gap energy (Eg) of the (Me3Hy)[PbI3] crystals was
calculated to be 3.20 eV (Figure S19b).
The obtained value is similar to the size of the energy band gap of
other 1D iodide hybrid perovskites (2.58–3.18 eV),42 but higher compared to organic or inorganic
3D analogues such as MAPbI3 (1.57 eV), FAPbI3 (1.48 eV), or CsPbI3 (1.73 eV).43
The temperature-dependent emission spectra of (Me3Hy)[PbI3] crystals measured in the range of 80–270 K in 5 K steps at 405 nm is plotted in Figure 9a. It can be seen that the emission bands cover a wide spectral region and exhibit two maxima, weaker and stronger, located at 520 and 700 nm, respectively. It was found that, the most intense luminescence was obtained at 80 K. Heating the sample causes a gradual decrease of its intensity until complete quenching at 190 K (Figure 9b) due to the thermally activated nonradiative decay channels.44 The large Stokes shift between the excitonic absorption and the nearest emission band (120 nm) (Figure S20), the large fwhm of the main emission band (over 200 nm), and the strong temperature quenching of luminescence lead to the conclusion that the origin of the transition recorded in Figure 9a is associated with self-trapped excitons (STEs).10,44−47 The presence of two bands indicates the existence of two kinds of defects with different depths. It is worth noting that excitation of the sample with the 405 nm line or with laser diodes of higher energy, that is, 266 and 375 nm, results in the absence of an emission band that could be assigned to FE (free exciton) and BE (bounded exciton), as shown in Figure S21. Since the position of the emission bands does not change with increasing temperature, the chromaticity coordinates were determined for the measurement performed at 80 K and presented on the CIE diagram (Figure 9c).
Figure 9.

Temperature-dependence emission spectra (a), integral emission intensity (b) and the CIE chromaticity coordinates (c) of (Me3Hy)[PbI3] crystals. Inset presents the photograph of the glowing sample.
In order to study the thermal kinetics of (Me3Hy)[PbI3] crystals, the following expression was
used: 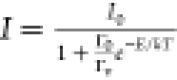 , where I is the intensity, I0 is the initial intensity at LT, Γv denotes the radiative decay rate, Γ0 is
the attempt rate for thermal quenching, and ΔE is the activation energy for thermal quenching.48 This equation has been recalculated as
, where I is the intensity, I0 is the initial intensity at LT, Γv denotes the radiative decay rate, Γ0 is
the attempt rate for thermal quenching, and ΔE is the activation energy for thermal quenching.48 This equation has been recalculated as 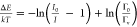 , in order to determine the activation energy
of the investigated material (ΔE is the slope
of
, in order to determine the activation energy
of the investigated material (ΔE is the slope
of 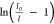 as a function of 1/kT and
as a function of 1/kT and 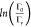 is a constant). The calculated activation
energy of (Me3Hy)[PbI3] crystals was determined
to be 65 meV (Figure S22).49
is a constant). The calculated activation
energy of (Me3Hy)[PbI3] crystals was determined
to be 65 meV (Figure S22).49
The luminescence decay profiles of (Me3Hy)[PbI3] crystals recorded at the 405 nm excitation line,
monitored at 520
and 700 nm at 80 K, corresponding to the two peaks observed in the
emission spectrum, are presented in Figure S23. It was found that analyzed compound exhibits curves that can be
fitted by a double exponential function regardless of the monitored
wavelength using the following expression  , where I(t) is the emission intensity, A1 and A2 are fitting constants, and τ1 and τ2 are their corresponding lifetimes. The lifetimes
determined for both emission peaks observed at 520 and 700 nm are
τ1 ≈ 260 ns and τ2 ≈
725 ns. Two components visible in the luminescence decays indicate
the existence of non-radiation energy transfers in the (Me3Hy)[PbI3] crystals. The obtained values are very similar
for both bands, which proves the same nature of the defects.
, where I(t) is the emission intensity, A1 and A2 are fitting constants, and τ1 and τ2 are their corresponding lifetimes. The lifetimes
determined for both emission peaks observed at 520 and 700 nm are
τ1 ≈ 260 ns and τ2 ≈
725 ns. Two components visible in the luminescence decays indicate
the existence of non-radiation energy transfers in the (Me3Hy)[PbI3] crystals. The obtained values are very similar
for both bands, which proves the same nature of the defects.
Conclusions
We synthesized a new perovskitoid 1,1,1-trimethylhydrazinium lead iodide, composed of 1D face-shared chains of [PbI6]4– octahedra along the [001] direction and disordered organic cations, to evaluate its structural, thermal, phonon, and emission properties using a variety of techniques. DSC measurement revealed the presence of two PTs at 322 (320) K and 207 (202) K upon cooling (heating), and the TGA measurement demonstrated that the material is stable up to 300 °C.
DSC, single-crystal XRD, and vibrational studies revealed that the transformation from the RT P63/m phase to the HT P63/mmc phase has a second-order nature and is associated with minor structural changes. In contrast, the PT to the LT orthorhombic (Pbca) phase has a first-order nature and is characterized by a significant change in entropy. It is associated with the ordering (locking) of the organic cations, rearrangement and strengthening of hydrogen bonds, deformation of the lead-iodide octahedral chains, and an increase in the number of inequivalent structural units. The abrupt character of this PT displays the desired switchable dielectric behavior between low (off) and high (on) dielectric states, designating it as a potentially switchable material.
The high-pressure Raman data indicated the presence of two high-pressure PTs, in the 2.8–3.2 and 6.4–6.8 GPa ranges. The low-pressure PT is associated with the ordering of the organic cations, compression of PbI6 octahedra, and distortion of the lead-iodide framework, while the second PT is most likely related to the partial and reversible amorphization.
The band gap energy of the crystals, as determined by optical measurements, is 3.20 eV. At LTs, they emit a reddish-orange broad-band emission that is related to STEs. The activation energy of the emission was found to be 65 meV.
Acknowledgments
The authors would like to acknowledge Prof. Marek Drozd from the Institute of Low Temperature and Structure Research, PAS for performing TGA measurements and the National Science Centre, Poland, for financial support in the course of the realization of a project entitled, Synthesis and physicochemical characterization of novel layered and perovskite-like hybrid organic–inorganic lead halides with protonated alkyl derivatives of hydrazine” (no. UMO-2020/37/N/ST5/02257).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c03287.
Powder XRD, DSC trace, frequency dependencies of the complex dielectric permittivity, complex electrical modules, packing diagrams for phases I, II, and III, RT and temperature-dependent Raman and IR spectra, IR spectrum, positions and bandwidths of selected IR and Raman bands as a function of temperature, pictures of crystals during HP Raman experiment, diffuse reflectance spectrum, emission spectra measured under 266 and 375 nm excitation, crystal data and structure refinement for I and II phases, selected geometric parameters for phases I and II, assignment of IR and Raman bands, Raman pressure intercepts and pressure coefficients (PDF), and crystal structure of (Me3Hy)[PbI3] at 230 K (CIF) and 375 K (CIF) (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Yoo J. J.; Seo G.; Chua M. R.; Park T. G.; Lu Y.; Rotermund F.; Kim Y.; Moon C. S.; Jeon N. J.; Correa-Baena V.; Bulović S. S.; Shin M. G.; Bawendi M. G.; Seo J. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. 10.1038/s41586-021-03285-w. [DOI] [PubMed] [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Liao W.-Q.; Zhang Y.; Hu C.; Mao J.; Ye H.; Li P.; Huang S. D.; Xiong R.-G. A Lead-Halide Perovskite Molecular Ferroelectric Semiconductor. Nat. Commun. 2015, 6, 7338. 10.1038/ncomms8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokhi S.; Gao W.; Wang Y.; Anandan P. R.; Rahaman Z.; Singh S.; Wang D.; Cazorla C.; Yuan G.; Liu J.; Wu T. Emergence of Ferroelectricity in Halide Perovskites. Small Methodas 2020, 4, 2000149. 10.1002/smtd.202000149. [DOI] [Google Scholar]
- Liu S.; Zheng F.; Koocher N. Z.; Takenaka H.; Wang F.; Rappe A. M. Ferroelectric Domain Wall Induced Band Gap Reduction and Charge Separation in Organometal Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 693–699. 10.1021/jz502666j. [DOI] [PubMed] [Google Scholar]
- Xiao X.; Li W.; Fang Y.; Liu Y.; Shao X.; Yang R.; Zhao J.; Dai X.; Zia R.; Huang J. Benign Ferroelastic Twin Boundaries in Halide Perovskites for Charge Carrier Transport and Recombination. Nat. Commun. 2020, 11, 2215. 10.1038/s41467-020-16075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick P. D.; Íñiguez N. C.; Haynes A. R.; Bristowe I. First-Principles Study of Ferroelastic Twins in Halide Perovskites. J. Phys. Chem. Lett. 2019, 10, 1416. 10.1021/acs.jpclett.9b00202. [DOI] [PubMed] [Google Scholar]
- Mączka M.; Gągor A.; Pikul A.; Stefańska D. Novel Hypophosphite Hybrid Perovskites of [CH3NH2NH2][Mn(H2POO)3] and [CH3NH2NH2][Mn(H2POO)2.83(HCOO)0.17] Exhibiting Antiferromagnetic Order and Red Photoluminescence. RSC Adv. 2020, 10, 19020–19026. 10.1039/d0ra03397a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mączka M.; Gagor A.; Zarȩba J. K.; Stefanska D.; Drozd M.; Balciunas S.; Šimėnas M.; Banys J.; Sieradzki A. Three-Dimensional Perovskite Methylhydrazinium Lead Chloride with Two Polar Phases and Unusual Second-Harmonic Generation Bistability above Room Temperature. Chem. Mater. 2020, 32, 4072–4082. 10.1021/acs.chemmater.0c00973. [DOI] [Google Scholar]
- Mączka M.; Ptak M.; Gągor A.; Stefańska D.; Sieradzki A. Layered Lead Iodide of [Methylhydrazinium]2PbI4 with a Reduced Band Gap: Thermochromic Luminescence and Switchable Dielectric Properties Triggered by Structural Phase Transitions. Chem. Mater. 2019, 31, 8563–8575. 10.1021/acs.chemmater.9b03775. [DOI] [Google Scholar]
- Mączka M.; Ptak M.; Gągor A.; Stefańska D.; Zarȩba J. K.; Sieradzki A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. 10.1021/acs.chemmater.9b05273. [DOI] [Google Scholar]
- Stoumpos C. C.; Mao L.; Malliakas C. D.; Kanatzidis M. G. Structure-Band Gap Relationships in Hexagonal Polytypes and Low-Dimensional Structures of Hybrid Tin Iodide Perovskites. Inorg. Chem. 2017, 56, 56–73. 10.1021/acs.inorgchem.6b02764. [DOI] [PubMed] [Google Scholar]
- Li X.; He Y.; Kepenekian M.; Guo P.; Ke W.; Even J.; Katan C.; Stoumpos C. C.; Schaller R. D.; Kanatzidis M. G. Three-Dimensional Lead Iodide Perovskitoid Hybrids with High X-Ray Photoresponse. J. Am. Chem. Soc. 2020, 142, 6625–6637. 10.1021/jacs.0c00101. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Xu Y.; Zhang H.; Xiao B.; Liu X.; Dong J.; Cheng Y.; Zhang B.; Jie W.; Kanatzidis M. G. Optical and Electronic Anisotropies in Perovskitoid Crystals of Cs3Bi2I9 Studies of Nuclear Radiation Detection. J. Mater. Chem. A 2018, 6, 23388–23395. 10.1039/c8ta09525f. [DOI] [Google Scholar]
- Zhang F.; Lu H.; Larson B. W.; Xiao C.; Dunfield S. P.; Reid O. G.; Chen X.; Yang M.; Berry J. J.; Beard M. C.; Zhu K. Surface Lattice Engineering through Three-Dimensional Lead Iodide Perovskitoid for High-Performance Perovskite Solar Cells. Chem 2021, 7, 774–785. 10.1016/j.chempr.2020.12.023. [DOI] [Google Scholar]
- Kong T.; Xie H.; Zhang Y.; Song J.; Li Y.; Lim E. L.; Hagfeldt A.; Bi D. Perovskitoid-Templated Formation of a 1D@3D Perovskite Structure toward Highly Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2101018. 10.1002/aenm.202101018. [DOI] [Google Scholar]
- Liu G.; Xu S.; Zheng H.; Xu X.; Xu H.; Zhang L.; Zhang X.; Kong F.; Pan X. Boosting Photovoltaic Properties and Intrinsic Stability for MA-Based Perovskite Solar Cells by Incorporating 1,1,1-Trimethylhydrazinium Cation. ACS Appl. Mater. Interfaces 2019, 11, 38779–38788. 10.1021/acsami.9b13701. [DOI] [PubMed] [Google Scholar]
- Sun W.; Zou J.; Wang X.; Wang S.; Du Y.; Cao F.; Zhang L.; Wu J.; Gao P. Enhanced Photovoltage and Stability of Perovskite Photovoltaics Enabled by a Cyclohexylmethylammonium Iodide-Based 2D Perovskite Passivation Layer. Nanoscale 2021, 13, 14915–14924. 10.1039/d1nr03624f. [DOI] [PubMed] [Google Scholar]
- Chen S.; Shang R.; Hu K. L.; Wang Z. M.; Gao S. [NH2NH3][M(HCOO)3] (M = Mn2+, Zn2+, Co2+ and Mg2+): Structural Phase Transitions, Prominent Dielectric Anomalies and Negative Thermal Expansion, and Magnetic Ordering. Inorg. Chem. Front. 2014, 1, 83–98. 10.1039/c3qi00034f. [DOI] [Google Scholar]
- Mączka M.; Sobczak S.; Kryś M.; Leite F. F.; Paraguassu W.; Katrusiak A. Mechanism of Pressure-Induced Phase Transitions and Structure-Property Relations in Methylhydrazinium Manganese Hypophosphite Perovskites. J. Phys. Chem. C 2021, 125, 10121–10129. 10.1021/acs.jpcc.1c01998. [DOI] [Google Scholar]
- Zienkiewicz J. A.; Kowalska D. A.; Fedoruk K.; Stefanski M.; Pikul A.; Ptak M. Unusual Isosymmetric Order-Disorder Phase Transition in the New Perovskite-Type Dimethylhydrazinium Manganese Formate Exhibiting Ferrimagnetic and Photoluminescent Properties. J. Mater. Chem. C 2021, 9, 6841–6851. 10.1039/d1tc01014j. [DOI] [Google Scholar]
- Zienkiewicz J. A.; Kucharska E.; Ptak M.. Mechanism of Unusual Isosymmetric Order-Disorder Phase Transition in [Dimethylhydrazinium]Mn(HCOO)3 Hybrid Perovskite Probed by Vibrational Spectroscopy. Materials. 2021, 14 (). DOI: 10.3390/ma14143984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mączka M.; Gągor A.; Ptak M.; Paraguassu W.; da Silva T. A.; Sieradzki A.; Pikul A. Phase Transitions and Coexistence of Magnetic and Electric Orders in the Methylhydrazinium Metal Formate Frameworks. Chem. Mater. 2017, 29, 2264–2275. 10.1021/acs.chemmater.6b05249. [DOI] [Google Scholar]
- Sheldrick G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae C. F.; Sovago I.; Cottrell S. J.; Galek P. T. A.; McCabe E.; Pidcock M.; Platings G. P.; Shields J. S.; Stevens M.; Towler P. A.; Wood P. A. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. 10.1107/S1600576719014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mączka M.; Zaręba J. K.; Gągor A.; Stefańska D.; Ptak M.; Roleder K.; Kajewski D.; Soszyński A.; Fedoruk K.; Sieradzki A. [Methylhydrazinium]2PbBr4, a Ferroelectric Hybrid Organic-Inorganic Perovskite with Multiple Nonlinear Optical Outputs. Chem. Mater. 2021, 33, 2331–2342. 10.1021/acs.chemmater.0c04440. [DOI] [Google Scholar]
- Xue C.; Yao Z.-Y.; Zhang J.; Liu W.-L.; Liu J.-L.; Ren X.-M. Extra Thermo- and Water-Stable One-Dimensional Organic- Inorganic Hybrid Perovskite [N-Methyldabconium]PbI3 Showing Switchable Dielectrics, Conductivity and Bright Yellow-Green Emission. Chem. Commun. 2018, 54, 4321–4324. 10.1039/C8CC00786A. [DOI] [PubMed] [Google Scholar]
- Peng H.; Liu Y.; Huang X.; Liu Q.; Yu Z.; Wang Z.-X.; Liao W.-Q. Homochiral One-Dimensional ABX3 Lead Halide Perovskites with High-Tc Quadratic Nonlinear Optical and Dielectric Switchings. Mater. Chem. Front. 2021, 5, 4756–4763. 10.1039/d1qm00223f. [DOI] [Google Scholar]
- Gaber A. A. M.; Wentrup C. Pyrolysis of Hydrazine Derivatives and Related Compounds with N-N Single Bonds. J. Anal. Appl. Pyrolysis 2017, 125, 258–278. 10.1016/j.jaap.2017.03.016. [DOI] [Google Scholar]
- Fujiwara H.; Egawa T.; Konaka S. Electron Diffraction Study of Thermal Decomposition Products of Trimethylamine: Molecular Structure of CH3-N=CH2. J. Mol. Struct. 1995, 344, 217–226. 10.1016/0022-2860(94)08440-S. [DOI] [Google Scholar]
- Trzebiatowska M.; Mączka A.; Gagor A. Pyrrolidinium-Based Cyanides: Unusual Architecture and Dielectric Switchability Triggered by Order – Disorder Process. Inorg. Chem. 2020, 59, 8855. 10.1021/acs.inorgchem.0c00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mączka M.; Nowok A.; Zarȩba J. K.; Stefańska D.; Gągor A.; Trzebiatowska M.; Sieradzki A. Near-Infrared Phosphorescent Hybrid Organic – Inorganic Perovskite with High-Contrast Dielectric and Third-Order Nonlinear Optical Switching Functionalities. ACS Appl. Mater. Interfaces 2022, 14, 1460–1471. 10.1021/acsami.1c20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mączka M.; Stefańska D.; Ptak M.; Gągor A.; Pikul A.; Sieradzki A. Cadmium and Manganese Hypophosphite Perovskites Templated by Formamidinium Cations: Dielectric, Optical and Magnetic Properties. Dalton Trans. 2021, 50, 2639–2647. 10.1039/d0dt03995k. [DOI] [PubMed] [Google Scholar]
- Xue C.; Wang S.; Liu W.-L.; Ren X.-M. Two-Step Structure Phase Transition, Dielectric Anomalies and Thermochromic Luminescence Behavior in a Direct Bandgap 2D Corrugated Layer Lead Chloride Hybrid of [(CH3)4N]4Pb3Cl10. Chem.—Eur. J. 2019, 25, 5280–5287. 10.1002/chem.201806032. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Son G.; Na T.; Choi S. Synthesis and Physical and Chemical Properties of Hypergolic Chemicals Such as N,N,N-Trimethylhydrazinium and 1-Ethyl-4-Methyl-1,2,4-Triazolium Salts. Appl. Sci. 2015, 5, 1547–1559. 10.3390/app5041547. [DOI] [Google Scholar]
- Goedken V. L.; Vallarino L. M.; Quagliano J. V. Cationic Ligands. Coordination of the 1,1,1-Trimethylhydrazinium Cation to Nickel(II). Inorg. Chem. 1971, 10, 2682–2685. 10.1021/ic50106a011. [DOI] [Google Scholar]
- Ciupa-Litwa A.; Ptak M.; Kucharska E.; Hanuza J.; Mączka M. Vibrational Properties and DFT Calculations of Perovskite-Type Methylhydrazinium Manganese Hypophosphite. Molecules 2020, 25, 5215. 10.3390/molecules25215215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mączka M.; Zienkiewicz J. A.; Ptak M. Comparative Studies of Phonon Properties of Three-Dimensional Hybrid Organic-Inorganic Perovskites Comprising Methylhydrazinium, Methylammonium, and Formamidinium Cations. J. Phys. Chem. C 2022, 126, 4048–4056. 10.1021/acs.jpcc.1c09671. [DOI] [Google Scholar]
- Mączka M.; Ptak M.; Vasconcelos D. L. M.; Giriunas L.; Freire P. T. C.; Bertmer M.; Banys J.; Simenas M. NMR and Raman Scattering Studies of Temperature-and Pressure-Driven Phase Transitions in CH3NH2NH2PbCl3 Perovskite. J. Phys. Chem. C 2020, 124, 26999–27008. 10.1021/acs.jpcc.0c07886. [DOI] [Google Scholar]
- Wu K.; Bera A.; Ma C.; Du Y.; Yang Y.; Li L.; Wu T. Temperature-Dependent Excitonic Photoluminescence of Hybrid Organometal Halide Perovskite Films. Phys. Chem. Chem. Phys. 2014, 16, 22476–22481. 10.1039/c4cp03573a. [DOI] [PubMed] [Google Scholar]
- Kubelka P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. 10.1364/JOSA.38.000448. [DOI] [PubMed] [Google Scholar]
- Qian J.; Guo Q.; Liu L.; Xu B.; Tian W. A Theoretical Study of Hybrid Lead Iodide Perovskite Homologous Semiconductors with 0D, 1D, 2D and 3D Structures. J. Mater. Chem. A 2017, 5, 16786–16795. 10.1039/c7ta04008c. [DOI] [Google Scholar]
- Eperon G. E.; Stranks S. D.; Menelaou C.; Johnston M. B.; Herz L. M.; Snaith H. J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. 10.1039/c3ee43822h. [DOI] [Google Scholar]
- Maqbool S.; Sheikh T.; Thekkayil Z.; Deswal S.; Boomishankar R.; Nag A.; Mandal P. Third Harmonic Upconversion and Self-Trapped Excitonic Emission in 1D Pyridinium Lead Iodide. J. Phys. Chem. C 2021, 125, 22674. 10.1021/acs.jpcc.1c07639. [DOI] [Google Scholar]
- Nazarenko O.; Kotyrba M. R.; Yakunin S.; Aebli M.; Rainò G.; Benin B. M.; Wörle M.; Kovalenko M. V. Guanidinium-Formamidinium Lead Iodide: A Layered Perovskite- Related Compound with Red Luminescence at Room Temperature. J. Am. Chem. Soc. 2018, 140, 3850–3853. 10.1021/jacs.8b00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko O.; Kotyrba M. R.; Wörle M.; Cuervo-Reyes E.; Yakunin S.; Kovalenko M. V. Luminescent and Photoconductive Layered Lead Halide Perovskite Compounds Comprising Mixtures of Cesium and Guanidinium Cations. Inorg. Chem. 2017, 56, 11552–11564. 10.1021/acs.inorgchem.7b01204. [DOI] [PubMed] [Google Scholar]
- Adnan M.; Rao K. N.; Acharyya J. N.; Kumar D.; Dehury K. M.; Prakash G. V. Synthesis, Structural, Linear, and Nonlinear Optical Studies of Inorganic–Organic Hybrid Semiconductors (R–C6H4CHCH3NH3)2PbI4,(R=CH3, Cl). ACS Omega 2019, 4, 19565–19572. 10.1021/acsomega.9b01704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorenbos P. Thermal Quenching of Eu2+ 5d-4f Luminescence in Inorganic Compounds. J. Phys.: Condens. Matter 2005, 17, 8103–8111. 10.1088/0953-8984/17/50/027. [DOI] [Google Scholar]
- Stefańska D.; Bondzior B.; Vu T. H. Q.; Miniajluk-Gaweł N.; Dereń P. J. The Influence of Morphology and Eu3+ Concentration on Luminescence and Temperature Sensing Behavior of Ba2MgWO6 Double Perovskite as a Potential Optical Thermometer. J. Alloys Compd. 2020, 842, 155742. 10.1016/j.jallcom.2020.155742. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


