Figure 5. The two orientations of PEX13 are bridged by the YG domain.
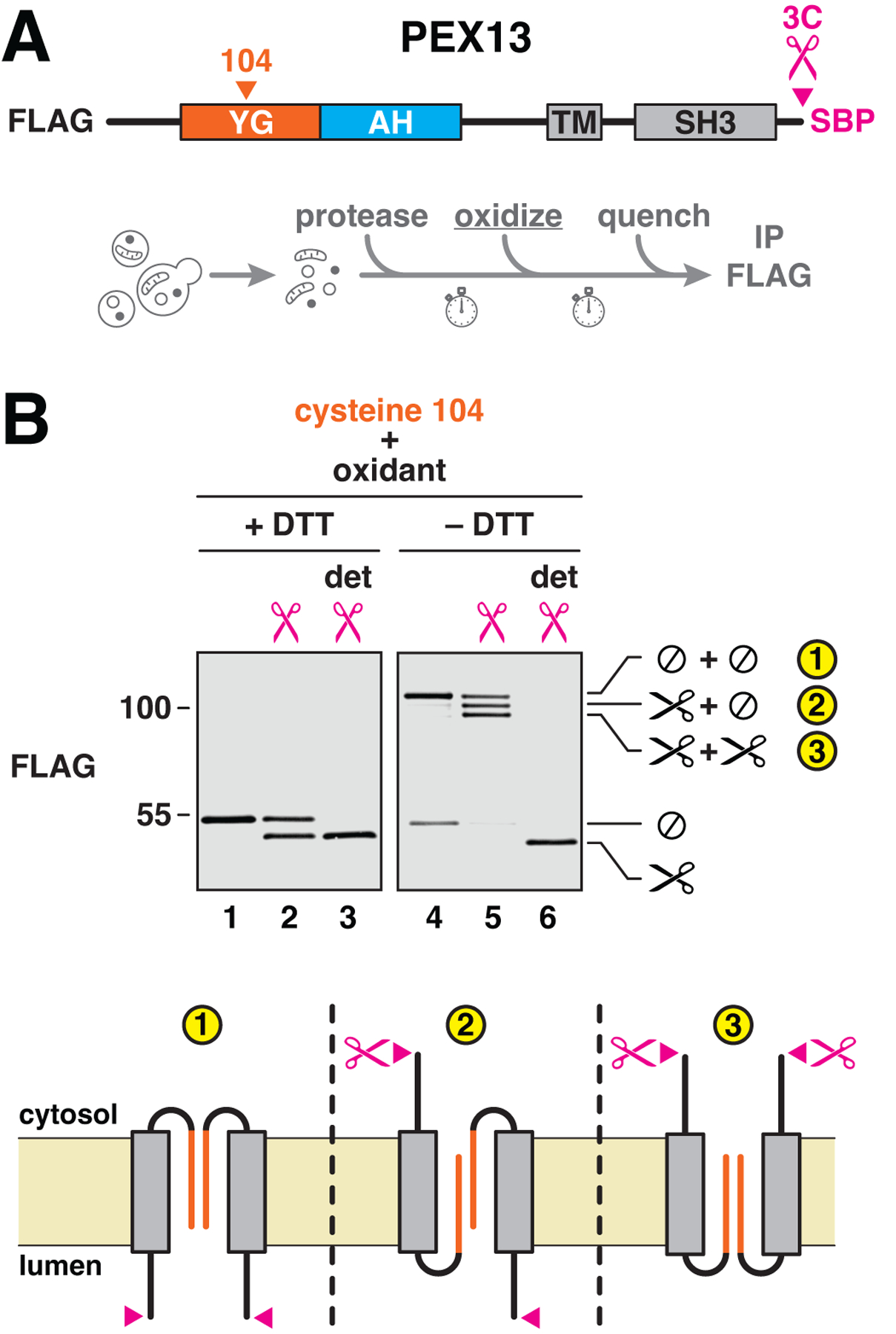
(A) To test whether the two orientations of PEX13 associate with each other, a single cysteine and a 3C protease–cleavage site were incorporated into FLAG-tagged yeast PEX13 as shown. An SBP tag was included at the C terminus to enhance the size shift after proteolytic cleavage. The resulting construct was integrated into yeast. Intact membranes from the corresponding strain were treated with the protease to reveal the protein’s two orientations, oxidized with Aldrithiol-4 to induce disulfide-bridge formation, and quenched to inactivate the protease and oxidant. (B) Disulfide-linked dimers were visualized by reducing (+DTT) or nonreducing (–DTT) SDS-PAGE and immunoblotting for the FLAG tag. Where indicated, detergent (det) was included during protease cleavage. Cleaved (scissors) and uncleaved (⊘) species are marked on the right. The topologies of the three observed dimers (numbered) are depicted below.
