Abstract
The mechanism by which Helicobacter pylori induces apoptosis remains unclear. In a previous study using biopsy samples, we found a significant correlation between the urease activity of an H. pylori strain and the apoptosis level induced by this strain. Therefore, in this study, we investigated whether urease and/or the ammonia generated by urease can induce apoptosis. Human gastric epithelial cell lines were cocultured with H. pylori, and the levels of apoptosis and ammonia production were measured. The medium was supplemented (or not supplemented) with urea and cytokines. While a large amount of ammonia (>30 mM) accumulated in the coculture containing urease-positive H. pylori and urea, no significant degree of apoptosis occurred. In the presence of tumor necrosis factor alpha (TNF-α), however, a marked acceleration of apoptosis was found in this coculture. Such enhancement of apoptosis was also induced by the addition of 4 to 8 mM ammonia to the cell culture without either H. pylori or urea but containing TNF-α. These results suggested that ammonia accelerates cytokine-induced apoptosis in gastric epithelial cells, while ammonia or urease molecules alone are unable to induce a significant degree of apoptosis.
Apoptosis is a physiological suicide mechanism which maintains homeostasis, in which cell death naturally occurs during tissue turnover. In the gastric epithelium, apoptosis may also play an essential role in maintaining tissue integrity, and the rate of new cell production by proliferation is matched by the rate of cell loss by apoptosis (4). Moss et al. (11) reported that the number of apoptotic cells in the gastric epithelium increases with Helicobacter pylori infection and decreases after the eradication of the bacterium. A previous study (8) also found an increase in apoptosis in epithelial cells of the stomach in H. pylori-infected patients as well as a decrease in apoptosis after eradication of this bacterium.
The mechanism by which H. pylori infection induces apoptosis has yet to be elucidated; however, urease, a predominant bacterial product of H. pylori, is generally considered to be mainly responsible for this infection (17). Therefore, the ammonia generated from the hydrolysis of urea by H. pylori urease may be one of the most likely inducers. Indeed, Tsujii et al. (21) showed that the long-term administration of ammonia induces mucosal atrophy in the stomach, thus suggesting an increased cell loss due to apoptosis. Moreover, Kohda et al. used gastric biopsy specimens from humans and found that there was a significant correlation between the urease activity of the H. pylori strain and the level of apoptosis induced by this bacterial strain (8). This raised the possibility that urease and/or ammonia causes the apoptosis of gastric epithelial cells in H. pylori infection.
In the stomach, however, it is considered that the degree of apoptosis induction may depend not only on bacteria and bacterial products but also on the associated inflammatory response. The proinflammatory cytokines gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) have been reported to augment the apoptosis induced by H. pylori (23). Therefore, in the present study we investigated whether urease and/or ammonia can induce apoptosis in the presence or absence of such proinflammatory cytokines, by using an in vitro cell culture system.
MATERIALS AND METHODS
Bacterial strains.
H. pylori strain 60 (cagA+) and strain 78 (cagA+) were isolated from gastric biopsy materials of gastroduodenal ulcer and duodenal ulcer patients, respectively, who were treated at Tokai University Hospital, Isehara, Japan. HPT73 is a urease-negative mutant of H. pylori constructed by allelic exchange mutagenesis from its corresponding wild-type strain, CPY3401 (19). A plasmid, pHPT54, which was used as the donor DNA to transform wild-type H. pylori, has a disruption in the ureB gene. These H. pylori strains were supplied by T. Nakazawa, Yamaguchi University, Ube, Japan. The bacteria were grown in brucella broth containing 5% fetal calf serum (FCS) in a microaerophilic atmosphere (5% O2) at 37°C, according to a method previously described (6). Bacteria in a logarithmic phase of growth were used. To determine the urease activity of H. pylori, the freshly prepared bacteria were suspended at various densities in RPMI 1640 medium containing 5% FCS, 20 mM HEPES, and 20 mM urea (pH 7.4). After incubation at 37°C for 30 to 240 min, the culture supernatant was collected to measure the concentration of ammonia after centrifugation at 3,000 rpm for 10 min in a himac CF7D centrifuge (Hitachi, Tokyo, Japan).
Agents.
Urea and a 25% ammonia solution were purchased from Wako Pure Chemical Industries, Osaka, Japan. Ammonium chloride was obtained from Kanto Chemical Co., Tokyo, Japan. Recombinant human TNF-α and recombinant human IFN-γ were obtained from R & D Systems, Inc., Minneapolis, Minn. Recombinant H. pylori urease B subunit was prepared according to the method reported by Hu et al. (5). Briefly, the ureB gene was subcloned on an expression vector, and Escherichia coli DH5α was transformed by this vector. Next, the recombinant gene product generated in the bacteria was obtained through lysozyme treatment of bacteria and then centrifugation. An analysis by Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed the purity to be approximately 90% (data not shown).
Cocultivation experiments.
A human gastric epithelial cell line, MKN 45, was obtained from the Japanese Center Research Resources Bank (Tokyo, Japan). This cell line shares some properties with primary gastric epithelial cells, such as adhesion to H. pylori and the resultant interleukin-8 production (6). The cells were grown in RPMI 1640 medium containing 5% FCS at 37°C in 5% CO2 for maintenance and used for experiments just after reaching confluency in the culture dishes. For the cell culture experiments, 105 cells and 107 CFU of H. pylori were suspended in 1 ml of RPMI 1640 medium (pH 7.4) supplemented with 5% FCS, 20 mM HEPES, and 20 mM urea and incubated in a 24-well culture dish at 37°C in a humidified 5% CO2 atmosphere. The recombinant urease B was added to the culture at a concentration of 200 μg/ml. Eight hours after incubation, both the cells and culture supernatant were collected to examine the degree of apoptosis and the concentration of ammonia, respectively. To detach the adherent cells, the dish was treated with 0.25% trypsin at 37°C for 5 min before harvest. The pH of the medium was measured by using a pH meter (pHBOY-P2; Shindengen Co., Tokyo, Japan). For the treatment of cells with cytokines, the cells were preincubated with 40 ng of IFN-γ/ml for 18 h in RPMI 1640 medium devoid of urea and H. pylori, and then the cells were added to 40 ng of TNF-α/ml at the beginning of cell culture. In the bacteria-free culture, RPMI 1640 medium containing 5% FCS and 20 mM HEPES (pH 7.4) was added to ammonia solution or ammonium chloride just before use. Then, 105 MKN 45 cells were suspended with 1 ml of this medium and incubated for 8 h at 37°C in a 5% CO2 atmosphere.
Analysis of apoptosis.
Apoptosis was examined by cell cycle analysis using flow cytometry (10). Briefly, single cells were fixed by exposure to 75% ethanol at −20°C for 24 h and then were treated with 0.1 mg of RNase A/ml at 37°C for 50 min. After being washed, the cells were stained with propidium iodide and then analyzed using a flow cytometer. The cells in a discrete subpopulation of signals under the G0/G1 cell cycle region (subdiploid cells) were designated as undergoing apoptosis, as the DNA was fragmented in the nuclei of these cells and such fragmentation is considered a hallmark of apoptosis. Apoptosis was also examined by an Annexin V FITC kit (Immunotech, Marseille, France). It is well known that in the early phase of apoptosis, phosphatidylserine, which is located in the inner leaflet of the plasma membrane, becomes exposed on the cell surface (9). In addition, annexin V binds preferentially to phosphatidylserine with a high affinity (22). In order to examine the degree of apoptosis using the kit, freshly prepared cells were mixed with fluorescein isothiocyanate-conjugated annexin V in a binding buffer. After incubation for 10 min, the cells were analyzed by flow cytometry. In order to discriminate between the different stages of apoptosis, such as the apoptotic and the secondary necrotic populations, the vital dye propidium iodide was added to the assay mixture.
Determination of ammonia concentration.
The concentration of ammonia in the culture supernatants was determined by the indophenol reaction, according to the modified method originally reported by Okuda et al. (14), using an Ammonia-Test-Wako kit (Wako Pure Chemical Industries). This method determines total ammonia, including NH3 and NH4+. Briefly, the deproteinized supernatant was added to phenol and sodium nitroprusside and subsequently was mixed with a solution consisting of NaOH, Na2HPO4 · 12H2O, and antiformin. After allowing the mixture to stand at 37°C for 20 min, absorbance was measured on a spectrophotometer at 630 nm. The calibration curve indicated a good linear relationship between absorbance and the standard ammonia concentration (r = 0.95; data not shown).
Statistics.
The experiments were carried out in triplicate in either two or three independent series, and the results were expressed as the mean ± standard deviation (SD). Comparisons between tests were done by using Student's t test, with statistical significance considered to be a P value of <0.05.
RESULTS
Assay of urease activity of H. pylori strains.
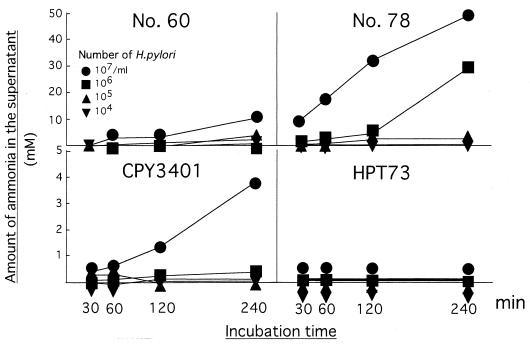
To determine the urease activity of the H. pylori strains, the bacteria were suspended in a medium supplemented with urea, and then the amount of ammonia that accumulated in the medium was continuously measured after incubation (Fig. 1). In the clinical isolates of the H. pylori strains, strain 78 had a much higher urease activity than did strain 60, since the amount of ammonia in the supernatant was five times higher in strain 78 than that in strain 60 when it was assayed 240 min after incubation at a density of 107 CFU/ml. A comparative analysis of the urease-negative mutant HPT73 and its wild type, CPY3401, confirmed the absence of urease activity in HPT73, while a moderate but significant degree of urease activity was observed in CPY3401. These results were confirmed by three series of independent experiments; one representative experiment is shown in Fig. 1.
FIG. 1.
Urease activity of H. pylori strains. Each strain of H. pylori was suspended at various densities in the medium, as indicated by the symbols, and then 100 μl of the supernatant was collected from the culture medium to measure the concentration of ammonia at the indicated time after incubation. Each symbol represents the mean value of triplicate supernatant samples.
Induction of apoptosis in cocultivation experiments.
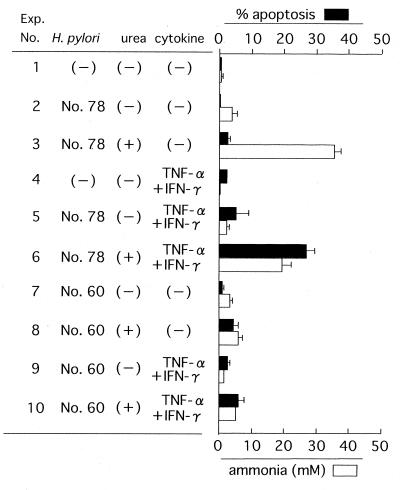
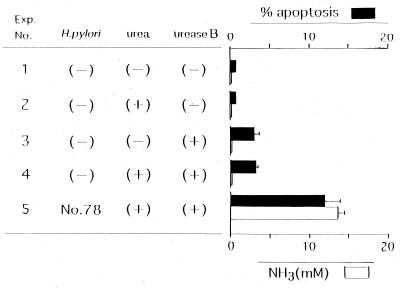
MKN 45 cells were cocultivated with H. pylori in the presence of urea and/or cytokines. Next, the degree of apoptosis of these cells and the amount of ammonia in the supernatant were measured after incubation for 8 h (Fig. 2). When urea was not added to the culture containing H. pylori strain 78, the amount of ammonia was low and the degree of apoptosis was minimal (experiment number 2), although the urease molecules were involved in this coculture as a bacterially associated form. Without urea or cytokines, strain 78 was unable to induce a detectable level of apoptosis in the coculture with KATO III cells, a gastric adenocarcinoma cell line (reference 6 and data not shown). While a high concentration of ammonia (>30 mM) accumulated in the coculture with strain 78 in the presence of urea, a significant degree of apoptosis did not occur (experiment number 3). In a different experiment (Fig. 3), the recombinant urease B subunit, which has no enzyme activity (compare experiment number 5 with number 4), induced a minimal level of apoptosis (∼3%). However, its apoptosis level was far lower than the apoptosis level obtained in the culture containing a sufficient amount of ammonia (Fig. 3, experiment number 5). This difference in apoptosis levels between experiment numbers 4 and 5 was statistically significant (P < 0.05). The findings thus suggested that neither the urease B subunit nor ammonia alone was sufficient to induce a significant degree of apoptosis in this human gastric epithelial cell line. Treatment of MKN 45 cells with a combination of TNF-α and IFN-γ alone did not induce a significant degree of apoptosis (Fig. 2, experiment number 4). However, the addition of strain 78 together with urea to the cytokine-containing culture markedly augmented the degree of apoptosis, which was accompanied by an elevation in the ammonia level (experiment number 6). A considerable decrease in the amount of ammonia in the number 6 experiment compared with the amount of ammonia in the number 3 experiment is thought to be caused by the addition of cytokines, which may lead to some inhibition of urease activity in the culture. Thus the ammonia level appeared to play a critical role in accelerating cytokine-induced apoptosis, because the deprivation of urea and the resultant decrease in the ammonia level considerably prevented this enhancement of apoptosis (experiment number 5).
FIG. 2.
Cocultivation experiment with MKN 45 cells. MKN 45 cells were cocultivated in triplicate with H. pylori in the presence of urea or cytokines, as indicated. Eight hours after incubation, the percent apoptosis ([number of apoptotic cells × 100]/total number of cells) and the concentration of ammonia in the culture supernatant were measured. The values represent the mean of three independent culture samples, and the bars represent SDs.
FIG. 3.
Cocultivation experiment using recombinant urease B subunit. MKN 45 cells were cocultivated in triplicate with recombinant urease B subunit in the presence of urea or H. pylori, as indicated. All cultures were treated with TNF-α and IFN-γ. Eight hours after incubation, the percent apoptosis and the concentration of ammonia were determined. The bars represent SDs.
A statistically significant difference (P < 0.05) was recognized in the degree of apoptosis between the number 5 and number 6 experiments. The difference in the ammonia level between them was also statistically significant (P < 0.05). There was only a marginal level of ammonia production, even in the presence of urea, in the coculture with strain 60 (experiment numbers 8 and 10), which coincided with the absence of acceleration in cytokine-induced apoptosis of the coculture. Taken together, these results suggested that ammonia enhances cytokine-induced apoptosis, whereas ammonia alone is unable to induce a significant degree of apoptosis.
Ammonia as an accelerator for apoptosis.
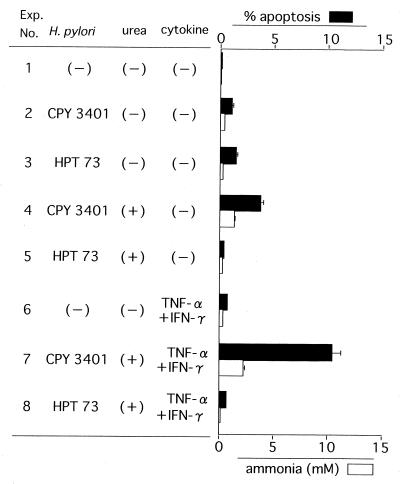
To further confirm the results presented above, we conducted a coculture experiment using MKN 45 cells with either a ureB-disrupted mutant HPT73 or its wild type, CPY3401 (Fig. 4). In the absence of urea, without which there is little production of ammonia, no increased apoptosis was found in the CPY3401 culture (experiment number 2) in comparison to the HPT73 culture (experiment number 3). This experiment again indicated the inability of urease molecules to induce apoptosis. In the coculture with urea, a small but significant amount of ammonia (around 2 to 3 mM) was generated by CPY 3401, which may be responsible for a low degree of apoptosis (experiment number 4), while no significant ammonia accumulation nor apoptosis induction was found in the corresponding HPT73 coculture (experiment number 5). When cytokines were introduced into these cocultures, the level of apoptosis in the CPY3401 coculture was 10 times higher than that in the HPT73 coculture (P < 0.01; compare experiment numbers 7 and 8). These results thus supported the theory that urease accelerates cytokine-induced apoptosis through the generation of ammonia.
FIG. 4.
Cocultivation experiment using urease-disrupted mutant. MKN 45 cells were cocultivated in triplicate with HPT73, a urease-disrupted mutant of H. pylori, or CPY3401, its wild-type strain, in the presence of urea or cytokine as indicated, in the same manner as described in the legend for Fig. 2. The values represent the mean of three independent culture samples, and the bars represent SDs.
Effect of ammonia in a bacteria-free system.
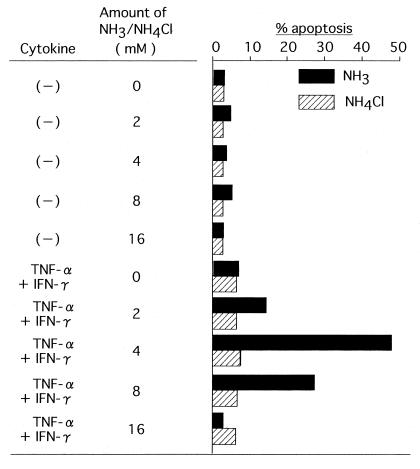
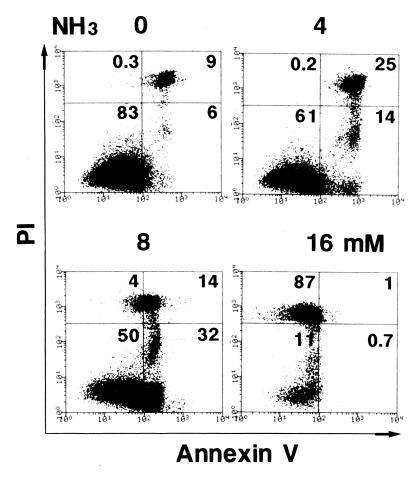
While the results obtained so far strongly suggest the critical role of ammonia in cytokine-induced apoptosis, it is still possible that ammonia may exert its effect in conjunction with urea, urease, or some other bacterial products. To rule out this possibility, an ammonia solution as the source of ammonia was added to a cell culture which contained no bacteria or urea (Fig. 5). The acceleration of cytokine-induced apoptosis reached its peak in the cultures containing 4 to 8 mM NH3, which again suggested the crucial role that ammonia plays in H. pylori-mediated apoptosis, while this degree of apoptosis (more than 40%) was considerably higher than that obtained in a coculture with H. pylori in which the ammonia level was about 10 to 20 mM (Fig. 2, experiment number 6). The ammonia alone induced a marginal but not remarkable degree of apoptosis (∼3%) in this experiment. An analysis of apoptosis by using annexin V and propidium iodide showed that a decrease rather than an increase of apoptosis in the culture to which more than 8 mM ammonia was added was due to a predominant induction of primary necrosis, which can be detected by staining with propidium iodide alone (Fig. 6). In the presence of 4 mM NH3, the degree of apoptosis reached a plateau at around 8 h after incubation and decreased thereafter in this annexin V assay (data not shown).
FIG. 5.
Cell culture with NH3 or NH4Cl. MKN 45 cells were treated in triplicate with various amounts of NH3 or NH4Cl, as indicated, in the absence or presence of cytokines. Eight hours after the treatment, the percent apoptosis was determined. Columns represent the mean of triplicate cultures. The SDs were all within 10% of the mean.
FIG. 6.
Apoptosis analysis using annexin V and propidium iodide. MKN 45 cells were treated with various amounts of NH3 solution, as indicated on the top of each panel, in the presence of TNF-α and IFN-γ. After 8 h of treatment, the level of apoptotic cells was analyzed by flow cytometry by using annexin V and propidium iodide. The value in the upper right corner of each quadrant indicates the percentage of apoptotic cells.
Effects of NH4+ and pH on apoptosis.
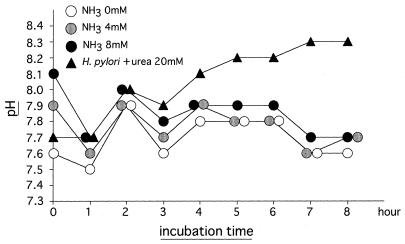
Some ammonia (NH3) molecules bind to H2O and thus generate NH4OH, which may then dissociate into NH4+ and OH− in gastric juice. Acidic conditions promote this reaction. Therefore, it is likely that NH4+ may exert an accelerating effect on cytokine-induced apoptosis. To address this problem, NH4Cl was added to the bacteria-free culture as a source of NH4+. As shown in Fig. 5, no significant acceleration of apoptosis was found in the culture to which NH4Cl was added, thus contradicting the possibility mentioned above. Finally, we examined the effect of exogenously added ammonia on the pH of the culture medium (Fig. 7). The addition of 4 and 8 mM ammonia solutions to the culture medium raised the initial pH of 7.6 to 7.9 and 8.1, respectively. These pH values then declined to 7.7 after 8 h of incubation. In the pH 8.1 medium prepared by adding NaOH instead of NH3, no significant acceleration of apoptosis occurred (data not shown). Moreover, the degree of apoptosis was slightly lower in the coculture with H. pylori (Fig. 2, experiment number 6) than the degree of apoptosis in the culture with 4 mM NH3 added (Fig. 5), although the former system always showed higher pH values than the latter system at 1 h and thereafter during the 8 h of incubation (Fig. 7). As a result, the possibility that ammonia augments apoptosis through the elevation of pH in the medium could thus be ruled out.
FIG. 7.
Measurement of pH of the culture medium. In the presence of TNF-α and IFN-γ, MKN 45 cells were treated with 0, 4, or 8 mM NH3 solution in the same manner as described in the legend for Fig. 5. In addition, the cells were cocultured with H. pylori strain 78 in the presence of 20 mM urea, TNF-α, and IFN-γ in the same manner as described in the legend for Fig. 2. During the incubation for 8 h, the pH of the culture supernatant was measured every hour using a pH meter. The symbols represent the mean of triplicate cultures. SDs were all within 10% of the mean.
DISCUSSION
The induction of apoptosis by H. pylori has been demonstrated in two types of studies: the identification of apoptotic cells in tissue sections from H. pylori-infected individuals and the induction of apoptosis in gastric epithelial cells in culture (16). However, it still remains to be clarified how H. pylori promotes apoptosis. A previous study using gastric biopsy samples from humans (8) found that the tissue colonized by H. pylori strains with high levels of urease activity showed a higher level of apoptosis than tissue colonized by H. pylori strains with low levels of urease activity. This finding suggests that an acceleration of apoptosis by H. pylori is caused by the urease and/or the ammonia generated from hydrolysis of urea by urease. Attempts to test this idea have been made in cocultivation experiments to induce apoptosis using H. pylori, urea, and gastric epithelial cell lines, in the presence or absence of cytokines. As a result, it was demonstrated that the ammonia generated from the urea markedly accelerated the apoptosis induced by TNF-α while neither ammonia nor urease molecules bound to bacteria could induce any significant degree of apoptosis in the absence of TNF-α.
Neithercut et al. (12) reported that the median ammonia concentration in gastric juice of H. pylori-infected subjects was 3.4 mM (range, 1.0 to 13.0 mM), which was higher than that in uninfected subjects (0.64 mM [range, 0.02 to 1.4 mM]). In the present study, 2 to 4 mM exogenously added ammonia raised the frequency of apoptosis from around 3% at background to 10 to 40%, respectively (Fig. 5). It is thus likely that such promotion of apoptosis by ammonia in vitro also plays an important role in enhancement of apoptosis in the mucosa of the H. pylori-infected stomach. The alkalinity of ammonia is not likely to be involved in the apoptosis promotion, as indicated in Fig. 7.
NH3 is transformed to NH4+ and both molecules are at equilibrium in water. The relative concentration of these two forms is pH dependent; the ratio of NH3 to NH4+ shows a 10-fold increase for each unit rise in pH. Considering that the acceleration of apoptosis was predominantly exerted by NH3 and not NH4+, as shown in the present study, apoptosis is thought to occur to a greater extent in gastric mucosa with less acidity, such as the mucosa of atrophic gastritis, in which a high prevalence of H. pylori infection has been reported (7). Moreover, the involvement of NH3 as a pathogenic factor for atrophic gastritis has been demonstrated by the oral administration of NH3 solution to rats (21). Taken together, these results suggest that an acceleration of apoptosis by NH3 is closely involved in the initiation and especially the progression of atrophic lesions in H. pylori-infected individuals. On the other hand, at pH 7.4 to 7.6, 99% of ammonia molecules are in the form of NH4+. This change from NH3 to NH4+ occurs quickly in water. It thus suggests that NH3 can exert its apoptosis-inducing effect on cells just a short time after being generated by urease or being added to the culture, and it then loses its effect very soon. The apoptosis level was significantly lower in the culture with both H. pylori and urea added to it (Fig. 2, experiment number 6) than in the culture with NH3 alone added to it (Fig. 5), although the ammonia level in the supernatant was much higher in the former culture than in the latter. This discrepancy between the levels of apoptosis and ammonia can be explained by the presumption that almost all the ammonia in the former culture consists of NH4+.
A variety of signals have been identified which can initiate apoptosis, including growth factor withdrawal, cell cycle perturbations, DNA damage, oxidative stress, nitric oxide, immune-mediated processes, and the ligands for specific cell death receptors such as TNF-α and Fas ligand (15). Among these signals, TNF-α is a predominant cytokine produced in the gastric mucosa of patients with H. pylori infection (2, 13), and it is able to induce apoptosis in a variety of cells. IFN-γ is known to exhibit synergistic biological effects with several other cytokines, including TNF-α (1), and is produced by lymphocytes when they come in contact with H. pylori (18). It is therefore likely that these cytokines are closely involved in the induction of apoptosis in the gastric mucosa. In the present study we therefore treated the cells with a combination of these two cytokines to prime the cells for the acceleration of apoptosis by ammonia.
The mechanism by which ammonia accelerates such cytokine-mediated apoptosis has yet to be elucidated. Accumulating evidence suggests that there are two independent apoptosis pathways that converge on the activation of downstream caspases, which are key substrate cleavage enzymes, and apoptotic death (3). The first pathway involves the ligation of such death receptors as the TNF-α receptor and Fas, and the second pathway targets mitochondria and releases cytochrome c from them. It is also possible that a cross talk exists between these pathways. Tsujii et al. (20) reported that ammonia inhibits mitochondrial respiration in gastric mucosal cells in vitro. This raises the possibility that ammonia primarily affects the mitochondria to release cytochrome c, whose amount is not sufficient to induce a significant degree of apoptosis but is enough to accelerate the death receptor-mediated signaling through a cross talk mechanism. Wagner et al. (23) reported that H. pylori alone induced a twofold increase in DNA fragmentation of the cells by apoptosis in vitro and a 2.7- to 6.0-fold increase in DNA fragmentation when TNF-α, IFN-γ, or agonistic anti-Fas antibody was coincubated with H. pylori; these findings thus appeared comparable to the findings obtained in the present study. Moreover, the reported findings indicated that a bacterial factor originating from the cytosol is responsible for such apoptosis induction. This factor can be eliminated by heat and trypsin digestion and is not correlated with the action of vacuolating cytotoxin, suggesting that it is a protein product relevant to the urease molecules. In the present study, using a pair of urease-negative and -positive H. pylori strains, however, no significant increase in apoptosis was found in the urease-positive culture, compared with the urease-negative culture, in the absence of urea.
REFERENCES
- 1.Crabtree J E. Immune and inflammatory responses to Helicobacter pylori infection. Scand J Gastroenterol. 1996;31(Suppl. 215):3–10. [PubMed] [Google Scholar]
- 2.Crabtree J E, Shallcross T M, Heatley R V, Waytt J I. Mucosal tumor necrosis factor α and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green D R. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 4.Hall P A, Coates P J, Ansari A, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 5.Hu L-T, Foxall P A, Russell R, Mobley H L T. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992;60:2657–2666. doi: 10.1128/iai.60.7.2657-2666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabir A M A, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41:49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karnes W E, Samloff I M, Siurala M, Kekki M, Sipponen P, Kim W R, Walsh J H. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167–174. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]
- 8.Kohda K, Tanaka K, Aiba Y, Yasuda M, Miwa T, Koga Y. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut. 1999;44:456–462. doi: 10.1136/gut.44.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin S J, Reutelingsperger C P M, McGahon A J, Rader J, Van Schie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus. Inhibition by overexpression of Bc1-2 and Abl. J Exp Med. 1995;182:1545–1557. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori T, Ando K, Tanaka K, Ikeda Y, Koga Y. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood. 1997;89:3565–3573. [PubMed] [Google Scholar]
- 11.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neithercut W D, Milne A, Chittajallu R S, Nujumi A M E, McColl K E L. Detection of Helicobacter pylori infection of the gastric mucosa by measurement of gastric aspirate ammonium and urea concentrations. Gut. 1991;32:973–976. doi: 10.1136/gut.32.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen H, Andersen L P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992;33:738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuda H, Fujii S, Kawashima Y. A direct colorimetric determination of blood ammonia. Tokushima J Exp Med. 1965;12:11–23. [PubMed] [Google Scholar]
- 15.Que F G, Gores G J. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238–1243. doi: 10.1053/gast.1996.v110.pm8613014. [DOI] [PubMed] [Google Scholar]
- 16.Shirin H, Moss S F. Helicobacter pylori induced apoptosis. Gut. 1998;43:592–594. doi: 10.1136/gut.43.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smoot D T, Mobley H L T, Chippendale G R, Lewison J F, Resau J H. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990;58:1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarkkanen J, Kosunen T U, Saksela E. Contact of lymphocytes with Helicobacter pylori augments natural killer cell activity and induces production of gamma interferon. Infect Immun. 1993;61:3012–3016. doi: 10.1128/iai.61.7.3012-3016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujii M, Kawano S, Tsuji S, Fusamoto H, Kamada T, Sato N. Mechanism of gastric mucosal damage induced by ammonia. Gastroenterology. 1992;102:1881–1888. doi: 10.1016/0016-5085(92)90309-m. [DOI] [PubMed] [Google Scholar]
- 21.Tsujii M, Kawano S, Tsuji S, Ito T, Nagano K, Sasaki Y, Hayashi N, Fusamoto H, Kamada T. Cell kinetics of mucosal atrophy in rat stomach induced by long-term administration of ammonia. Gastroenterology. 1993;104:796–801. doi: 10.1016/0016-5085(93)91015-a. [DOI] [PubMed] [Google Scholar]
- 22.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C P M. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–52. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 23.Wagner S, Beil W, Westermann J, Logan R P H, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]