Abstract
Ocular nerve palsies are among the most common cranial neuropathies in neurological practice. Nerves can get affected anywhere along their path from the brainstem to the orbit. There can be isolated involvement of multiple cranial nerves together. The etiologies differ according to the type of presentation. The steps toward the diagnosis need to be strategically planned and must be based on clinical localization. It is crucial to make proper localization to plan further investigations and thus treatment of the etiology. This review covers the approach toward the diagnosis, etiologies involved, and management of ocular cranial neuropathies.
Keywords: Abducens palsy, diplopia, ocular nerve palsy, oculomotor palsy, trochlear palsy
INTRODUCTION
Ocular cranial nerves (CNs), that is, oculomotor (III), trochlear (IV), and abducens (VI), represent the efferent visual pathway and are responsible for adequate extraocular movements. This review article will help in approaching the diagnosis, evaluation, and management of patients with ocular CN palsies.
To understand the approach to diagnosis, one must remember the anatomical course of the nerve as etiology differs according to the site of localization of the lesion. Hence, we have to understand the individual nerve anatomy before discussing further.
NEUROANATOMY
Ocular nerves arise from the brainstem from their respective nucleus/nuclei, which give rise to fascicules (axons from the nucleus to the exit from the brainstem).[1] After exiting from the brainstem, the nerves travel through respective cisterns (cisternal segment), further to the cavernous sinus (CS), and finally into the orbit through a superior orbital fissure (SOF) supplying their respective muscles.[1,2]
Oculomotor nerve (III CN): III CN arises from oculomotor nuclei which lie in the upper part of the midbrain at the level of the superior colliculus. The nuclei are arranged in a specific manner around the periaqueductal area[3] which is clinically important for the localization of lesions [Box 1].[1] Fascicles arise from their respective nucleus and travel along the substance of the midbrain to cross the red nucleus, superior cerebellar peduncle fibers, substantia nigra, and crus cerebri.[2] The nerve exits from the interpeduncular fossa into the subarachnoid space and travels anteriorly between the posterior cerebral artery and superior cerebellar artery, further parallel to the posterior communication (PCOM) artery which lies superiorly.[4] As the nerve goes anteriorly, it travels just medial to the uncus of the temporal lobe. Thereafter, it pierces the lateral wall of the CS just above the trochlear nerve after which it divides into superior and inferior divisions just before it enters the SOF.[5] Superior division supplies superior rectus (SR) and levator palpebrae superioris (LPS) muscles, while inferior division supplies medial rectus (MR), inferior rectus (IR), and inferior oblique (IO) and carries parasympathetic fibers to ciliary muscles and sphincter pupillae.[1,6,7]
Box 1.
Important points to remember regarding Oculomotor nuclear complex
| Oculomotor subnuclei innervate the individual ocular muscle. |
| MR nuclei are spread over the nuclear complex. |
| There is only a single nucleus that supplies LPS on both sides. |
| SR nuclei are medial most. |
| The axons from SR nuclei decussate across the midline at the level of the nuclear complex. |
Trochlear nerve (IV CN): IV CN arises from the trochlear nucleus which lies at the lower part of the midbrain at the level of the inferior colliculus.[2] It has a single nucleus for the respective nerve on two sides, ventrolateral to the cerebral aqueduct and dorsal to the medial longitudinal fasciculus (MLF).[5] Fascicles arise and run posterior-laterally and then medially, around periaqueductal gray matter to reach the dorsal midbrain, where they decussate in the anterior medullary velum.[2] The nerve emerges from the midbrain dorsally just inferior to the inferior colliculus and runs anteriorly into the quadrigeminal, ambient, crural, and pontomesencephalic cistern over the lateral part of the brainstem lying close to the edge of tentorium cerebelli.[8] It passes between the posterior cerebral artery (dorsally) and the superior cerebellar artery (ventrally), and subsequently enters the lateral wall of the CS by piercing the dura below the entry of III CN.[5] The trochlear nerve runs above the ophthalmic division of the trigeminal nerve in the CS and further enters the orbit through SOF to supply superior oblique (SO) muscle. Thus, the trochlear nerve supplies contralateral SO.[1,7,8]
Abducens nerve (VI CN): Abducens nerve arises from its nucleus which lies under the floor of the fourth ventricle in the dorsal part of the lower part of the pons.[7] The facial colliculus (formed by the genu of the facial nerve) lies dorsal to the nucleus. The nuclear complex contains two types of neurons: lateral rectus (LR) motor neurons which supply LR muscle and the interneurons, the axons of which cross the midline and ascend via contralateral to end in contralateral oculomotor nuclei and are thus responsible for maintaining coordinated movements of both eyes in horizontal gaze.[1,3] LR motor neurons give rise to axons which travel anteriorly within the substance of pons medial to the fascicle for the facial nerve through the medial lemniscus to exit the brainstem at the pontomedullary junction lateral to corticospinal bundles.[2] The further course of the sixth nerve is unique as it bends upwards perpendicularly and travels anterior to the brainstem close to the clivus, coming in close contact with the basilar artery as well as with the hypoglossal nerve. It ascends further and bends anteriorly to enter the Dorello's canal under the petroclinoid ligament.[1] The nerve is tethered in the Dorello's canal, medial to petrous apex, thus making it susceptible to damage. Moving further, it enters the CS and runs lateral to the internal carotid artery (ICA) and medial to an ophthalmic division of the trigeminal nerve.[5] It carries sympathetic fibers for pupils while its course in the CS. Eventually, the nerve enters SOF to enter orbit and supply ipsilateral LR muscle.[1,9]
DIAGNOSTIC APPROACH
Diagnosis is made clinically in most cases based on localization and evolution of symptoms because the etiologies differ according to the site of the lesion. If a clinician is good enough to localize the lesion, then half of the work is done, and imaging and other investigations would merely confirm the clinical suspicion. Thus, detailed history and meticulous examination are imperative in making the diagnosis.
Anatomical localization of lesion: To localize the lesion, one must answer the questions in Box 2. These specific points would make the process of localization much easier. Now we will go through the specific points for localization for each ocular nerve and also discuss the relevant etiology wherever needed.
Box 2.
Questions to answer before localization
| Questions to answer before localization: |
| Isolated or multiple ocular nerves involvement? |
| Unilateral or bilateral? |
| Pupillary involvement or not? |
| What is the first symptom to start? Onset, duration, and progression? |
| The sequence of structural involvement? Tell us about where the disease started and how it spreads. |
| Horner’s or not? |
| Proptosis or not? |
| Local eye symptoms like chemosis, excessive lacrimation or not? |
| Associated vision loss? |
| Associated with other cranial nerve involvement or not? |
| Associated headache or not? |
| Associated with seizures or not? |
| Associated papilledema or not? |
| Associated with long tract involvement or not? |
| Associated cerebellar signs or signs of meningeal irritation and/or other associated symptoms like fever, anorexia, and weight loss? |
-
Oculomotor nerve palsy:
- The first step is to know whether it is unilateral or bilateral, isolated or multiple nerves. Bilateral involvement is more commonly seen when there is a lesion at the level of the nuclear complex or subarachnoid space. But one must remember that ophthalmoplegia due to nuclear lesions is often not complete and are asymmetrical. Moreover, if there is bilateral ptosis with unilateral involvement of IR, MR, and IO, the lesion is at an ipsilateral nuclear complex as a single nucleus supplies both LPS. In addition, nuclear lesions lead to bilateral upgaze restriction as the subnucleus for SR is medial most and the emerging axons decussate at the nuclear level, so any unilateral lesion will damage the ipsilateral SR subnucleus with contralateral crossing fibers. Isolated MR palsy rarely occurs in nuclear lesions as the MR neurons lie in three distinct regions within the nuclear complex.[1]
- Bilateral III CN palsy with varying combinations can occur with lesions at the exit from the midbrain or even in CS/SOF lesions if the disease is fulminant to affect both sides.
- Unilateral oculomotor palsy is more difficult to localize particularly if it is isolated with no other signs. However, unilateral oculomotor palsy with other long tract involvement indicates a fascicular lesion [Figure 1]. Varying manifestations can be seen based on the course of the nerve as it crosses the red nucleus, superior cerebellar peduncle, substantia nigra, and cerebral peduncle, thus resulting in various syndromes.[10] Infarction is the commonest etiology reported with tumors, cavernomas, and demyelination [Table 1].[11,12]
- The most common site for isolated involvement is between the exit from the midbrain and entry in the CS [Figure 2]. Various etiologies could affect the nerve, like aneurysms, ischemia, infiltration, infectious processes like tuberculosis [Figure 2], inflammatory diseases like sarcoidosis, immunoglobulin G4-related (IgG4) disease, hypertrophic pachymeningitis, vasculitis, avulsion because of trauma, displacement of nerve by hemorrhagic contusion or hematoma, and even benign condition like ophthalmoplegic migraine [Figure 3].[13] It is to be noted here that aneurysmal oculomotor palsy commonly involves the pupil, while diabetic oculomotor palsy usually spares the pupil.[14]
- Uncal herniation could lead to ipsilateral oculomotor palsy, which usually begins with dilatation of the pupil leading to anisocoria (Hutchison's pupil)[15] which, if found, is an ominous sign in an unconscious patient and warrants an urgent computed tomography (CT) of the head to look for impending herniation.
- If there is a combination of III with varying combinations of IV, VI, and ophthalmic (V1), maxillary (V2), and mandibular (V3) division of the trigeminal nerve, the lesion is either in the CS or SOF.
- Divisional palsy results in paresis of specific muscles supplied by either division of III CN. If there is preferential involvement of SR and LPS involvement, the lesion is after the divisions were formed i.e., into the orbit.
- Myasthenia gravis is a close mimicker and must be ruled out in all cases [Figure 4].
Trochlear nerve palsy: Isolated CN IV palsy is rare. Nuclear lesions lead to contralateral SO palsy as fibers decussate in the anterior medullary velum. Fascicular involvement is not alone and can be associated with ipsilateral Horner's syndrome and internuclear ophthalmoplegia because of the involvement of sympathetic fibers and MLF.[1] Involvement of brachium of superior colliculus results in contralateral afferent pupillary defect with contralateral III CN palsy.[1,7] Lesions in the CS, SOF, and orbit will be explained in the multiple CN palsies section.
-
Abducens nerve palsy:
- Isolated abducens palsy is commonly seen as a false localizing sign (usually bilateral but asymmetrical) because of raised intracranial pressure.[9]
- Abducens nucleus lesions could never lead to isolated LR restriction because they also give rise to interconnecting fibers to MLF. So, damage to the nucleus leads to ipsilateral horizontal gaze palsy, thus no diplopia.[1]
- The nerve is prone to various diseases once it exits pons because it has a long course. It can get involved in infectious meningitis, carcinomatous meningitis, hypertrophic pachymeningitis, and sarcoidosis.[18] Tethering of the nerve in the Dorello's canal makes it prone to distortion and stretch injury because of raised intracranial pressure, for example, tubercular meningitis, cryptococcal meningitis, benign intracranial hypertension, and cerebral venous sinus thrombosis.[2] Also, it runs in close association with tentorium cerebelli where any degree of raised pressure might result in compression of the nerve.
-
Multiple CN palsies:
- III, IV, VI, and V: If there is a combination of III with varying combinations of IV, VI, and ophthalmic (V1), maxillary (V2), and mandibular (V3) division of the trigeminal nerve, the lesion is either in the CS or SOF. V1 is the first to enter the CS (with the exit of III, IV, and VI) followed by V2, while V3 enters at last. Accordingly, CS lesions are classified into the anterior, middle, and posterior cavernous syndrome.[19] This distinction is important because the etiologies differ as per each part of CS.[20]
- II, III, IV, VI, and V1: Involvement of optic nerve (CN II) along with varying combinations of III, IV, VI, and V1 localizes the lesion to SOF/orbital apex. Nerve palsies are associated with chemosis, proptosis, and excessive lacrimation. Common etiologies are craniofacial trauma, inflammatory disorders like Tolosa–Hunt syndrome (THS), IgG4 disease, sarcoidosis, Wegner's granulomatosis, orbital tumors, mucormycosis, and aspergillosis.[21,22]
- VI and ipsilateral Horner's syndrome: The lesion localizes to VI CN within the cavernous sinus. There is no other localization of this presentation and the syndrome is called Parkinson syndrome.[23] This syndrome also gives us an idea about the etiology. VI CN is closest to the ICA, so any lesion arising from ICA will involve VI before affecting other ocular nerves like carotid aneurysm[24] and carotid-cavernous fistula.[25] However, if the patient has the involvement of III/IV/VI/V before VI, then the etiology is arising from the wall of the cavernous sinus.
- VI and XII: Combined abducens and hypoglossal nerve palsy is considered to be an ominous sign and only localizes to the clivus near the lower brainstem [Figure 7]. The syndrome has been described concerning nasopharyngeal carcinomas invading the base of the skull and has also been called Godtfredsen syndrome or Clival syndrome [Figure 8].[26]
- VI, V, and VII: Lesions of petrous apex lead to spread to trigeminal ganglion as well as abducens nerve which lies in close association (called Gradenigo's syndrome).[27]
- Bilateral complete ophthalmoplegia: Bilateral involvement of all the ocular nerves can be localized to the nucleus or fasciculus (brainstem infarction, demyelination, or tumors),[28] or nerves in subarachnoid space [Figure 9] (tubercular meningitis and Miller Fisher syndrome),[29] or CS (carotid dissection, pituitary apoplexy, skull base metastasis, mucormycosis, and head trauma).[29] Bilateral ocular palsies because of brainstem lesions are associated with a depressed sensorium/comatose state and quadriparesis and are associated with poor prognosis.[28] Myasthenia gravis is a close mimicker and must be ruled out in all cases.
Figure 1.
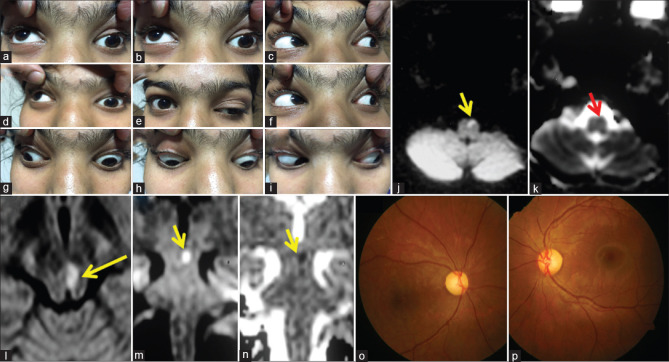
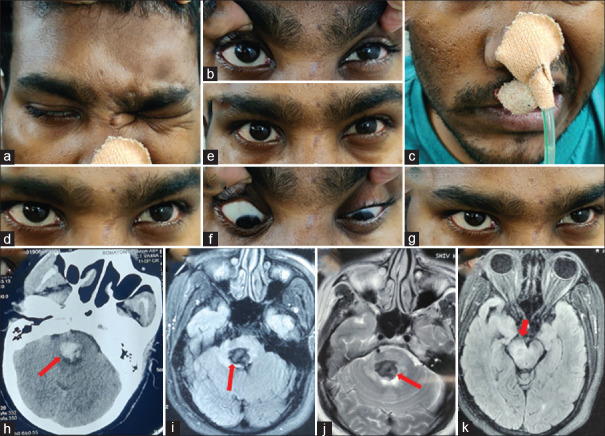
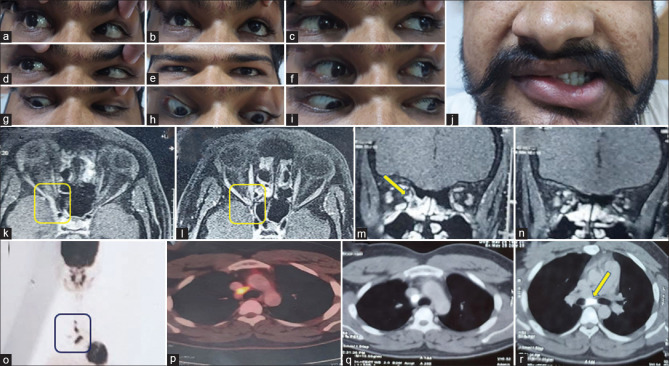
A 15-year-old girl presented with acute onset binocular horizontal diplopia with drooping of the left eyelid (a–i). She had a history of acute onset weakness of the right upper and lower limb which was treated with aspirin at another center after an MRI showed an acute infarct in the left ventral medulla (DWI: j and ADC: k). She was worked up for etiologies of stroke with normal complete blood count, renal and liver function tests, thyroid function tests, procoagulant workup including APLA, vasculitic workup, echocardiography, and angiography of intracranial and extracranial neck vessels. When she presented to us, her examination revealed left ptosis with restriction of left MR, IR, IO, and bilateral SR with normal pupils, and brisk deep tendon reflexes in the right upper and lower limb. Surprisingly, her visual acuity was 6/60 in the right eye and finger-counting at 3 feet in the left eye with a pale disc bilaterally (fundus photography: p). She also revealed her difficulty in looking at the board in her class because of which she started sitting in the front row. What will be the approach to this patient? Because of the involvement of the anterior visual pathway in her, one can get confused about localization. Another confusing point is brisk reflexes on the right side of the body which can be possible if we consider the lesion at the level of the left cerebral peduncle leading to ipsilateral oculomotor palsy and contralateral corticospinal signs. However, the point to note here is bilateral SR restriction which takes us more toward lesion at the nucleus rather than fascicle. Brisk reflexes were likely because of an old insult (left medullary infarct). Repeat MRI showed diffusion restriction in the left side of the midbrain (DWI: l, m, and ADC: n) supporting the localization. We considered a strong possibility of mitochondrial cytopathy because of young age, recurrent stroke-like presentations, and optic atrophy. Muscle biopsy revealed red ragged fibers which were COX deficient on enzyme stain. DWI: Diffusion weighted image, ADC: Apparent diffusion coefficient, APLA: Antiphospholipid antibodies
Table 1.
Etiologies of third cranial nerve palsy based on anatomical localization
| Anatomical localization | Etiologies |
|---|---|
| Nuclear | Infarction Hemorrhage Cavernoma Demyelination: Multiple sclerosis, NMOSD, ADEM Tumors: Lymphoma, glioma, metastasis Trauma Neurocysticercosis, tuberculoma |
| Fascicle | Infarction Hemorrhage Cavernoma Tumors: Lymphoma, glioma, metastasis Demyelination: Multiple sclerosis, NMOSD, ADEM Neurocysticercosis, tuberculoma, toxoplasma, listeria |
| Subarachnoid space | Aneurysmal of PCOM, basilar, internal carotid, posterior cerebral, superior cerebellar artery Microvascular ischemia (diabetes, hypertension, dyslipidemia, coronary artery disease, atherosclerosis) Meningeal inflammation: tubercular meningitis, cryptococcal meningitis, neuroborreliosis, carcinomatous/lymphomatous meningitis Hypertrophic meningitis: Sarcoidosis, IgG4 disease, Wegner’s granulomatosis, Sjogren’s syndrome Postinfectious neuropathy Ophthalmoplegic migraine Subarachnoid hemorrhage Cerebral venous sinus thrombosis Benign intracranial hypertension Hydrocephalous Vascular ectasias Tumors: Meningioma, chordoma, metastasis Head trauma Neurosurgical procedures Uncal herniation Miller Fisher syndrome |
| Cavernous sinus | Inflammatory: THS, sarcoidosis, IgG4 disease, Wegner’s granulomatosis Tumors: pituitary macroadenoma, meningioma, lymphoma, nasopharyngeal carcinoma, metastasis Infection: Mucormycosis, aspergillosis, tuberculoma Aneurysm from the internal carotid artery, carotid-cavernous fistula Pituitary apoplexy Mucocele of the sphenoid sinus |
| Orbit | Infection: Aspergillosis, mucormycosis, pyogenic orbital cellulitis, tuberculosis of orbit Tumors Inflammatory, granulomatous: Sarcoidosis, IgG4 disease Orbital trauma |
| Localization unknown | Migraine, congenital, postviral palsy, idiopathic palsies, postradiation, Miller Fisher syndrome |
Figure 2.
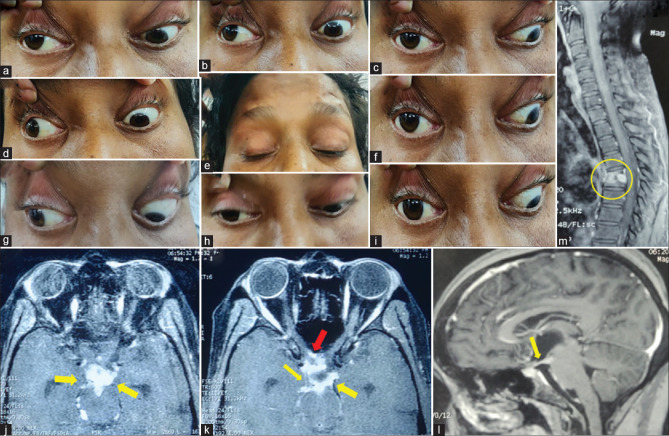
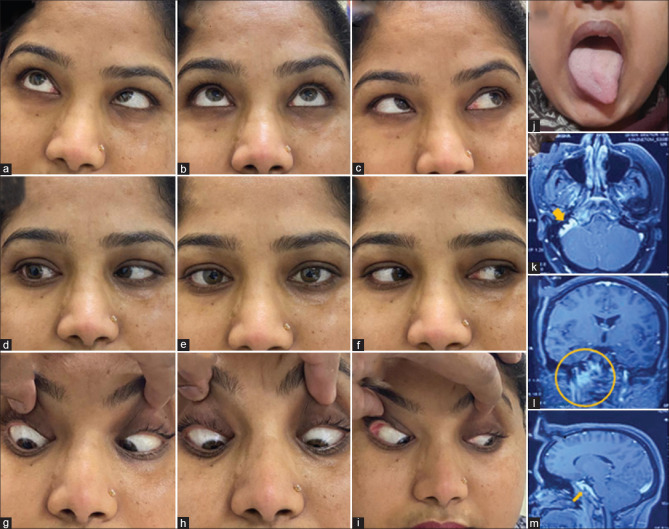
A 35-year-old female got admitted for evaluation of fever, anorexia, and weight loss for 2 months. She was evaluated outside and started on antitubercular drugs because of probable pulmonary tuberculosis. However, she has recently developed drooping of both upper eyelids (e). On examination, she had complete ptosis bilaterally with asymmetrical oculomotor palsy with pupillary involvement and left LR restriction (a–i). The localization in this patient could be nuclear as she has bilateral third CN palsy (ptosis and SR restriction are bilateral). Gadolinium-enhanced MRI revealed no involvement of the midbrain but there were dense exudates (yellow arrows in j–l, TIW) entrapping oculomotor nerves at their exit in the interpeduncular fossa and course further in subarachnoid space also abutting optic nerves (red arrow in k) and chiasma. MRI spine showed evidence of Pott's spine at D5 and D6 (yellow circle, m). She was started on dexamethasone with the continuation of antitubercular treatment
Figure 3.
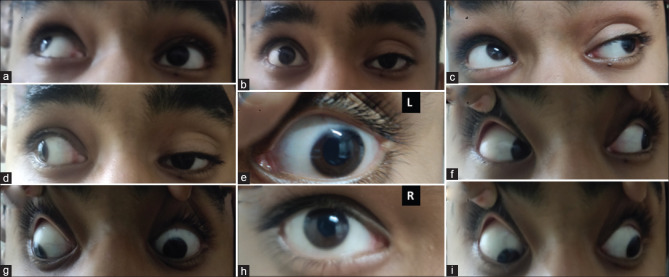
A 15-year-old male presented with a history of acute onset drooping of left eye with a deviation of left eyeball laterally and headache since morning. There was no history of headaches in the past. Examination revealed dilated left pupil (e) with sluggish light reflex and left SR, IR, IO, MR restriction with ptosis. Because of painful III CN palsy with pupillary involvement in a young male, the possibility of an aneurysm was considered and gadolinium-enhanced MRI brain and orbit along with constructive interference in steady state with MR angiography was performed. Imaging was unremarkable. CT angiography was done to look out for any aneurysm which might have been missed; however, it was normal. The other routine and serological workup along with cerebrospinal fluid (CSF) pressure and CSF workup were completely normal. He started improving spontaneously. The final diagnosis of ophthalmoplegic migraine was made and he was started on propranolol. He gradually recovered
Figure 4.
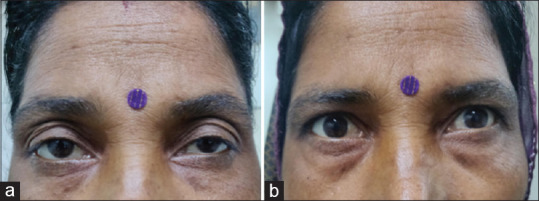
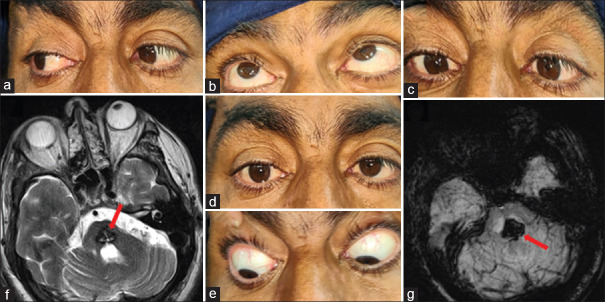
A 32-year-old female with a history of drooping of upper eyelids for 2 months. It was bilateral, asymmetrical (left > right) without diplopia, but had fluctuations in the symptoms. Examination showed fatiguability on upgaze, no restriction of extraocular movements, and normal pupils. The case can be easily confused with the last case; however, pupillary sparing will lead us to consider the possibility of the neuromuscular junction or muscle disease. The neostigmine test was positive (b), which confirmed the diagnosis of ocular myasthenia. Acetylcholine antibody test came positive
Figure 5.
A 40-year-old male with a history of hypertension presented with weakness of the right upper and lower limb along with diplopia and severe ataxia. Examination showed left VI and VII CN palsy with impairment of left horizontal gaze (c: unable to look in left lateral gaze) along with severe gait and stance ataxia, cerebellar speech with oculopalatal myoclonus. MRI brain showed pontine bleed (red arrows in f: FLAIR and g: susceptibility-weighted image)
Figure 6.
A 32-year-old male presented with acute onset weakness of the left upper and lower limb with dysarthria and ataxia. Examination revealed bilateral horizontal gaze palsy, right facial palsy (LMN) with stance, and gait ataxia. Noncontrast-enhanced CT head showed hyperintensity in pons likely of acute hemorrhage. MRI brain FLAIR (i) and T2 (j) sequences showed a hypodense lesion in the dorsal aspect of the pons, which showed blooming in the susceptibility-weighted image. Considering the young age of the patient without a history of vascular risk factors, the high possibility of cavernoma was kept
Figure 7.
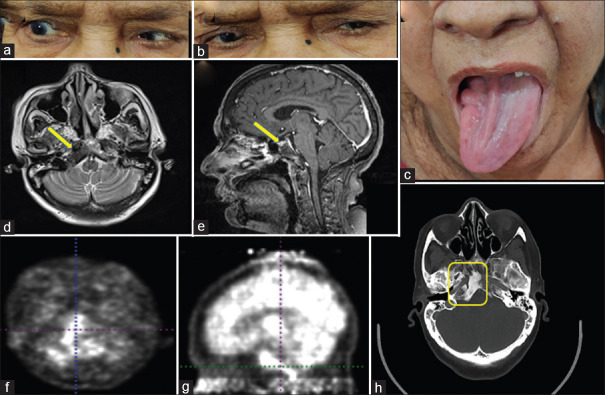
A 38-year-old female with a history of rheumatoid arthritis, on methotrexate and intermittent steroids, presented with a history of headache and recurrent diplopia which improves completely on taking steroids. She had two episodes in the last 4 months. She presented with the third episode because of diplopia on tapering steroids, cushingoid habitus, and increased frequency of headache. On examination, she had right VI (d) and right XII CN palsy (Fig. 7j). Because of a combination of VI and XII CN palsy, the lesion around the clivus was suspected with the possibility of inflammatory etiology (IgG4 disease, sarcoidosis) in view of steroid responsiveness. Neoplastic etiology was also considered because of the high incidence of nasopharyngeal carcinoma in the localization suspected. Contrast-enhanced MRI brain and base of the skull was done, which suggested soft tissue mass lesion in the right skull base region (yellow arrows and circle) with bony erosion of the right petrous apex, basi-occiput, and sphenoid. The lesion was seen extending into carotid space, prevertebral, and nasopharyngeal space causing effacement of the fossa of Rosenmuller inferiorly and clivus posterio-superiorly. Evaluation revealed raised serum IgG4 levels (994 mg/dL normal <800 mg/dL). A tissue biopsy was deferred by an otorhinologist because of the difficult approach at the site of the lesion. FDG-PET scan showed FDG avid lesion and bone scan using TcMDP three-phase study showed increased perfusion and osteoblastic activity in the right petrous apex with subtle erosive changes in clivus. She was restarted on steroids with a cover of injectable antibiotics. She showed mild improvement and is on close follow-up
Figure 8.
A 68-year-old female with lung carcinoma presented with dysarthria (more to linguals) in the last 3 months which has been static since then. She had no other significant history. On further examination, she had right abducens palsy (a) and right hemi tongue atrophy with deviation to the right side (c). Localizing her symptoms to right VI CN and right XII CN without any headache or any other long tract signs, we consider the possibility of the lesion at the clivus (Godtfredsen syndrome) or nearby subarachnoid space. Contrast-enhanced MRI of the brain and base of the skull was done to confirm the hyperostotic clival metastasis (d and e) which was further confirmed on FDG-PET CT scan which shows increased uptake (f-h)
Figure 9.
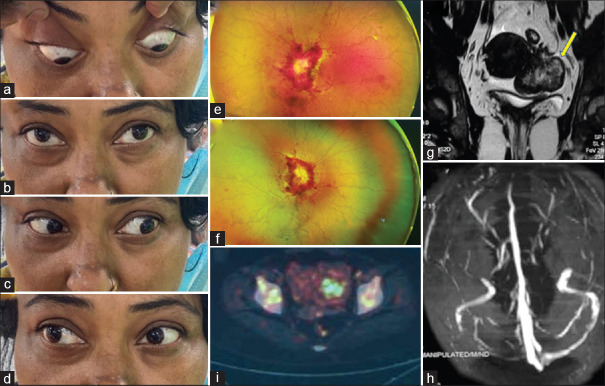
A 42-year-old female presented with headache for the past one-and-a-half month and visual loss for 20 days, and left followed by right binocular diplopia. A clinical picture suggested raised intracranial pressure but the etiology seems to be fulminant. The possibility of carcinomatous meningitis, secondary CNS lymphoma was kept initially. Examination showed severe anemia, bilateral grade V papilledema with peripapillary hemorrhages and tortuous vessels (e and f) and multiple cranial nerves palsies (bilateral VI, CN left partial III CN, right VII CN, bilateral IX and X CN, right XII CN). Contrast-enhanced MRI brain was unremarkable except for minimal tortuosity of optic nerves. However, MR venography revealed thrombosis of the right transverse and sigmoid sinuses. Lumbar puncture showed raised ICP of 31 cm H2O. Cerebrospinal fluid showed malignant epithelial cells of epithelial origin. Ultrasonography (USG) abdomen and pelvis revealed a bilateral adnexal mass with both solid and cystic components confirmed by MRI pelvis (g). USG-guided fine-needle aspiration cytology of adnexal mass confirmed carcinoma. FDG-PET reported FDG avid lesion in the right adnexa with variably FDG avid skeletal lesions (i). She also had hypercalcemia of malignancy which was managed with intravenous hydration and anti-hypercalcemic measures. She was planned for radiotherapy; however, relatives were not ready for further treatment when counseled regarding the disease
Etiology
Various etiologies responsible for ocular nerve palsies are listed in [Tables 1, 2, 3]. The most frequent isolated ocular palsy is the sixth nerve palsy while pure trochlear nerve palsies are the least common.[30] Microvascular ischemia is the most common cause of oculomotor and abducens palsy, with 58.1% and 69.8%, respectively.[30,31] However, head injury is the most common etiology for trochlear palsy followed by ischemia.[30,31] No cause could be found after extensive evaluation in 6.1% of cases.[31] However, the young population has a different scenario and vascular ischemia tends to be rare in them.[30] Congenital palsy, head trauma, and postinfection tend to predominate at the young age of <20 years.[30] Ischemia is mainly seen at ≥50 years of age when hypertension, diabetes, coronary artery disease, and dyslipidemia are common and are important risk factors. Phuljhele et al.[31] reported that 18.2% (63 out of 345 cases) had preexistent risk factors and 40.8% were diagnosed after the presentation. Acute onset, painful oculomotor palsy is a neurological emergency (especially at a young age) and immediate angiography of intracranial vessels must be done to rule out aneurysm. It has been emphasized in the literature to follow the rule of pupillary involvement to distinguish between ischemic and aneurysmal oculomotor palsy. But it has to be noted that pupillary sparing is not universal in ischemia and the pupil can get involved in 17% of patients.[32] Keane et al.[33] reported up to 53% of cases. On the contrary, pupil sparing has been reported only in 1–8% of cases of PCOM aneurysm associated with III CN palsy.[33,34] Other etiologies common for isolated oculomotor palsy are aneurysmal compression, trauma, compression from neoplasm, stroke, pituitary apoplexy, THS, ophthalmoplegic migraine [Figure 4], giant cell arteritis, and entrapment by exudates in tubercular meningitis.[32]
Table 2.
Etiologies of fourth cranial nerve palsy based on anatomical localization
| Anatomical localization | Etiologies |
|---|---|
| Nuclear | Trauma Infarction Hemorrhage Cavernoma Demyelination Tumors |
| Fascicle | Trauma Infarction Hemorrhage Cavernoma Demyelination Tumors |
| Subarachnoid space | Aneurysmal of the superior cerebellar artery Trauma Microvascular ischemia (diabetes, hypertension, dyslipidemia, coronary artery disease, atherosclerosis) Meningeal inflammation: tubercular meningitis, cryptococcal meningitis, neuroborreliosis Hypertrophic meningitis: Sarcoidosis, IgG4 disease, Wegner’s granulomatosis Hydrocephalous Benign intracranial hypertension Tumors: Nerve sheath tumor, Schwannoma, meningioma, ependymoma, carcinomatous/lymphomatous meningitis, metastasis Iatrogenic trauma during neurosurgical procedures Miller Fisher syndrome |
| Cavernous sinus | Inflammatory: THS, sarcoidosis, IgG4 disease, Wegner’s granulomatosis Tumors: pituitary macroadenoma, meningioma, lymphoma, metastasis Infection: Mucormycosis, aspergillosis, tuberculoma Aneurysm from the internal carotid artery, carotid-cavernous fistula Pituitary apoplexy |
| Orbit | Infection: Aspergillosis, mucormycosis, pyogenic orbital cellulitis, tuberculosis of orbit Tumors Inflammatory, granulomatous: Sarcoidosis, IgG4 disease Orbital trauma |
| Localization unknown | Migraine, congenital, postviral palsy, idiopathic palsies, postradiation, Miller Fisher syndrome |
Table 3.
Etiologies of abducens cranial nerve palsy based on anatomical localization
| Anatomical localization | Etiologies |
|---|---|
| Nuclear | Congenital Demyelination Infarction Hemorrhage Cavernoma Tumors Wernicke–Korsakoff syndrome |
| Fascicle | Demyelination Infarction Tumors Hemorrhage Trauma |
| Subarachnoid space | Aneurysmal of the vertebral artery, posterior-inferior cerebellar artery Microvascular ischemia (diabetes, hypertension, dyslipidemia, coronary artery disease, and atherosclerosis) Meningeal inflammation: tubercular meningitis, cryptococcal meningitis, neuroborreliosis, Lyme’s disease, syphilis, CMV meningitis, skull base osteomyelitis Non-localizing signs: Benign intracranial hypertension, meningitis, Cerebral venous sinus thrombosis, intracranial space-occupying lesion Trauma Tumors: Nerve sheath tumor, Schwannoma, meningioma, ependymoma, Clival tumor, skull base tumor, nasopharyngeal tumor, carcinomatous/lymphomatous meningitis, metastasis Inflammatory: Sarcoidosis, IgG4 disease, Wegner’s granulomatosis, necrotizing vasculitis, systemic lupus erythematosus Arnold–Chiari malformation Miller Fisher syndrome Spontaneous intracranial hypotension |
| Petrous apex | Infection: Otitis media, mastoiditis Nasopharyngeal carcinoma Thrombosis of transverse/sigmoid sinus Trauma Paget’s disease |
| Cavernous sinus | Aneurysm from the internal carotid artery Carotid-cavernous fistula Cavernous carotid artery dolichoectasia Inflammatory: THS, sarcoidosis, IgG4 disease, Wegner’s granulomatosis Tumors: Nasopharyngeal carcinoma, pituitary macroadenoma, meningioma, lymphoma, meningioma, metastasis Infection: Mucormycosis, aspergillosis, tuberculoma Pituitary apoplexy |
| Orbit | Infection: Aspergillosis, mucormycosis, pyogenic orbital cellulitis, tuberculosis of orbit Tumors Inflammatory, granulomatous: Sarcoidosis, IgG4 disease Orbital trauma |
| Localization unknown | Migraine, congenital, postviral palsy, Wernicke–Korsakoff syndrome idiopathic palsies, post-radiation, Miller Fisher syndrome, infectious mononucleosis, pregnancy, trauma |
The fourth nerve palsy is the least common, and the isolated palsy is the most congenital or traumatic.[35] Trauma and ischemia are the most common etiologies for isolated trochlear palsy.[30] It could go undiagnosed for years because of compensated head tilt ameliorating the symptoms. The incidence was found to be higher in males, which might be because of more incidence of head trauma. A Korean nationwide study reported two peaks of presentation: 0–4 years of age and 75–78 years of age.[36] Vascular disease was the commonest etiology in the second peak.[36]
Isolated sixth nerve palsy is frequently seen in medical emergencies in patients with hydrocephalous, raised intracranial pressure because of tubercular meningitis, cryptococcal meningitis, benign intracranial hypertension, and cerebral venous sinus thrombosis. It is often bilateral but asymmetrical as the nerve gets involved in the subarachnoid space and one must never miss looking for papilledema which confirms the localization.[24] Other causes are direct infiltration by carcinomatous meningitis, skull base metastasis, entrapment by tubercular exudates, and inflammatory disorders, for example, sarcoid, vasculitis, and mastoiditis.[2,24]
Acquiring an etiological diagnosis for CS syndromes (CSS) is exhaustive due to the lack of tissue diagnosis and varied etiological possibilities with treatment protocols [Figures 10 and 11]. A definitive diagnosis might not be achieved in all cases; 86% were diagnosed in series by Bhatkar et al.[37] Neoplasm (metastasis and pituitary macroadenoma), fungal infections, and THS were the most common etiologies reported in the latest Indian data, accounting for 28.8%, 24.6%, and 23.2% of cases, respectively.[37] Fungal infections were the main etiology in middle CSS, while inflammatory causes have been common in anterior CSS and tumors in posterior CSS.[20]
Figure 10.
A 38-year-old male presented with right frontal headache, throbbing character, pain relieved transiently with oral analgesics. He noticed 10–12 days later a binocular double vision in horizontal gaze. His headache and double vision spontaneously resolved over 20–25 days after the intake of oral analgesics. However, he further developed drooping of the right upper lid associated with redness, tearing, swelling, and pain. On evaluation, he had only a perception of light in the right eye with complete ophthalmoplegia and proptosis (a–i). Keeping clinical localization as superior orbital fissure because of III, IV, VI, and II CN involvement, differentials thought were fungal infection (mucormycosis, aspergillosis) and inflammatory disorders (sarcoid, IgG4 disease). Contrast-enhanced MRI brain showed contrast enhancement and thickening of the right cavernous sinus (yellow arrows in j-l) extending to the right orbital apex. Sinuses were normal in MRI as well as in dedicated CT for paranasal sinuses. One week later, the lesion extended to encase cavernous ICA and into the sellar fossa and right sphenoid sinus. He was started on empirical liposomal Amphotericin (5 mg/kg), and trans sphenoidal biopsy of soft tissue lesion was done. The patient showed improvement with a decrease in eyelid swelling, and ophthalmoplegia but the vision did not improve. Workup for etiology revealed a negative fungal profile, normal serum galactomannan, high serum IgG levels, and normal cerebrospinal fluid workup. Biopsy revealed non-specific inflammation. Whole-body FDG-PET showed increased uptake in the right cavernous sinus, bilateral salivary glands, pancreas, and renal cortices. Hence, the possibility of IgG4 disease was strong. The plan was to start steroids for salvaging the vision; however, he tested positive for both hepatitis C and B. Owing to chronic active hepatitis C, he was started on Sofosbuvir + Daclatasvir and Tenofovir was started for chronic hepatitis B. Because of active viral replication, steroids were started later on
Figure 11.
A 22-year-old gentleman presented with acute onset painless horizontal binocular diplopia with no history of diurnal variation or fatiguability since 3 weeks. The next day he had also developed right-sided ptosis and had noticed that he was not able to move his right eyeball in any direction completely. History was suggestive of right-sided LMN facial palsy 2 years back with aberrant regeneration which had persisted till date. At the time of presentation, he had bilateral upper eyelid swelling more on the lateral aspect with restriction of eye movements in all directions in the right eye with pupillary sparing and right LMN facial palsy with the rest of the neurological and systemic examination being normal. Hence, evaluation was done for the syndrome of acute onset painless external ophthalmoparesis with an old history of LMN facial nerve paresis which did not reveal any evidence of neuromuscular junction disorder. Gadolinium-enhanced MRI of the brain and orbit showed an ill-defined lesion in the right cavernous sinus and orbital apex which showed mild contrast enhancement (k). The lesion was FDG avid in FDG-PET of the whole body, with FDG avid enlarged mediastinal and paratracheal lymph nodes (fig. 0-r). The possibility of sarcoidosis was kept which was further confirmed by raised serum ACE levels of 90.1 (normal values: 8–63). Endobronchial ultrasound-guided transbronchial needle aspiration showing epithelioid cell granulomas. Ziehl–Neelsen stain and Bactec culture was negative. The patient was started on oral steroids and azathioprine. He was asymptomatic at 6 months follow-up
MANAGEMENT
The management of ocular CN palsies depends on the ocular CN involved and its etiology. Contrast-enhanced MRI brain and orbits must be done in all cases. An angiogram to rule out an aneurysm is performed in all cases with painful oculomotor palsy. Steps toward further evaluation are described in [Box 3].
Box 3.
Summary of investigations in ocular cranial palsies
| Step 1. Neuroimaging in the form of Gadolinium-enhanced MRI brain and orbit (with fat-suppressed imaging) and use of newer modalities like constructive interference in steady state to trace individual cranial nerves. An angiogram is needed if there is suspicion of an aneurysm. Step 2: ESR, C reactive protein, apart from complete blood count, renal and liver function tests, lipid profile, blood sugar, HbA1c, ECG, blood pressure monitoring. ANCA, ANA, ACE levels, IgG4 levels (for inflammatory disorders). Detailed nasal examination by an otorhinologist and nasal smear in all cases of CSS and orbital lesions (to rule out fungal etiology). If needed, nasal endoscopy can be done on a case-to-case basis. CT paranasal sinuses to look for involvement of sinuses. Immediate debridement must be done, with maxillectomy/fronto-ethmoidectomy/orbital exenteration depending on the extent of the disease. Empirical amphotericin B can be started based on the intraoperative findings. CT chest and abdomen or fluorodeoxyglucose/positron emission tomography (FDG-PET) scan of the whole body to rule out malignancy. PET also helps in looking for evidence of inflammation/infection elsewhere. For example, IgG4 diseases, sarcoidosis, and tuberculosis. A tissue biopsy can be taken from the cranial or extracranial site with the help of CT/PET. |
Treatment must be directed toward the management of risk factors like diabetes, hypertension, and dyslipidemia. Ischemic craniopathy resolves spontaneously in 6–12 weeks.[38,39] Treatment of aneurysms by coiling or clipping is done as an emergency procedure. However, the extent of oculomotor nerve recovery following either procedure is still debatable.[40] Posttraumatic palsies have fewer chances for recovery, especially complete palsies.[18]
Diplopia may be treated with an occluding spectacle or contact lens,[41] by generating ptosis with botulinum toxin.[42] The use of Fresnel's prism over the paretic eye or non-dominant eye helps eliminate binocular diplopia at the behest of mild blurring of vision.[41] The use of topical pilocarpine can alleviate photophobia and can give temporary cosmetic benefits to individuals bothered by severe anisocoria. Surgical intervention is recommended when diplopia remains static or does not improve even after 6 months to a year of appropriate treatment. The objectives are to mitigate the symptoms of diplopia, alignment of the eye to the primary gaze, correction of ptosis, and address the associated cosmetic concerns.[43] A detailed methodology of the various surgical methods used by strabismologists is beyond the scope of this review.
Steroids are the mainstay of treatment in inflammatory diseases, like THS, IgG4RD, ANCA vasculitis, sarcoidosis along with other immunomodulatory drugs. They are also used in infective causes like TB meningitis to reduce leptomeningeal inflammation and reduction of exudates entrapping CNs.[44]
CONCLUSION
Various etiologies are responsible for ocular motor palsies. However, a thorough history and meticulous clinical examination along with knowledge of neuroanatomy are key to diagnosis. Imaging plays an important role in diagnostic evaluation. Ischemia is the common etiology overall; however, trauma and congenital causes are the most common in young adults along with aneurysms and inflammatory disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brazis PW, Masdeu JC, Biller J, editors. The localization of lesions affecting the ocular motor system. Localization in Clinical Neurology. (7th) 2017:187–334. [Google Scholar]
- 2.Laine FJ. Cranial nerves III, IV, and VI. Top Magn Reson Imaging. 1996;8:111–30. doi: 10.1097/00002142-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Büttner-Ennever JA. The extraocular motor nuclei: Organization and functional neuroanatomy. Prog Brain Res. 2006;151:95–125. doi: 10.1016/S0079-6123(05)51004-5. [DOI] [PubMed] [Google Scholar]
- 4.Martins C, Yasuda A, Campero A, Rhoton AL., Jr Microsurgical anatomy of the oculomotor cistern. Neurosurgery. 2006;58(4 Suppl 2) doi: 10.1227/01.NEU.0000204673.55834.BE. ONS-220-7; discussion ONS-7-8. [DOI] [PubMed] [Google Scholar]
- 5.Miller NR. The ocular motor nerves. Curr Opin Neurol. 1996;9:21–5. doi: 10.1097/00019052-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Park HK, Rha HK, Lee KJ, Chough CK, Joo W. Microsurgical anatomy of the oculomotor nerve. Clin Anat. 2017;30:21–31. doi: 10.1002/ca.22811. [DOI] [PubMed] [Google Scholar]
- 7.Sanders M. Walsh and Hoyt's Clinical Neuro-Ophthalmology. 4th Edition, Volume 5. J Neurol Neurosurg Psychiatry. 1996;61:236–7. [Google Scholar]
- 8.Joo W, Rhoton AL., Jr Microsurgical anatomy of the trochlear nerve. Clin Anat. 2015;28:857–64. doi: 10.1002/ca.22602. [DOI] [PubMed] [Google Scholar]
- 9.Joo W, Yoshioka F, Funaki T, Rhoton AL., Jr Microsurgical anatomy of the abducens nerve. Clin Anat. 2012;25:1030–42. doi: 10.1002/ca.22047. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt D. [Classical brain stem syndrome. Definitions and history] Ophthalmologe. 2000;97:411–7. doi: 10.1007/s003470070090. [DOI] [PubMed] [Google Scholar]
- 11.Marx JJ, Thömke F. Classical crossed brain stem syndromes: Myth or reality? J Neurol. 2009;256:898–903. doi: 10.1007/s00415-009-5037-2. [DOI] [PubMed] [Google Scholar]
- 12.Richards BW, Jones FR, Jr, Younge BR. Causes and prognosis in 4,278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113:489–96. doi: 10.1016/s0002-9394(14)74718-x. [DOI] [PubMed] [Google Scholar]
- 13.Adams ME, Linn J, Yousry I. Pathology of the ocular motor nerves III, IV, and VI. Neuroimaging Clin N Am. 2008;18:261–82. doi: 10.1016/j.nic.2007.11.001. preceding x-x. [DOI] [PubMed] [Google Scholar]
- 14.Lajmi H, Hmaied W, Ben Jalel W, Chelly Z, Ben Yakhlef A, Ben Zineb F, et al. Oculomotor palsy in diabetics. J Fr Ophtalmol. 2018;41:45–9. doi: 10.1016/j.jfo.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 15.PJ Koehler EW. Fixed and dilated: the history of a classic pupil abnormality. J Neurosurg. 2015;122:453–63. doi: 10.3171/2014.10.JNS14148. [DOI] [PubMed] [Google Scholar]
- 16.Brogna C, Fiengo L, Türe U. Achille Louis Foville's atlas of brain anatomy and the Defoville syndrome. Neurosurgery. 2012;70:1265–73. doi: 10.1227/NEU.0b013e31824008e7. discussion 73. [DOI] [PubMed] [Google Scholar]
- 17.CASERO. The Millard-Gubler syndrome. Am J Ophthalmol. 1948;31:344. [PubMed] [Google Scholar]
- 18.Kumar N, Kaur S, Raj S, Lal V, Sukhija J. Causes and outcomes of patients presenting with diplopia: A hospital-based study. Neuroophthalmology. 2021;45:238–45. doi: 10.1080/01658107.2020.1860091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferson G. On the saccular aneurysms of the internal carotid artery in the cavernous sinus. Br J Surg. 2005;26:267–302. [Google Scholar]
- 20.Bhatkar S, Goyal MK, Takkar A, Modi M, Mukherjee KK, Singh P, et al. Which classification of cavernous sinus syndrome is better-Ishikawa or Jefferson? A prospective study of 73 patients. J Neurosci Rural Pract. 2016;7(Suppl 1):S68–71. doi: 10.4103/0976-3147.196448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bone I, Hadley DM. Syndromes of the orbital fissure, cavernous sinus, cerebello- pontine angle, and skull base. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 3):iii29–38. doi: 10.1136/jnnp.2005.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CT, Chen YR. Traumatic superior orbital fissure syndrome: Current management. Craniomaxillofac Trauma Reconstr. 2010;3:9–16. doi: 10.1055/s-0030-1249369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vishnu VY, Petluri G, Gupta V, Lal V, Khurana D, Goyal MK. Parkinson syndrome: A precise localization for abducens palsy. J Neurol Sci. 2014;336:288–9. doi: 10.1016/j.jns.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Elder C, Hainline C, Galetta SL, Balcer LJ, Rucker JC. Isolated abducens nerve palsy: Update on evaluation and diagnosis. Curr Neurol Neurosci Rep. 2016;16:69. doi: 10.1007/s11910-016-0671-4. [DOI] [PubMed] [Google Scholar]
- 25.Leonard TJ, Moseley IF, Sanders MD. Ophthalmoplegia in carotid cavernous sinus fistula. Br J Ophthalmol. 1984;68:128–34. doi: 10.1136/bjo.68.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane JR. Combined VIth and XIIth cranial nerve palsies: A clival syndrome. Neurology. 2000;54:1540–1. doi: 10.1212/wnl.54.7.1540. [DOI] [PubMed] [Google Scholar]
- 27.Motamed M, Kalan A. Gradenigo's syndrome. Postgrad Med J. 2000;76:559–60. doi: 10.1136/pmj.76.899.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurtell MJ, Halmagyi GM. Complete ophthalmoplegia. Stroke. 2008;39:1355–7. doi: 10.1161/STROKEAHA.107.504761. [DOI] [PubMed] [Google Scholar]
- 29.Keane JR. Bilateral ocular paralysis: Analysis of 31 inpatients. Arch Neurol. 2007;64:178–80. doi: 10.1001/archneur.64.2.178. [DOI] [PubMed] [Google Scholar]
- 30.Akagi T, Miyamoto K, Kashii S, Yoshimura N. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol. 2008;52:32–5. doi: 10.1007/s10384-007-0489-3. [DOI] [PubMed] [Google Scholar]
- 31.Phuljhele S, Dhiman R, Sharma M, Kusiyait SK, Saxena R, Mahalingam K, et al. Acquired ocular motor palsy: Current demographic and etiological profile. Asia Pac J Ophthalmol (Phila) 2020;9:25–8. doi: 10.1097/01.APO.0000617940.70112.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang C, Leavitt JA, Hodge DO, Holmes JM, Mohney BG, Chen JJ. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23–8. doi: 10.1001/jamaophthalmol.2016.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keane JR. Third nerve palsy: Analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662–70. doi: 10.1017/s0317167100010866. [DOI] [PubMed] [Google Scholar]
- 34.Kissel JT, Burde RM, Klingele TG, Zeiger HE. Pupil-sparing oculomotor palsies with internal carotid-posterior communicating artery aneurysms. Ann Neurol. 1983;13:149–54. doi: 10.1002/ana.410130207. [DOI] [PubMed] [Google Scholar]
- 35.Bagheri A, Fallahi MR, Abrishami M, Salour H, Aletaha M. Clinical features and outcomes of treatment for fourth nerve palsy. J Ophthalmic Vis Res. 2010;5:27–31. [PMC free article] [PubMed] [Google Scholar]
- 36.Jung EH, Kim SJ, Lee JY, Cho BJ. The incidence and presumed aetiologies of fourth cranial nerve palsy in Korea: A 10-year nationwide cohort study. Eye (Lond) 2021;35:3012–9. doi: 10.1038/s41433-020-01374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatkar S, Goyal MK, Takkar A, Mukherjee KK, Singh P, Singh R, et al. Cavernous sinus syndrome: A prospective study of 73 cases at a tertiary care centre in Northern India. Clin Neurol Neurosurg. 2017;155:63–9. doi: 10.1016/j.clineuro.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Jung JS, Kim DH. Risk factors and prognosis of isolated ischemic third, fourth, or sixth cranial nerve palsies in the Korean population. J Neuroophthalmol. 2015;35:37–40. doi: 10.1097/WNO.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 39.Teuscher AU, Meienberg O. Ischaemic oculomotor nerve palsy. Clinical features and vascular risk factors in 23 patients. J Neurol. 1985;232:144–9. doi: 10.1007/BF00313889. [DOI] [PubMed] [Google Scholar]
- 40.Kassis S, Jouanneau E, Tahon F, Salkine F, Perrin G, Turjman F. Recovery of third nerve palsy after endovascular treatment of posterior communicating artery aneurysms. World Neurosurg. 2010;73:11–6. doi: 10.1016/j.surneu.2009.03.042. discussion e2. [DOI] [PubMed] [Google Scholar]
- 41.Anilkumar SE NK. Prisms in the treatment of diplopia with strabismus of various etiologies. Ind J Ophthal. 2022;70:609–12. doi: 10.4103/ijo.IJO_939_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talebnejad MR, Sharifi M, Nowroozzadeh MH. The role of Botulinum toxin in management of acute traumatic third-nerve palsy. J AAPOS. 2008;12:510–3. doi: 10.1016/j.jaapos.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Singh A, Bahuguna C, Nagpal R, Kumar B. Surgical management of third nerve palsy. Oman J Ophthalmol. 2016;9:80–6. doi: 10.4103/0974-620X.184509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadagopan KA, Wasserman BN. Managing the patient with oculomotor nerve palsy. Curr Opin Ophthalmol. 2013;24:438–47. doi: 10.1097/ICU.0b013e3283645a9b. [DOI] [PubMed] [Google Scholar]