Summary
This was a two-stage phase II trial of a mTORC1/2 inhibitor (mTORC: mammalian target of rapamycin complex) Sapanisertib (TAK228) in patients with rapalog-resistant pancreatic neuroendocrine tumors (PNETs) (NCT02893930). Approved rapalogs such as everolimus inhibit mTORC1 and have limited clinical activity, possibly due to compensatory feedback loops. Sapanisertib addresses the potential for incomplete inhibition of the mTOR pathway through targeting of both mTORC1 and mTORC2, and thus to reverse resistance to earlier rapamycin analogues. In stage 1, patients received sapanisertib 3 mg by mouth once daily on a continuous dosing schedule in 28-day cycle. This trial adopted a two-stage design with the primary objective of evaluating objective tumor response. The first stage would recruit 13 patients in order to accrue 12 eligible and treated patients. If among the 12 eligible patients at least 1 patient had an objective response to therapy, the study would move to the second stage of accrual where 25 eligible and treated patients would be enrolled. This study activated on February 1, 2017, the required pre-determined number of patients (n = 13) had entered by November 5, 2018 for the first stage response evaluation. The accrual of this trial was formally terminated on December 27, 2019 as no response had been observed after the first stage accrual. Treatment-related grade 3 adverse events were reported in eight (61%) patients with hyperglycemia being the most frequent, in three patients (23%). Other toxicities noted in the trial included fatigue, rash diarrhea, nausea, and vomiting. The median PFS was 5.19 months (95% CI [3.84, 9.30]) and the median OS was 20.44 months (95% CI [5.65, 22.54]). Due to the lack of responses in Stage 1 of the study, the study did not proceed to stage 2. Thus the potential to reverse resistance was not evident.
Keywords: PNET, mTORC1/2 inhibitor, Sapanisertib
Pancreatic neuroendocrine tumors (PNETs) are a rare (one per 100, 000 individuals per year), heterogenous group of slow growing tumors representing approximately 1.3% of all cases of pancreatic cancer in incidence and 10% of cases in prevalence [1, 2]. Two-thirds of patients diagnosed with PNET have unresectable metastatic disease and therefore require systemic therapy.
The mammalian target of rapamycin (mTOR) is an intracellular serine/threonine kinase that regulates cell cycle progression, protein translation, metabolism, cellular proliferation and survival of normal cells integrating growth factors and nutrient signals [3, 4]. It is activated by two main upstream factors, namely phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt). Deregulation of PI3K-Akt-mTOR signaling pathway is reported as dysregulated in neuroendocrine tumors (NETs [5, 6]. mTOR exists in two distinct multiprotein complexes: mTORC1 and mTORC2, which differ in their binding ligands, and their sensitivity to rapamycin and its active analogs [7]. mTORC1 promotes cell growth through two key effectors: p70S6 Kinase 1 (S6K1) and eIF4E-binding protein 1 (4EBP1). mTORC2 promotes cell proliferation and survival through activation of AKT by phosphorylation. Everolimus, a rapamycin analog, is a potent inhibitor of mTOR pathway, with significant activity in advanced NETs [8]. Everolimus specifically inhibits mTORC1 [9], it has been hypothesized that drug resistance to everolimus may occur through AKT activation by means of mTORC2 and IGF1/IGFR signaling activation due to inhibition of the S6K negative feedback [10, 11].
Sapanisertib (INK128/MLN0128/TAK228), is a second generation mTOR inhibitor drug that directly binds to the ATP binding site of mTOR, thereby potently inhibiting both mTORC1 and mTORC2 [12], and overcoming the everolimus-resistant phosphorylation of 4EBP1 and AKT [13, 14]. Unlike rapalogs, sapanisertib has strong cytotoxic activity towards tumors [15–17]. Sapanisertib and paclitaxel with or without trastuzumab was evaluated in patients with advanced solid malignancies. The most common types of cancers in this study were lung (21%), ovarian (12%), and breast, endometrial, and esophageal (9% each). Sapanisertib was well tolerated in this study and exhibited anti-tumor activity in a range of tumor types. Out of 54 patients, eight experienced partial responses and six had stable disease lasting over 6 months [18]. Sapanisertib was also well tolerated and displayed preliminary therapeutic activity in patients with refractory multiple myeloma, non-Hodgkin’s lymphoma, and Waldenström’s macroglobulinemia. Almost half of the patients in the study achieved stable disease [19].
Chamberlain et al. developed a patient-derived xenograft model of PNET (PDX-PNET) which was used to evaluate everolimus and sapanisertib. Treatment of PDX-PNETs with either agent strongly inhibited growth. As seen in patients, some PDX-PNETs developed resistance to everolimus. However, sapanisertib, a more potent inhibitor of the mTOR pathway, caused tumor shrinkage in most everolimus-resistant tumors [20]. Thus, preclinical data from the use of this model suggested that sapanisertib may be an effective new treatment option for patients with everolimus-resistant PNET.
Thus, based on the potential ability of a dual mTORC1/ mTORC2 to address mTORC2-mediated escape mechanisms and resistance and the activity of sapanisertib noted in a PDX-NET model, we initiated a prospective two-stage optimal design Phase II study of sapanisertib in patients whose disease had progressed on or after treatment with mTOR inhibitors.
The primary objective of this study was to evaluate objective tumor response. Secondary objectives were to estimate Progression Free Survival (PFS), describe response duration, and to evaluate toxicity and disease control rate (DCR). Herein, we present results of the stage 1 analysis.
Patients and methods
Patients
Patients 18 years of age or more, with unresectable or metastatic, histologically confirmed low or intermediate grade (Klimstra Criteria) pancreatic neuroendocrine tumor (PNET) with radiological evidence of disease progression since last treatment (per investigator assessment) were eligible. Patients were required to have refractory disease to treatment with an mTOR inhibitor. Prior or concurrent therapy with somatostatin analogs was permitted, provided dosing was stable for at least 2 months prior to study start and throughout the study. Other key inclusion criteria were ECOG performance status ≤ 1, measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 [21], and adequate bone marrow and organ function. Patients with diabetes mellitus were allowed if fasting blood glucose (FBG) ≤ 130 mg/dL (mmol/L) or HbA1c ≤ 7%. Key exclusion criteria included prior treatment with a PI3K or AKT inhibitor for PNETs, more than three previous systemic treatment regimens for PNETs, or discontinuation of prior mTOR inhibitor therapy due to toxicity.
The study was reviewed by regulatory authorities and approved by the institutional review board of each participating center. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki guidelines for Good Clinical Practice, as defined by the International Conference on Harmonization.
Study treatment
This was a prospective, multicenter, two-stage, phase II study of single-agent sapanisertib. In stage 1 (single-arm, open-label), patients received sapanisertib 3 mg by mouth once daily on a continuous dosing schedule in 28-day cycles. In order to allow more predictable absorption of sapanisertib after oral administration and to allow scale-up manufacturing of sapanisertib, Takeda developed new sapanisertib capsules containing milled API for clinical studies in 1 mg, 3 mg, and 5 mg strengths. Treatment continued until radiological evidence of disease progression (per RECIST v1.1), intolerable toxicity, death, withdrawal of consent, or extraordinary medical circumstances.
For patients with clinically significant adverse events (AEs) related to study treatment, dose interruptions and dose reduction to 2 mg initially, followed by an abbreviated 5 days/week schedule at the 2 mg dose at second occurrence, were permitted.
Study design
This trial adopted a two-stage design with the primary objective of evaluating objective tumor response (complete response (CR) + partial response (PR)). The null hypothesis was a 5% true response rate versus the alternative hypothesis of a 20% true response with sapanisertib. The first stage would recruit 13 patients in order to accrue 12 eligible and treated patients. If among the 12 eligible patients at least 1 patient had an objective response to therapy, the study would move to the second stage of accrual where 27 additional patients would be enrolled to achieve an accrual of 25 eligible and treated patients. If at the end of the second stage 4 or more patients attain objective response to therapy, the null hypothesis would be rejected and the agent would be recommended for further evaluation. This design has type I error of 0.094, 90.2% power and a probability of rejection at the first stage under the null hypothesis of at least 54%.
Safety and efficacy assessments
ECOG performance status, physical examination, vital signs, weight, laboratory evaluations, and glucose (fasting and at home) were continuously monitored. AEs were recorded from the first dose to 30 days following the last dose of sapanisertib and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Assessments of antitumor activity were performed in all patients who received at least one cycle (28 days) of sapanisertib. Tumor response was assessed locally during screening and every 8 weeks following treatment initiation, per Response Evaluation Criteria In Solid Tumors (RECIST) guideline v1.1.
Statistical analyses
All analyses were performed based on eligible patients who started protocol treatment except for the toxicity analysis which was based on all patients who started protocol treatment regardless of eligibility. Descriptive statistics (frequency, percentage, median, and/or range) were used to characterize patient demographics, disease characteristics, and adverse events (AEs) with AEs evaluated using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Exact binomial 95% confidence intervals were computed for the objective response rate (CR + PR) and disease control rate (CR + PR + stable disease (SD)) among all eligible patients who started protocol treatment. Tumor response was evaluated using Response Evaluation Criteria In Solid Tumors (RECIST) guideline (version 1.1). The time-to-event curves were estimated using the Kaplan–Meier method, with the 95% confidence interval estimated using the log–log transformation. Progression Free Survival (PFS) was defined as time from study registration to disease progression or to death without progression, whichever occurred first. If date of death was greater than 4 months after the date of last documented to be free of progression or if patients were alive and free of progression at the time of the analysis, patients were censored at the time of last disease assessment. If such a date was not available, patients were censored at the time of registration. Overall survival was defined as time from study registration to death from any cause, censoring cases who were alive at the date of last contact. Duration of response was defined as the time from the onset of response (CR or PR, whichever status was recorded first) to first documentation of disease progression. Patients with responses but without documented disease progression were censored at the time of last disease evaluation. Patients without a response were not included in this analysis.
All p-values are two-sided with a level < 0.05 considered statistically significant.
Results
Patient characteristics
The analysis was based on data available as of May 11, 2021. This study activated on February 1, 2017 and was suspended on November 5, 2018 because the required pre-determined number of patients (n = 13) had entered for the first stage response evaluation. The accrual of this trial was formally terminated on December 27, 2019 as no response had been observed after the first stage accrual.
All 13 patients enrolled to this trial started protocol treatment. One patient was ineligible due do baseline scans done after study registration. Table 1 summarizes patient demographics and disease characteristics at baseline for the 12 eligible patients who started study treatment. The median age was 62.5 years, ranging between 36 and 85 years. The majority of patients were females (58.3%), White (81.8%), and non-Hispanic/Latino (83.3%). All patients had metastatic confirmed pancreatic neuroendocrine tumors, with 7 intermediate grade and 5 low-grade. Over half (66.6%) had tumor stage at either T2 or T3 (n = 4 each). Disease sites of involvement are tabulated in Table 2.
Table 1.
Patient Demographics and Disease Characteristics at Baseline (N = 12)
| Variable | N | % |
|---|---|---|
| Age (in years, median (minimum, maximum)) | 62.5 (36, 85) | |
| Gender | ||
| Female | 7 | 58.3 |
| Male | 5 | 41.7 |
| Race | ||
| Asian | 1 | 9.1 |
| Black or African American | 1 | 9.1 |
| White | 9 | 81.8 |
| Not Reported | 1 | - |
| Ethnicity | ||
| Hispanic or Latino | 2 | 16.7 |
| Not Hispanic or Latino | 10 | 83.3 |
| ECOG Performance Status | ||
| 0 | 6 | 50.0 |
| 1 | 6 | 50.0 |
| Histology—Pancreatic neuroendocrine tumors | 12 | 100.0 |
| Histology Grade | ||
| I ntermediate Grade | 7 | 58.3 |
| Low Grade | 5 | 41.7 |
| Clinical Tumor T Stage | ||
| T0 | 1 | 8.3 |
| T2 | 4 | 33.3 |
| T3 | 4 | 33.3 |
| T4 | 1 | 8.3 |
| TX | 2 | 16.7 |
| Clinical Regional Lymph Node N Stage | ||
| N0 | 4 | 33.3 |
| N1 | 6 | 50.0 |
| NX | 2 | 16.7 |
| Clinical Distant Metastasis M Stage | ||
| M1 | 11 | 100.0 |
| Unknown | 1 | - |
| Tumor Functional -Yes | 1 | 8.3 |
| Disease Unresectable—Yes | 11 | 91.7 |
| Metastatic Neoplasm Confirmed Diagnosis | 12 | 100.0 |
| Prior mTOR Inhibitor Administered (with Everolimus) | 12 | 100.0 |
| Prior Strong Protein Kinase Inhibitor Cytochrome Inducer Administered | 0 | 0.0 |
| Prior Chemotherapy Somatostatin Analog Therapy Administered | 8 | 66.7 |
| Somatostatin Analog Therapy Administration Continuinga | 6 | 75.0 |
| Prior Sunitinib Chemotherapy Administeredb | 1 | 25.0 |
| Prior PI3K or AKT Inhibitor Administered | 0 | 0.0 |
| Prior Systemic Therapy Administered | 8 | 66.7 |
| Prior Radiation Therapy Administered | 2 | 16.7 |
| Prior Surgery Performed | 6 | 50.0 |
| Prior Hepatic Artery Embolization Performed | 3 | 25.0 |
| Prior Hepatic Cryoablation Performed | 0 | 0.0 |
| Prior Hepatic Radiofrequency Ablation Performed | 1 | 8.3 |
| Prior Transarterial Chemoembolization Performed | 1 | 8.3 |
| Prior Bland Embolization Performed | 0 | 0.0 |
| Prior Therapy Other Administered | 0 | 0.0 |
Denominator, n = 8
Missing, n = 8; denominator, n = 4
Missing, n = 3
Table 2.
Disease Site(s) of Involvement
| Disease Neuroendocrine Tumor Anatomic Site Involvement | N | % |
|---|---|---|
| Adjacent to kidney | 1 | 2.8 |
| Adjacent Viscera/Vessels | 2 | 5.6 |
| Bone | 1 | 2.8 |
| Liver | 12 | 33.3 |
| Lung | 3 | 8.3 |
| Mediastinum/Hila: More superior node | 1 | 2.8 |
| Periaortic lymph node | 1 | 2.8 |
| Peritoneum | 3 | 8.3 |
| Primary Tumor/Pancreas | 8 | 22.2 |
| Regional Lymph Nodes | 4 | 11.1 |
Treatment and tolerability
The total number of treatment cycles given ranged between 1 to 10, with mean at 4.1 and median at 3. Table 3 shows the incidence of reasons off treatment by the total number of treatment cycles given. Among the 12 patients reported here, four were off treatment due to disease progression, three went off study due to AEs.
Table 3.
Reasons Off Treatment by Total Number of Treatment Cycles Given
| Off Treatment Reason | Total Number of Cycles Given | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 10 | Total | ||
| N | N | N | N | N | N | N | % | |
| Adverse event/side effects/complications | 1 | 1 | 1 | 0 | 0 | 0 | 3 | 25.0 |
| Death on study | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8.3 |
| Disease progression- relapse during active treatment | 0 | 0 | 0 | 1 | 1 | 2 | 4 | 33.3 |
| Patient withdrawal/refusal after beginning protocol therapy | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 16.7 |
| Other | 1a | 0 | 1b | 0 | 0 | 0 | 2 | 16.7 |
| Total | 2 | 2 | 3 | 2 | 1 | 2 | 12 | 100.0 |
Off treatment for medical decision
off treatment due to symptoms demonstrating disease progression and/or intolerance of sapanisertib
The incidence of all grades of treatment-related adverse events are summarized in Table 4 among patients who started protocol treatment (n = 13). Amongst all these toxicities, the worst grade was reported as grade 3 (n = 8, 61%) with hyperglycemia the most common one (n = 3, 23%). One patient was reported with treatment unrelated lethal AE. Other grade 1–2 AEs such as fatigue (31%), rash (15%), gastrointestinal AEs such as oral mucositis (46%), diarrhea (23%), nausea (46%) and vomiting (8%) were observed.
Table 4.
Treatment-Related Toxicities
| Toxicity Type | Treatment Arm | |||
|---|---|---|---|---|
| A (n = 13) | ||||
| Grade | ||||
| 1,2 | 3 | 4 | 5 | |
| n (%) | n (%) | n (%) | n (%) | |
| Anemia | 2 (15) | - | - | - |
| Abdominal pain | 2 (15) | - | - | - |
| Diarrhea | 3 (23) | 1 (8) | - | - |
| Mucositis oral | 6 (46) | - | - | - |
| Nausea | 6 (46) | - | - | - |
| Vomiting | 1 (8) | 1 (8) | - | - |
| Fatigue | 4 (31) | 1 (8) | - | - |
| Cholesterol high | 2 (15) | - | - | - |
| Lymphocyte count decreased | - | 1 (8) | - | - |
| Anorexia | 2 (15) | - | - | - |
| Hyperglycemia | 7 (54) | 3 (23) | - | - |
| Pruritus | 2 (15) | - | - | - |
| Rash maculo-papular | 2 (15) | 1 (8) | - | - |
Efficacy
Table 5 summarizes the best overall response among the 12 eligible patients who started protocol treatment. No CR or PR was observed. Eight patients were reported with SD, two with disease progression, one with unevaluable response, and one with insufficient evaluation. Per the pre-determined rules, if at least 1 patient had an objective response to therapy among the 12 eligible and treated patients, the study would move to the second stage of accrual. Given no response was observed, the study accrual was completely terminated after the first stage of accrual. The response rate was, thus, 0% with 95% confidence interval (CI) (0.0, 0.26); the disease control rate was 66.7% with 95% CI (0.35, 0.90).
Table 5.
Best Overall Response
| Response | N | % |
|---|---|---|
| Complete response | 0 | 0.0 |
| Partial response | 0 | 0.0 |
| Stable disease | 8 | 66.7 |
| Progression | 2 | 16.7 |
| Insufficient evaluationa | 1 | 8.3 |
| Unevaluableb | 1 | 8.3 |
| Total | 12 | 100.0 |
Symptomatic deterioration reported but died prior to documented disease progression
Did not complete one cycle of treatment
Figure 1 displays the PFS curve with the 95% confidence interval. The median PFS was 5.19 months (95% CI [3.84, 9.30]). The median follow-up time was 23.62 months among the three patients who are still alive. Figure 2 shows the survival curve with the 95% confidence interval. The median OS was 20.44 months (95% CI [5.65, 22.54]). As no response was observed in this trial, duration of response could not be calculated.
Fig. 1.
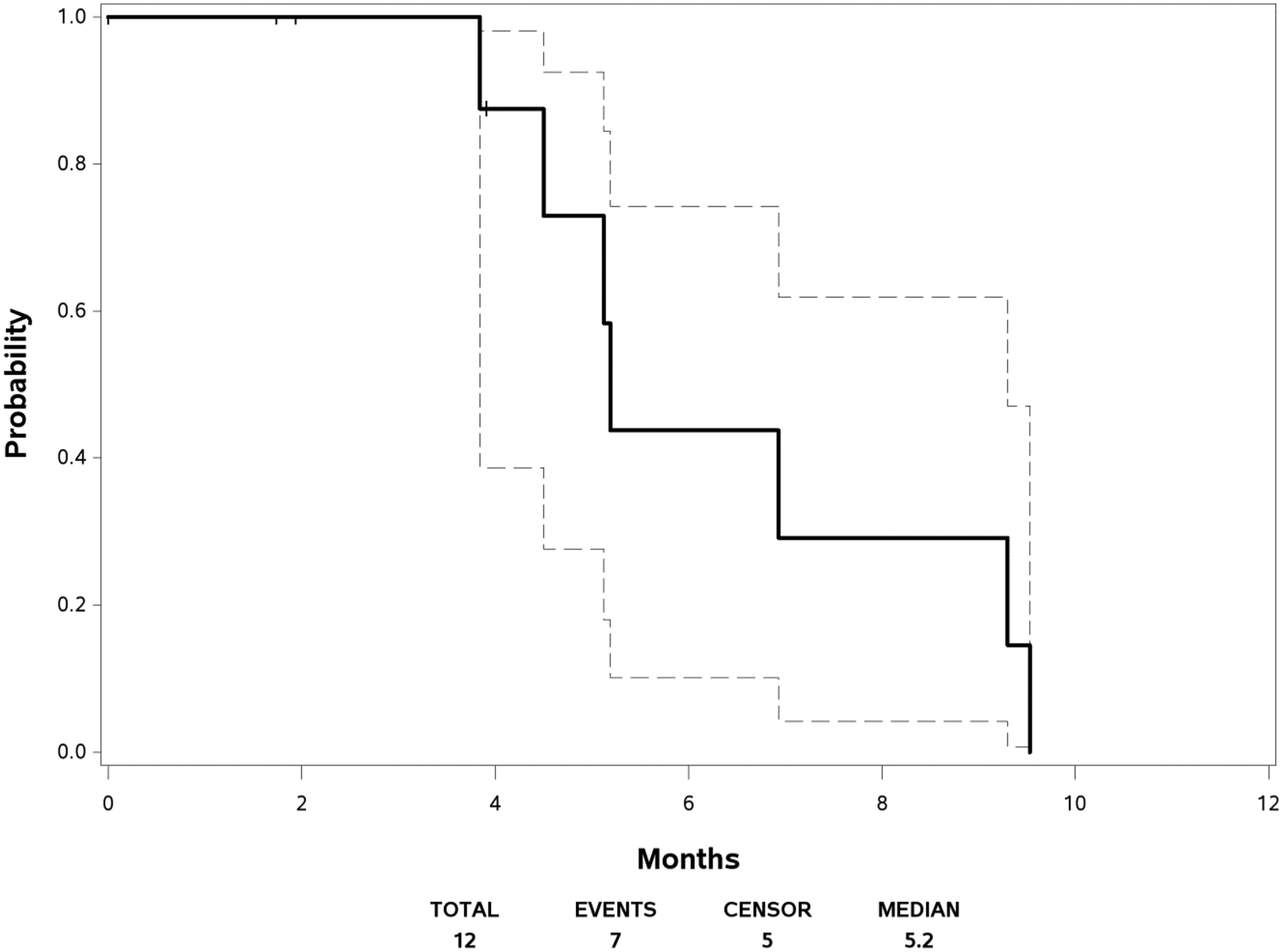
Progression-Free Survival
Fig. 2.
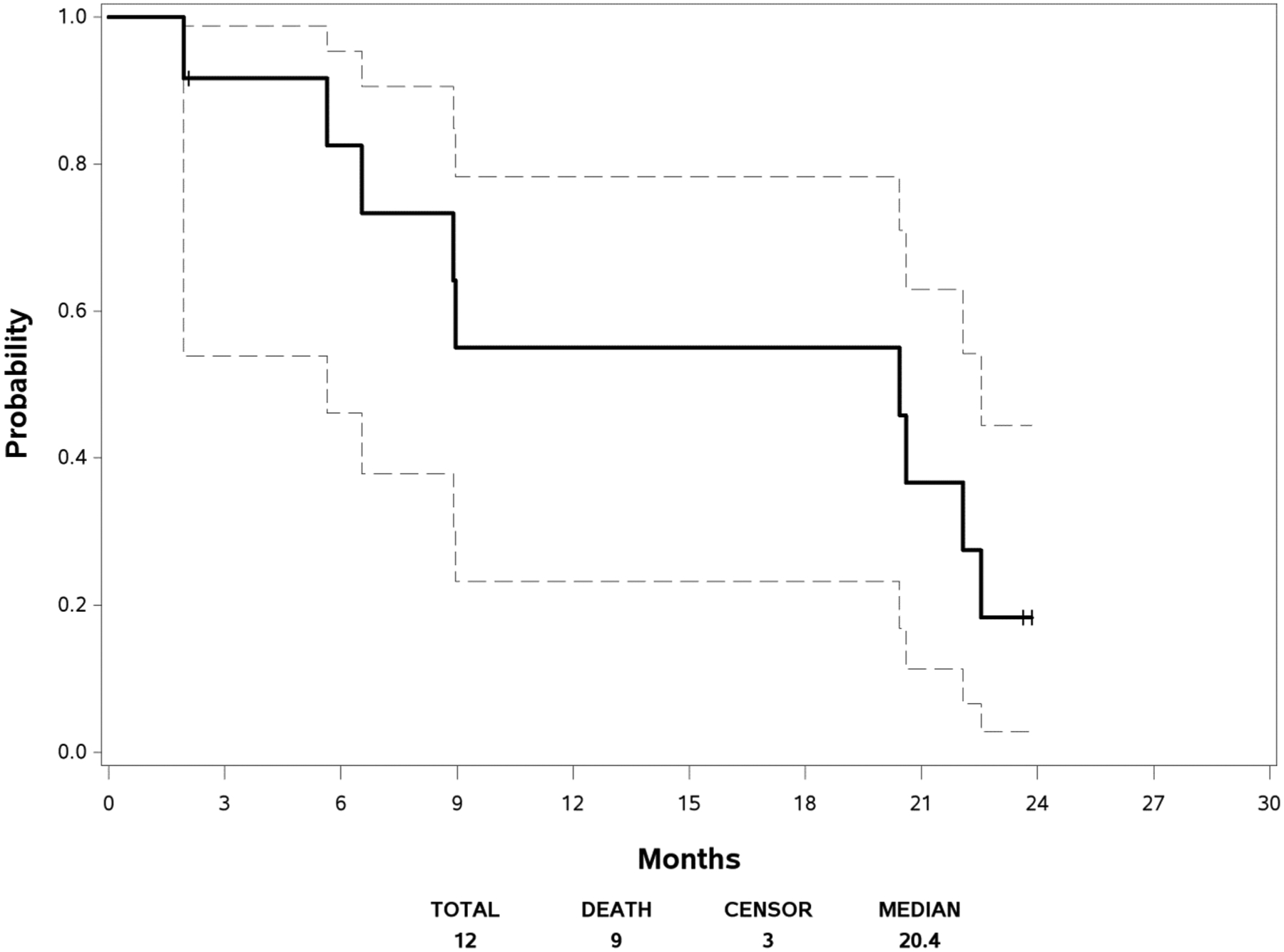
Overall Survival
Discussion
The aim of this two-stage, phase II study was to evaluate the efficacy of once daily sapanisertib in patients with advanced PNETs whose disease had progressed on or after treatment with mTOR inhibitors. At the interim futility analysis, performed after 12 eligible patients, no responses were observed. This was below the prespecified threshold of at least one patient having an objective response, so the study therefore did not proceed to stage 2.
While no responses were observed in the eligible patients, the disease control rate was 66.7% and the median PFS was 5.19 months. This compares unfavorably to a mPFS of 12.6 months with sunitinib in previously treated pNET [22]. In the RADIANT-3 trial, the mPFS was 11.0 months with everolimus as compared with 4.6 months with placebo [23]. This modest antitumor activity suggests that while sapanisertib potently inhibits both mTORC1 and mTORC2, and can overcome the phosphorylation of 4EBP1 and AKT in everolimus-resistant preclinical models, alternate pathways may drive disease progression in clinical practice. In different cancer models, mTORC1/2 inhibition promotes reorganization of integrin/focal adhesion kinase mediated adhesomes, induction of IGFR/IR-dependent PI3K activation and AKT phosphorylation, via an integrin/FAK/IGFR-dependent process [24]. Moreover, mTOR KIs induce overactivation of the MEK/ERK pathway through PI3K-independent feedback loops, similarly to those described for dual PI3K/mTOR inhibitors [25]. Specifically, we conclude that sapanisertib has low potential to reverse everolimus resistance.
The dose of sapanisertib has been investigated in various studies. It has been studied for the treatment of advanced solid and liquid tumors and has demonstrated an acceptable safety profile and preliminary therapeutic activity in two phase I trials [26, 27]. Both studies used capsules of TAK-228 comprising unmilled active pharmaceutical ingredient, which patients took with a light meal.
There were improvements made to the TAK-228 manufacturing process that included a physical milling step following granulation to improve particle size distribution and enable automated capsule filling for scaling-up manufacturing [28, 29]. A phase I study (NCT02412722) evaluated the safety, tolerability, pharmacokinetics and antitumor activity of single-agent TAK-228 (milled capsules), administered daily (QD) or weekly (QW) and in combination with paclitaxel in patients with advanced solid tumors. Maximum tolerated doses for milled TAK-228 were 3 mg (TAK-228 QD), and 30 mg (TAK-228 QW), slightly lower than those previously reported for unmilled TAK-228. In patients with solid tumors, common DLTs were mucosal inflammation, asthenia, stomatitis, fatigue, rash and hyperglycemia [30]. A variety of mutations in the MTOR gene have been described in different cancers [31–33]: some of these mutations, located in the kinase domain such as the methionine 2327 isoleucine substitution (M2327I), can increase the catalytic activity of mTOR and thus of both mTORC1 and mTORC2 complexes. In these cases, the concentrations of mTOR KIs such as MLN0128 required to inhibit mTORC1/mTORC2 substrates are higher than those required for wild-type mTOR kinase [34]. Thus, whether dosing issues impacted the clinical activity of sapanisertib remains unknown.
Hyperglycemia is a well-documented class effect of mTOR inhibitors, with grade ≥ 3 events occurring in up to 15% of patients in previous phase III clinical trials [35–37]. We recorded a 23% incidence of Grade 3 hyperglycemia in our study. Other toxicities observed were fatigue (31%), rash (15%), gastrointestinal AEs such as oral mucositis (46%), diarrhea (23%), nausea (46%) and vomiting (8%) were observed. The safety profile described here for sapanisertib is consistent with previous studies [26, 27, 38] as well as clinical observations of other mTOR inhibitors [32–34].
Other agents such as Dactolisib (BEZ235) an oral dual PI3K/mTOR inhibitor [39] and AZD2014, a highly selective dual inhibitor of the mammalian rapamycin (mTORC1 and mTORC2) [40] have failed to demonstrate greater efficacy as compared to mTORC1 inhibition alone by everolimus in patients with P-NETs and renal cell carcinoma, respectively.
In conclusion, given the modest efficacy of sapanisertib observed in stage 1 of our study that was insufficient to trigger the initiation of stage 2; and results that are consistent with studies of other dual TORC pathway inhibitors in neuroendocrine tumors, we believe that alternate approaches to the treatment of neuroendocrine tumors should be investigated. A better understanding of the biology of neuroendocrine neoplasms will lead to the development of new therapeutic strategies that will expand treatment options for these patients.
Funding
This study was conducted by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the award numbers: U10CA180821, U10CA180820, U10CA180794, and UG1CA233320. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Milan SA, Yeo CJ (2012) Neuroendocrine tumors of the pancreas. Curr Opin Oncol 24(1):46e55. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Eisner MP, Leary C et al. (2007) Population-based study of islet cell car- cinoma. Ann Surg Oncol 14:3492e3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Averous J, Proud CG (2006) When translation meets transformation: the mTOR story. Oncogene 25:6423–6435 [DOI] [PubMed] [Google Scholar]
- 4.Bjornsti MA, Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4:335–348. 10.1038/nrc1362 [DOI] [PubMed] [Google Scholar]
- 5.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N (2011) DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331:1199–1203. 10.1126/science.1200609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G, Pederzoli P, Croce CM, Scarpa A (2010) Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 10(3):457e468. [DOI] [PubMed] [Google Scholar]
- 8.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Ho€rsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM et al. (2011) Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumors associated with carcinoid syndrome (RADIANT-2): a randomized, placebo-controlled, phase 3 study. Lancet 378:2005e2012. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Z, Sarbassov D, Samudio IJ et al. (2007) Rapamycin derivatives reduce mTORC2 signaling and inhibit AKTactivation in AML. Blood 109:3509e3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N (2011) AKTinhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19:58–71. 10.1016/j.ccr.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M (2000) A rapamycin-sensitive pathway downregulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol 14:783–794. 10.1210/mend.14.6.0446 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ et al. (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM (2009) Activesite inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycinresistant functions of mTORC1. J Biol Chem 284:8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D (2010) Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell 17:249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, Vu C, Lilly MB, Mallya S, Ong ST et al. (2010) Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med 16:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh AC, Ruggero D (2010) Targeting eukaryotic translation initiation factor 4E (eIF4E) in cancer. Clin Cancer Res 16:4914–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burris III HA, Kurkjian CD, Hart L, Pant S, Murphy PB, Jones SF, Neuwirth R, Patel CG, Zohren F, Infante JR (2017) TAK-228 (formerly MLN0128), an investigational dual TORC1/2 inhibitor plus paclitaxel, with/without trastuzumab, in patients with advanced solid malignancies. Cancer Chemother. Pharm 80:261–273 [DOI] [PubMed] [Google Scholar]
- 19.Ghobrial IM, Siegel DS, Vij R, Berdeja JG, Richardson PG, Neuwirth R, Patel CG, Zohren F, Wolf JL (2016) TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: A phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenstrom’s macroglobulinemia. Am J Hematol 91:400–405 [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain CE, German MS, Yang K, Wang J, VanBrocklin H, Regan M, Shokat KM, Ducker GS, Kim GE, Hann B, Donner DB, Warren RS, Venook AP, Bergsland EK, Lee D, Wang Y, Nakakura EK (2018) A Patient-derived Xenograft Model of Pancreatic Neuroendocrine Tumors Identifies Sapanisertib as a Possible New Treatment for Everolimus-resistant Tumors. Mol Cancer Ther 17(12):2702–2709. 10.1158/1535-7163.MCT-17-1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247 [DOI] [PubMed] [Google Scholar]
- 22.Faivre S, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, Vinik A, Van Cutsem E, Bang YJ, Lee SH, Borbath I, Lombard-Bohas C, Metrakos P, Smith D, Chen JS, Ruszniewski P, Seitz JF, Patyna S, Lu DR, Ishak KJ, Raymond E (2017) Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol 28(2):339–343. 10.1093/annonc/mdw561 [DOI] [PubMed] [Google Scholar]
- 23.Yao JC, Shah MH, Ito T et al. (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364(6):514–523. 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon SO, Shin S, Karreth FA, Blenis J, Karreth FA, Jedrychowski MP et al. (2017) Focal adhesion- and IGF1R-dependent survival and migratory pathways mediate tumor resistance to mTORC1/2 inhibition. Mol Cell. 10.1016/j.molcel.2017.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoang B, Frost P, Shi Y, Belanger E, Benavides A, Pezeshkpour G, et al. (2010) Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood. L. Formisano, et al. Critical Rev Oncol/Hematol 147(2020):102886 9 10.1182/blood-2010-05-285726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghobrial IM, Siegel DS, Vij R et al. (2016) TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: A phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non- Hodgkin lymphoma, or Waldenström’s macroglobulinemia. Am J Hematol 91:400–405 [DOI] [PubMed] [Google Scholar]
- 27.Voss M, Gordon MS, Mita M et al. (2015) 354 Phase I study of investigational oral mTORC1/2 inhibitor MLN0128: Expansion phase in patients with renal, endometrial, or bladder cancer. Eur J Cancer 51:S72 [Google Scholar]
- 28.Khadka P, Ro J, Kim H et al. (2014) Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm 9:304–316. 10.1016/j.ajps.2014.05.005 [DOI] [Google Scholar]
- 29.Loh ZH, Samanta AK, Sia Heng PW (2015) Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm 10:255–274. 10.1016/j.ajps.2014.12.006 [DOI] [Google Scholar]
- 30.Burris H, Hart L, Kurkjian C et al. (2012) A phase 1, open-label, dose-escalation study of oral administration of the investigational agent MLN0128 in combination with paclitaxel (P) in patients (pts) with advance
- 31.Grabiner BC, Nardi V, Birsoy K, Possemato R, Shen K, Sinha S et al. (2014) A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 10.1158/2159-8290.CD-13-0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T, Nakashima A, Guo L, Coffman K, Tamanoi F (2010) Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene. 10.1038/onc.2010.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh AP, Marshall CB, Coric T, Shim E, Kirkman R, Ballestas ME et al. (2015) Point mutations of the mTOR-RHEB pathway in renal cell carcinoma. Oncotarget [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E et al. (2011) mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKTsignaling. Cancer Discov. 10.1158/2159-8290.CD-11-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infante JR, Tabernero J, Cervantes A et al. (2013) Abstract C252: A phase 1, dose-escalation study of MLN0128, an investigational oral mammalian target of rapamycin complex 1/2 (mTORC1/2) catalytic inhibitor, in patients (pts) with advanced non-hematologic malignancies. Mol Cancer Ther 12(Suppl):C252. 10.1158/1535-7163.TARG-13-C252 [DOI] [Google Scholar]
- 36.Hudes G, Carducci M, Tomczak P et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281 [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, Escudier B, Oudard S et al. (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer 116:4256–4265 [DOI] [PubMed] [Google Scholar]
- 38.Yao JC, Shah MH, Ito T et al. (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salazar R, Garcia-Carbonero R, Libutti SK, Hendifar AE, Custodio A, Guimbaud R, Lombard-Bohas C, Ricci S, Klümpen HJ, Capdevila J et al. (2018) Phase II study of BEZ235 versus everolimus in patients with mammalian target of rapamycin inhibitor-naïve advanced pancreatic neuroendocrine tumors. Oncologist 23:766–e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powles T, Wheater M, Din O, Geldart T, Boleti E, Stockdale A, Sundar S, Robinson A, Ahmed I, Wimalasingham A et al. (2016) A randomised phase 2 study of AZD2014 versus everolimus in patients with VEGFrefractory metastatic clear cell renal cancer. Eur Urol 69:450–456. 10.1016/j.eururo.2015.08.035 [DOI] [PubMed] [Google Scholar]


