Abstract
Purpose
To construct machine learning models for predicting progression free survival (PFS) and overall survival (OS) with esophageal squamous cell carcinoma (ESCC) patients.
Methods
204 ESCC patients were randomly divided into training cohort (n = 143) and test cohort (n = 61) according to the ratio of 7:3. Two radiomics models were constructed by radiomics features, which were selected by LASSO Cox model to predict PFS and OS, respectively. Clinical features were selected by univariate and multivariate Cox proportional hazards model (p < 0.05). Combined radiomics and clinical model was developed by selected clinical and radiomics features. The receiver operating characteristic curve, Kaplan Meier curve and nomogram were used to display the capability of constructed models.
Results
There were 944 radiomics features extracted based on volume of interest in CT images. There were six radiomics features and seven clinical features for PFS prediction and three radiomics features and three clinical features for OS prediction; The radiomics models showed general performance in training cohort and test cohort for prediction for prediction PFS (AUC, 0.664, 0.676. C-index, 0.65, 0.64) and OS (AUC, 0.634, 0.646.C-index, 0.64, 0.65). The combined models displayed high performance in training cohort and test cohort for prediction PFS (AUC, 0.856, 0.833. C-index, 0.81, 0.79) and OS (AUC, 0.742, 0.768. C-index, 0.72, 0.71).
Conclusion
We developed combined radiomics and clinical machine learning models with better performance than radiomics or clinical alone, which were used to accurate predict 3 years PFS and OS of non-surgical ESCC patients. The prediction results could provide a reference for clinical decision.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13014-022-02186-0.
Keywords: Esophageal squamous cell carcinoma, Radiomics, Progression free survival, Overall survival, Machine learning
Introduction
Esophageal cancer (EC) is the seventh incidence and sixth mortality malignant tumors in the world, Easten Asia shows the highest regional incidence rates in the worldwide [1]. Especially in China, which is the main cause of the heavy burden for Easten Asia [1].The main subtype of EC in China is esophageal squamous cell carcinoma (ESCC), which has a proclivity for earlier lymphatic spread, and is associated with a poorer prognosis [2]. Due to most ESCC patients are diagnosed at an advanced stage, the 5 years overall survival rate is less than 20% [3]. Furthermore, most patients with advanced or medial stage ESCC always lost the operation opportunity [4, 5].Therefore, chemoradiotherapy (CRT) or neoadjuvant chemoradiotherapy (NCRT) are effectively strategies to treat with ESCC [6]. Even though there are different treatment strategies, the recurrence and metastasis are still the main factors that affects the prognosis and patient’s survival [7]. Currently, the methods to predict prognosis of ESCC patients are mostly based on clinic risk factors, pathology and image, such as the patients characteristics like age, gender, treatment response, the tumor characteristics like location, size, differential, TNM stage et al., the pathology characteristics like lymphovascular invasion, the hematology test results like leukocyte, platelet [8–12]. Nevertheless, the prediction of treatment outcomes based on images or clinic risk factors alone is too simply to represent the actually therapeutic effects.
Radiomics is considered as one of the most vital technical to predict the efficacy of ESCC treatment. At present, the radiomics extracted signatures are mostly based on computer tomography (CT) imaging, magnetic resonance imaging (MRI) or18F-flurodeoxyglucose positron emission tomography (18F-FDG PET) /CT imaging [13]. Nakajo et al [14] found texture features intensity variability (IV) and size-zone variability (SZV), and volumetric parameters metabolic tumor volume (MTV) and total lesion glycolysis (TLG) can predict tumor response. And the positive and negative predictive values for non-responders were 77% and 69% in MTV, 76%and 100% in TLG, 78% and 67% in IV and78% and82% in SZV, respectively. But none of them was an independent factor for the prediction of prognosis in the EC patients. Furthermore, Li et al [15] recruited 134 ESCC treated by CRT patients to evaluate the prognostic value of metabolic parameters of pre-treatment and interim 18F-FDG PET/CT for overall survival (OS). And they found that maximum of standard uptake value (SUVmax2), MTV1, △MTV, TLG1, TLG2 and △TLG were associated with OS. However, the model with more robustness is urgently needed develop to predict the prognosis and OS of ESCC patients. Chu et al [16] developed an optimal model to predict disease-free survival (DFS) and overall survival (OS) in patients with ESCC and demonstrated MR image combined with clinical features had superior performance in both training and test groups for predicting DFS ([C-Index], 0.714, 0.729) and OS ([C-Index], 0.730, 0.712). Peng et al. [17] combined CT radiomics features and clinical risk factors to determine recurrence-free survival (RFS) and OS after surgery in patients with ESCC. They revealed that the C-indices of the RFS radiomics nomograms were 0.758, 0.722 and 0.684, and the C-indices of the OS radiomics nomograms were 0.884, 0.809 and 0.729 in the training cohort, internal validation cohort and external validation cohort, respectively.
This study is aimed to develop and test the radiomics models to predict 3 years progression free survival (PFS) and OS of no-surgical ESCC patients based on contrast enhanced CT(CECT) images, which combined radiomics features and clinical features. The models could be used in individualized evaluation in pre-treatment and providing decision-making reference.
Materials and methods
Patients
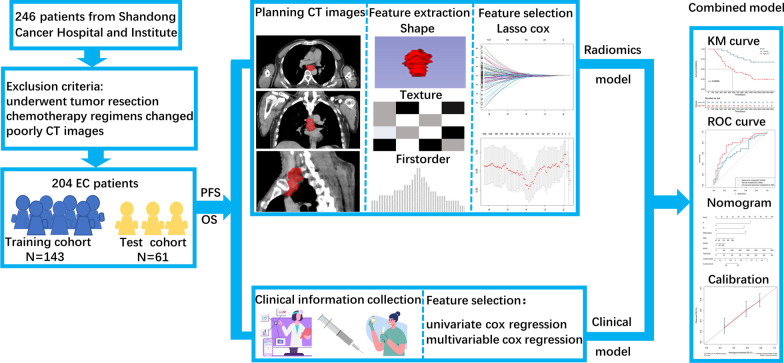
Our study recruited ESSC patients from February 2012 and December 2018, who were treated by chemoradiotherapy (CRT) in Shan Dong first medical university affiliated tumor hospital. Inclusion criteria were as follows: (1) age ≥ 18; (2) Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; (3) histopathologically confirmed squamous cell carcinoma; (4) cT3-4N0M0/cT1-4N + M0 or cM1 (positive nonregional lymph nodes and irradiated during radiotherapy) in accordance with AJCC 7th edition; (5) treated by 3-dimensional conformal radiation therapy(3D-CRT) or intensity-modulated radiation therapy (IMRT) with radiation total doses ≥ 50 Gy using conventional fractionated radiotherapy, chemotherapy cycles ≥ 4, chemotherapy with cisplatin plus fluorouracil (PF) or docetaxel (DP). Exclusion criteria were as follows: (1) patients changed chemotherapy regimens during definitive chemoradiotherapy; (2) CT images quality were poorly; (3) patients who underwent radical surgical treatment. This study was approved by the ethics committee of Shandong First Medical University affiliated tumor hospital according to the Declaration of Helsinki. Due to the research was a retrospective scientific study, there was no informed consent form in our investigation. All the patients were randomly divided into training cohort and test cohort according to the proportion of 7:3.
The acquisition of CT images and the region of interest
All the ESCC patients were scanned with Philips Big Core CT (Phillips Medical Systems, 96 Highland Heights, OH). The scanning parameters were as follows: tube voltage: 120KvP, tube current: 53-400 mA, each scanning period: 2.8 s, the interval time: 1.8 s. The CT images were reconstructed with a 512 × 512 image matrix and a voxel size of 0.9766 mm × 0.9766 mm × 3 mm. The image thickness was 3 mm. Patients were fixed by a vacuum cushion in the scanning process. Afterwards, the intravenous CECT images of each patient for the development of treatment planning.
The volume of interest (VOI) was defined as the gross tumor volume (GTV), which was the visible primary tumor (GTVp) and metastatic lymph nodes (GTVnd) detected by CECT. A radiologist with more than 10-year work experience delineated the VOI according to the National Comprehensive Cancer Network (NCCN) guideline. And another senior radiologist reviewed the delineation.
The collection of clinical features
The clinical features were collected, which involved: age, gender, tumor location, TNM stage, differentiation, therapeutic model, radiotherapy technology and dose, chemotherapy plan and cycles, the hematology test results, radiation pneumonia (RP), radiation esophagitis (RE), nausea or vomiting (NV), cardiac disorders, clinical response, Objective Response Rate (ORR), Disease Control Rate (DCR). Patients were followed up every 1 to 3 months after completion of chemotherapy for the first 2 years and every 6 to 12 months thereafter.
Tumor’s location and clinical TNM stage was evaluated by the medical imaging examination, such as CT, PET-CT. All the patients were treated with four different CRT therapeutic models, which included induction chemotherapy followed by concurrent chemoradiotherapy (I-CCRT), concurrent chemoradiotherapy followed by consolidation chemotherapy (CCRT-C), induction chemotherapy followed by concurrent chemoradiotherapy and consolidation chemotherapy (I-CCRT-C), sequential chemoradiation (SCRT). The ECOG PS of ESCC patients were assessed by the patient’s performance. The differentiation of cancer cell was estimated by the pathological examination. Some hematology test values were involved in our research, such as carcinoembryonic antigen (CEA), Cytokeratin-19-fragment CYFRA21-1 (Cyfra21). Others hematology test results were also classified to different grades by the Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE 4.0), which included anemia, leukopenia, thrombocytopenia, neutropenia, aspartate aminotransferase (AST), alanine transaminase (ALT), total bilirubin (TBIL). What’s more, the RE, RP, NV and Cardiac disorders were classified or showed in the study. Such as ORR, DCR, response, the efficacy was evaluated by the Response Evaluation Criteria in Solid Tumors Version 1.0. And clinical response was classified as complete response (CR), partial response (PR), no response (NR), or progressive disease (PD). PFS was defined as the period from the start of the anticancer treatment to the time of the first diagnostic progression or death or last follow-up. OS was defined from the start of the initial antitumor treatment to the date of death from any cause, regardless of disease status or last follow-up.
Feature extraction
We extracted the radiomics features from the basal CT before any therapy and the clinical parameters collected from the first follow-up CTs. Patients were followed up every 1 to 3 months after completion of chemotherapy for the first 2 years and every 6 to 12 months thereafter. Total of 944 features based on patient CT images were extracted by Radiomics software based on 3D slicer, which were divided into two categories: without preprocessing and after wavelet transform. In addition, these features include 14 shape features, 180 first-order features and 750 texture features. The resampled voxel sizes were set to 3 × 3 × 3 mm3 to standardize the slice thickness. The radiomics features were generated from the original, wavelet-filtered, and Laplacian of Gaussian (LoG)-filtered images. And the Log-kernel size was set to 3 × 3. The features included shape, shape2D, First-order and texture feature. Texture features included Gray Level Cooccurrence Matrix (GLCM), Gray Level Dependence Matrix (GLDM), Gray Level Run Length Matrix (GLRLM), Gray Level Size Zone Matrix (GLSZM) and Neighborhood Gray-tone Difference Matrix (NGTDM) [18].
Feature selection and model development
A total of 204 ESCC patients were divided into training and test cohorts to evaluate 3 years PFS and OS. Based on the training cohort, radiomics features were selected by the least absolute shrinkage and selection operator (LASSO) Cox model with tenfold cross validation, respectively. According to the selected radiomics features, two radiomics models with good prediction performance for PFS and OS were established.
Clinical features were selected by univariate and multivariate Cox proportional hazards model (p < 0.05). The selected clinical features were added into the multivariable Cox proportional hazards model based on radiomics features to improve the predictive ability. Finally, two combined models were established by selected radiomics and clinical features to predict PFS and OS.
The optimum cutoff value was the median of predicted value. Consequently, patients were divided into a high-risk group and a low-risk group in the training set. After the survival curves of the two groups were evaluated by the Kaplan Meier (KM) method, the differences between the survival curves were tested by the log-rank test (p < 0.05). The prediction ability of the survival rate was evaluated by the concordance index (C-index) and receiver operator characteristic (ROC) curve. Nomograms and calibration curve were built based on the two clinical and radiomics models. Calibration curves were calculated to evaluate the consistency between the nomogram-predicted results and recorded survival results. The flowchart of survival model construction is presented in Fig. 1.
Fig. 1.
The flow chart of this study. PFS, progression free survival; OS, overall survival; KM, Kaplan–Meier; ROC, receiver operator characteristic
Statistical analysis
Feature extraction was implemented in 3DSlicer (Version 4.11, https://www.slicer.org/). Statistical analyses were performed using R software (Version 3.4.0, https://www.r-project.org/). The Kruskal–Wall test performed in MATLAB (2013b, https://www.mathworks.com/) was used to analyze the different groups, and P values less than 0.05 were considered statistically significant. All statistical tests were two-sided.
Results
Patient demographics and clinicopathological characteristics
A total of 204 patients were analyzed to predict 3 years PFS and OS, respectively. The clinicopathological characteristics for survival analysis (training and test cohort) are shown in Additional file 5: Table S1. There were no significant differences between training cohort and test cohort of the clinical variables, except ECOG PS and Differentiation in PFS prediction model and response, DCR, ORR in OS prediction model. All of the advanced non-surgical ESCC patients were followed up for the full length of the follow-up period. In end of the last follow-up, 87 patients (60.84%) in the training set and 38 patients (62.30%) in the test set had disease progression. The survival time of 61 patients (42.66%) in the training set and 35 patients (57.38%) in the test was less than three years. There was no statistically significant difference in PFS and OS between the two groups (χ2 = 0.038, P = 0.845; χ2 = 3.719, P = 0.054, respectively).
The establishment of models to predict PFS
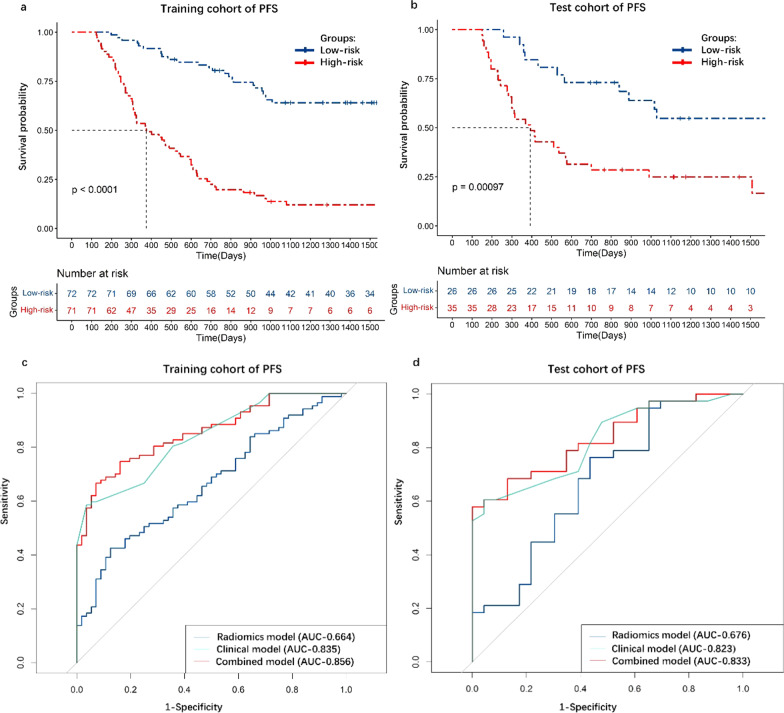
There were 944 radiomics features extracted from the VOI of CT images. Six radiomics features were selected by LASSO Cox model (Additional file 1: Figure S1a, 1b). Radiomics model was built by these features. Univariate and multivariate Cox hazard regression models were used to select clinical features, including ECOG PS, N stage, differentiation, RE, ORR, DCR, clinical response, (p < 0.05, respectively). The analysis of all clinical features was shown in Table S2. The radiomics features were shown in Additional file 7: Table S3. Combined model was built by selected radiomics and clinical features.
As the KM curves shown (Fig. 2a, b), selected radiomics and clinical features discriminated between high-risk group and low-risk group. In the training cohort, the C-index of radiomics model, clinical model, combined model was 0.65, 0.79 and 0.81, the AUC was 0.664, 0.835 and 0.856 respectively (Fig. 2c). In the test cohort, the C-index of radiomics model, clinical model, combined model was 0.64, 0.78 and 0.79, the AUC was 0.676, 0.823 and 0.833, respectively (Fig. 2d). The nomogram was constructed by combined model (Additional file 2: Fig. S2a). Then, the calibration curves of the nomogram for PFS showed that the predicted value of 3 years of PFS was roughly consistent with the actual value (Additional file 2: Fig. S2b, 2c).
Fig. 2.
The KM curve and ROC curve of PFS prediction model. a KM curve in training cohort. b KM curve in test cohort. c ROC curve in training cohort. d ROC curve in test cohort. PFS, progression free survival; OS, overall survival; KM, Kaplan–Meier; ROC, receiver operator characteristic
The establishment of models to predict OS
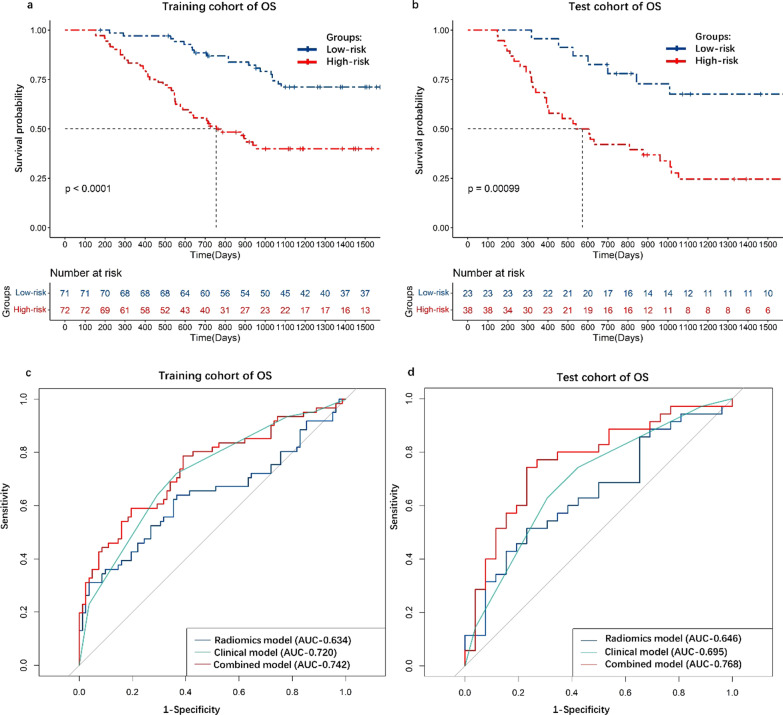
Radiomics model was built by three radiomics features which were selected by LASSO Cox model (Additional file 3: Figure S3a, 3b). Univariate and multivariate Cox hazard regression models were used to choose clinical features, including N stage, M stage and differentiation (p < 0.05, respectively). The analysis of all clinical features was shown in Additional file 5: Table S2. The radiomics features were shown in Additional file 8: Table S4. Combined model was built by selected radiomics and clinical features.
As the KM curves shown (Fig. 3a, b), selected radiomics and clinical features discriminated between high-risk group and low-risk group. In the training cohort, the C-index of radiomics model, clinical model, combined model was 0.64, 0.69, 0.72, respectively and the AUC was 0.634, 0.720, 0.742, respectively (Fig. 3c). In the test cohort, the C-index of radiomics model, clinical model, combined model was 0.65, 0.64 and 0.71, respectively. The AUC was 0.646, 0.695 and 0.768, respectively (Fig. 3d). The nomogram was constructed by combined model (Additional file 4: Fig. S4a). Then, the calibration curves of the nomogram for OS showed that the predicted value of 3 years of OS was roughly consistent with the actual value (Additional file 4: Fig. S4b, 4c).
Fig. 3.
The KM curve and ROC curve of PFS prediction model. a KM curve in training cohort. b KM curve in test cohort. c ROC curve in training cohort. d ROC curve in test cohort. PFS, progression free survival; OS, overall survival; KM, Kaplan–Meier; ROC, receiver operator characteristic
Discussion
Radiomics studies in EC most focused on the prediction of lymph node metastasis, radiation-induced diseases in the earlier [19–21]. What’s more, there were still few researches in the prediction of both PFS and OS [16, 22–24]. The establishment of accurate prediction models of PFS and OS were conducive to clinical decision-making and are expected to improve the survival rate of advanced ESCC patients. Therefore, in the present study, we constructed and validated machine learning models to predict PFS and OS of non-surgical ESCC patients, which incorporated the clinical variables and CECT images. The C-index and AUC showed that combined models had a better performance than radiomics or clinic models alone. The results demonstrated that incorporated the clinical variables enhanced the combined models’ predictive efficacy both in PFS and OS. In other word, the pre-treatment CECT images would not provide enough information to predict the treatment outcomes and the clinical data are essential for patient’s survival prediction.
As for the prediction of PFS and OS, the combined models performed well with the prognostic accuracy over 70% based on clinical and radiomics features. The C-index of PFS prediction radiomics model, clinical model, combined model in the test cohort was 0.64, 0.78, 0.79 and AUC was 0.676, 0.823, 0.833, respectively. The C-index of OS prediction radiomics model, clinical model, combined model in the test cohort was 0.65, 0.64, 0.71 and the AUC was 0.646, 0.695, 0.768, respectively. Bohanes et al. [25] found that gender and age had significant influence for the treatment outcomes of EC patients. But, in our study, age and sex were not involved in the model’s development as they were no statistically significant by univariate Cox regression. TNM stage was the most commonly prognosis prediction method in clinical. MES et al. [26] and Zhao et al. [27] combined TNM stage and other clinical factors to improve the prognostic predictive. In the PFS and OS prediction, N stage was filtered to develop clinical model in our study. But for the prediction of OS, M stage was also selected to develop models. Li et al. [28] used deep learning to predict the treatment response to CCRT for ESCC patients which also included the M stage in the progress of model development.
Jayaprakasam et al. [22] established radiomics model to predict PFS based on 72 ESCC patient’s PET/CT images and the AUC was 0.73 in the test cohort. They first included the PET responders into survey. But, PET/CT examination was highly expensive than CT or MRI and the sample was small in the study. Luo et al. [23] also developed a nomogram model for predicting local PFS based on CT images and C-index was 0.723 in test cohort. This study included clinical response to develop model and obtained a fine result. Liu et al. [29] have found that clinical complete response after neoadjuvant CRT was significantly correlated with survival of patients with ESCC. So, we also selected the clinical response, ORR and DCR into model’s building, which significantly enhanced the model’s prediction efficacy of PFS. After selecting, the tumor differentiation was also chosen to develop the PFS and OS prediction models. Barbetta et al. [30] found that poor tumor differentiation was an independent risk factor for recurrence in EC patients. Qiu et al. [31]incorporated radiomics and clinical features (including tumor differentiation) to predict postoperative recurrence risk of ESCC patients.And the C-index of test cohort was 0.724 in their combined model. In previous study, researchers had proved that ECOG PS had significant prognostic effects on clinical response and survival [32, 33].And RE was regarded as one of the factors affecting patient’s prognosis [34]. ECOG PS was used to evaluated the patient’s physical performance before treatment and RE was the radiation-induced esophageal disease after treatment. In our study, ECOG PS and RE were also selected to predict PFS.
Except the tumor differentiation and N stage described above, M stage was also selected to develop the OS prediction models. Shi et al. [35] concluded that metastatic lesions were closely related to the prognosis of patients. Li et al. [28] used deep learning to predict the treatment response to CCRT for ESCC patients and the M stage was also involved in the progression of model development. Due to the heterogeneity of tumor, the treatment outcomes of patients might be different even with the same clinical features [11, 36, 37].
Radiomics is defined as the high-throughput extraction of image features from radiographic images [38, 39]. Radiomic features provide abundant additional information predictive of underlying tumor biology and behavior [40]. These signatures can be used alone or with other patient related data (e.g., pathological data, genomic data, clinical data) to predict tumor phenotyping, treatment response prediction and prognosis. Our study finial selected one shape texture, two GLSZM textures, two GLDM textures and one GLCM texture to develop PFS prediction model and one shape texture, one GLSZM texture, one GLDM texture to construct OS prediction model. Wavelet transformed features contain more information and are more difficult to explain than first-order and shape features, but also reflect more complex information about tumor heterogeneity [16]. Therefore, the prediction results based on Wavelet transformed features are consistent with the cognition of clinical outcomes.
Although our research developed the survival prediction model with good accuracy, there are still some limitations in our study. Due to it is a retrospective study, some clinical variables are not comprehensive enough. Furthermore, all of the recruited EC patients were confirmed ESCC by pathology examination, which were the advanced stage and lost the surgery opportunity. Therefore, the constructed model may be limited in esophageal adenocarcinoma (EAC) or surgery patients. Due to the treatment plan is so vital for patient’s survival, our study recruited the patients treated with CRT, which might limit the model’s therapy decision. Adding the genomics features in the model is willing to improve the accuracy of the treatment outcomes. What’s more, the further study of Delta-radiomics model could provide better prediction for the PFS and OS [41].
Conclusion
This research displays a great performance for the prediction ESCC patients PFS and OS by combining the pre-treatment CECT radiomics signatures with clinical features. The prediction results will provide further decision-marking reference for clinician.
Supplementary Information
Additional file 1: Fig. S1. Radiomics feature s selected by LASSO Cox for PFS prediction.
Additional file 2: Fig. S2. PFS prediction Nomogram and calibration curve of combined model.
Additional file 3: Fig. S3. Radiomics features selected by LASSO Cox for OS prediction.
Additional file 4: Fig. S4. OS prediction Nomogram and calibration curves of combined model.
Additional file 5: Table S1. Patient characteristics in training (n = 143) and test cohorts (n = 61).
Additional file 6: Table S2. The clinical characteristics analysis of PFS and OS in the training cohort.
Additional file 7: Table S3. Radiomics features selected with LASSO Cox for PFS prediction.
Additional file 8: Table S4. Radiomics features selected with LASSO Cox for OS prediction.
Acknowledgements
Not applicable.
Abbreviations
- EC
Esophageal cancer
- ESCC
Esophageal squamous cell carcinoma
- CRT
Chemoradiotherapy
- NCRT
Neoadjuvant chemoradiotherapy
- CT
Computer tomography
- MRI
Magnetic resonance imaging
- 18F-FDG PET
18F-flurodeoxyglucose positron emission tomography
- OS
Overall survival
- RFS
Recurrence-free survival
- PFS
Progression free survival
- CECT
Contrast enhanced CT
- ECOG PS
Eastern Cooperative Oncology Group performance status
- 3D-CRT
3-Dimensional conformal radiation therapy
- IMRT
Intensity-modulated radiation therapy
- PF
Plus fluorouracil
- DP
Plus docetaxel
- VOI
Volume of interest
- GTV
Gross tumor volume
- GTVp
Primary tumor
- GTVnd
Metastatic lymph nodes
- RP
Radiation pneumonia
- RE
Radiation esophagitis
- NV
Nausea or vomiting
- ORR
Objective response rate
- DCR
Disease control rate
- I-CCRT
Induction chemotherapy followed by concurrent chemoradiotherapy
- CCRT-C
Concurrent chemoradiotherapy followed by consolidation chemotherapy
- I-CCRT-C
Induction chemotherapy followed by concurrent chemoradiotherapy and consolidation chemotherapy
- SCRT
Sequential chemoradiotherapy
- CEA
Carcinoembryonic antigen
- AST
Aspartate aminotransferase
- ALT
Alanine transaminase
- TBIL
Total bilirubin
- ULN
Upper limit of normal value
- CR
Complete response
- PR
Partial response
- NR
No response
- PR
Progressive disease
- GLCM
Gray level cooccurrence matrix
- GLDM
Gray level dependence matrix
- GLRLM
Gray level run length matrix
- GLSZM
Gray level size zone matrix
- NGTDM
Neighborhood gray-tone difference matrix
- LASSO
Least absolute shrinkage and selection operator
- KM
Kaplan Meier
- C-index
Concordance index
- ROC
Receiver operator characteristic
- ECA
Esophageal adenocarcinoma
Author contributions
Conceptualization, CY and MC; methodology, LZ.; software, XM; validation, CY; formal analysis, LZ; investigation, CY; resources, HD; data curation, XM; writing—original draft preparation, CY; writing—review and editing, LZ; visualization, MC; supervision, YY; funding acquisition, YY and MC. YY and MC contributed equally to this study. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by National Nature Science Foundation of China (81800156, 81974467), Shandong Province Key R&D Program (2018GSF118031), Shandong Medical Association Clinical Research Fund—Qilu special project (YXH2022ZX02197), Taishan Scholars Project of Shandong Province (ts201712098).
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Shandong First Medical University affiliated tumor hospital according to the Declaration of Helsinki (2022006022). Patient consent was waived due to the research was a retrospective scientific study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yongbin Cui, Email: yongbincui100@163.com.
Zhengjiang Li, Email: lzjsdsfdx@126.com.
Mingyue Xiang, Email: xiangmy123@163.com.
Dali Han, Email: dalihan_sdch@163.com.
Yong Yin, Email: yongyinsd@126.com.
Changsheng Ma, Email: machangsheng_2000@126.com.
References
- 1.Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 22019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Liu H, Chen Y. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, Open-Label Clinical. Trial J Clin Oncol. 2018;36(27):2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omloo JM, Lagarde SM, Hulscher JB, et al.. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246(6): 992–1000; discussion-1. [DOI] [PubMed]
- 6.Wang X, Liu X, Li D. Concurrent selective lymph node radiotherapy and S-1 plus cisplatin for esophageal squamous cell carcinoma: a phase II study. Ann Surg Oncol. 2019;26(6):1886–1892. doi: 10.1245/s10434-019-07264-4. [DOI] [PubMed] [Google Scholar]
- 7.Welsh J, Settle SH, Amini A. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118(10):2632–2640. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Liang D, Du L. Clinical characteristics and survival of 5283 esophageal cancer patients: A multicenter study from eighteen hospitals across six regions in China. Cancer Commun (Lond) 2020;40(10):531–544. doi: 10.1002/cac2.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang WY, Chen XX, Chen WH. Nomograms for predicting risk of locoregional recurrence and distant metastases for esophageal cancer patients after radical esophagectomy. BMC Cancer. 2018;18(1):879. doi: 10.1186/s12885-018-4796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang S, Ou J, Liu J. Application of contrast-enhanced CT radiomics in prediction of early recurrence of locally advanced oesophageal squamous cell carcinoma after trimodal therapy. Cancer Imaging. 2021;21(1):38. doi: 10.1186/s40644-021-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, Liu Z, Li C. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit Health. 2022;4(1):e8–e17. doi: 10.1016/S2589-7500(21)00215-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Zhou Z, Tian D. A validated nomogram integrating hematological indicators to predict response to neoadjuvant therapy in esophageal squamous cell carcinoma patients. Ann Transl Med. 2021;9(8):703. doi: 10.21037/atm-21-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traverso A, Wee L, Dekker A. Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys. 2018;102(4):1143–1158. doi: 10.1016/j.ijrobp.2018.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajo M, Jinguji M, Nakabeppu Y. Texture analysis of (18)F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2017;44(2):206–214. doi: 10.1007/s00259-016-3506-2. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Zschaeck S, Lin Q. Metabolic parameters of sequential 18F-FDG PET/CT predict overall survival of esophageal cancer patients treated with (chemo-) radiation. Radiat Oncol. 2019;14(1):35. doi: 10.1186/s13014-019-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu F, Liu Y, Liu Q, et al.. Development and validation of MRI-based radiomics signatures models for prediction of disease-free survival and overall survival in patients with esophageal squamous cell carcinoma. Eur Radiol. 2022;32(9):5930-5942. [DOI] [PubMed]
- 17.Peng H, Xue T, Chen Q, et al. Computed tomography-based radiomics nomogram for predicting the postoperative prognosis of esophageal squamous cell carcinoma: a multicenter study. Acad Radiol. 2022;S1076-6332(22)00070-8. [DOI] [PubMed]
- 18.Stefano A, Leal A, Richiusa S, et al.. Robustness of PET radiomics features: impact of co-registration with MRI. Appl Sci. 2021;11(21):10170.
- 19.Wu L, Yang X, Cao W. Multiple level CT radiomics features preoperatively predict lymph node metastasis in esophageal cancer: a multicentre retrospective study. Front Oncol. 2019;9:1548. doi: 10.3389/fonc.2019.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu J, Shen C, Qin J. The MR radiomic signature can predict preoperative lymph node metastasis in patients with esophageal cancer. Eur Radiol. 2019;29(2):906–914. doi: 10.1007/s00330-018-5583-z. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Gao Z, Li C. Computed tomography-based delta-radiomics analysis for discriminating radiation pneumonitis in patients with esophageal cancer after radiation therapy. Int J Radiat Oncol Biol Phys. 2021;111(2):443–455. doi: 10.1016/j.ijrobp.2021.04.047. [DOI] [PubMed] [Google Scholar]
- 22.Jayaprakasam VS, Gibbs P, Gangai N, et al. Can (18)F-FDG PET/CT radiomics features predict clinical outcomes in patients with locally advanced esophageal squamous cell carcinoma? Cancers (Basel). 2022;14(12):3035. [DOI] [PMC free article] [PubMed]
- 23.Luo HS, Chen YY, Huang WZ. Development and validation of a radiomics-based model to predict local progression-free survival after chemo-radiotherapy in patients with esophageal squamous cell cancer. Radiat Oncol. 2021;16(1):201. doi: 10.1186/s13014-021-01925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Wu LL, Zhang Y, et al. Establishing a survival prediction model for esophageal squamous cell carcinoma based on CT and histopathological images. Phys Med Biol. 2021;66(14):5015. [DOI] [PubMed]
- 25.Bohanes P, Yang D, Chhibar RS. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30(18):2265–2272. doi: 10.1200/JCO.2011.38.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mes SW, van Velden FHP, Peltenburg B. Outcome prediction of head and neck squamous cell carcinoma by MRI radiomic signatures. Eur Radiol. 2020;30(11):6311–6321. doi: 10.1007/s00330-020-06962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Gong J, Xi Y. MRI-based radiomics nomogram may predict the response to induction chemotherapy and survival in locally advanced nasopharyngeal carcinoma. Eur Radiol. 2020;30(1):537–546. doi: 10.1007/s00330-019-06211-x. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Gao H, Zhu J. 3D deep learning model for the pretreatment evaluation of treatment response in esophageal carcinoma: a Prospective Study (ChiCTR2000039279) Int J Radiat Oncol Biol Phys. 2021;111(4):926–935. doi: 10.1016/j.ijrobp.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Liu SL, Xi M, Yang H. Is there a correlation between clinical complete response and pathological complete response after neoadjuvant chemoradiotherapy for esophageal squamous cell cancer? Ann Surg Oncol. 2016;23(1):273–281. doi: 10.1245/s10434-015-4764-0. [DOI] [PubMed] [Google Scholar]
- 30.Barbetta A, Sihag S, Nobel T. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J Thorac Cardiovasc Surg. 2019;157(3):1249–59.e5. doi: 10.1016/j.jtcvs.2018.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Q, Duan J, Deng H, et al. Development and validation of a radiomics nomogram model for predicting postoperative recurrence in patients with esophageal squamous cell cancer who achieved pCR after neoadjuvant chemoradiotherapy followed by surgery. Front Oncol. 2020;10:1398. [DOI] [PMC free article] [PubMed]
- 32.Song T, Wan Q, Yu W. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget. 2017;8(58):98974–98984. doi: 10.18632/oncotarget.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghazy HF, El-Hadaad HA, Wahba HA. Metastatic esophageal carcinoma: prognostic factors and survival. J Gastrointest Cancer. 2022;53(2):446–450. doi: 10.1007/s12029-021-00610-4. [DOI] [PubMed] [Google Scholar]
- 34.Simone CB., II Thoracic radiation normal tissue injury. Semin Radiat Oncol. 2017;27(4):370–377. doi: 10.1016/j.semradonc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Shi Z, Zhu X, Ke S. Survival impact of concurrent chemoradiotherapy for elderly patients with synchronous oligometastatic esophageal squamous cell carcinoma: a propensity score matching and landmark analyses. Radiother Oncol. 2021;164:236–244. doi: 10.1016/j.radonc.2021.09.033. [DOI] [PubMed] [Google Scholar]
- 36.KALASEKAR S M, VANSANT-WEBB C H, EVASON K J. Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities . Cancers (Basel), 2021, 13(21). [DOI] [PMC free article] [PubMed]
- 37.Yang F, Wang Y, Li Q. Intratumor heterogeneity predicts metastasis of triple-negative breast cancer. Carcinogenesis. 2017;38(9):900–909. doi: 10.1093/carcin/bgx071. [DOI] [PubMed] [Google Scholar]
- 38.Lambin P, Rios-Velazquez E, Leijenaar R. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar V, Gu Y, Basu S. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sah BR, Owczarczyk K, Siddique M. Radiomics in esophageal and gastric cancer. Abdom Radiol (NY) 2019;44(6):2048–2058. doi: 10.1007/s00261-018-1724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefano A, Comelli A, Bravata V. A preliminary PET radiomics study of brain metastases using a fully automatic segmentation method. BMC Bioinform. 2020;21(Suppl 8):325. doi: 10.1186/s12859-020-03647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Radiomics feature s selected by LASSO Cox for PFS prediction.
Additional file 2: Fig. S2. PFS prediction Nomogram and calibration curve of combined model.
Additional file 3: Fig. S3. Radiomics features selected by LASSO Cox for OS prediction.
Additional file 4: Fig. S4. OS prediction Nomogram and calibration curves of combined model.
Additional file 5: Table S1. Patient characteristics in training (n = 143) and test cohorts (n = 61).
Additional file 6: Table S2. The clinical characteristics analysis of PFS and OS in the training cohort.
Additional file 7: Table S3. Radiomics features selected with LASSO Cox for PFS prediction.
Additional file 8: Table S4. Radiomics features selected with LASSO Cox for OS prediction.
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.