Abstract
Obesity usually is accompanied by inflammation of fat tissue, with a prominent role of visceral fat. Chronic inflammation in obese fat tissue is of a lower grade than acute immune activation for clearing the tissue from an infectious agent. It is the loss of adipocyte metabolic homeostasis that causes activation of resident immune cells for supporting tissue functions and regaining homeostasis. Initially, the excess influx of lipids and glucose in the context of overnutrition is met by adipocyte growth and proliferation. Eventual lipid overload of hypertrophic adipocytes leads to endoplasmic reticulum stress and the secretion of a variety of signals causing increased sympathetic tone, lipolysis by adipocytes, lipid uptake by macrophages, matrix remodeling, angiogenesis, and immune cell activation. Pro-inflammatory signaling of adipocytes causes the resident immune system to release increased amounts of pro-inflammatory and other mediators resulting in enhanced tissue-protective responses. With chronic overnutrition, these protective actions are insufficient, and death of adipocytes as well as senescence of several tissue cell types is seen. This structural damage causes the expression or release of immunostimulatory cell components resulting in influx and activation of monocytes and many other immune cell types, with a contribution of stromal cells. Matrix remodeling and angiogenesis is further intensified as well as possibly detrimental fibrosis. The accumulation of senescent cells also may be detrimental via eventual spread of senescence state from affected to neighboring cells by the release of microRNA-containing vesicles. Obese visceral fat inflammation can be viewed as an initially protective response in order to cope with excess ambient nutrients and restore tissue homeostasis but may contribute to tissue damage at a later stage.
Keywords: Obesity, Adiposity, Visceral fat, Inflammation, Adipocyte hypertrophy, Adipocyte hyperplasia, Adipose tissue macrophages, Resident immune cells, Cytokines, Crown-like structures
Background
Fat tissue is interspersed with resident immune cells as are all other solid tissues of the body. Activation of such immune cells usually is accompanied by local or systemic inflammation of varying intensity. A key characteristic of inflammation is the increased production of cytokines, chemokines, and other immune mediators which bind to cognate receptors present on/in numerous cell types in the local tissue and throughout the body. In addition to the role of resident immune cells, most all other cell types of a tissue can be activated to release pro-inflammatory mediators, usually at lower levels that seen for “professional” immune cells. Mild responses include local insulin resistance, oxidative stress and altered cell metabolism. Higher degrees of inflammation are characterized by the activation and infiltration of circulating immune cells which may cause local pain, edema, or fever [1, 2].
Triggers of resident immune cell activation not only comprise microbial or other infections but also “sterile” stimuli such as metabolic, physical, or toxic stress leading to excess production of oxygen radicals, reactive lipids, protein aggregates, stress proteins or cell necrosis followed by the release of diverse immunostimulatory compounds termed damage-associated molecular patterns [1].
The present review describes changes in visceral fat tissue in response to chronic overnutrition, the signals and cell types involved in the early stages of tissue inflammation, and the progression to full-blown inflammation characterized by tissue damage and infiltration of circulating immune cells.
Main text
Resident immune cells in lean visceral fat tissue
In visceral fat, members of the innate as well as adaptive immune system have been identified. These include macrophages, dendritic cells, granulocytes, innate lymphoid cells (ILCs and natural killer (NK) cells), and also T and B cells [3] (Fig. 1). Single-cell transcriptome analysis of mouse lean visceral adipose tissue leukocytes identified 15 distinct subpopulations [4]. In normal non-inflamed tissue, these cells are not only immune guardians against infection but also support proper tissue function. Many of these findings originate from studies in experimental models, but where analyzed, similar physiological functions of immune cells have also been reported in humans [5]. For instance, macrophages exhibit functional heterogeneity which includes the removal of dead or apoptotic fat cells, remodeling the extracellular matrix and promoting angiogenesis [5]. A subtype of macrophages supports the control of lipid metabolism by uptake and digestion of lipids [6, 7]. Furthermore, the secretion of IL-27 appears to be a major pathway of promoting thermogenesis in fat cells [8]. Macrophages contribute to the regulation of thermogenesis in response to cold exposure [9]. There is no homogeneous distribution of macrophage subtypes. For instance, in human subcutaneous tissue, spatial mapping identified macrophages with a M1-like phenotype associated with niches of adipocyte progenitor cells while macrophages with a non-inflammatory phenotype were dispersed throughout the fat tissue [10].
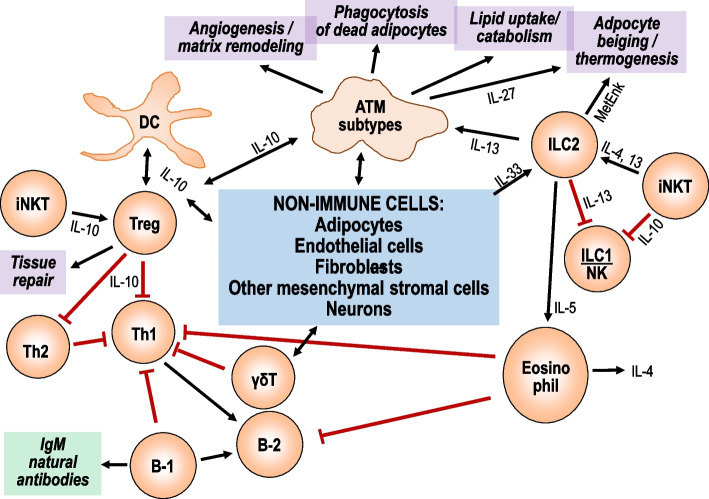
Fig. 1.
Network and physiological functions of resident immune cells in lean visceral adipose tissue. In the absence of metabolic or inflammatory stress resident immune cells interact among themselves and with adipocytes and stromal cells to maintain proper tissue functions. There are no signature cytokines defining the maintenance state of resident immune cells. Rather, the concept of a buffered system applies, without polarization towards a Th1/M1- or Th2/M2-like pattern or towards another biased state of immune reactivity. Cytokines, chemokines, acute phase proteins, and other immune mediators are released in small amounts mostly from resident immune cells but also from mesenchymal stromal cells and adipocytes. Several macrophage subtypes promote matrix remodeling and angiogenesis, phagocytose dead cell and lipid aggregates, and promote adipocyte thermogenesis. ILC2 also supports adipocyte thermogenesis and stimulates physiological eosinophil functions. Regulatory T cells promote tissue repair and interact with macrophages and other immune cell types to maintain a non-inflammatory state. Low-level secretion of immune mediators by macrophages, dendritic cells, and other immune cell types such as ILC2s, iNKTs, Th2 cells, γδT cells, B-1b cells, and eosinophils helps to prevent immune cell activation. For better readability, only a few key intercellular signals are included in the scheme. ATM, adipose tissue macrophage; DC, dendritic cell; IL, interleukin; ILC, innate lymphoid cell; iNKT, innate natural killer T cell; MetEnk, methionine-enkephalin peptides; NK, natural killer cell
ILC2 cells contribute to the regulation of energy expenditure by promoting the differentiation of beige adipocytes from adipocyte precursors or beiging of white fat cells in visceral tissues via upregulation of uncoupling protein 1 (UCP1 by enkephalin peptides [11, 12]. ILC2-derived IL-13 helps to prevent pro-inflammatory activation of macrophages, dendritic cells, ILC1, and natural killer cells. By secreting IL-5, ILC2 cells promote anti-inflammatory eosinophil activity.
Conventional dendritic cells exhibit a tolerogenic phenotype, characterized by IL-10 production and suppression of Th1-promoting activity by upregulated expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) [13]. Regulatory T cells (Treg) represent the major CD4-positive T cell type, they participate in tissue repair and preserve Glut-4 expression by adipocytes [14, 15]. Interestingly, the majority of Tregs appear to be oligoclonal in mice as indicated by distinct T cell receptor repertoires. There may be MHC II-dependent antigen recognition involved, as suggested by the close association with resident macrophages and dendritic cells [16]. The primary function of Tregs probably is to keep other immune cell types in a neutral physiological state, i.e., preventing immune activation either towards a pro-inflammatory or a Th2-like state.
Resident B-1b lymphocytes secrete natural IgM antibodies and promote adipose physiological functions by suppressing B-2 cells, in mice and humans [17]. In addition, B-1 cells comprise the major cell type of fat-associated lymphoid clusters which appear to contribute to humoral immune responses to peritoneal antigens [18]. Lymphoid clusters in mice and humans are also a rich source of Th2-like cytokines released from innate Th2-like lymphoid cells [19, 20]. Fat-associated lymphoid clusters such as milky spots on the omentum surface probably serve immune functions of the peritoneal cavity rather than supporting physiological fat tissue functions. Indeed, the numbers of milky spots increase during peritoneal inflammation in response to local TNFα and innate natural killer T cell activity [20, 21]. Studies in mice suggest that sympathetic innervation is promoted by γδT cells by signaling via the IL-17 receptor C to induce TGFß1 production by parenchymal cells [22]. Further, sympathetic neuron-associated macrophages (SAMs) regulate neuron growth and modulate adrenergic signaling [23].
Based mostly on animal studies, the continuous release of anti-inflammatory mediators from macrophages, dendritic cells, Th2-cells, γδT cells, eosinophils, mucosa-associated invariant T cells (MAIT), and invariant natural killer T cells appears to further help maintain metabolic homeostasis [14, 24–32] (Fig. 1). The support of tissue functions by resident immune cells involves interactions with non-immune tissue cells including adipocytes, endothelial cells, neurons, fibroblasts, and other mesenchymal stromal cells [3, 33, 34].
In the absence of immunologic stimuli, immune mediator secretion from resident immune cells and other fat tissue cells is low. The local immune milieu is well buffered, i.e., there is neither a dominance of Th1/M1-like nor Th2/M2-like immune reactivity. In this context, it is important to note that there is interdependence of Th1/M1- and Th2/M2-associated cytokines. For instance, the Th1-type cytokine TNFα stimulates the production of the Th2/Treg-associated cytokine IL-10 which in turn downregulates TNFα. Further, pro-inflammatory TNFα and IL-17A induce counterregulatory IL-33 for the stimulation of anti-inflammatory Tregs and ILC2s [15, 35, 36].
Taken together, in lean visceral adipose tissue, there is a physiological network of adipocytes, stromal cells, and immune cells. The resident immune system is not dormant but supports overall tissue functions. There are no signature cytokines defining the maintenance state of resident immune cells. Rather, the concept of a buffered system applies, without polarization towards a Th1/M1- or Th2/M2-like pattern or towards another biased state of immune reactivity. Cytokines, chemokines, acute phase proteins, and other immune mediators are released in small amounts mostly from resident immune cells but also from mesenchymal stromal cells and adipocytes [37, 38].
From lean to obese visceral fat tissue
The primary cause of progression from lean to obese visceral fat tissue is excess calorie intake, including digestible carbohydrates. Human metabolic control usually is geared in such a way that a calorie surplus is not disposed of by generating additional thermal energy but is stored to a large degree as triglycerides in adipocytes. Excess calorie consumption causes an increase of circulating insulin levels after and between meals. Being an anabolic hormone, insulin suppresses lipolysis and promotes fat storage in adipocytes already at concentrations that are in the high normal range or which are slightly elevated. Pharmacological or experimental lowering of insulin levels indeed ameliorates obesity which indicates that the support of lipogenesis by insulin is obesogenic (reviewed by [39]). These regulatory effects of insulin do not apply for all adipocytes. In subcutaneous tissue about half of mature adipocytes are insulin responsive, the two other subtypes exhibit little or no increased transcriptional activity when exposed to hyperinsulinemia [10].
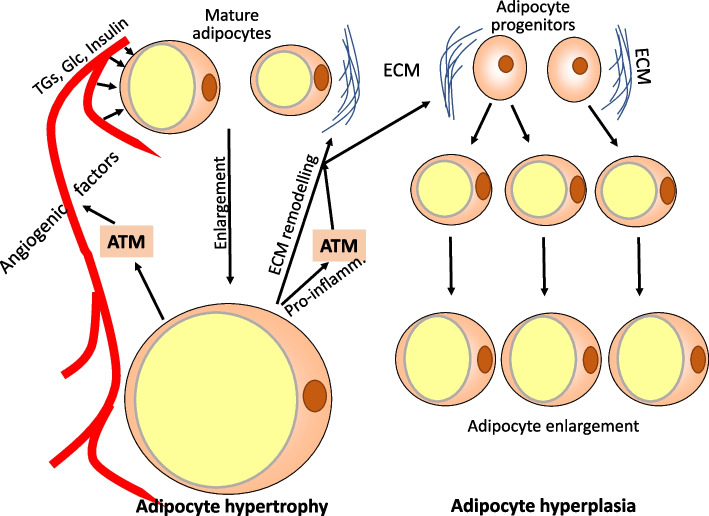
Anabolic activity of visceral fat tissue in response to overnutrition involves adipocyte enlargement and hyperplasia to accommodate for increased requirements of energy storage, i.e., clearance of excess lipids and glucose from the blood. Lipogenesis leads to enlargement of mature adipocytes because of more fat stored in one large lipid droplet organelle. The gain in cell volume may be several thousandfolds, with a cell diameter increase from <20 up to 300 μm [40]. There is also differentiation and growth of preadipocytes, but in visceral fat hyperplasia contributes less to the increase of fat mass than adipocyte hypertrophy [41]. The formation of new fat-laden adipocytes from precursor cells appears to begin when enlarged mature adipocytes reach a critical cell size and release mediators stimulating preadipocyte growth and differentiation [42, 43]. Fat cell hyperplasia thus is a second pathway of coping with excess circulating nutrients (Fig. 2) [43].
Fig. 2.
Response of visceral fat tissue to excess calories by adipocyte hypertrophy and hyperplasia. In response to high levels of circulating glucose, triglycerides, and the anabolic hormone insulin mature adipocytes take up increased amounts of nutrients and store excess energy as triglycerides in one large lipid droplet organelle. The cell size may increase 10–15-fold in diameter. Enlarged adipocytes secrete factors favoring angiogenesis and remodeling of the extracellular matrix and release of growth factors which is essential for mesenchymal stem cells, adipocyte progenitors, and preadipocytes to differentiate into lipid-storing mature adipocytes. In parallel, macrophages are stimulated to support angiogenesis and matrix remodeling. ATM, adipocyte tissue macrophages; TGs, triglycerides; Glc, glucose; ECM, extracellular matrix; Pro-inflamm., pro-inflammatory mediators
Studies in mice indicate that a major obstacle to fat tissue expansion in response to high-fat diet feeding is the collagen network of the extracellular matrix. A major source of collagen is perivascular cells in response to signaling via the platelet-derived growth factor 1α [44]. Most relevant for limiting fat tissue expansion is the extracellular matrix of niches rich in adipocyte precursors. Interestingly, these niches harbor potentially pro-inflammatory macrophages [10] and induction of acute local inflammation, for instance by injection of low-dose lipopolysaccharide enhances fibrolysis and remodeling of the extracellular matrix, and promotes angiogenesis to allow for efficient adipocyte hyperplasia. Enlarged adipocytes initiate fat tissue remodeling by secreting angiogenic factors such as such as fibroblast growth factor-2, vascular endothelial growth factor, human growth factor, and other mediators such as extracellular matrix proteases (Fig.2) [41]. Efficient remodeling requires activation of pro-inflammatory macrophages by hypertrophic adipocytes which appears to be a physiological response needed for fat tissue growth because downregulation of pro-inflammatory reactivity prevents proper adipocyte hyperplasia [41, 45, 46]. Thus, at least initially, inflammation in adipose tissue is a physiological adaptive response which improves fat tissue plasticity and consequently preserves metabolic control and insulin sensitivity [47]. A similar important role of inflammatory reactions, such as activation of the NLRP3 inflammasome, has been reported to drive postburn white adipose tissue remodeling [48].
Storage of energy in form of triglycerides also occurs in other fat tissues of the body, notably subcutaneous fat. The adipogenic activity and the ability to mobilize preadipocytes in response to overeating have been reported to be delayed in subcutaneous fat and therefore may be insufficient to lower the metabolic stress of visceral fat tissue during excess calorie intake [43]. However, this is different in persons with true metabolic healthy obesity, i.e., defined by an absence of metabolic syndrome criteria (except for increased waist circumference) and of insulin resistance calculated as HOMA-IR [49]. In these persons, the growth of visceral fat and adipocyte enlargement is only moderate, and excess nutrients are primarily handled by enlargement and hyperplasia of adipocytes in subcutaneous fat tissue, primarily in the superficial layer [43, 50–52].
In sum, the primary fat tissue response to excess calorie intake includes enlargement of adipocytes, differentiation of new mature cells from pre-adipocytes or stem cells, all supported by remodeling of the extracellular matrix, and of angiogenesis for appropriate blood supply. Growth of visceral fat tissue is not possible without appropriate remodeling of the vasculature and the extracellular matrix surrounding preadipocytes and small adipocytes. Enlarged adipocytes initiate these changes by secreting factors promoting angiogenesis and matrix remodeling. These adaptive responses are characteristic of metabolically healthy obesity.
Obese visceral fat tissue inflammation in response to disturbed local metabolic homeostasis
A recent overview of inflammatory responses to non-infectious stimuli in various tissues of the body has concluded that there appear to be three types of perturbation causing an inflammatory response which, at least initially, are considered protective [2] The suggested hierarchy of perturbations is loss of regulation, loss of function and loss of structure. This concept is applied here to obese fat tissue, and the current section considers loss of regulation.
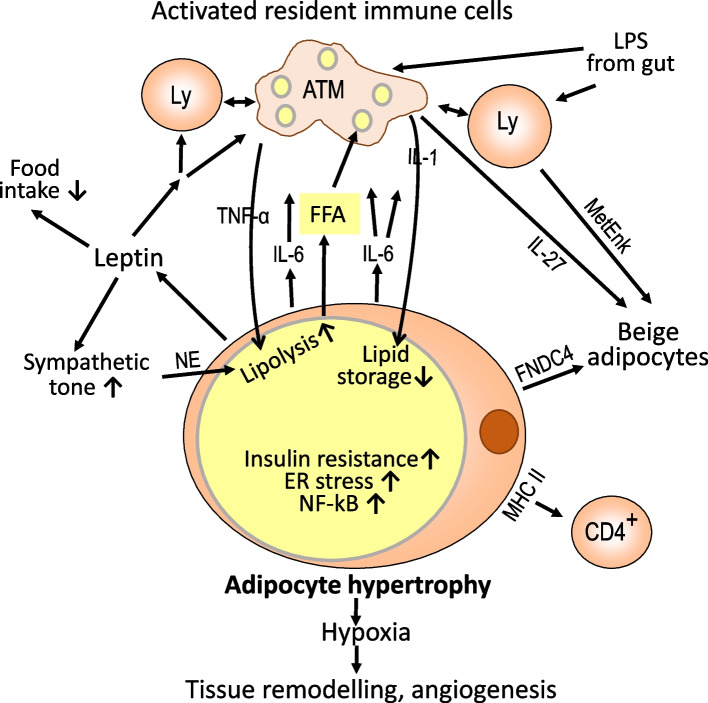
In those visceral adipose regions where the adaptive response to excess energy influx has reached a limit, metabolic homeostasis is lost, and activation of resident immune cells occurs. In detail, strongly enlarged adipocytes fail to maintain metabolic homeostasis of lipid storage versus lipolysis because the lipid overload leads to endoplasmic reticulum stress, increased expression of the inflammation regulator NF-kB and the production of inflammation-inducing signals such as IL-6 [40, 53]. The secretion of pro-inflammatory mediators in response to loss of metabolic homeostasis has been termed metaflammation [54].
Enlarged adipocytes exhibit additional responses to caloric stress. For instance, adipocytes respond to high ambient nutrient concentrations with the release of leptin and other hormones which target the brain to limit food intake and increase the sympathetic tone. Adrenaline and noradrenaline are released from nerve endings in adipose tissue and activate lipolysis by signaling via ß-adrenergic receptors of adipocytes. Sympathetic neuron-associated macrophages may function as rate-limiters by degrading noradrenaline via monoamine oxidase A [23]. The locally increased concentration of non-esterified fatty acids is expected to activate pro-inflammatory macrophage functions. This may involve co-secretion of adipocyte fatty acid binding protein (FABP4), induction of FABP4 in macrophages, and signaling via toll-like receptors TLR4 and TLR2. Free fatty acids do not directly bind to TLR4, but lipid metabolism within macrophages is affected by the influx of free fatty acids which has pro-inflammatory consequences if there is simultaneous activation of TLR4. The latter may result from increased levels of lipopolysaccharide released from gut microbiota in the context of gut leakiness during an obesogenic diet [55–60]. Further, recent studies suggest a role of adenine nucleotide translocase 2 in mediating free fatty acid-induced mitochondrial dysfunction, increased oxygen radical production and NF-kB activation in fat tissue macrophages [61]. The secretion of leptin by enlarged adipocytes not only limits food intake and promotes lipolysis in visceral fat but also engages leptin receptors present on most immune cells. This results in modest activation of immune reactivity of resident immune cells towards a Th1/M1-type pro-inflammatory bias [54, 62].
Another pathway of promoting local inflammation in response to adipocyte enlargement is activated by rapid fat tissue growth in the presence of insufficient angiogenesis which lowers capillary density and increases diffusion distance for oxygen eventually resulting in a hypoxic environment of enlarged adipocytes. Adipose is among the most vascularized tissues with each adipocyte surrounded by capillaries [63]. Lowering ambient oxygen concentration in adipocyte culture caused a switch from oxidative phosphorylation to anaerobic glycolysis and changed the expression of more than 1000 genes [64]. One major mediator of this response is hypoxia-inducible factor 1α [55]. Pro-inflammatory mediators secreted by mature adipocytes during hypoxia include chemokines and cytokines such as PAI-1, CCL5, and IL-6 as well as micro RNAs [65–68] (Fig. 3). A subset of macrophages is closely associated with the vasculature and characterized by the expression of lymphatic vessel endothelial hyaluronan receptor 1. These macrophages support angiogenesis by producing tissue remodeling growth factors and metalloproteinases [21, 69]. Hypoxia does not homogeneously affect visceral fat tissue but is a regional phenomenon as concluded from immunohistochemical staining for hypoxia-inducible factor 1α. The colocalization of enhanced numbers of macrophages and T cells supports the pro-inflammatory property of hypoxia [70].
Fig. 3.
Local inflammation in response to disturbed adipocyte metabolic homeostasis. When enhanced lipid storage via adipocyte enlargement and differentiation of progenitor cells fails to maintain metabolic homeostasis, local inflammatory changes occur in order to dispose of excess lipid and regain metabolic control. For one, lipid-laden adipocytes experience endoplasmic reticulum stress and increased expression of NFkB leading to the release of pro-inflammatory mediators such as IL-6. Additional pro-inflammatory signals are delivered by the release of free fatty acids, leptin, lipopolysaccharides, and other products of an unbalanced microbiota in the context of a leaky gut. Activated resident immune cells release amounts of pro-inflammatory mediators sufficient to promote lipolysis and suppress lipid storage in part via induction of insulin resistance. In addition, there is an uptake of lipids by macrophages and storage in small lipid droplets. Leptin interacts with receptors in the brain to limit food intake and increase the sympathetic tone. The increased local release of noradrenaline also promotes lipolysis. Another pro-inflammatory condition results from hypoxia due to local enlargement of adipocytes. The concomitant release of enzymes and factors promoting tissue remodeling and angiogenesis may be considered a healing response. Enlarged adipocytes overexpress MHC class II antigens and appear to present antigens to CD4-positive T cells. Another pathway of limiting energy storage is the induction of adipocyte beiging by transdifferentiation or growth from progenitors and the disposal of excess energy by thermogenesis. For better readability, only a few key intercellular signals are included in the scheme. FNDC4, fibronectin type III domain-containing protein 4; FFA, free fatty acids; LPS, lipopolysaccharide; NE, norepinephrine/noradrenaline; NFkB, nuclear factor kappa B
A third pathway of pro-inflammatory activation of resident immune cells is suggested by the finding that hypertrophic adipocytes overexpress major histocompatibility antigens class II (MHCII) and produce costimulatory molecules for effective antigen presentation to CD4 positive T cells. Although antigens presented have not been identified, it is remarkable that mice with genetic depletion of MHC II in adipocytes gain weight as control mice but do not develop adipose tissue inflammation and insulin resistance [71].
An additional pathway of lowering the metabolic stress in obese visceral fat tissue is the transdifferentiation of white adipocytes to beige adipocytes and the formation of new beige adipocytes from precursor cells (Fig. 3). Beige adipocytes contain several smaller lipid droplet organelles and more mitochondria than hypertrophic adipocytes for burning free fatty acids to generate heat. Secretion of IL-27 from macrophages promotes thermogenesis in fat cells [8], as does the release of enkephalin peptides from ILC2 cells [11, 12]. The major mediator of beiging in visceral fat released by adipocytes in obesity is fibronectin type III domain-containing protein 4 (FNDC4) which probably targets the receptor GRP116. There is a positive association between the expression of FNDC4 and obesity-associated inflammation [72]. In line with a role in regaining normal tissue homeostasis, FNDC4 exhibits anti-inflammatory properties in macrophages [73].
Taken together, loss of metabolic homeostasis in fat tissue is sufficient to initiate a local pro-inflammatory response. Secretion of pro-inflammatory mediators from macrophages and other immune cells substantially exceeds the release from adipocytes [38]. This may be viewed as an attempt to restore proper energy balance [74]. The locally enhanced concentrations of mediators like TNFα, IL-1, and IL-6 act back on adipocytes and suppress further lipid storage by inhibiting lipoprotein lipase, needed for lipid uptake, and by promoting lipolysis and fatty acid release via several pathways. These include the local induction of insulin resistance in insulin-sensitive adipocytes resulting from engaging TNFα or other pro-inflammatory mediators including microRNAs and subsequent impairment of insulin signaling for lipolysis inhibition [26, 75]. Further support comes from increased activation of extracellular signal-regulated kinase (ERK) stimulating beta3 adrenergic receptor-mediated lipolysis via protein kinase A [76]. Inflammatory stress induces kinase activity of inositol-requiring protein 1 (IRE-1), a component of the endoplasmic reticulum stress response, which is also followed by enhanced lipolysis [77]. In addition, there is upregulation of lysosomal biogenesis, increased uptake and turnover of lipids, and increased formation of lipid droplets in macrophages, all of which can be considered an attempt to lower the lipid load of adipocytes [78]. Finally, burning of free fatty acids via promoting thermogenesis/beiging is supported by the release of IL-27 from macrophages, enkephalin peptides from ILC2s, and FNDC4 from adipocytes. The interaction between the various cell types in adipose tissue can also be described as crosstalk since there is signaling between cells in both directions. Crosstalk not only involves the secretion of soluble mediators but also of particulate structures such as extracellular vesicles or mitochondria [79, 80].
The scenario described relates to observations in animal models. In humans, the direct demonstration an early phase of inflammatory reactions induced by metabolically stressed enlarged adipocytes during overnutrition would require repeated biopsies of visceral fat tissue, but the mechanisms detailed above also apply to human cells. In mice, high-fat diet feeding studies observed an early period of 4–8 weeks with adipocyte enlargement, limited local immune activation, vasculogenesis, matrix remodeling, and clearance of a low number of dead adipocytes by local macrophages [81].
Influx of immune cells into obese visceral fat inflammation in response to functional/structural tissue damage
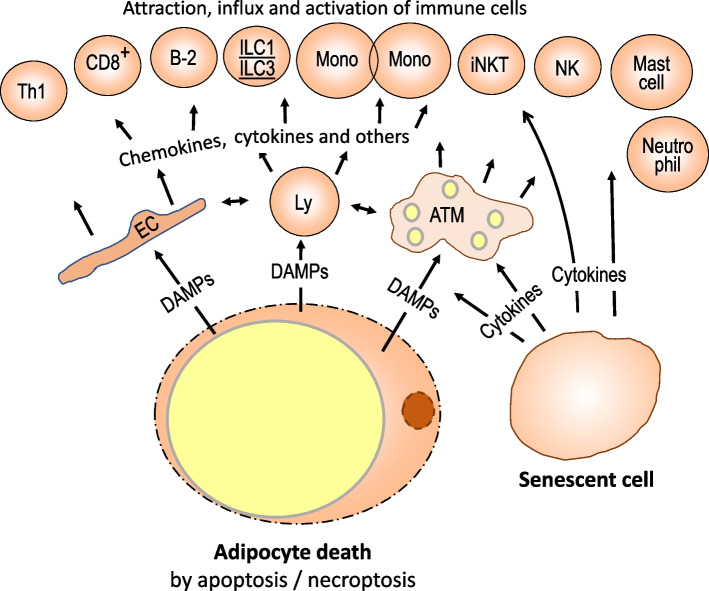
A general characteristic of tissue damage is the loss of structural integrity, i.e., molecular cues are presented on cell surfaces or are released that are usually sequestered and not accessible to the immune system. Many of these molecules are immunostimulatory damage-associated molecular patterns (DAMPs), they include stress proteins, high mobility group box 1 protein (HMGB1), DNA, some lipids, and mitochondrial structures, among many others. DAMP receptors (also called pattern recognition receptors) are present on innate immune cells and in part also on adaptive immune cells and non-immune cells such as epithelial cells, endothelial cells, or fibroblasts. DAMP receptors include toll-like receptors, C-type lectin receptors, cytoplasmic NLR receptors, and several DNA sensors. Signaling via these receptors leads to the production of pro-inflammatory cytokines and other mediators [82, 83].
In visceral fat tissue, major sources of DAMPs are apoptotic/necroptotic/pyroptotic adipocytes. Dead adipocytes accumulate in obese visceral tissue and attract resident macrophages giving the image of crown-like structures resulting in phagocytic activity and proliferation. Apoptotic adipocytes express surface proteins favoring phagocytosis by M2-type macrophages [84]. Treg cells also associate with crown-like structures and probably support non-inflammatory macrophage functions [14]. However, probably because of the size difference of macrophages and hypertrophic dying adipocytes, there is also lysosomal exocytosis, and the released DAMPs stimulate pro-inflammatory immune activities which are more M1- than M2-like [85]. Immune activation by DAMPs appears to exceed pro-inflammatory signaling caused by metabolically stressed adipocytes because there is an influx of monocytes and other immune cells which outnumber resident immunocytes [55, 84]. In mouse fat tissue, induction of inflammasome and caspase-1 activity for the release of IL-1 and IL-18 is required for the recruitment of circulating immune cells and their pro-inflammatory activation [86]. Secretion of macrophage chemotactic protein 1 (MCP-1) also contributes to monocyte attraction [87, 88].
Another functional/structural change in obese visceral tissue is the accumulation of senescent cells, mostly macrophages, pre-adipocytes, mature adipocytes, and endothelial cells, probably in response to high metabolic activity, concomitant high oxygen radical production, mitochondrial dysfunction, and DNA damage. These cells exhibit impaired cell functions and an irreversible proliferative arrest in association with the secretion of a variety of pro-inflammatory cytokines, chemokines, proteases, and vesicles containing microRNAs, DNA, lipids, and protein. Peptides secreted in the context of the senescence-associated secretory phenotype (SASP) not only stimulate adipocytes and activate resident immune cells but also help recruit circulating immune cells to fat tissue followed by their activation [58, 89–91].
Structural damage also ensues if physiological remodeling of the extracellular matrix of obese visceral fat tissue is insufficient to adapt to tissue growth and enhanced angiogenesis. Collagen accumulates around adipocytes and in fiber bundles leading to decreased tissue plasticity. This leads to an adipocyte-mediated release of endotrophin, a cleavage product of collagen VI, which enhances local inflammatory responses [92–94].
The findings described above suggest that the recruitment of immune cells and their accumulation occurs in response to structural damage of visceral fat tissue (Fig. 4). The dominant immune cells in the infiltrate are monocytes developing into tissue macrophages. Concomitantly, there is an influx of other immune cell types, including T and B cells, ILC1s, ILC3s, NK cells, mast cells, and neutrophils [25, 30, 94–99]. Since the fat tissue is not homogeneous with regard to vascularization, hypoxia, and adipocyte death, there is regional diversity of the inflammatory state.
Fig. 4.
Severe visceral fat tissue inflammation in response to structural disruption. Excessive enlargement of adipocytes in response to chronic overnutrition eventually causes structural damage with dying adipocytes and cell senescence as hallmarks. The phagocytotic capacity of macrophages is overwhelmed and released DAMPS strongly activate resident immune and endothelial cells resulting in the attraction of virtually all types of immune cells. Their pro-inflammatory activation also stimulates anti-inflammatory activities. Another structural change is the accumulation of senescent cells, mostly macrophages, pre-adipocytes, mature adipocytes, and endothelial cells. Senescent cells secrete pro-inflammatory mediators and enhance the accumulation of immune cells from circulation. ATM, adipose tissue macrophages; DAMP, damage-associated molecular pattern; Mono, monocytes; EC, endothelial cell; CD8+, CD8-positive T cells
DAMPs and free fatty acids do not exhibit the same strong immunostimulatory activity as seen for bacterial or viral components. Therefore, the inflammation quality is characterized by lesser pro-inflammatory signaling which is related but not identical to classical Th1/M1-type activity, and there is an upregulated lipid metabolism [6, 55, 100, 101]. Th1/M1-like inflammatory activity is also promoted by local strong hypoxic conditions in the context of anaerobic glycolytic immunometabolism [102, 103].
In inflamed obese visceral tissue infiltrated by immune cells, there is an overall dominance of pro-inflammatory activity. This favors pro-inflammatory versus tissue-supporting or anti-inflammatory macrophages, Th1 versus Th2 promoting dendritic cells, Th1 versus Treg cells, B-2 versus B-1b lymphocytes, ILC1s versus ILC2s and iNKT cells, mast cells and neutrophils versus eosinophils, and CD8+ - versus γδT cells [5, 25, 28, 29, 70, 104] (Fig. 4). However, it must be considered that any Th1/M1-type pro-inflammatory activity gives rise to an antagonistic anti-inflammatory response so that both, pro- and anti-inflammatory activities, including Th1- and Th2-associated cytokines, are upregulated. This situation is best researched for macrophages, which remain the prominent immune cell type in inflamed obese visceral tissue with structural damage, largely due to the recruitment of monocytes from circulation. Most infiltrated macrophages are polarized towards a pro-inflammatory phenotype which only partially resembles classic M1-like activity characterized by the secretion of IL-1ß, IL-18, TNFα, chemokines, and proteases [100]. As discussed above, pro-inflammatory cytokines such as TNFα elicit the production of anti-inflammatory cytokines such as IL-10 or of prostaglandin E2. A fraction of macrophages exhibits a M2-like or a mixed M1/M2 phenotype [85, 105, 106]. There is regional diversity between macrophages within and outside crown-like structures, and in other human obese visceral adipose tissue [85]. For instance, macrophages with adipogenic and angiogenic gene expression patterns are distributed more evenly in the visceral fat tissue while lipid-laden pro-inflammatory macrophages are associated with dead adipocytes [85].
Obesity induced by long-term feeding of a high-fat diet in mice also changes the major phenotype of dendritic cells in visceral fat towards a pro-inflammatory profile. There is secretion of IFNα from plasmacytoid dendritic cells [58]. In parallel, the number of regulatory T cells, supporting the maintenance function of immune cells, is decreasing. The loss of Treg lymphocytes from obese visceral tissue appears to be a direct consequence of IFNα action [58]. The lower number of regulatory T cells may be the major reason accounting for a pro-inflammatory shift in several other immunocytes. Early changes include an influx of pro-inflammatory T cells and of B lymphocytes. In high-fat diet-induced obesity of mice both cell types appear to precede peak macrophage infiltration [107, 108]. IFN γ secretion by CD4- and CD8-positive T lymphocytes as well as of NK cells and ILC1s probably is a strong activator of pro-inflammatory macrophage activity. Stimulation of T-cells for IFN γ production probably is supported by the pro-inflammatory B2 subset of B lymphocytes while the percentage of anti-inflammatory B1 cells is decreased [55, 97, 104, 107–111]. There is also activation of MAIT cells which promotes macrophage activation by secretion of TNFα and IL-17 [112].
In the context of visceral obese fat tissue inflammation, there is also an increase of activated neutrophils. These cells release extracellular traps which interact with other immune cells to promote pro-inflammatory responses and possibly contribute to remodeling of the matrix because of the protease content of traps, in addition to promoting insulin resistance [113, 114]. Obese visceral fat tissue also harbors increased numbers of mast cells [115] but it is not clear whether these cells promote or dampen inflammation [116].
The immune cell influx in response to structural damage of fat tissue appears to exhibit tissue-protective and also detrimental properties. Fat tissue repair such as elimination of dying adipocytes, enhanced lipolysis, tissue remodeling, and angiogenesis represent beneficial functions of infiltrated and resident immune cells. However, animal studies indicate that matrix remodeling during chronic inflammation eventually may lead to fibrosis, i.e., excess incorporation of fibrils such as collagen vi into the extracellular scaffold of adipocytes which limits adipose plasticity and metabolic function [117]. An alternative view suggests that a rigid extracellular membrane prevents excessive enlargement of adipocytes and supports metabolic homeostasis [118].
Senescent cells in inflamed tissue probably also have beneficial and as well as detrimental effects. In animal models, beneficial effects include the orchestration of tissue remodeling through the secretion of pro-inflammatory factors. Senescent cells positively impact health span, liver, and vascular tissue fibrosis, and wound healing [119, 120]. However, if senescent cells are not cleared within days or weeks by innate immune cells, they accumulate and spread senescence to neighboring and distant cells, mostly via secretion of microRNA-containing vesicles with the consequence of a pro-fibrotic state and deficient tissue function in hypertrophic obesity mice [46, 121–123]. Obesity and hyperinsulinemia also drive the senescence of adipocytes or visceral fat macrophages in humans [91, 124]. In obese mice, genetic or pharmacological elimination of senescent cells promoted adipogenesis and decreased the influx of monocytes into abdominal fat [89, 125]. When human obese visceral tissue containing senescent cells was transplanted into immunodeficient mice, lower glucose tolerance and increased insulin resistance were observed. These detrimental effects were suppressed by clearing the human fat tissue from senescent cells by treatment with a selenolytic cocktail prior to transplantation [90].
Severe visceral obesity often is accompanied by systemic low-grade inflammation, insulin resistance, glucose intolerance, and other measures of metabolic disturbances. This does not simply appear to be a spill-over effect because there seem to be contributions of other organs such as the liver, the hypothalamus, and the gut microbiota [126–128]. Overnutrition and excess systemic nutrients cause changes in the liver related to those described for visceral fat. There is enhanced lipid uptake by several cell types followed by disturbed metabolic homeostasis as evident from endoplasmic reticulum stress in hepatocytes. Eventually, this leads to structural tissue damage such as death of hepatocytes and fibrosis. Loss of metabolic homeostasis and tissue damage is accompanied by activation of the resident immune system. Pro-inflammatory responses are carried by Kupffer cells, stellate cells, many infiltrated immune cell types, other stromal cell types, and also by hepatocytes [129–136]. In animal models, immune intervention trials often have led to improved metabolic control with or without decreased adiposity indicating a pathogenic role of inflammatory immune reactivity [137–139]. However, most studies do not allow to distinguish between effects mediated at the level of the liver, pancreas, vasculature, gut, brain, or adipose tissue. A detailed discussion of diet-induced inflammatory changes outside the visceral fat tissue and of immune intervention studies is outside the scope of this paper.
Conclusions (Table 1)
Table 1.
Key messages and gaps of knowledge
|
• In normal non-inflamed fat tissue, resident immune cells interact with adipocytes and stromal cells for metabolic homeostasis, thermogenesis, and cell turnover. • Adipocytes deal with excess ambient nutrients by increased lipid storage (hypertrophy) and proliferation (hyperplasia). Resident pro-inflammatory macrophages support matrix remodeling required for adipocyte hyperplasia. • Excessive enlargement of adipocytes causes metabolic stress and the release of pro-inflammatory mediators which activate resident immune cells for enhanced production of immune mediators, resulting in enhanced sympathetic tone, lipolysis in adipocytes, lipid uptake in macrophages, matrix remodeling, and angiogenesis. • Chronic overnutrition eventually leads to structural/functional damage, i.e., adipocyte death and accumulation of senescent cells. Both are strong immunostimulatory processes causing the influx of monocytes and many other immune cell types from circulation. There is further enhanced matrix remodeling including fibrosis, angiogenesis, lipid uptake by macrophages, clearing of the tissue from cell debris, and spread of senescent state from affected to neighboring cells by the release of microRNA-containing vesicles. • Although tissue inflammation serves the restoration of a physiological functional state it is not known at which stage and by what quality chronic inflammation may mediate detrimental effects. • The role of senescent cells in supporting or damaging tissue functions requires further research. • Subtypes of visceral obesity remain to be defined, with age, sex and genetic background as important parameters. The protective versus detrimental functions of inflammation may differ between subtypes. • Molecular mechanisms are often deduced from animal studies. Differences between animal models and obese humans must be taken into account. |
Inflammation of tissues in the absence of infectious, toxic or allergenic agents in general is caused by the local expression of immunostimulatory molecules in the context of metabolic or physical tissue damage. The activation of resident immune cells as well as the influx of immune cells from circulation into stressed tissue can be interpreted as an attempt to regain the previous physiological balance [2, 74]. Loss of metabolic tissue homeostasis appears to be sufficient to produce a “physiological“ local inflammatory response that enforces restoration of homeostasis. Loss of structure (tissue damage) elicits a more intense form of inflammation with influx of circulating immune cells, again primarily supporting tissue functions [2].
In obese visceral fat tissue, adaptive or repair functions of macrophages and other activated immune cells include support of matrix remodeling and angiogenesis by secretion of proteases and growth factors to accommodate for adipocyte enlargement and hyperplasia, lipid uptake and catabolism to lower lipid load, stimulation of thermogenesis for lipid burning, promotion of lipolysis and local insulin resistance to reduce lipid storage, and clearance from dead adipocytes and senescent cells. Concomitant fibrosis may be regarded as protective or detrimental, and a low density of senescent cells may favor matrix remodeling. The increase of crown-like structures and the accumulation of senescent cells suggest that repair functions become overwhelmed. Whether pro-inflammatory activities carried by the immune cell infiltrate from circulation eventually contribute to tissue damage remains to be analyzed.
Finally, subtypes of visceral obesity remain to be defined, and not all of them may be represented by animal models. Subtypes may differ with respect to metabolic characteristics, age, sex or genetic background. The protective versus detrimental functions of inflammation may differ between subtypes.
Acknowledgements
I thank Stephan Martin, University of Düsseldorf, Germany, and Fraser W. Scott, the Ottawa Hospital Research Institute and the University of Ottawa, Canada, for reviewing the manuscript, and Kerstin Kempf, Düsseldorf Catholic Hospital Group, Germany, for helping with preparing the manuscript.
Abbreviations
- CCL
C-C motif chemokine ligand
- DAMP
Damage-associated molecular pattern
- ERK
Extracellular signal-regulated kinase
- FABP4
Fatty acid binding protein
- FFA
Free fatty acids
- FNDC4
Fibronectin type III domain-containing protein 4
- Glut
Glucose transporter
- HMGB1
High mobility group box 1 protein
- HOMA-IR
Homeostasis model assessment of insulin resistance
- IFN
Interferon
- IL
Interleukin
- ILC
Innate lymphoid cell
- iNKT
Innate NK T cell
- IRE-1
Inositol-requiring protein 1
- MAIT
Mucosa-associated invariant T cell
- MCP-1
Macrophage chemotactic protein 1
- MHC
Major histocompatibility complex
- NF-kB
Nuclear factor “kappa-light-chain-enhancer” of activated B cells
- NK
Natural killer
- NLRP3
NOD-, LRR-, and pyrin domain-containing protein 3
- PAI
Plasminogen activator inhibitor
- PPARγ
Peroxisome proliferator-activated receptor gamma
- SAM
Sympathetic neuron-associated macrophage
- SASP
Senescence-associated secretory phenotype
- Th
Helper T cell
- TGF
Tumor growth factor
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- Treg
Regulatory T cell
- UCP
Uncoupling protein
Author’s contributions
HK conceived and wrote all the material in this review. All authors read and approved the final manuscript.
Funding
The work was supported by Gesellschaft von Freunden und Förderern der Heinrich-Heine-Universität Düsseldorf e.V.
Availability of data and materials
Data for this review were identified by searches of MEDLINE, PubMed, and references from relevant articles using the search terms “visceral fat tissue,” “fat inflammation,” “obesity inflammation,” “resident immune cells,” and “adipose tissue macrophages.” In order to limit the number of references, more recently published papers referring to several previously published articles were cited, if possible. Only articles published in English were selected.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- 3.Psaila AM, Vohralik EJ, Quinlan KGR. Shades of white: new insights into tissue-resident leukocyte heterogeneity. FEBS J. 2022;289:308. doi: 10.1111/febs.15737. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock A, Brown EJ, Garabedian ML, Pena S, Sharma M, Lafaille J, et al. Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells. Immunometabolism. 2019;1:e190008. [DOI] [PMC free article] [PubMed]
- 5.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Grijalva A, Skowronski A, van EM SMJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178:686. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Li D, Cao G, Shi Q, Zhu J, Zhang M, et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature. 2021;600:314. doi: 10.1038/s41586-021-04127-5. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MS, Jun H. The Adipose Tissue Macrophages Central to Adaptive Thermoregulation. Front Immunol. 2022;13:884126. doi: 10.3389/fimmu.2022.884126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backdahl J, Franzen L, Massier L, Li Q, Jalkanen J, Gao H, et al. Spatial mapping reveals human adipocyte subpopulations with distinct sensitivities to insulin. Cell Metab. 2021;33:1869. doi: 10.1016/j.cmet.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Painter JD, Akbari O. Type 2 Innate Lymphoid Cells: Protectors in Type 2 Diabetes. Front Immunol. 2021;12:727008. doi: 10.3389/fimmu.2021.727008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macdougall CE, Wood EG, Loschko J, Scagliotti V, Cassidy FC, Robinson ME, et al. Visceral Adipose Tissue Immune Homeostasis Is Regulated by the Crosstalk between Adipocytes and Dendritic Cell Subsets. Cell Metab. 2018;27:588. doi: 10.1016/j.cmet.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao Q, Gu J, Zhou J, Wang Q, Li X, Deng Z, et al. Tissue Tregs and Maintenance of Tissue Homeostasis. Front Cell Dev Biol. 2021;9:717903. doi: 10.3389/fcell.2021.717903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolodin D, van PN LC, Magnuson AM, Cipolletta D, Miller CM, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmon DB, Srikakulapu P, Kaplan JL, Oldham SN, McSkimming C, Garmey JC, et al. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin Resistance. Arterioscler Thromb Vasc Biol. 2016;36:682. doi: 10.1161/ATVBAHA.116.307166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 20.Benezech C, Luu NT, Walker JA, Kruglov AA, Loo Y, Nakamura K, et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol. 2015;16:819. doi: 10.1038/ni.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trim WV, Lynch L. Immune and non-immune functions of adipose tissue leukocytes. Nat Rev Immunol. 2022;22:371. doi: 10.1038/s41577-021-00635-7. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, et al. gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 2020;578:610. doi: 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23:1309. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 27.Schmidleithner L, Thabet Y, Schonfeld E, Kohne M, Sommer D, Abdullah Z, et al. Enzymatic Activity of HPGD in Treg Cells Suppresses Tconv Cells to Maintain Adipose Tissue Homeostasis and Prevent Metabolic Dysfunction. Immunity. 2019;50:1232. doi: 10.1016/j.immuni.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Kane H, Lynch L. Innate Immune Control of Adipose Tissue Homeostasis. Trends Immunol. 2019;40:857. doi: 10.1016/j.it.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed DS, Isnard S, Lin J, Routy B, Routy JP. GDF15/GFRAL Pathway as a Metabolic Signature for Cachexia in Patients with Cancer. J Cancer. 2021;12:1125. doi: 10.7150/jca.50376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitoh S, Van WK, Nakajima O. Crosstalk between Metabolic Disorders and Immune Cells. Int J Mol Sci. 2021;22:10017. [DOI] [PMC free article] [PubMed]
- 31.Toubal A, Lehuen A. Role of MAIT cells in metabolic diseases. Mol Immunol. 2021;130:142. doi: 10.1016/j.molimm.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Munoz-Rojas AR, Mathis D. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol. 2021;21:597. doi: 10.1038/s41577-021-00519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens HY, Bowles AC, Yeago C, Roy K. Molecular Crosstalk Between Macrophages and Mesenchymal Stromal Cells. Front Cell Dev Biol. 2020;8:600160. doi: 10.3389/fcell.2020.600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21:704. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 35.van der Poll T, Jansen J, Levi M, ten Cate H, ten Cate JW, van Deventer SJ. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med. 1994;180:1985. doi: 10.1084/jem.180.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Hao J, Zeng J. Sauter ER. SnapShot: FABP Functions. Cell. 2020;182:1066. doi: 10.1016/j.cell.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 38.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16:232. doi: 10.1186/s12916-018-1225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenkula KG, Erlanson-Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. 2018;315:R284–R295. doi: 10.1152/ajpregu.00257.2017. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075. doi: 10.1007/s00125-016-3933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marques BG, Hausman DB, Martin RJ. Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am J Physiol. 1998;275:R1898–R1908. doi: 10.1152/ajpregu.1998.275.6.R1898. [DOI] [PubMed] [Google Scholar]
- 43.Haczeyni F, Bell-Anderson KS, Farrell GC. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev. 2018;19:406. doi: 10.1111/obr.12646. [DOI] [PubMed] [Google Scholar]
- 44.Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, et al. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 2015;29:1106. doi: 10.1101/gad.260554.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wernstedt AI, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127:74. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Q, An YA, Kim M, Zhang Z, Zhao S, Zhu Y, et al. Suppressing adipocyte inflammation promotes insulin resistance in mice. Mol Metab. 2020;39:101010. doi: 10.1016/j.molmet.2020.101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinaik R, Barayan D, Jeschke MG. NLRP3 Inflammasome in Inflammation and Metabolism: Identifying Novel Roles in Postburn Adipose Dysfunction. Endocrinology. 2020;161:bqaa116. [DOI] [PMC free article] [PubMed]
- 49.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019;129:4022. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulet N, Esteve D, Bouloumie A, Galitzky J. Cellular heterogeneity in superficial and deep subcutaneous adipose tissues in overweight patients. J Physiol Biochem. 2013;69:575. doi: 10.1007/s13105-012-0225-4. [DOI] [PubMed] [Google Scholar]
- 52.Kosaka K, Kubota Y, Adachi N, Akita S, Sasahara Y, Kira T, et al. Human adipocytes from the subcutaneous superficial layer have greater adipogenic potential and lower PPAR-gamma DNA methylation levels than deep layer adipocytes. Am J Physiol Cell Physiol. 2016;311:C322–C329. doi: 10.1152/ajpcell.00301.2015. [DOI] [PubMed] [Google Scholar]
- 53.Lemmer IL, Willemsen N, Hilal N, Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 55.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 56.Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prentice KJ, Saksi J, Hotamisligil GS. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J Lipid Res. 2019;60:734. doi: 10.1194/jlr.S091793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li HL, Wu X, Xu A, Hoo RL. A-FABP in Metabolic Diseases and the Therapeutic Implications: An Update. Int J Mol Sci. 2021;22:9386. [DOI] [PMC free article] [PubMed]
- 59.Zhou H, Urso CJ, Jadeja V. Saturated Fatty Acids in Obesity-Associated Inflammation. J Inflamm Res. 2020;13:1. doi: 10.2147/JIR.S229691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, et al. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018;27:1096. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Moon JS, da Cunha FF, Huh JY, Andreyev AY, Lee J, Mahata SK, et al. ANT2 drives proinflammatory macrophage activation in obesity. JCI. Insight. 2021;6:e147033. [DOI] [PMC free article] [PubMed]
- 62.Kiernan K, MacIver NJ. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front Immunol. 2020;11:622468. doi: 10.3389/fimmu.2020.622468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 65.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 66.Skurk T, Mack I, Kempf K, Kolb H, Hauner H, Herder C. Expression and secretion of RANTES (CCL5) in human adipocytes in response to immunological stimuli and hypoxia. Horm Metab Res. 2009;41:183. doi: 10.1055/s-0028-1093345. [DOI] [PubMed] [Google Scholar]
- 67.Trayhurn P, Alomar SY. Oxygen deprivation and the cellular response to hypoxia in adipocytes - perspectives on white and brown adipose tissues in obesity. Front Endocrinol (Lausanne). 2015;6:19. doi: 10.3389/fendo.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mori MA, Ludwig RG, Garcia-Martin R, Brandao BB, Kahn CR. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019;30:656. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho CH, Koh YJ, Han J, Sung HK, Jong LH, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 70.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond). 2008;32:451. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 71.Song J, Deng T. The Adipocyte and Adaptive Immunity. Front Immunol. 2020;11:593058. doi: 10.3389/fimmu.2020.593058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fruhbeck G, Fernandez-Quintana B, Paniagua M, Hernandez-Pardos AW, Valenti V, Moncada R, et al. FNDC4, a novel adipokine that reduces lipogenesis and promotes fat browning in human visceral adipocytes. Metabolism. 2020;108:154261. doi: 10.1016/j.metabol.2020.154261. [DOI] [PubMed] [Google Scholar]
- 73.Bosma M, Gerling M, Pasto J, Georgiadi A, Graham E, Shilkova O, et al. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nat Commun. 2016;7:11314. doi: 10.1038/ncomms11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meizlish ML, Franklin RA, Zhou X, Medzhitov R. Tissue Homeostasis and Inflammation. Annu Rev Immunol. 2021;39:557. doi: 10.1146/annurev-immunol-061020-053734. [DOI] [PubMed] [Google Scholar]
- 75.Fuchs A, Samovski D, Smith GI, Cifarelli V, Farabi SS, Yoshino J, et al. Associations Among Adipose Tissue Immunology, Inflammation, Exosomes and Insulin Sensitivity in People With Obesity and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161:968. doi: 10.1053/j.gastro.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong S, Song W, Zushin PH, Liu B, Jedrychowski MP, Mina AI, et al. Phosphorylation of Beta-3 adrenergic receptor at serine 247 by ERK MAP kinase drives lipolysis in obese adipocytes. Mol Metab. 2018;12:25. doi: 10.1016/j.molmet.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foley KP, Chen Y, Barra NG, Heal M, Kwok K, Tamrakar AK, et al. Inflammation promotes adipocyte lipolysis via IRE1 kinase. J Biol Chem. 2021;296:100440. doi: 10.1016/j.jbc.2021.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu L, Liu W, Bai F, Xu Y, Liang X, Ma C, et al. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front Immunol. 2021;12:708978. doi: 10.3389/fimmu.2021.708978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Z, Xu A. Adipose Extracellular Vesicles in Intercellular and Inter-Organ Crosstalk in Metabolic Health and Diseases. Front Immunol. 2021;12:608680. doi: 10.3389/fimmu.2021.608680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai Z, Huang Y, He B. New Insights into Adipose Tissue Macrophages in Obesity and Insulin Resistance. Cells. 2022;11:1424. [DOI] [PMC free article] [PubMed]
- 81.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 82.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 83.Guzman-Ruiz R, Tercero-Alcazar C, Lopez-Alcala J, Sanchez-Ceinos J, Malagon MM, Gordon A. The potential role of the adipokine HMGB1 in obesity and insulin resistance. Novel effects on adipose tissue biology. Mol Cell Endocrinol. 2021;536:111417. doi: 10.1016/j.mce.2021.111417. [DOI] [PubMed] [Google Scholar]
- 84.Haase J, Weyer U, Immig K, Kloting N, Bluher M, Eilers J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 85.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dommel S, Bluher M. Does C-C Motif Chemokine Ligand 2 (CCL2) Link Obesity to a Pro-Inflammatory State? Int J Mol Sci. 2021;22:1500. [DOI] [PMC free article] [PubMed]
- 89.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18:e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Wang B, Gasek NS, Zhou Y, Cohn RL, Martin DE, et al. Targeting p21(Cip1) highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 2022;34:75. doi: 10.1016/j.cmet.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matacchione G, Perugini J, Di ME, Sabbatinelli J, Prattichizzo F, Senzacqua M, et al. Senescent macrophages in the human adipose tissue as a source of inflammaging. Geroscience. 2022;44:1941-60. [DOI] [PMC free article] [PubMed]
- 92.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clement K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest. 2019;129:4032. doi: 10.1172/JCI129192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qi Y, Hui X. The shades of grey in adipose tissue reprogramming. Biosci Rep. 2022;42:BSR20212358. [DOI] [PMC free article] [PubMed]
- 95.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 98.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hildreth AD, Ma F, Wong YY, Sun R, Pellegrini M, O'Sullivan TE. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol. 2021;22:639. doi: 10.1038/s41590-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Snodgrass RG, Boss M, Zezina E, Weigert A, Dehne N, Fleming I, et al. Hypoxia Potentiates Palmitate-induced Pro-inflammatory Activation of Primary Human Macrophages. J Biol Chem. 2016;291:413. doi: 10.1074/jbc.M115.686709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee YS, Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35:307. doi: 10.1101/gad.346312.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T, et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016;23:685. doi: 10.1016/j.cmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li C, Menoret A, Farragher C, Ouyang Z, Bonin C, Holvoet P, et al. Single cell transcriptomics based-MacSpectrum reveals novel macrophage activation signatures in diseases. JCI. Insight. 2019;5:e126453. [DOI] [PMC free article] [PubMed]
- 106.Caslin HL, Bhanot M, Bolus WR, Hasty AH. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol Rev. 2020;295:101. doi: 10.1111/imr.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 108.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 110.O'Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, et al. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism. 2012;61:1152. doi: 10.1016/j.metabol.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferno J, Strand K, Mellgren G, Stiglund N, Bjorkstrom NK. Natural Killer Cells as Sensors of Adipose Tissue Stress. Trends Endocrinol Metab. 2020;31:3. doi: 10.1016/j.tem.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 112.Toubal A, Kiaf B, Beaudoin L, Cagninacci L, Rhimi M, Fruchet B, et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun. 2020;11:3755. doi: 10.1038/s41467-020-17307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Freitas DF, Colon DF, Silva RL, Santos EM, Guimaraes VHD, Ribeiro GHM, et al. Neutrophil extracellular traps (NETs) modulate inflammatory profile in obese humans and mice: adipose tissue role on NETs levels. Mol Biol Rep. 2022;49:3225. doi: 10.1007/s11033-022-07157-y. [DOI] [PubMed] [Google Scholar]
- 115.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97:E1677–E1685. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- 116.Goldstein N, Kezerle Y, Gepner Y, Haim Y, Pecht T, Gazit R, et al. Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes. Cells. 2020;9:1508. [DOI] [PMC free article] [PubMed]
- 117.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Datta R, Podolsky MJ, Atabai K. Fat fibrosis: friend or foe? JCI Insight. 2018;3:e122289. [DOI] [PMC free article] [PubMed]
- 119.Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner KD, et al. Defined p16(High) Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 2020;32:87. doi: 10.1016/j.cmet.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28:1556. doi: 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fang J, Li L, Cao X, Yue H, Fu W, Chen Y, et al. Transmissible Endoplasmic Reticulum Stress Mediated by Extracellular Vesicles from Adipocyte Promoting the Senescence of Adipose-Derived Mesenchymal Stem Cells in Hypertrophic Obesity. Oxid Med Cell Longev. 2022;2022:7175027. doi: 10.1155/2022/7175027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh C, Koh D, Jeon HB, Kim KM. The Role of Extracellular Vesicles in Senescence. Mol Cells. 2022;45:603. doi: 10.14348/molcells.2022.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Q, Hagberg CE, Silva CH, Lang S, Hyvonen MT, Salehzadeh F, et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat Med. 2021;27:1941. doi: 10.1038/s41591-021-01501-8. [DOI] [PubMed] [Google Scholar]
- 125.Sierra-Ramirez A, Lopez-Aceituno JL, Costa-Machado LF, Plaza A, Barradas M, Fernandez-Marcos PJ. Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging (Albany NY). 2020;12:11337. doi: 10.18632/aging.103607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 127.Nogueiras R, Sabio G. Brain JNK and metabolic disease. Diabetologia. 2021;64:265. doi: 10.1007/s00125-020-05327-w. [DOI] [PubMed] [Google Scholar]
- 128.Regnier M, Van HM, Knauf C, Cani PD. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J Endocrinol. 2021;248:R67–R82. doi: 10.1530/JOE-20-0473. [DOI] [PubMed] [Google Scholar]
- 129.Gallego-Duran R, Montero-Vallejo R, Maya-Miles D, Lucena A, Martin F, Ampuero J, et al. Analysis of Common Pathways and Markers From Non-Alcoholic Fatty Liver Disease to Immune-Mediated Diseases. Front Immunol. 2021;12:667354. doi: 10.3389/fimmu.2021.667354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol. 2022;18:461-72. [DOI] [PubMed]
- 131.Mashek DG. Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD. Mol Metab. 2021;50:101115. doi: 10.1016/j.molmet.2020.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flessa CM, Kyrou I, Nasiri-Ansari N, Kaltsas G, Kassi E, Randeva HS. Endoplasmic reticulum stress in nonalcoholic (metabolic associated) fatty liver disease (NAFLD/MAFLD). J Cell Biochem. 2022;123:1585-606. [DOI] [PubMed]
- 133.Horn CL, Morales AL, Savard C, Farrell GC, Ioannou GN. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatol Commun. 2022;6:12. doi: 10.1002/hep4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22:429-43. [DOI] [PMC free article] [PubMed]
- 135.Ramadori P, Kam S, Heikenwalder M. T cells: Friends and foes in NASH pathogenesis and hepatocarcinogenesis. Hepatology. 2022;75:1038. doi: 10.1002/hep.32336. [DOI] [PubMed] [Google Scholar]
- 136.Shaker ME. The contribution of sterile inflammation to the fatty liver disease and the potential therapies. Biomed Pharmacother. 2022;148:112789. doi: 10.1016/j.biopha.2022.112789. [DOI] [PubMed] [Google Scholar]
- 137.Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48:1038. doi: 10.1007/s00125-005-1764-9. [DOI] [PubMed] [Google Scholar]
- 138.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest. 2017;127:83. doi: 10.1172/JCI88884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.da Cruz Nascimento SS, Carvalho de Queiroz JL, Fernandes de MA, de Franca Nunes AC, Piuvezam G, Lima Maciel BL, et al. Anti-inflammatory agents as modulators of the inflammation in adipose tissue: A systematic review. Plos one. 2022;17:e0273942. doi: 10.1371/journal.pone.0273942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this review were identified by searches of MEDLINE, PubMed, and references from relevant articles using the search terms “visceral fat tissue,” “fat inflammation,” “obesity inflammation,” “resident immune cells,” and “adipose tissue macrophages.” In order to limit the number of references, more recently published papers referring to several previously published articles were cited, if possible. Only articles published in English were selected.