Abstract
Background
A considerable proportion of people experience lingering symptoms after Coronavirus Disease 2019 (COVID-19). The aim of this study was to investigate the frequency, pattern and functional implications of cognitive impairments in patients at a long-COVID clinic who were referred after hospitalisation with COVID-19 or by their general practitioner.
Methods
Patients underwent cognitive screening and completed questionnaires regarding subjective cognition, work function and quality of life. Patients' cognitive performance was compared with that of 150 age-, sex-, and education-matched healthy controls (HC) and with their individually expected performance calculated based on their age, sex and education.
Results
In total, 194 patients were assessed, on average 7 months (standard deviation: 4) after acute COVID-19.44–53 % of the patients displayed clinically relevant cognitive impairments compared to HC and to their expected performance, respectively. Moderate to large impairments were seen in global cognition and in working memory and executive function, while mild to moderate impairments occurred in verbal fluency, verbal learning and memory. Hospitalised (n = 91) and non-hospitalised (n = 103) patients showed similar degree of cognitive impairments in analyses adjusted for age and time since illness. Patients in the cognitively impaired group were older, more often hospitalised, had a higher BMI and more frequent asthma, and were more often female. More objective cognitive impairment was associated with more subjective cognitive difficulties, poorer work function and lower quality of life.
Limitations
The study was cross-sectional, which precludes causality inferences.
Conclusions
These findings underscore the need to assess and treat cognitive impairments in patients at long-COVID clinics.
Keywords: COVID-19, Cognitive impairment, Quality of life, Work function
1. Introduction
Recent evidence indicates that a substantial proportion of people with Coronavirus Disease 2019 (COVID-19) experience lingering physical and/or cognitive symptoms. Long COVID or post-COVID-19-syndrome is, according to the National Institute of Health and Care Excellence (NICE), defined as; symptoms persisting longer than 12 weeks after the acute onset of COVID-19 (https://www.nice.org.uk/guidance/ng188). Most common long COVID symptoms are fatigue, dyspnea and cognitive impairment, such as difficulties with memory and concentration (Bliddal et al., 2021; Ceban et al., 2022). In particular, the prevalence of cognitive sequelae, also known as cognitive COVID, ranges from 12 % to 80 % across studies (Lopez-Leon et al., 2021; Office for National Statistics, 2021). In a small longitudinal study of patients hospitalised with COVID-19, we found that greater cognitive impairment was associated with greater depression and anxiety symptoms after 3 months (Miskowiak et al., 2021) and 1 year (Miskowiak et al., 2022). In fact, the level of cognitive impairment after 3 months was related to the severity of mood symptoms after 1 year (Miskowiak et al., 2022). This may indicate a role of cognitive impairment in the common neuropsychiatric sequelae of COVID-19 (Taquet et al., 2021). Cognitive impairment is not uncommon following respiratory illnesses in general. However, the incidence of cognitive- and mental health consequences of COVID-19 appears to be higher even after 2 years (Taquet et al., 2022; Hewitt et al., 2022). Specifically, two studies found higher risk of developing dementia after pneumonia caused by the SARS-CoV-2 virus compared with the risk of dementia following pneumonias caused by other viruses (Taquet et al., 2021; Qureshi et al., 2022). In keeping with this, poorer cognitive outcomes after 6 months were found in COVID-19 patients admitted to intensive care units (ICU) compared to patients admitted to the ICU for other reasons (Nersesjan et al., 2022).
A large longitudinal neuroimaging study of 782 participants including 394 participants with mild to moderate COVID-19 showed a significant illness-related reduction of grey matter thickness and volume in the left parahippocampal gyrus, orbitofrontal cortex and insula extending to the anterior cingulate cortex and temporal pole – brain areas directly linked to the olfactory and gustatory systems but also higher functions (Douaud et al., 2022). These structural changes were hypothesized at least partially to result from direct virus invasion of the central nervous system via the olfactory bulb (Douaud et al., 2022). Other studies suggest that exaggerated and long-lasting inflammatory responses also contribute to impairments in working memory and executive function (Ceban et al., 2022; Alnefeesi et al., 2020). In contrast, cerebral oxygen starvation may play a more prominent role in the COVID-associated difficulties in hippocampus-based verbal learning and memory because hippocampal neurons are particularly susceptible to oxygen deprivation (Couzin-Frankel, 2020). Finally, the observation of white matter deficits indicates that microhaemorrhages can also contribute to cognitive complications of COVID-19 (Lersy et al., 2021). Complex and varying interactions between these different pathophysiological mechanisms - rather than any single mechanism alone - are likely to explain the cognitive sequelae of COVID-19 (Boldrini et al., 2021).
Studies of cognitive COVID have produced discrepant findings regarding the frequency and pattern of cognitive impairments. While a few studies found negligible cognitive decline in COVID-19 patients (Mattioli et al., 2021; Whiteside et al., 2022), other studies found high frequencies of impairment across executive function, attention and memory (Bungenberg et al., 2022; Costas-Carrera et al., 2022; Crivelli et al., 2022; Jaywant et al., 2021; Krishnan et al., 2022; Lopez-Leon et al., 2021; Mazza et al., 2021; Miskowiak et al., 2021; Miskowiak et al., 2022). The discrepancies are likely due to differences in the populations (hospitalised or non-hospitalised patients, differences in age, levels of education or comorbidities) and methodologies, including sampling strategies and cognition measurement tools with varying sensitivity to cognitive decline in younger populations (Nielsen et al., 2022). Large populations have been studied through electronic health records, which has provided insight into risks of developing neuropsychiatric disorders following COVID-19 (Taquet et al., 2022). However, a methodological limitation in most studies of cognitive COVID with cognitive testing is that they were generally conducted in small samples, with few exceptions (Hampshire et al., 2020). Nevertheless, meta-analysis of cognitive functions in >25,268 individuals from individual studies indicates that 22 % of individuals exhibit lingering memory- and attention difficulties after COVID-19 (Ceban et al., 2022). However, no study to date has investigated the prevalence and pattern of cognitive impairments in patients at long-COVID clinics.
The aim of this study was to investigate the pattern, frequency, and functional implications of cognitive impairments in a large sample of patients assessed at a long-COVID clinic at the Copenhagen University Hospital, Bispebjerg, either as a standard clinical follow-up appointment 3–4 months after hospitalisation at the hospital department or after referral by their general practitioner due to lingering physical symptoms. Specifically, we aimed to investigate the following questions: (I) what is the frequency of clinically relevant cognitive impairments in patients in a long-COVID clinic, (II) what is the pattern and severity of these cognitive impairments, (III) do cognitive impairments differ between hospitalised and non-hospitalised patients, (IV) are there differences in clinical and demographic variables between patients with and without cognitive impairments after COVID-19, and (V) do patients' objective cognitive functions scale with their subjective cognitive difficulties in daily life, quality of life and work function?
2. Materials and methods
2.1. Participants and recruitment
Patients at the long-COVID clinic at Bispebjerg Hospital took part in the study as part of their clinical assessments. Patients were referred to the clinic either (i) as part of their standard 3–4-month follow-up assessment after hospitalisation with COVID-19 at the Bispebjerg Hospital or (ii) due to referral from their general practitioner in the capital region of Denmark due to lingering physical symptoms after COVID-19, most commonly respiratory problems. All patients with sufficient Danish language fluency, as evaluated by the health care personnel, took part in the cognitive assessment. An additional inclusion criterion for the present study was absence of pre-existing neurological disorder.
Patients tested positive for SARS-CoV-2 on a PCR-test between March 2020 and April 2021. This period includes mainly first wave cases as well as expectedly the Alpha variant (B.1.1.7) since this variant was first identified in Denmark November 2020 and classified as the dominant variant in March 2021 (Statens Serum Institut, 2022). Patients underwent cognitive testing from June 2020 until December 2021. The study was approved by the regional ethics committee in the Capital Region of Denmark (protocol no. H-20035553) and all patients gave written informed consent.
The healthy control participants are from a pre-established normative data set (Ott et al., 2021a; Ott et al., 2021b). They were recruited through blood banks in the Capital Region of Denmark, while waiting to give blood. The rationale for recruiting the control group this way was to avoid a bias of high education or superior cognitive function to represent the background population.
2.2. Procedures
Cognitive screening was conducted as part of the comprehensive clinical assessments of patients at the long—COVID clinic at Copenhagen University Hospital, Bispebjerg. Objective cognitive functions were assessed with the brief (<20 min) Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D; Jensen et al., 2015; Ott et al., 2021a). The SCIP-D is a performance-based cognition test battery with 5 subtests, that measure: verbal learning and memory, working memory, verbal fluency, and processing speed. Danish demographically adjusted norms exist for the SCIP, which enables accurate estimation for the individual patient of their expected score, based on their age, sex and education level (Ott et al., 2021a). Further, the SCIP was selected because of its high sensitivity to cognitive deficits in general and specifically its documented sensitivity of cognitive sequalae of COVID-19 (Miskowiak et al., 2021). Furthermore, objective executive function was assessed with the Trail Making Test Part B in n = 189 of the patients (TMT-B; Army Battery, 1944), for which age- and education level adjusted norms also exist (Ott et al., 2021a). Subjective cognitive functions were assessed with the Cognitive Failures Questionnaire (CFQ; Broadbent et al., 1982). Pre-existing SCIP data was available for the n = 150 age-, sex- and education-matched healthy control (HC) participants from a previous norm study (Ott et al., 2021a).
Patients also completed a set of questionnaires including the Work Productivity and Activity Impairment Questionnaire (WPAI; Reilly et al., 1993), and EQ-5D-5L Quality of life questionnaire (EQ5D; Lloyd and Pickard, 2019) and underwent assessment of physical functions (the findings regarding physical functions will be reported separately for clarity reasons).
2.3. Statistical analyses
Statistical analyses were conducted using IBM SPSS statistics 25 for windows (IBM Corporation, Armonk, New York). Statistical significance was set to an alpha-level of p < 0.05 (two-tailed). To examine whether data were normally distributed, the Shapiro-Wilk's test was used.
Research question (I) regarding the frequency of clinically relevant global or selective cognitive impairment in the patients was investigated through two approaches, (A) by comparing the patients' actual cognitive performance with what would be their expected performance calculated with regression-based formulas based on their age, sex and level of education, and (B) by comparing patients' cognitive performance to a healthy control group (n = 150) matched on age, sex and education from a preestablished normative data set (Ott et al., 2021a). As for comparison (A), the regression-based formulas enable estimates of demographically corrected normative scores applicable at an individual level (Duff, 2012). Thus, the formulas estimate whether each patient's cognition score is similar to or deviates from what would be expected of an individual with homogeneous demographic characteristics. Distinctively, the reliable change indexes (RCI) are standardized scores for the deviation of the observed scores from the predicted scores calculates as; (observed score − predicted score) / SEE, where SEE is the standard error of the estimate for the regression equation (Attix et al., 2009). The cut-off score for clinically relevant global cognitive impairment was defined as performance ≥ 1 SD below the expected SCIP total score or ≥1 SD below the normative HC mean for the SCIP total, depending on approach of comparison (A) or (B), respectively. Selective cognitive impairments were defined as performance ≥ 1 SD below the expected score or below the normative HC mean on ≥2 of the six individual tests in comparison by approach (A) or (B), respectively. Regarding the measure of executive functioning (TMT-B), we could solely compare with expected scores based on age, sex and level of education, since this data was not collected within the healthy control group.
Research question (II) regarding the pattern and severity of cognitive impairments was investigated through the two complementary approaches by comparing (A) expected scores, based on demographically adjusted norms, with actual cognitive scores of the patients as well as with (B) comparisons with norms from the healthy control group. Comparisons for normally distributed data were performed with independent samples t-tests and paired samples t-test. For non-normally distributed data, comparisons were performed with Mann-Whitney U tests and paired samples Wilcoxon test.
Research question (III) regarding the impact of COVID-19 severity, hospitalised and non-hospitalised patients was compared on objective cognitive performance, subjective cognition, experienced work function and quality of life. These analyses would be adjusted for any demographic variables for which hospitalised and non-hospitalised differed.
Research question (IV) about possible differences in clinical and demographic variables between patients with and without cognitive impairments after COVID-19 was examined through comparison on these variables between patients with selective or global impairments (‘impaired group’) and without cognitive impairments (‘intact group’).
Research question (V) regarding associations between the patients' objective cognitive functions and subjective cognitive difficulties, quality of life and work function were analysed with Pearson's correlations or Spearman's rho for normally and non-normally distributed data, respectively. The RCI for the patients' SCIP total scores and TMT-B scores were used as measures of ‘global cognitive impairment’ and ‘executive dysfunction’ in these analyses, respectively. Executive function was investigated along with global cognitive function since executive dysfunction previously has been associated with poorer work function and quality of life (Miskowiak et al., 2021; Poletti et al., 2021).
3. Results
3.1. Participant characteristics
A total of 194 of 301 patients (64.5 %) in the long-COVID clinic were eligible (sufficient Danish fluency and absence of pre-existing neurological disease) and were included in the study (56 % females; mean age 51 ± 15). Of these, 91 patients were referred to the clinic as a standard follow-up assessment after hospitalisation with COVID-19 and 103 patients were referred by their general practitioner due to lingering physical symptoms after COVID-19. The time between illness and neuropsychological assessment was an average of 7 (±4) months. Table 1 presents demographic characteristics of the patients as well as of an age-, sex- and education-matched sample of n = 150 healthy control participants, recruited through blood banks in the Capital Region of Denmark, from a pre-established normative data set (Ott et al., 2021a; Ott et al., 2021b).
Table 1.
Demographics, clinical characteristics, quality of life, work function, objective and subjective cognition data from patients and a matched control group as well as the expected cognitive scores based on patients' age, sex and education.
| Patients (n = 194) | Expected scores based on age, sex, and education | Healthy controls (n = 150) | p-Value all patients actual vs. expected | p-Value all patient vs. healthy controls | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 50.8 (15.4) | 50.9 (9.0) | 0.69 | ||
| Sex, no. females (%) | 108 (56) | 84 (56) | 0.95 | ||
| Years of education, mean (SD) | 15.3 (3.9) | 14.9 (3.0) | 0.21 | ||
| BMI (kg/m2) | 27.2 (5.5) | ||||
| Clinical characteristics | 2.1 (2.2) | ||||
| Charlson comorbidity score | 34 (19) | ||||
| Asthma, no (%) | 217 (113) | ||||
| Days since illness, mean (SD) | |||||
| Cognition | 71.0 (13.4) | 77.2 (4.5) | 76.7 (9.3) | <0.001 | <0.001 |
| SCIP Total Score, mean (SD) | |||||
| VLT-L, mean (SD) | 21.3 (3.8) | 22.7 (1.2) | 22.9 (3.1) | <0.001 | 0.001 |
| WMT, mean (SD) | 18.3 (3.8) | 20.0 (0.8) | 20.1 (2.6) | <0.001 | <0.001 |
| VFT, mean (SD) | 14.8 (5.5) | 16.4 (1.4) | 15.9 (4.5) | <0.001 | 0.04 |
| VLT-D, mean (SD) | 6.5 (2.3) | 7.4 (0.7) | 7.3 (1.9) | <0.001 | 0.005 |
| PMT, mean (SD) | 10.0 (2.7) | 10.7 (1.2) | 10.6 (2.3) | <0.001 | 0.101 |
| TMT-B, mean (SD) | 100.6 (50.0) | 73.3 (17.7) | – | <0.001 | – |
| CFQ Total | 63.5 (17.0) | – | – | – | – |
| Proportion with severe complaints, scores ≥ 43, number (%) | 164 (90) | ||||
| Patients (n = 194) | |
|---|---|
| EQ-5D-5L Quality of life questionnaire | |
| Movement | 1.5 (0.9) |
| Personal care | 1.3 (0.6) |
| Usual activity | 2.4 (1.2) |
| Pain | 2.3 (1.0) |
| Anxiety/depression | 1.8 (0.9) |
| Work productivity and activity impairment | |
| Percent work time missed due to health (absenteeism) | 20.1 [0.0, 100.0] |
| Percent impairment while working due to health (presenteeism) | 30.8 [0.0, 100.0] |
| Percent overall work impairment due to health | 8.1 [0.0, 54.6] |
| Percent activity impairment due to health | 41.0 [0.0, 100.0] |
Data is presented as mean (SD) or number (percentage). WPAI, Work Productivity and Activity Impairment data is reported as median [minimum, maximum]. Abbreviations: no, number, BMI, body mass index, SD, standard deviation. SCIP, Screen for Cognitive Impairment in Psychiatry; SD, standard deviation VLT-L, verbal learning test - learning; WMT, working memory test; VFT, verbal fluency test; VLT-D, verbal learning test – delayed recall; PMT, psychomotor speed test; TMT-B, Trail Making Test B; CFQ, Cognitive Failures Questionnaire. Data for BMI was available for 192 of the 194 patients. Data for asthma was only available for 175 of the 194 patients. Charlson Comorbidity Score was only available for 160 of the 194 patients. CFQ data was only available for 182 of the 194 patients. Data for subtest PMT and consequently SCIP total was available for 193 out of the 194 patients and 149 out of the 150 healthy controls. TMT-B data was only available for 187 of the 194 patients. Data for EQ-5D-5L and WPAI was only available for 170–173 of the 194 patients.
Table 2 displays demographic and clinical characteristics of the patients divided in groups of hospitalised (47 %) and non-hospitalised (53 %) patients. Compared with non-hospitalised patients, hospitalised patients were older (age, mean ± SD: hospitalised: 57 ± 14, non-hospitalised: 45 ± 14), had higher BMI (mean ± SD, hospitalised: 30 ± 6, non-hospitalised: 25 ± 4), had higher Charlson comorbidity score (mean ± SD, hospitalised: 2.8 ± 2.4, non-hospitalised: 1.4 ± 1.6) and higher frequency of asthma (mean ± SD, hospitalised: 21 %, non-hospitalised: 9 %). In addition, the time between illness and cognitive assessment was longer in non-hospitalised patients compared to hospitalised patients (months, mean ± SD: hospitalised: 5 ± 3, non-hospitalised: 9 ± 6), who were automatically followed up 3–4 months after their hospital discharge.
Table 2.
Demographics and cognition data for hospitalised and non-hospitalised patients.
| Patients | Hospitalised (n = 91) | Non-hospitalised (n = 103) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 57.2 (14.0) | 45.2 (14.4) | <0.001 |
| Sex, no. females (%) | 49 (54) | 59 (57) | 0.63 |
| Years of education, mean (SD) | 15.1 (4.0) | 15.6 (3.9) | 0.32 |
| BMI (kg/m2), mean (SD) | 29.5 (6.0) | 25.1 (4.0) | <0.001 |
| Clinical characteristics | |||
| Charlson comorbidity score, mean (SD) | 2.8 (2.3) | 1.5 (1.8) | <0.001 |
| Asthma, no (%) | 22 (26) | 12 (13) | 0.04 |
| Days since illness, mean (SD) | 162 (78) | 266 (117) | <0.001 |
| Cognition | |||
| SCIP Total Score, mean (SD) | 66.2 (14.4) | 75.2 (11.0) | 0.06 |
| VLT-L, mean (SD) | 20.1 (4.1) | 22.4 (3.2) | 0.12 |
| WMT, mean (SD) | 17.6 (4.3) | 18.9 (3.2) | 0.55 |
| VFT, mean (SD) | 13.5 (5.2) | 15.9 (5.5) | 0.07 |
| VLT-D, mean (SD) | 5.9 (2.5) | 7.1 (1.9) | 0.07 |
| PMT, mean (SD) | 9.1 (2.9) | 10.9 (2.3) | 0.46 |
| TMT-B, mean (SD) | 114.9 (52.3) | 88.5 (44.7) | 0.79 |
Data is presented as mean (SD) or number (percentage). Data for BMI was available for 192 of the 194 patients. Data for asthma was only available for 175 of the 194 patients. Charlson Comorbidity Score was only available for 160 of the 194 patients.
3.2. Question (I): what is the frequency of clinically relevant cognitive impairments in these patients?
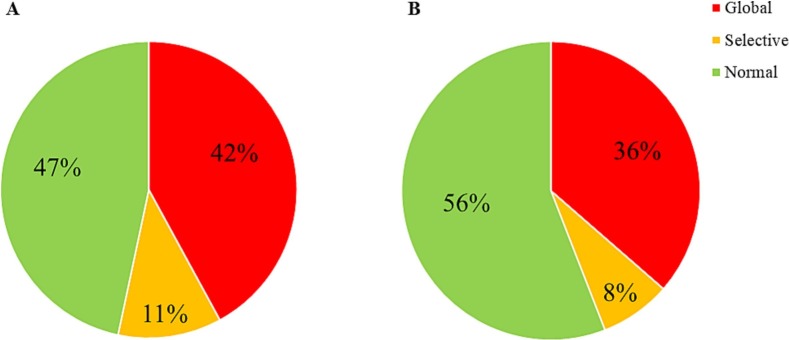
Fig. 1 illustrates the frequency of clinically relevant global and selective cognitive impairment in the patients investigated through approach (A) and (B). In comparison with estimated expected performance (A), 81 patients (42 %) met the criteria for global cognitive impairment, while 22 patients (11 %) showed selective cognitive impairment and 90 (47 %) were cognitively normal. The comparison with the matched healthy control group (B), yielded 70 patients (36 %) as globally impaired, 15 (8 %) as selectively impaired and 108 (56 %) as cognitively normal. Hence, the two different methodologies for estimating the frequency of clinically relevant cognitive impairment, yielded 44–53 % of the patients with cognitive impairment.
Fig. 1.
Proportion of patients with clinically relevant global or selective cognitive impairments in comparison with (A) normative scores adjusted for age, sex and education estimated with regression models and with (B) an age-, sex- and education-matched healthy control group (n = 150). Cut-off for global impairment defined as ≥1 SD below estimated expected score in (A) and ≥1 SD below the normative mean from the healthy control group in (B). Cut-off for selective impairment defined as ≥1 SD below estimated expected score or ≥1 SD below the HC mean on ≥2 of the six cognitive tests in comparison with group A or B, respectively. One of the 194 patients excluded from this analysis due to one incomplete subtest and consequently no SCIP total score; the analysis was thus conducted based on n = 193 patients.
3.3. Question (II): what is the pattern and severity of the cognitive impairments?
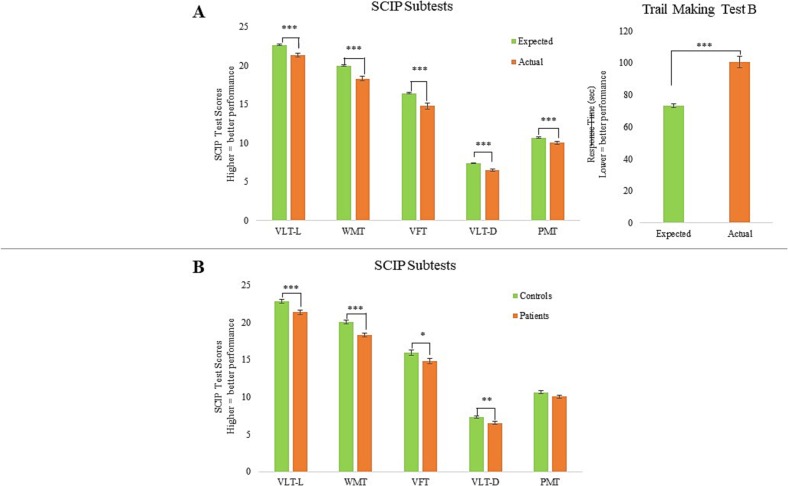
Fig. 2 illustrates the pattern and severity of cognitive impairments investigated through approach (A) and (B). Approach (A), comparing patients' actual- and expected performance based on their demographics, showed that on average, patients displayed global cognitive impairment on the SCIP with a large effect size (SCIP Total: p < 0.001, Cohen's d = 0.62). Further impairment with large effect size was observed in working memory and executive function (WMT: p < 0.001, Cohen's d = 0.62; TMT-B: p < 0.001, Cohen's d = 0.73). Impairments with moderate effect sizes were observed within the following cognitive domains; verbal learning, verbal fluency, delayed verbal memory and psychomotor speed (VLT-L: p < 0.001, Cohen's d = 0.50; VFT: t = 4.49, df = 195, p < 0.001, Cohen's d = 0.40; VLT-D: p < 0.001, Cohen's d = 0.43; PMT: p < 0.001, Cohen's d = 0.34).
Fig. 2.
Pattern of cognitive impairments in patients (n = 194) on average 7 months (SD = 4) months after COVID-19 in comparison (A) with normative scores adjusted for age, sex and education estimated with regression models and (B) with an age-, sex- and education-matched healthy control group (n = 150). Most pronounced impairments were seen in working memory (WMT) and executive function (TMT-B). Graphs represent the mean and error bars the standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001.
A similar pattern of impairments was found using approach (B), comparing the patients with the demographically matched healthy control group. Specifically, patients displayed global cognitive impairment on the SCIP with moderate effect size (p < 0.001, Cohen's d = 0.49) and impairment with moderate effect size on working memory (WMT: p < 0.001, Cohen's d = 0.55). Impairments with small to moderate effect sizes were found within verbal learning, verbal fluency and delayed verbal memory (VLT-L: p = 0.001, Cohen's d = 0.46; VFT: t = 2.07, df = 341, p = 0.04, Cohen's d = 0.22; VLT-D: p = 0.005, Cohen's d = 0.38). However, patients' performance in psychomotor speed appeared to be unimpaired compared to the healthy control group (PMT: p = 0.1).
Taken together, there appears to be a pattern of most prominent impairment in the higher cognitive domains, working memory and executive function, minimal to no impairment in psychomotor speed and intermediate non-specific impairments in the remaining cognitive domains.
3.4. Question (III): do cognitive impairments differ between hospitalised and non-hospitalised patients?
To investigate whether severity of illness had impact on the extent of cognitive impairment, hospitalised and non-hospitalised patients were compared. Groups differed in two important variables that likely influence cognitive status: age (years, mean ± SD: hospitalised 57 ± 14, non-hospitalised: 45 ± 14) and time since illness (days, mean ± SD: hospitalised: 162 ± 78, non-hospitalised: 266 ± 117). Analyses were therefore adjusted for age and time since illness, which revealed no differences in cognitive performance between hospitalised patients and non-hospitalised patients. Notably, hospitalised and non-hospitalised patients also showed no significant differences in subjective cognitive difficulties (CFQ), work function (WPAI) or quality of life (EQ-5D-5L) (p > 0.08).
3.5. Question (IV): are there differences in clinical and demographic variables between patients with and without cognitive impairments?
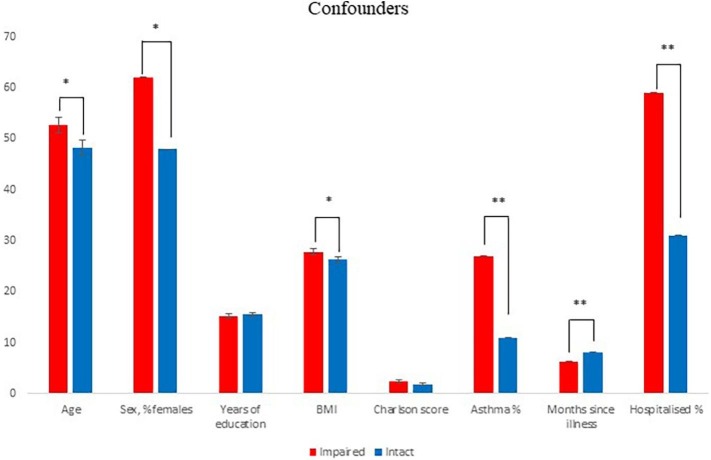
Fig. 3 displays demographic and clinical characteristics in the patients divided in groups of cognitively impaired (53 %) and intact (47 %) patients, presenting possible confounders and/or contributors to cognitive impairments after COVID-19. Intact or impaired cognition status was established in comparisons with the patients' individually expected performance based on their age, sex and level of education. Compared with cognitively intact patients, cognitively impaired patients were older (age, mean ± SD: impaired: 53 ± 16, intact: 48 ± 14, p = 0.04), had higher BMI (mean ± SD, impaired: 28 ± 6, intact: 26 ± 5, p = 0.04), had a higher frequency of asthma (%, impaired: 27 %, intact: 11 %, p = 0.01) and of hospitalisation with COVID-19 (%, impaired: 57 %, intact: 34 %, p = 0.002), were more often female (%, impaired: 62 %, intact: 48 %, p = 0.05), and had a shorter time since illness (months, mean ± SD: impaired: 6 ± 3, intact: 8 ± 4, p = 0.002). In contrast, the groups showed no difference in years of education (p = 0.5) or physical comorbidities (p = 0.2).
Fig. 3.
Demographics and clinical characteristics of patients with cognitive impairment (n = 103) compared to patients with intact cognition (n = 90) after COVID-19. Intact/not-intact data available for 193 of the 194 patients. Data for BMI was only available for 191 of the 193 patients. Data for asthma was only available for 174 of the 193 patients. Charlson Comorbidity Score was only available for 159 of the 194 patients. Graphs represent the mean and error bars the standard error of the mean. *p < 0.05; **p < 0.01.
3.6. Question (IV): how does patients' objective cognitive impairment relate to their subjective cognitive difficulties, quality of life and work function?
More global objective cognitive impairment correlated weakly with greater subjective cognitive difficulties in daily life (Pearson's correlation; SCIP total deviation from expected scores; r = −0.23, p = 0.002). More executive dysfunction also correlated with more subjective cognitive difficulties (Spearman's rho; TMT-B deviation from expected scores; r = −0.21, p = 0.005). Longer time between illness and cognitive assessment correlated with both less global cognitive impairment (Spearman's rho; r = 0.26, p < 0.001) and less executive dysfunction (Spearman's rho; r = 0.18, p = 0.02) across all patients. For non-hospitalised patients, longer time since illness was also related to less global cognitive impairment (Spearman's rho; r = 0.23, p < 0.02). For hospitalised patients, the degree of cognitive impairment showed no correlation with length of hospitalisation (p > 0.3), possibly due to the little variation in the time since hospital discharge among patients (5 ± 3 months).
For quality of life measurements (EQ-5D-5L), more global cognitive impairment correlated with poorer mobility/movement (Spearman's rho; r = −0.17, p = 0.03) and more pain (Spearman's rho; r = −0.16, p = 0.04). Further, executive dysfunction correlated with poorer mobility/movement (Spearman's rho; r = −0.16, p = 0.04) and with more anxiety and depression (Spearman's rho; r = −0.18, p = 0.02).
Regarding work function (WPAI), more global cognitive impairment correlated with more impairment while working (Spearman's rho; presenteeism: r = −0.20, p = 0.04) and more activity impairment (Spearman's rho; r = −0.19, p = 0.02). More executive dysfunction correlated with more time away from work (absenteeism; Spearman's rho; r = −0.25, p = 0.01) and with more overall work impairment (Spearman's rho; r = −0.22, p = 0.03).
4. Discussion
In this large study of 194 patients at a long-COVID clinic in Copenhagen, we found clinically relevant cognitive impairment ranging from 44 % to 53 % of the patients 7 months (SD = 4) after COVID-19, depending on the approach for estimating clinically relevant cognitive impairment (comparison with a healthy control group or demographically adjusted norms, respectively). Impairments were observed with moderate to large effect sizes in a global measure of cognition and in working memory and executive function, while mild to moderate impairments were observed in verbal fluency, verbal learning and memory. Hospitalised (n = 91) and non-hospitalised (n = 103) patients showed similar degree of cognitive impairments in analyses adjusted for differences in age and time since illness. Comparisons between cognitively impaired (53 %) and non-impaired (47 %) patients showed that impaired patients were older, had more often been hospitalised, had a shorter time since acute COVID-19, had a higher BMI and more frequent asthma, and were more often female but showed no differences in education levels or other physical comorbidities. More objective cognitive impairment was weakly associated with greater subjective cognitive difficulties in daily life, poorer work function and lower quality of life.
The observed broad pattern of cognitive impairments in patients after COVID-19 is consistent with previous performance-based studies as well as electronic health records (Alemanno et al., 2021; Bungenberg et al., 2022; Krishnan et al., 2022; Costas-Carrera et al., 2022; Crivelli et al., 2022; Mazza et al., 2021). In contrast, one study of 120 patients with mild to moderate COVID-19 found no lingering cognitive impairment after 4 months (Mattioli et al., 2021). The absence of cognitive impairments might be due to the milder cases of COVID-19 and slightly younger patients (48 years, range: 26–65) (Mattioli et al., 2021). Our findings are also consistent with previous evidence for associations between objective cognitive impairments and subjective cognitive difficulties, poorer work function and quality of life (Mendez et al., 2021; Miskowiak et al., 2021; Poletti et al., 2021; Miskowiak et al., 2022). In contrast, two studies found no association between objective and subjective cognitive difficulties (Gouraud et al., 2021; Whiteside et al., 2022), possibly due to a more conservative cut off score for cognitive impairment (performance < 2 SD below normative standard scores) which allowed for less variance in the data (Whiteside et al., 2022), and different test batteries with no assessment of executive function (Gouraud et al., 2021).
Most pronounced impairment was found in working memory and executive function – a domain associated with more anxiety, depression and overall work impairment in our cohort. Indeed, working memory and executive functions are cognitive domains of key importance in real life and work functioning, where cognitive flexibility, ability to switch between tasks, planning and initiation are critical skills (Garcia-Molina et al., 2012). This may explain the finding that greater executive dysfunction predicted poorer quality of life and psychosocial functioning after COVID-19 (Poletti et al., 2021). Consistent with our finding that hospitalisation and asthma were associated with cognitive impairment, other studies have found poorer cognition in hospitalised patients compared to non-hospitalised patients, indicating severity of acute illness as impacting degree of cognitive impairment (Bungenberg et al., 2022; Mattioli et al., 2022). Our finding that there were more women in the cognitively impaired than in the intact group (62 % vs 48 %) is consistent with broader observations of most frequent long COVID symptoms in women (Sylvester et al., 2022), although the reasons for this difference are unclear.
A better understanding of the biological processes contributing to cognitive COVID is important to inform potential treatment strategies. Previous studies examining inflammatory parameters in COVID-19 patients have reported elevations in proinflammatory markers, such as cytokines and D-dimers, which have been associated with poorer cognitive function and depression (Ceban et al., 2022; Mazza et al., 2021). Inflammation has also been observed in patients with similar cognitive impairment following other respiratory diseases (Bailey et al., 2021). Specifically, marked increases in IL-6, TNFα, and IL-1β; cytokines with profound impact on working memory and attention, suggest a cytokine storm that implicates hyperinflammation as possible cause for damaging the frontal lobes, causing the observed executive dysfunction and impairment in working memory (Alnefeesi et al., 2020; Ceban et al., 2022). In this regard, COVID-19 patients with impaired working memory and executive function might benefit from anti-inflammatory treatment. The observed pattern of broad cognitive impairments in our cohort is in line with demonstrated decline in grey matter thickness and volume in frontal and left parahippocampal regions after COVID-19 (Douaud et al., 2022). These structural brain changes and cognitive impairments after COVID-19 likely result from a complex interaction between exaggerated inflammatory responses, microhaemorrhages, oxygen deprivation, and perhaps direct virus invasion of the brain (Boldrini et al., 2021; Couzin-Frankel, 2020; Douaud et al., 2022).
Our finding that around half of patients in a long-COVID clinic present with substantial cognitive impairments indicates a clear need to screen for and treat cognitive impairments in patients who have had COVID-19, regardless of whether they were hospitalised. It is unclear how long and to what extent these cognitive COVID symptoms persist. We found a negative association between the severity of cognitive impairment and time since illness, suggesting some improvement over time. However, a previous smaller study by our group of hospitalised patients revealed no cognitive improvement from 3 months to 1 year after hospital discharge (Miskowiak et al., 2022). Larger longitudinal studies with longer follow-up times are needed to elucidate the duration of cognitive COVID. Further, studies are needed to investigate whether newer SARS-CoV-2 variants associated with milder symptoms, such as Omicron, and being vaccinated may result in less frequent and milder cognitive sequalae of COVID (Hampshire et al., 2020; Ceban et al., 2022). Treatment options targeting cognitive COVID are only just starting to emerge. In particular, one study found that an 8-week personalized multidisciplinary rehabilitation program, involving cognitive rehabilitation with the Guttmann, NeuroPersonalTrainer (GNTP), along with respiratory and physical rehabilitation, ameliorated patients' deficits in executive functions (Albu et al., 2021). Another potential treatment is the multifunctional hormone erythropoietin (EPO), which could potentially reverse cognitive impairments after COVID-19 because it can improve respiration, aid neuroprotection, and increase neuroplasticity (Ehrenreich et al., 2020).
A strength of the study was the large cohort, that has provided new insights into prevalence and pattern of cognitive impairment in patients in long-COVID clinics. However, notably the study does not assess the prevalence of cognitive COVID in the general population, but specifically in a long-COVID clinic among patients who have either been hospitalised with COVID-19 or who seek help from their general practitioner due to lingering physical symptoms after COVID-19. Another strength is the assessment of not only objective but also subjective cognitive function, work function and quality of life, which enabled insight into how these are associated in patients with long-COVID. The study was cross-sectional, as conclusions regarding causal mechanisms or relations cannot be drawn. Longitudinal studies are therefore needed to investigate causes and trajectory of long-COVID symptoms.
In conclusion, we found the frequency of clinically relevant cognitive impairment ranging in approximately half of the patients 7 months (SD = 4) after COVID-19. Impairment was observed in a global measure of cognition as well as in working memory, executive function, verbal fluency, verbal learning and memory. More objective cognitive impairment was associated with more subjective cognitive difficulties, poorer work function and quality of life. The findings underscore the clinical importance of screening for cognitive impairments and offering cognitive rehabilitation treatment to patients referred to long-COVID clinics.
Role of funding sources
The funding bodies had no role in the study design, data collection, analysis and interpretation of data, writing of the report or the decision to submit the article for publication.
CRediT authorship contribution statement
KWM and SJ defined the aim and hypotheses of this study. KWM, JKP, DVG, TKR, HH, CHD, DP and SJ were involved in conducting the study and assessing the patients. JKP and KWM conducted the statistical analyses and wrote the first draft. All authors contributed to and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest in relation to the current manuscript. Outside of the present work, KWM reports having received consultancy fees from Janssen-Cilag and Lundbeck; JKP, DVG, DP, HH, CHD and SJ report no conflicts of interest outside of the present work.
Acknowledgements
The authors thank the Department of Pulmonology Medicine and Respiratory Research Unit, Bispebjerg University Hospital, for the financial support for the study. KWM would like to thank the Lundbeck Foundation for her five-year Lundbeck Foundation Fellowship (grant no. R215-2015-4121).
References
- Albu S., Zozaya N.Rivas, Murillo N., Garcia-Molina A., Chacon C.A.Figueroa, Kumru H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: a prospective, observational cohort study. Disabil. Rehabil. 2021:1–8. doi: 10.1080/09638288.2021.1977398. [DOI] [PubMed] [Google Scholar]
- Alemanno F., Houdayer E., Parma A., Spina A., Del Forno A., Scatolini A., Angelone S., Brugliera L., Tettamanti A., Beretta L., Iannaccone S. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnefeesi Y., Siegel A., Lui L.M.W., Teopiz K.M., Ho R.C.M., Lee Y., Nasri F., Gill H., Lin K., Cao B., Rosenblat J.D., McIntyre R.S. Impact of SARS-CoV-2 infection on cognitive function: a systematic review. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.621773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attix D.K., Story T.J., Chelune G.J., Ball J.D., Stutts M.L., Hart R.P., Barth J.T. The prediction of change: normative neuropsychological trajectories. Clin. Neuropsychol. 2009;23:21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]
- Bailey Erin K., Steward Kayla A., VandenBussche Jantz Alicia B., Kamper Joel E., Mahoney Elaine J., Duchnick Jennifer J. Neuropsychology of COVID-19: anticipated cognitive and mental health outcomes. Neuropsychology. 2021;35:335–351. doi: 10.1037/neu0000731. [DOI] [PubMed] [Google Scholar]
- Battery Army Individual Testcollab. War Department, Adjutant General’s Office; Washington, DC: 1944. Manual of Directions and Scoring. [Google Scholar]
- Bliddal S., Banasik K., Pedersen O.B., Nissen J., Cantwell L., Schwinn M., Tulstrup M., Westergaard D., Ullum H., Brunak S., Tommerup N., Feenstra B., Geller F., Ostrowski S.R., Gronbaek K., Nielsen C.H., Nielsen S.D., Feldt-Rasmussen U. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021;11:13153. doi: 10.1038/s41598-021-92045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini Maura, Canoll Peter D., Klein Robyn S. How COVID-19 Affects the brain. JAMA Psychiatry (Chicago, Ill.) 2021;78:682–683. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Bungenberg J., Humkamp K., Hohenfeld C., Rust M.I., Ermis U., Dreher M., Hartmann N.K., Marx G., Binkofski F., Finke C., Schulz J.B., Costa A.S., Reetz K. Long COVID-19: objectifying most self-reported neurological symptoms. Ann. Clin. Transl. Neurol. 2022;9:141–154. doi: 10.1002/acn3.51496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., Di Vincenzo J.D., Cao B., Lin K., Mansur R.B., Ho R.C., Rosenblat J.D., Miskowiak K.W., Vinberg M., Maletic V., McIntyre R.S. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas-Carrera A., Sanchez-Rodriguez M.M., Canizares S., Ojeda A., Martin-Villalba I., Prime-Tous M., Rodriguez-Rey M.A., Segu X., Valdesoiro-Pulido F., Borras R., Peri J.M., Vieta E. Neuropsychological functioning in post-ICU patients after severe COVID-19 infection: the role of cognitive reserve. Brain Behav. Immun. Health. 2022;21 doi: 10.1016/j.bbih.2022.100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. The mystery of the pandemic's 'happy hypoxia. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- Crivelli L., Calandri I., Corvalan N., Carello M.A., Keller G., Martinez C., Arruabarrena M., Allegri R. Cognitive consequences of COVID-19: results of a cohort study from South America. Arq. Neuropsiquiatr. 2022;80:240–247. doi: 10.1590/0004-282X-ANP-2021-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Keating P., Winkler A.M., Collins R., Matthews P.M., Allen N., Miller K.L., Nichols T.E., Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch. Clin. Neuropsychol. 2012;27:248–261. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich Hannelore, Weissenborn Karin, Begemann Martin, Busch Markus, Vieta Eduard, Miskowiak Kamilla W. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol. Med. (Cambridge, Mass.) 2020;26 doi: 10.1186/s10020-020-00186-y. 58-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Molina A., Tormos J.M., Bernabeu M., Junque C., Roig-Rovira T. Do traditional executive measures tell us anything about daily-life functioning after traumatic brain injury in spanish-speaking individuals? Brain Inj. 2012;26:864–874. doi: 10.3109/02699052.2012.655362. [DOI] [PubMed] [Google Scholar]
- Gouraud C., Bottemanne H., Lahlou-Laforet K., Blanchard A., Gunther S., Batti S.E., Auclin E., Limosin F., Hulot J.S., Lebeaux D., Lemogne C. Association between psychological distress, cognitive complaints, and neuropsychological status after a severe COVID-19 episode: a cross-sectional study. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.725861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire Adam, Trender William, Chamberlain Samuel R., Jolly Amy, Grant Jon E., Patrick Fiona, Mazibuko Ndaba, Williams Steve, Barnby Joseph M., Hellyer Peter, Mehta Mitul A. medRxiv: 2020.10.20.20215863; 2020. Cognitive Deficits in People Who Have Recovered From COVID-19 Relative to Controls: An N=84,285 Online Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt Kelsey C., Marra David E., Block Cady, Cysique Lucette A., Drane Daniel L., Haddad Michelle M., Łojek Emilia, McDonald Carrie R., Reyes Anny, Eversole Kara, Bowers Dawn. Central nervous system manifestations of COVID-19: a critical review and proposed research agenda. J. Int. Neuropsychol. Soc. 2022;28:311–325. doi: 10.1017/S1355617721000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46:2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Stottrup M.M., Nayberg E., Knorr U., Ullum H., Purdon S.E., Kessing L.V., Miskowiak K.W. Optimising screening for cognitive dysfunction in bipolar disorder: validation and evaluation of objective and subjective tools. J. Affect. Disord. 2015;187:10–19. doi: 10.1016/j.jad.2015.07.039. [DOI] [PubMed] [Google Scholar]
- Krishnan K., Miller A.K., Reiter K., Bonner-Jackson A. Neurocognitive profiles in patients with persisting cognitive symptoms associated with COVID-19. Arch. Clin. Neuropsychol. 2022;37(4):729–737. doi: 10.1093/arclin/acac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersy F., Benotmane I., Helms J., Collange O., Schenck M., Brisset J.C., Chammas A., Willaume T., Lefebvre N., Solis M., Hansmann Y., Fabacher T., Caillard S., Mertes P.M., Pottecher J., Schneider F., Meziani F., Fafi-Kremer S., Kremer S. Cerebrospinal fluid features in patients with coronavirus disease 2019 and neurological manifestations: correlation with brain magnetic resonance imaging findings in 58 patients. J. Infect. Dis. 2021;223:600–609. doi: 10.1093/infdis/jiaa745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A., Pickard A.S. The EQ-5D and the EuroQol group. Value Health. 2019;22:21–22. doi: 10.1016/j.jval.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Res. Sq. 2021 doi: 10.1038/s41598-021-95565-8. rs.3. rs-266574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Piva S., Stampatori C., Righetti F., Mega I., Peli E., Sala E., Tomasi C., Indelicato A.M., Latronico N., De Palma G. Neurologic and cognitive sequelae after SARS-CoV2 infection: different impairment for ICU patients. J. Neurol. Sci. 2022;432 doi: 10.1016/j.jns.2021.120061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J. Neurol. 2021;268:4422–4428. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza Mario Gennaro, Palladini Mariagrazia, De Lorenzo Rebecca, Magnaghi Cristiano, Poletti Sara, Furlan Roberto, Ciceri Fabio, Rovere-Querini Patrizia, Benedetti Francesco. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R., Balanza-Martinez V., Luperdi S.C., Estrada I., Latorre A., Gonzalez-Jimenez P., Feced L., Bouzas L., Yepez K., Ferrando A., Hervas D., Zaldivar E., Reyes S., Berk M., Menendez R. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 2021;290:621–631. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Fugledalen L., Jespersen A.E., Sattler S.M., Podlekareva D., Rungby J., Porsberg C.M., Johnsen S. Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: pattern, severity, and functional implications. Eur. Neuropsychopharmacol. 2022;59:82–92. doi: 10.1016/j.euroneuro.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesjan V., Fonsmark L., Christensen R.H.B., Amiri M., Merie C., Lebech A.M., Katzenstein T., Bang L.E., Kjaergaard J., Kondziella D., Benros M.E. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non-COVID-19 illness. JAMA Psychiatry. 2022;79(5):486–497. doi: 10.1001/jamapsychiatry.2022.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.L., Ludwigsen T., Johnsen S., Miskowiak K.W. Cognitive late sequelae of COVID-19. Ugeskr. Laeger. 2022;184 [PubMed] [Google Scholar]
- Office for National Statistics Technical article: updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021
- Ott C.V., Knorr U., Jespersen A., Obenhausen K., Roen I., Purdon S.E., Kessing L.V., Miskowiak K.W. Norms for the screen for cognitive impairment in psychiatry and cognitive trajectories in bipolar disorder. J. Affect. Disord. 2021;281:33–40. doi: 10.1016/j.jad.2020.11.119. [DOI] [PubMed] [Google Scholar]
- Ott Caroline V., Vinberg Maj, Kessing Lars V., Bowie Christopher R., Forman Julie L., Miskowiak Kamilla W. Effect of action-based cognitive remediation on cognitive impairment in patients with remitted bipolar disorder: a randomized controlled trial. Bipolar Disord. 2021;23:487–499. doi: 10.1111/bdi.13021. [DOI] [PubMed] [Google Scholar]
- Poletti S., Palladini M., Mazza M.G., Lorenzo R.De, Covid- BioB Outpatient Clinic Study group. Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2021;272(5):773–782. doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.I., Baskett W.I., Huang W., Naqvi S.H., Shyu C.R. New-onset dementia among survivors of pneumonia associated with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect. Dis. 2022;9 doi: 10.1093/ofid/ofac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly M.C., Zbrozek A.S., Dukes E.M. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- Statens Serum Institut Beskrivelse af udvalgte virusvarianter. 2022. https://covid19.ssi.dk/virusvarianter/virusvariantbeskrivelser
- Sylvester S.V., Rusu R., Chan B., Bellows M., O'Keefe C., Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr. Med. Res. Opin. 2022;38:1391–1399. doi: 10.1080/03007995.2022.2081454. [DOI] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet Maxime, Sillett Rebecca, Zhu Lena, Mendel Jacob, Camplisson Isabella, Dercon Quentin, Harrison Paul J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9:815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside D.M., Basso M.R., Naini S.M., Porter J., Holker E., Waldron E.J., Melnik T.E., Niskanen N., Taylor S.E. Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection part 1: cognitive functioning. Clin. Neuropsychol. 2022:1–23. doi: 10.1080/13854046.2022.2030412. [DOI] [PubMed] [Google Scholar]