Abstract
BACKGROUND
Wyburn-Mason syndrome (WMS) is a neurocutaneous disorder consisting of vascular malformations of the brain, eye, and skin. These include characteristically high-flow intracranial and intraorbital arteriovenous malformations (AVMs) that present commonly with visual deterioration, headache, and hemiplegia. Complete removal of these lesions is challenging. Most patients are followed closely, and intervention occurs only in the setting of worsening symptoms secondary to AVM growth or hemorrhage. Here the authors present the first known case of a patient with WMS and a pituitary macroadenoma.
OBSERVATIONS
A 62-year-old man with a 30-year history of WMS with right basal ganglia and orbital AVMs and right eye blindness presented for new-onset left-sided vision loss. A pituitary adenoma was identified compressing the optic chiasm and left optic nerve. Magnetic resonance imaging and digital subtraction angiography studies were obtained for surgical planning, and the patient underwent an endoscopic transnasal transsphenoidal resection, with significant postoperative vision improvement.
LESSONS
Given the variable presentation and poor characterization of this rare syndrome, patients with WMS presenting with new symptoms must undergo evaluation for growth and hemorrhage of known AVMs, as well as new lesions. Further, in patients undergoing intracranial surgery, extensive preoperative imaging and planning are crucial for safe and successful procedures.
Keywords: Wyburn-Mason, pituitary macroadenoma, Bonnet-Dechaume-Blanc, neurocutaneous disorders, vision loss, endoscopic transnasal transsphenoidal approach
ABBREVIATIONS: AVM = arteriovenous malformation, CSF = cerebrospinal fluid, DSA = digital subtraction angiography, MRI = magnetic resonance imaging, WMS = Wyburn-Mason syndrome
Wyburn-Mason, or Bonnet-Dechaume-Blanc, syndrome (WMS) is a rare, neurocutaneous disorder characterized predominantly by vascular malformations involving primarily the brain, eye, and skin.1 Wyburn-Mason detailed the syndrome in 1943 as a constellation of arteriovenous malformations (AVMs) of the midbrain, ipsilateral retinal vascular abnormalities, and cutaneous nevi with progressive neurological involvement.2 The condition is extremely rare, with approximately 100 cases reported in the literature, making epidemiological characterization difficult.3,4 However, to date, no correlation with age or sex has been noted.4 Since its initial characterization, the definition of WMS has been expanded to reflect the wide variety in lesion location and presenting symptoms.4 AVMs have been described throughout the optic tract beyond the retina (including the optic chiasm, optic nerve, and occipital lobe), as well as the basal ganglia, thalamus, hypothalamus, and cerebellum.3,4 These are supplied by either the anterior or posterior circulation and are often dynamic, which may explain the progressive nature of the syndrome in some patients.1,3,4 Additionally, maxillofacial AVMs have been reported.3 While generally a unilateral condition, cases involving the bilateral orbits have been described.5 Together, these potential AVMs place patients at risk of any number of severe complications, including blindness, intracranial hemorrhage, massive epistaxis, and gingival bleeding. The cutaneous symptoms are often subtle and only present in a minority of cases.1,4 When present, they vary in coloration and size.1,4
Neurological presentation is variable but related directly to the lesion size and location.4 The most common symptom is visual deterioration, with headache, hemiplegia, and homonymous hemianopsia relatively common as well.2,3,6,7 Onset generally occurs in adolescence.1,4 In the original series, Wyburn-Mason emphasized the progressive nature of the neurological symptoms as well as mental changes,2 although cognitive changes have only been found in approximately 30% of cases in more recent analysis.4 As such, the natural history of the syndrome is patient- and lesion-specific and generally unpredictable.4 Further, in contrast to typical AVMs, those associated with WMS have often been noted to be dynamic and change over the patient’s lifetime.8–11 This remodeling is likely related to the high-flow nature of the AVMs with hemodynamic changes that can lead to spontaneous thrombosis and decompensation.9,11–14 Given the rarity of the syndrome, there is no consensus regarding the annual rate of AVM rupture; however, series by Dayani and Sadun11 and Théron et al.15 found 5 out of 27 (18.5%) and 4 out of 25 (16%) patients, respectively, experienced AVM rupture, rates similar to those in nonsyndromic AVM.11,15,16 Therefore, the rate of rupture may be similar to that of standard AVMs, between 2% and 4% annually.16–19
Treatment is challenging; attempts to obliterate WMS-related AVMs have included open surgical and minimally invasive techniques including radiosurgery and photocoagulation. Complete removal is rarely achieved, often due to adverse lesion locations.1,4,20 Therefore, patients are generally followed closely and only treated when necessary, such as in the case of hemorrhage or growth leading to worsening symptoms.20
Herein we present the first case, to our knowledge, of a patient with WMS and an associated pituitary adenoma. We discuss the specific presentation of this case of WMS and describe the operative challenges of approaching and resecting a pituitary tumor in the setting of multiple known vascular malformations.
Illustrative Case
History
A 62-year-old man with a past medical history of type 2 diabetes mellitus, hyperlipidemia, and congenital right eye blindness presented with progressive left temporal hemianopsia. Thirty years before he presented to our clinic, AVMs involving the right basal ganglia and orbit, consistent with WMS, were identified as the likely cause of his right eye blindness. Of note, his father was also born with unilateral vision loss, although the patient was unaware of any formal diagnosis. The patient remained stable until the onset of left-sided vision loss 8 years prior to presentation. He was diagnosed with cataract and given a recommendation for neurovascular evaluation prior to cataract surgery.
Examination
At initial presentation, the patient endorsed 50% vision loss in his left eye, with diminished ability to read and drive, but denied other neurological symptoms. On physical examination, he was found to have visual acuity of no light perception with the right eye and 20/30 with the left eye. He could count fingers only in the nasal field of the left eye. The right eye was enophthalmic with partial upgaze deficit. There were dilated vessels of the conjunctiva and sclera. Fundus examination (limited due to cataract) showed funnel retinal detachment and dilated vessels (Fig. 1A). The left eye examination was normal except for moderate cataract and temporal pallor of the optic nerve head (Fig. 1B). Additionally, he had diminished rapid alternating movements in the left upper and lower extremity. He was found to have hypopigmented facial macules as well as an enlarged and pulsatile upper lip (Fig. 1C and D).
FIG. 1.

Imaging of the fundus demonstrating an abnormal vascular structure of the right eye (A) compared with the left eye (B). Hypopigmented facial macules on the right cheek and chin (C, arrowheads) and upper lip AVM (arrowhead). Prominent vasculature on right eyelid and nose (arrowheads) (D).
Sella protocol magnetic resonance imaging (MRI) revealed a 15 × 13–mm mildly enhancing sellar and suprasellar mass abutting the bilateral cavernous internal carotid arteries and optic chiasm with thinning of the left aspect of the optic chiasm and left prechiasmatic optic nerve (Fig. 2). Formal Humphrey visual field testing demonstrated complete loss of vision in the left temporal hemifield, with some superior nasal hemifield loss (Fig. 3A). Optical coherence tomography of the left eye showed nasal hemimacular thinning of the ganglion cell complex layer of the retina, consistent with retrograde degeneration of retinal ganglion cells injured at the chiasm (Fig. 3B and C). No symptoms of altered pituitary function were noted on endocrinological evaluation; however, given the significant new decline in visual function in the setting of preexisting right eye blindness, urgent transsphenoidal surgical removal of the mass was recommended.
FIG. 2.

Preoperative imaging of adenoma (arrowhead). Coronal postcontrast T1-weighted MR image (upper) shows minimal intercarotid distance (white bar). Sagittal postcontrast gradient-recalled echo sequence (lower).
FIG. 3.

Preoperative ophthalmic studies. Left eye visual field testing (A) demonstrated complete loss of the left temporal hemifield and incomplete loss of the superior nasal hemifield. Left eye OCT with (B) horizontal cross-section of the retina through the fovea showing narrowing of the nasal GCC layer (left side of image, between yellow and purple lines) and heat map (C) of GCC layer thickness showing thinning in the nasal hemimacula (left side of image). GCC = ganglion cell complex; OCT = optical coherence tomography; OS = oculus sinister.
Preoperative angiogram also demonstrated a dilated right ophthalmic artery with a large saccular aneurysm contiguous with a right orbital AVM, as well as dilated branches of the external carotid artery (Fig. 4A–C). Notably, there was no angiographic evidence of the previously identified right basal ganglia AVM, which was re-demonstrated on MRI (Fig. 4D and E). Evaluation of the right external carotid artery distribution revealed a tortuous and dilated superficial temporal artery, while the internal maxillary artery and its branches were normal. No AVMs or other malformations were noted (Fig. 4G and H).
FIG. 4.
Preoperative AVM imaging. DSA showing anteroposterior (A) and lateral (B and C) views of the orbital AVM without evidence of basal ganglia AVM. Axial postcontrast gradient-recalled echo sequence (D) showing basal ganglia AVM. Axial 3D time-of-flight sequence (E) demonstrating lack of blood flow within basal ganglia AVM. Axial 3D time-of-flight sequence (F) showing orbital AVM. DSA showing anteroposterior (G) and lateral (H) views of internal maxillary artery (white arrowhead), superficial temporal artery (black arrowhead), and sphenopalatine artery (H, white arrow). 3D = three-dimensional.
Operation
Endoscopic transnasal transsphenoidal resection with stereotactic neuronavigational guidance was performed in collaboration with otolaryngology. The base of the sphenoid sinus was removed and widened, allowing visualization of the sella, carotid protuberances, planum sphenoidale, with subsequent endoscopic opening of the sella using standard techniques. The mass was soft and mildly hemorrhagic; near gross-total resection and complete chiasmatic decompression were achieved. Vascular integrity was confirmed via microvascular Doppler; diaphragmatic depression confirmed that the chiasm was adequately decompressed. A small left superior cerebrospinal fluid (CSF) leak was repaired, and the sellar closure was bolstered with a posterior vascularized nasoseptal flap.
Postoperatively, the patient was neurologically stable with immediately improved left temporal hemianopsia. He was able to see movement approximately 30° lateral to the midline, whereas preoperatively he could not identify movement beyond midline. Two days postoperatively, the patient’s visual fields continued to improve. There was no evidence of CSF leak, and the patient was discharged without incident.
Histopathological Examination
Histopathological analysis of the pituitary mass was consistent with a gonadotroph pituitary adenoma. It was characterized by proliferation of neoplastic cells arranged in sheets and large, poorly formed lobules with no mitotic figures or necrosis. Immunohistochemical staining showed that the neoplastic cells were positive for steroidogenic factor 1 and variably positive for follicle-stimulating hormone and alpha human chorionic gonadotropin but was otherwise negative.
Postoperative Course
At the patient’s 1-month postoperative follow-up visit, he endorsed subjective improvement in the left visual field. Postoperative MRI demonstrated improvement in pituitary stalk deviation and decreased mass effect on the optic chiasm (Fig. 5A and B). Ophthalmological evaluation demonstrated objective improvement in the left visual field as well (Fig. 5C). At his 1-year postoperative visit, the patient endorsed continued improvement in his vision, there was no significant change in pituitary function, and surveillance MRI revealed no residual pituitary lesion. Additionally, the patient underwent uncomplicated left cataract extraction 7 months after surgery, which further improved his vision.
FIG. 5.
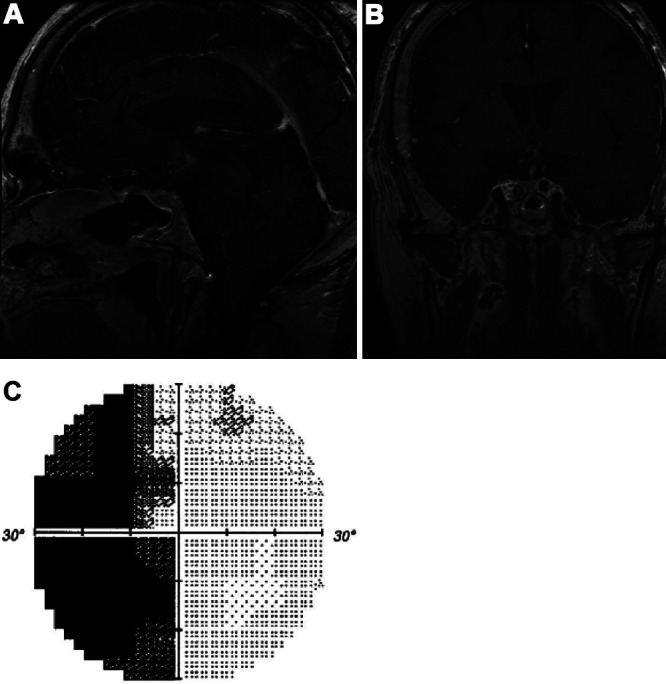
Postoperative findings. Sagittal (A) and coronal (B) postcontrast T1-weighted MRI sequences showing a reduction in the pituitary macroadenoma and in compression of the optic chiasm. Left eye visual field testing (C) demonstrating improvement in the superior nasal and temporal hemifields.
Discussion
We report the first, to our knowledge, case of concurrent WMS and pituitary adenoma requiring operative intervention due to deteriorating visual function. Given the rare nature of this condition, we first highlighted the unique characteristics of this patient’s specific case of WMS to contribute a new example to the limited literature discussing the syndrome. We then discussed the operative challenges of transsphenoidal surgery in the setting of patients with numerous vascular malformations and emphasized the importance of rapid action to preserve and enhance visual function.
Observations
Characterization of WSM
This patient exhibits a combination of neurological, ocular, and cutaneous lesions that are characteristic of WMS.1–4,11,15 He was found to have a deep cerebral and an orbital AVM during workup for severe right temporal headaches 30 years prior to the current presentation. This is consistent with the natural history of the syndrome, in which neurological symptoms related to the size and location of the underlying lesions are the primary presenting symptoms.3,4 Intracranial WMS-related AVMs are generally deep, but can be found throughout the brain and brainstem.1,4 In this case, the AVM was characterized on MRI as a tangle of blood vessels with associated flow voids and hemosiderin deposition involving the right basal ganglia, right thalamus, right caudate nucleus, and right corona radiata (Fig. 4D). Interestingly, digital subtraction angiography (DSA) did not reveal any evidence of an AVM in the region of the right basal ganglia. Time-of-flight magnetic resonance (MR) angiography sequences (Fig. 4E) corroborated the angiography findings and suggested no blood flow through the malformation. That the AVM was seen on MRI but not in angiographic studies may suggest spontaneous thrombosis without involution of the AVM prior to presentation. This phenomenon has been described in other cases of patients with WMS-related AVMs and is probably mechanistically similar to nonsyndromic AVMs: turbulent blood flow and subsequent vessel wall damage lead to an increased risk of thrombosis.13,14,21–23 Further supporting remote spontaneous thrombosis of the right basal ganglia AVM is the fact that, other than congenital right eye blindness and baseline headaches, the patient had been neurologically asymptomatic prior to his presentation in our clinic.
The patient’s congenital right eye blindness is likely secondary to his right orbital AVM, characterized on angiography by a dilated right ophthalmic artery with large saccular aneurysm, ultimately feeding into an orbital AVM (Fig. 4F). Notably, retinal lesions are the classic ocular association in WMS; however, they are not a requirement for the diagnosis. Wyburn-Mason’s original case series described 6 patients without retinal involvement2 and multiple case series since then have reported similar findings.11,12,15 There is a wide range of visual symptoms reported in the literature, including visual field defects, decreasing visual acuity, gaze defects, and proptosis.2,11,15 Complete vision loss is rarer, but has been documented.11,15 It is crucial when considering management of these ocular lesions to emphasize preservation of any visual function. Enlarging lesions that may result in further compression and disruption of vision might benefit from resection or ablation, whereas those that are stable may be better managed conservatively given the risk of damage to surrounding structures during interventions.
Cutaneous lesions are not required for diagnosis and, in fact, are found in a minority of cases.4 When present, these lesions can range from faint discolorations to port-wine stains and large AVMs.4,11 Of note, these malformations are generally ipsilateral to the cerebral and ocular lesions.4 In this patient, small hypopigmented macules were found bilaterally throughout his cheek, ear, and chin (Fig. 1C and D). Additionally, his upper lip was found to be enlarged and pulsatile, indicating another potential vascular malformation.
While patients present most often in childhood, some have exhibited symptoms at birth as well as later in life.4,15,24 Therefore, the presentation of this patient, with ocular symptoms at birth and neurological symptoms in adulthood, is unusual but not unheard of. Finally, the patient’s father was also congenitally blind in 1 eye, although he did not have any known neurological lesions or cutaneous abnormalities. At this time, WMS is thought to be nonhereditary,1,4 although Bhattacharya et al.3 did note two patients in their series of 15 who had first-degree family members with other cerebrofacial vascular lesions.
Finally, while pituitary adenomas have not been previously documented in patients with WMS, the limited number of cases in the literature argues that further investigation into potential links between these lesions may be warranted.
Management of Pituitary Adenoma
In a patient presenting with a novel visual field defect and known history of WMS, it is important to consider growth or hemorrhage of an AVM along with standard ophthalmological diagnoses. As our patient’s case demonstrates, the differential diagnosis must be kept broad to encompass new, potentially unrelated neurological issues. In this case, a gonadotroph pituitary adenoma was the underlying cause of the patient’s left temporal visual field deficit. With his worsening vision and clear compression of the optic chiasm, urgent surgical removal of the mass was indicated to preserve his remaining vision. However, the presence of the right hemispheric AVM required careful operative consideration and planning.
In contrast to standard operative planning for resection of pituitary lesions, DSA was necessary in this case to fully characterize the patient’s AVMs. It was crucial to ensure that the transsphenoidal operative corridor would be amenable to resection of the pituitary adenoma for multiple reasons. Certain types of pituitary adenoma have been found to reduce intercarotid distance, thereby complicating transsphenoidal surgery.25 With a minimum cavernous intercarotid distance of 8 mm at the posterior aspect of the sphenoidal sinus and 13 mm at the widest portion of the tumor, the operative corridor was narrow, but resection was deemed safe. Further, the abnormal nature of the vasculature in this syndrome meant that detailed vascular imaging of the carotid anatomy was essential to understand the relationship of the tumor to the internal carotid artery and its branches. The predisposition of these patients to vascular malformations in maxillofacial structures meant that delicate care had to be taken during the endonasal approach to avoid damage to any intranasal vascular abnormalities, which could result in severe epistaxis. Complete characterization of the arterial supply and venous return of the nasal corridor via DSA allows for the development of safe operative approaches. Here, the external carotid distribution was notable for a normal internal maxillary artery without any AVMs, allowing for a safe endonasal approach. Although no specific nasal vascular malformations were identified in this patient, his nasal turbinates were highly vascularized and required a higher-than-usual volume of epinephrine during sellar exposure and meticulous hemostasis was imperative. Intraoperative vascular Doppler also provided real-time reassurance that vascular structures were preserved. A nasoseptal flap buttressed with fibrin sealant (Tisseel, Baxter), thrombin-soaked Gelfoam (Pfizer), and Nasopore (Stryker) was utilized to prevent CSF leakage and to minimize the chance of a return to the operating room given the patient’s complex and delicate vasculature. Ultimately, the resection was performed successfully without intraoperative or postoperative complications.
Lessons
WMS is a rare syndrome characterized by intracranial, ocular, and facial AVMs that requires a multidisciplinary approach with thorough history, physical examination, laboratory studies, and imaging to determine optimal treatment strategies. In our patient, who was blind in 1 eye prior to presentation, new left hemifield visual loss on presentation necessitated a thorough workup for causes of deteriorating vision, which revealed a gonadotroph pituitary adenoma. Urgent multidisciplinary surgical intervention preceded by careful preoperative planning, including dedicated vascular imaging to avoid complications during approach and resection, resulted in improved left hemifield vision. Our case suggests that diagnoses other than growth or hemorrhage of orbital and intracranial AVMs must be considered when a patient with WMS presents with new vision changes.
Acknowlegments
Funding was received from the National Institutes of Health (grant no. 30 026877, awarded to H.E.M.) and Department of Defense.
Disclosures
Dr. Moss reported personal fees from Twenty Twenty Therapeutics, Verana Health, Genentech, and Springer, and legal firms outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Purger. Acquisition of data: Purger, Moss, Dodd. Analysis and interpretation of data: Hug, Purger, Dodd. Drafting the article: Hug, Purger, Dodd. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Hug. Statistical analysis: Purger. Study supervision: Purger, Dodd.
References
- 1.Rizzo R. Two rare neurocutaneous syndromes: Wyburn-Mason and Proteus. In: Curatolo P, Riva D, editors. Neurocutaneous Syndromes in Children. Éditions John Libbey Eurotext; 2006. pp. 73–79. [Google Scholar]
- 2. Wyburn-Mason R. Arteriovenous aneurysm of mid-brain and retina, facial nevi and mental changes. Brain. 1943;66(3) [Google Scholar]
- 3. Bhattacharya JJ, Luo CB, Suh DC, Alvarez H, Rodesch G, Lasjaunias P. Wyburn-Mason or Bonnet-Dechaume-Blanc as cerebrofacial arteriovenous metameric syndromes (CAMS). A new concept and a new classification. Interv Neuroradiol. 2001;7(1):5–17. doi: 10.1177/159101990100700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggieri M, Konez O, Rocco C. Wyburn-Mason syndrome. In: Ruggieri M, , Pascual-Castroviejo I, Di Rocco C, , editors. Neurocutaneous Disorders Phakomatoses and Hamartoneoplastic Syndromes. Springer Vienna; 2008. pp. 345–352. [Google Scholar]
- 5. Kim J, Kim OH, Suh JH, Lew HM. Wyburn-Mason syndrome: an unusual presentation of bilateral orbital and unilateral brain arteriovenous malformations. Pediatr Radiol. 1998;28(3):161. doi: 10.1007/s002470050319. [DOI] [PubMed] [Google Scholar]
- 6. Willinsky RA, Lasjaunias P, Terbrugge K, Burrows P. Multiple cerebral arteriovenous malformations (AVMs). Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology. 1990;32(3):207–210. doi: 10.1007/BF00589113. [DOI] [PubMed] [Google Scholar]
- 7. Paillas JE, Bonnal J, Righini C. [Encephalo-retino-facial angioma (Bonnet, Dechaume and Blanc syndrome)] Rev Neurol (Paris) 1959;101:698–707. [PubMed] [Google Scholar]
- 8. Dekking HM. Arteriovenous aneurysm of the retina with spontaneous regression. Ophthalmologica. 1955;130(2):113–115. doi: 10.1159/000302653. [DOI] [PubMed] [Google Scholar]
- 9. Gregersen E. Arteriovenous aneurysm of the retina. A case of spontaneous thrombosis and healing. Acta Ophthalmol (Copenh) 1961;39(5):937–939. doi: 10.1111/j.1755-3768.1961.tb07759.x. [DOI] [PubMed] [Google Scholar]
- 10. Augsburger JJ, Goldberg RE, Shields JA, Mulberger RD, Magargal LE. Changing appearance of retinal arteriovenous malformation. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215(1):65–70. doi: 10.1007/BF00413398. [DOI] [PubMed] [Google Scholar]
- 11. Dayani PN, Sadun AA. A case report of Wyburn-Mason syndrome and review of the literature. Neuroradiology. 2007;49(5):445–456. doi: 10.1007/s00234-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 12. Danis R, Appen RE. Optic atrophy and the Wyburn-Mason syndrome. J Clin Neuroophthalmol. 1984;4(2):91–95. [PubMed] [Google Scholar]
- 13. Elizalde J, Vasquez L. Spontaneous regression in a case of racemose haemangioma Archer’s type 2. Retin Cases Brief Rep. 2011;5(4):294–296. doi: 10.1097/ICB.0b013e3181f66a97. [DOI] [PubMed] [Google Scholar]
- 14. Brodsky MC, Hoyt WF. Spontaneous involution of retinal and intracranial arteriovenous malformation in Bonnet-Dechaume-Blanc syndrome. Br J Ophthalmol. 2002;86(3):360–361. doi: 10.1136/bjo.86.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Théron J, Newton TH, Hoyt WF. Unilateral retinocephalic vascular malformations. Neuroradiology. 1974;7(4):185–196. doi: 10.1007/BF00342696. [DOI] [PubMed] [Google Scholar]
- 16. Brown RD, Jr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68(3):352–357. doi: 10.3171/jns.1988.68.3.0352. [DOI] [PubMed] [Google Scholar]
- 17. Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg. 2013;118(2):437–443. doi: 10.3171/2012.10.JNS121280. [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Al-Shahi Salman R, McCulloch CE, Stapf C, Young WL. Untreated brain arteriovenous malformation: patient-level meta-analysis of hemorrhage predictors. Neurology. 2014;83(7):590–597. doi: 10.1212/WNL.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younge BR. Wyburn–Mason syndrome. In: Gomez MR, editor. Neurocutaneous Diseases. Elsevier; 1987. pp. 376–380. [Google Scholar]
- 21. Levine J, Misko JC, Seres JL, Snodgrass RG. Spontaneous angiographic disapparance of a cerebral arteriovenous malformation. Third reported case. Arch Neurol. 1973;28(3):195–196. doi: 10.1001/archneur.1973.00490210075011. [DOI] [PubMed] [Google Scholar]
- 22. Schatz H, Chang LF, Ober RR, McDonald HR, Johnson RN. Central retinal vein occlusion associated with retinal arteriovenous malformation. Ophthalmology. 1993;100(1):24–30. doi: 10.1016/s0161-6420(93)31701-x. [DOI] [PubMed] [Google Scholar]
- 23. Conforti P. Spontaneous disappearance of cerebral arteriovenous angioma. Case report. J Neurosurg. 1971;34(3):432–434. doi: 10.3171/jns.1971.34.3.0432. [DOI] [PubMed] [Google Scholar]
- 24. Rizzo R, Pavone L, Pero G, Chiaromonte I, Curatolo P. A neurocutaneous disorder with a severe course: Wyburn-Mason’s syndrome. J Child Neurol. 2004;19(11):908–911. [PubMed] [Google Scholar]
- 25. Mascarella MA, Forghani R, Di Maio S, et al. Indicators of a reduced intercarotid artery distance in patients undergoing endoscopic transsphenoidal surgery. J Neurol Surg B Skull Base. 2015;76(3):195–201. doi: 10.1055/s-0034-1396601. [DOI] [PMC free article] [PubMed] [Google Scholar]



