Abstract
Taste neophobia, the rejection of novel tastes or foods, involves an interplay of various brain regions encompassing areas within the central gustatory system, as well as nuclei serving other functions. Previous findings, utilising c-Fos imaging, identified several brain regions which displayed higher activity after ingestion of a novel taste as compared to a familiar taste. The present study extends this analysis to include additional regions suspected of contributing to the neurocircuitry involved in evoking taste neophobia. Our data show increased c-Fos expression in the basolateral amygdala, central nucleus of the amygdala, gustatory portion of the thalamus, gustatory portion of the insular cortex and the medial and lateral regions of the parabrachial nucleus. These results confirm the contribution of areas previously identified as active during ingestion of novel tastes and expose additional areas that express elevated levels of c-Fos under these conditions, thus adding to the neural network involved in the detection and initial processing of taste novelty.
Keywords: Unknown taste, Neophobic reaction, c-Fos, innate aversive response, Rat
Graphical Abstract

1. Introduction
Humans and non-human animals consume a novel food item much less avidly than they do when the same food is known to be familiar and safe (for reviews see Reilly, 2018a). Presumably, this is because the content of the new item is unknown and therefore potentially dangerous - hence the term taste (or food) neophobia for this phenomenon (e.g., Barnett, 1958; Domjan, 1977). If the novel food is followed by malaise (e.g., toxicosis, a novel drug state, or various types of internal pain) a conditioned taste aversion (CTA) is acquired and the food will be shunned on subsequent encounters (Lin, Arthurs & Reilly, 2017; Reilly & Schachtman, 2009; Revusky & Garcia, 1970). Taste neophobia is an important defensive mechanism in many animals, particularly those with varied diets, because it limits intake of a new food until its post-ingestive effects are experienced. With repeated exposures, taste neophobia follows its normal course and dissipates as the food is determined to be acceptable to the health and well-being of the individual (Reilly, 2018b). Taste neophobia can, however, become problematic if the initial avoidance response to the benign food item does not habituate. In humans, such exaggerated taste neophobia may lead to, for example, fussy/picky eating (Nicklaus & Monnery-Patris, 2018), some aspects of avoidant/restrictive food intake disorder (Dovey, 2018), or adversely influence the addition of new foods into the diets of those individuals with, for example, special needs (Williams & Seiverling, 2018).
Understanding of the neural substrates and mechanisms of taste neophobia is still developing. Our research has focused on elucidating the roles of the component nuclei of the central gustatory system of the rodent. In this system ascending taste information travels from the mouth to synapse in the rostral aspect of the nucleus of the solitary tract (rNST; Contreras, Beckstead & Norgren, 1982; Corson, Aldridge, Wilmoth & Erisir, 2012) and then ascends to the medial region of the parabrachial nucleus (mPBN) in the pons (Herbert, Moga & Saper, 1990; Norgren & Leonard, 1973). Efferent axons from the mPBN bifurcate and follow two routes to the forebrain. As its name indicates, axons in the thalamocortical pathway synapse in the gustatory thalamus (GT; Bester, Bourgeais, Villanueva, Besson & Bernard, 1999; Karimnamazi & Travers, 1998) which, in turn, projects to the gustatory cortex (GC; Kosar, Grill & Norgren, 1986; Nakashima, Uemura, Yasui, Ozaki, Tabata & Taen, 2000). The ventral pathway involves separate and reciprocal projections to, most notably, the bed nucleus of the stria terminalis (BNST; Alden, Besson & Bernard, 1994; Karimnamazi & Travers, 1998), to the central nucleus of the amygdala (CNA; Bernard, Alden & Besson, 1993; Karimnamazi & Travers, 1998), and to the lateral hypothalamus (LH; Halsell, 1992; Norgren, 1976).
The two nuclei of the dorsal pathway not only project to each other but also connect to nuclei of the ventral pathway which, in turn, project to the PBN and/or the NST. That is, taste-responsive cells in the GC send descending fibers to the BNST, CNA and LH as well as to the PBN and the NST (Kang & Lundy, 2009, 2010; Moga, Herbert, Hurley, Yasui, Gray & Saper, 1990; Ottersen, 1982; Pitkanen, Savander & Le Doux 1997; Shi & Cassell,1998; Veening, 1978; Veening, Swanson, & Sawchenko, 1984). The GT projects to the basolateral amygdala (BLA), the BNST and the CNA, (Halsell, 1992; Nakashima et al., 2000; Ottersen & Ben-Ari, 1979; Turner & Herkenham, 1991). Direct projection from the GT to the PBN or to the NST have not been demonstrated. However, suggesting an indirect connection, orthodromic stimulation of the GT influences activity in both the PBN and the NST (Cho, Mao & Li, 2008; Mao, Cho & Li, 2008). In addition, there is a connection between the GT and the medial amygdala (MeA; Nakashima et al., 2000; Turner & Herkenham, 1991). Finally, the BLA projects to the CNA (Pitkanen et al., 1997) and, in turn, the CNA projects to the PBN and NST (Kang & Lundy, 2009; Tokita, Inoue & Boughter, 2009; Whitehead, Bergula & Holliday, 2000).
The neural circuitry underlying taste memory processing extends beyond the central gustatory system. It includes, most notably, the nucleus accumbens (NAc; Grau-Perales, Expósito, Gómez-Chacón, Morón & Gallo, 2020; Ramírez-Lugo, Nunez-Jaramillo & Bermúdez-Rattoni, 2007), the nucleus basalis magnocellularis (NBM; Miranda, Ferreira, Ramırez-Lugo & Bermudez-Rattoni, 2003; Rodríguez-García & Miranda, 2016) and the perirhinal cortex (PrC; Morillas, Gómez-Chacón & Gallo, 2017; Ramos, 2020).
As this sketch indicates, there are numerous candidate structures that might contribute to the taste neophobia circuit of the brain. While the organization of this circuit is unknown it likely involves coordinated activity across multiple structures as the process progresses from detection of the unknown (novel) taste through to acceptance of the safe (and familiar) taste. The transition from novel to familiar taste ranges from seconds to hours to days. We are interested in the structures involved in the detection of taste novelty, which occurs over the first few minutes of taste intake. Our initial work with c-Fos (Lin, Roman, Arthurs & Reilly, 2012) revealed significantly more Fos immunoreactivity following the consumption of a novel (relative to familiar) saccharin solution in four (i.e., BLA, CNA, GC, and GT) of the seven nuclei examined. The goal of the present research was to replicate and extend the previous work by examining c-Fos immunoreactivity in the nuclei of the central gustatory system and related structures in rats voluntarily drinking a novel taste or, after repeated exposures, the same taste when familiar. The target areas were: rNST, mPBN, lPBN (lateral PBN), GT, GC, CNA, MeA, BLA, BNST, LH, NAcC (NAc core), NAcS (NAc shell), PrC and NBM. The long-term goal of our research is to understand the neural substrates and mechanisms of taste neophobia.
Rats received one (Group Novel; n = 6) or six (Group Familiar; n = 4) taste trials, all trials spaced three days apart. Each trial involved 15-min free access to 0.3% saccharin, except for except Trial 1 when intake was capped at 3 ml for Group Novel and Trial 6 when intake was capped at 3 ml for Group Familiar. Comparisons of saccharin-evoked c-Fos immunoreactivity across the fourteen target areas were used to identify the neural components engaged in the initial neophobic reaction to the ingestion of a small amount of an unknown taste.
2. Results
2.1. Behavioral
Saccharin intake for both groups over the course of the experiment are shown in Table 1. A one-way analysis of variance conducted on data obtained from Group Familiar over Trials 1 - 5 revealed a significant main effect of Trials, F(4,12) = 11.50, p < .001, . Follow-up statistics (Fisher LSD test) showed that consumption on Trial 1 was significantly lower than intake on Trials 2 - 5 (ps < .05). Demonstrating a steady recovery from neophobia, saccharin intake increased significantly from Trial 1 to Trial 2 (p < .05). and from Trial 2 to Trial 3 (p < .05). The initially novel saccharin was accepted as safe and familiar (i.e., habituation of taste neophobia occurred) as shown by comparable intake of saccharin on Trial 3 relative to Trial 4 and on Trial 4 relative to Trial 5 (ps > .05). So, the rats in Group Familiar drank ~14 ml of saccharin more at asymptote than on Trial 6.
Table 1.
Mean ( ± SE) 0.3% saccharin intake (ml) of rats in the Novel and Familiar groups across all trials of the experiment.
| Trial | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Novel | ||||||
| Mean | 2.92 | - | - | - | - | - |
| SE | 0.08 | |||||
| Familiar | ||||||
| Mean | 2.63 | 10.63 | 17.00 | 15.00 | 17.12 | 3.00 |
| SE | 2.13 | 2.25 | 1.37 | 1.87 | 0.66 | 0.00 |
Notes: Intake was capped at 3.00 ml on Trial 1 for rats in the Novel group and on Trial 6 for rats in the Familiar group.
2.2. Immunohistology
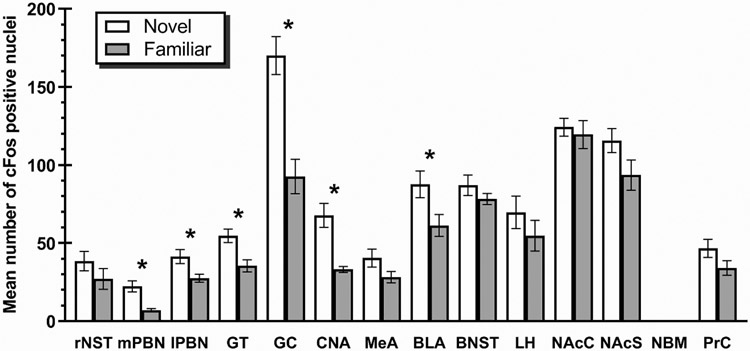
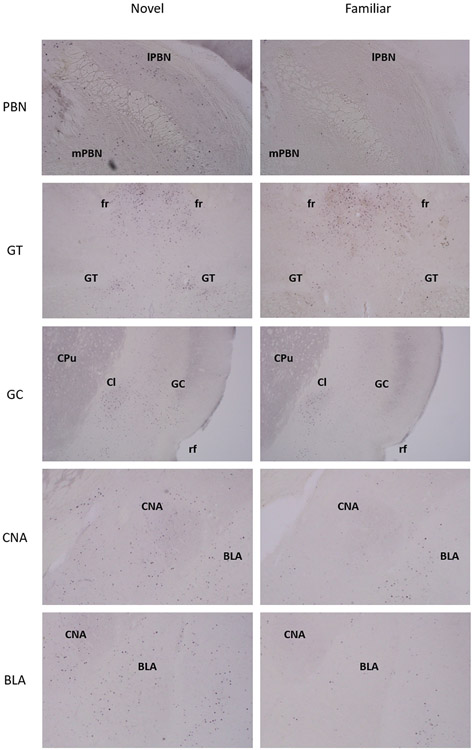
Depicted in Fig. 1 are the number of c-Fos positive cell counts across the 14 target areas in Group Novel (Trial 1) and Group Familiar (Trial 6). The NBM did not reveal a sufficient number of c-Fos positive cells in either group for statistical analysis and therefore was characterized as not containing c-Fos positive cells. Compared to rats in Group Familiar, the Group Novel animals showed increased c-Fos positive neurons in: mPBN, t(8)=3.42, p<0.01; lPBN, t(8) =2.32, p<0.05; BLA, t(8)=2.33, p<0.05; CNA, t(8)=3.56, p<0.01; GT, t(8)=3.10, p<0.05; and the GC, t(8)=4.407, p<0.01. Comparable levels of c-Fos expression were found in: rNST, t(8)=1.21, p>0.05; MeA, t(8)=1.58, p>0.05; BNST, t(8)=1.02, p>0.05; LH, t<1; NAc, t(8)=1.81, p>0.05; NAcS, t<1; and the PrC, t(8)=1.54, p>0.05. Fig. 2 shows digital photomicrographs of each of the target structures that displayed increased c-Fos expression following ingestion of a novel (left panels) or familiar (right panels) saccharin solution.
Fig 1.
Mean (±SE) number of c-Fos positive nuclei following ingestion of novel or familiar 0.3% saccharin solution in: rostral nucleus of the solitary tract (rNST), medial parabrachial nucleus (mPBN), lateral parabrachial nucleus (lPBN), gustatory thalamus (GT), gustatory cortex (GC), central nucleus of the amygdala (CNA), medial nucleus of the amygdala (MeA), basolateral nucleus of the amygdala (BLA), bed nucleus of the stria terminalis (BNST), lateral hypothalamus (LH), nucleus accumbens core (NAcC), nucleus accumbens shell (NAcS), nucleus basalis magnocellularis (NBM), perirhinal cortex (PrC). * Significant at p<0.05
Fig 2.
Representative digital images of c-Fos immunostaining in animals that displayed a significantly higher number of c-Fos positive cells in the novel group (left column) than the familiar group (right column) within the medial parabrachial nucleus (mPBN), lateral parabrachial nucleus (lPBN), gustatory thalamus (GT), gustatory cortex (GC), central nucleus of the amygdala (CNA), basolateral amygdala (BLA). Additional abbreviations: Cl, claustrum; CPu, caudate putamen; fr, fasciculus retroflexus; rf, rhinal fissure.
Some additional analyses were conducted to determine the distribution of Fos-labelled neurons in subcomponents of the GC and PBN (see Table 2). The same statistical pattern (significantly higher cell counts expressed following ingestion of novel versus familiar saccharin solution) was found in three of four subregions as revealed in the GC per se: dorsal agranular t(8)=2.730, p<0.05; dysgranular t(8)=7.057, p<0.01; and granular t(8)=4.694, p<0.01 - the ventral agranular subnucleus showed comparable expression in both groups (t<1). The mPBN was partitioned into 2 subregions and the lPBN into 5 subnuclei. Analyses revealed that both sub-regions of the mPBN expressed higher counts of c-Fos positive cells in the novel vs familiar group: medial t(8)=4.707, p<0.01; external t(8)=2.727, p<0.05. Within the lPBN only the ventral subnucleus expressed more c-Fos positive cells following ingestion of novel vs familiar saccharin: t(8)=3.317, p=0.02 - the remaining lPBN subnuclei did not reveal a statistically significant difference between novel and familiar groups: central t(8)=2.283, p>0.05; dorsal t<1; crescent t(8)=1.374 p>0.05; waist t(8)=1.711, p>0.05.
Table 2.
Target areas analyzed by number of sections, anterior-posterior (AP) range (Paxinos & Watson, 2005), and divisions, parts or sub-nuclei included.
| Target Area |
Sections | AP range (mm) |
Divisions, parts or sub-nuclei |
|---|---|---|---|
| rNST | 4 | −12.0 to −12.6 | lateral; medial; rostrolateral; ventral; ventrolateral |
| mPBN | 4 | −9.0 to −9.6 | medial; external |
| lPBN | 4 | −9.0 to −9.6 | central; crescent; dorsal; ventral; waist |
| GT | 3 | −3.5 to −4.0 | parvicellular region |
| GC | 6 | 1.8 to 0.2 | agranular, dorsal; agranular, ventral; dysgranular; granular |
| CNA | 5 | −1.8 to −3.0 | capsular; lateral; medial |
| MeA | 5 | −2.0 to −3.2 | anterodorsal, anteroventral |
| BLA | 5 | −2.0 to −3.2 | basolateral amygdaloid nucleus: anterior, posterior, ventral; lateral amygdaloid nucleus: dorsolateral, ventrolateral, ventromedial; basomedial amygdaloid nucleus: anterior, posterior |
| BNST | 4 | 0.3 to −0.3 | medial division, anterior; lateral division: posterior, ventral, dorsal; medial division, ventral |
| LH | 5 | −1.8 to −3.2 | magnocellular; peduncular; tuberal |
| NAcC | 5 | 1.8 to 0.2 | core |
| NAcS | 5 | 1.8 to 0.2 | shell |
| NBM | 2 | −1.2 to −1.4 | basal |
| PrC | 6 | −3.0 to −6.0 | perirhinal cortex |
Abbreviations: rNST, rostral nucleus of the solitary tract; mPBN, medial parabrachial nucleus; lPBN, lateral parabrachial nucleus; GT, gustatory thalamus; GC, gustatory cortex; CNA, central nucleus of the amygdala; MeA, medial nucleus of the amygdala; BLA, basolateral nucleus of the amygdala; BNST, bed nucleus of the stria terminalis; LH, lateral hypothalamus; NAcC, nucleus accumbens core; NAcS, nucleus accumbens shell; NBM, nucleus basalis magnocellularis; PrC, perirhinal cortex.
3. Discussion
Of the fourteen areas examined, increased Fos immunoreactivity was found in the BLA, CNA, GT, GC, mPBN and lPBN of rats given novel saccharin relative to animals that received familiar saccharin; no differential expression of c-Fos was found in the other eight areas (BNST, MeA, rNST, LH, NAc, NAcS, NBM and the PrC). Thus, these results replicate and extend the findings of Lin, Roman, Arthurs & Reilly. (2012). However, before moving on to examine these outcomes, it is necessary to address three issues relevant to interpretation of the results.
First, in that earlier study the rats drank 0.5% saccharin, a highly neophobic solution, to ensure a strong behavioral response. The mean amount consumed by Group Novel on that trial was 4.83 ( ± 1.70) ml; the Familiar rats were limited to 5 ml. But the variance (standard error) revealed that some Novel rats drank very little of the neophobic solution. Whilst the low volume consumed by these animals obviously demonstrated that their drinking behavior was a sensitive indicator of the detection of the novel solution, it also raised concern about the sufficiency of the taste exposure to support complete expression of c-Fos across the entire neural network that constitutes the taste neophobia circuit. This concern was addressed in the present experiment by using a lower, but nonetheless neophobia-inducing, concentration of saccharin (0.3%; e.g., Ramos, 2015) and by limiting maximum intake to 3 ml. The results show that 5 of the Novel animals drank the maximum of 3 ml whilst the sixth rat consumed 2.5 ml (all Familiar rats drank 3 ml). Thus, in the present experiment, the intake of saccharin on the critical trial was tightly matched in terms of volume and variance thereby permitting greater confidence in the quality of the comparisons of the evoked c-Fos expression in the Novel and Familiar rats.
The second issue concerns the nature of taste neophobia. Conventionally, taste neophobia is viewed as an innate avoidance response (e.g., Barnett,1958; Richter, 1953). By this analysis the detection of novelty influences only the amount consumed; palatability (or hedonic value) is not considered a factor in taste avoidance. However, Lin, Amodeo, Arthurs, and Reilly (2012) found that taste neophobia involves not only a reluctance to consume the novel taste but also that palatability is at its lowest level on initial exposure to a novel taste. Palatability was found to increase as familiarity with the taste increased with each benign exposure, until asymptote was achieved. Thus, the detection of taste novelty triggers an innate aversive response not an avoidance response - the novel edible is treated as distasteful, and ingestion is stalled.
Thirdly, the rate at which learning accrues to a taste stimulus is profoundly influenced by the novelty of that taste. This phenomenon, termed latent inhibition (Lubow, 1989), is readily shown in the acquisition of CTAs. Specifically, the development of a CTA takes significantly fewer taste-malaise pairings when the taste is novel relative to when the taste is familiar (e.g., Lubow, 2009; Roman, Lin, & Reilly, 2009). Thus, the speed at which CTAs are acquired can provide valuable information to aid interpretation of taste neophobia performance consequent to brain manipulations.
Turning to the c-Fos results. As noted, six brain areas showed increased immunoreactivity consequent to the ingestion of a small volume (3 ml) of a novel saccharin solution BLA, GC, GT, CNA, mPBN and lPBN. We interpret these results as definitive evidence that these structures are among the first neural components engaged in the processing of unknown taste stimuli.
Brain manipulations (e.g., lesions, pharmacological treatments) of the BLA result in an increased intake of novel, but not familiar, taste solutions (Lin Arthurs & Reilly, 2018; Lin, Roman, St. Andre & Reilly, 2009; Miranda, LaLumiere, Buen, Bermudez-Rattoni & McGaugh, 2003), which suggests that the experimental animals were responding as-if the novel taste was familiar to them. Supporting this interpretation, the same brain manipulations delay, but do not prevent, acquisition of CTAs to novel taste solutions but have no influence on CTA acquisition when the taste solution is familiar (e.g., Aggleton, Petrides, & Iversen, 1981; Roman, Lin, & Reilly, 2009; Shimai & Hoshishima, 1982; St. Andre & Reilly, 2007). Interestingly, brain manipulations of the GC results in the same type of deficits in taste neophobia (Gutiérrez, Téllez, Bermúdez-Rattoni, 2003; Lin, Arthurs & Reilly, 2018; Lin, Roman, St. Andre & Reilly, 2009) and CTA acquisition (Kiefer & Braun, 1977; Roman, Lin, & Reilly, 2009; St. Andre & Reilly, 2007). Furthermore, unilateral asymmetric lesions of the BLA and GC result in the same taste neophobia deficit as bilateral lesions of either structure (Lin & Reilly, 2012), indicating that the BLA and GC form a functional unit operating in unison but performing interdependent, not identical, roles in taste neophobia.
At first glance, the role of the GT in taste neophobia appears to be like that of the BLA and GC in so far as brain manipulations of the GT attenuate taste neophobia (Arthurs, Lin & Reilly, 2018; Arthurs & Reilly, 2013). However, because lesions of the GT have no effect on the acquisition of CTAs (Arthurs & Reilly, 2013; Mungarndee, Lundy, & Norgren, 2006; Reilly, Bornovalova, Dengler & Trifunovic, 2003), GT must be performing taste neophobia function different from that of the BLA and GC.
In addition to the present work, immunohistochemical research implicates the CNA in taste neophobia (e.g., Koh, Wilkins, & Bernstein, 2003; Lin, Roman, Arthurs & Reilly, 2012). There is, however, little indication in the literature what that role might involve. Although there appear to be no studies that have directly evaluated the effect of CNA manipulations on taste neophobia, a few reports have evaluated the effects of CNA lesions on CTA acquisition. These studies, reviewed in Reilly and Bornovalova (2005), consistently find little, if any, effect of the bilateral lesions on the amount consumed on the first taste-malaise trial (where any disruption of neophobia might be expected to be manifest) or on the rate of CTA acquisition across trials. Furthermore, in a latent inhibition study using excitotoxic lesions (to avoid damaging gustatory axons of passage) and which controlled the amount of solution consumed during taste familiarization and on the first taste-malaise pairing (to avoid any lesion-induced intake differences), St. Andre and Reilly (2007) found no effect of CNA lesions on the acquisition of CTA to a novel or to a familiar taste solution. Thus, it would appear that the CNA has a qualitatively different role in taste neophobia than the BLA, GC or GT. One, easily testable, hypothesis is that the CNA may be a component of the circuit responsible for modulating palatability/disgust responses. Supportive of this notion, Tanaka, Li, Mukae, and Tanabe (2021) reported increased aversive orofacial responses (indicative of disgust; e.g., Grill & Norgren, 1978; Parker, Limebeer & Rana, 2009) and elevated c-Fos in the CNA consequent to intraoral infusions of a bitter-tasting quinine solution. An assessment of palatability changes, using lick pattern analysis (Davis, 1989; Dwyer, 2012) during the initial occurrence of, and recovery from, taste neophobia in rats with CNA lesions would be particularly helpful in testing this hypothesis.
The most studied taste-guided behavior consequent to brain lesions of the PBN is taste aversion learning and multiple publications indicate that the PBN is essential for this behavior (for reviews see Norgren & Grigson, 1996; Reilly, 1999, 2009; Spector, 1995). Moreover, the findings that mPBN lesions spare, among other functions, flavour preference learning and conditioned aversions to aqueous capsaicin (a trigeminal stimulus) while preventing, in the same animals, CTA acquisition indicates a role for the mPBN in the associative mechanism that specifically links prior taste with subsequent malaise (Grigson, Reilly, Scalera, & Norgren, 1998; Grigson, Reilly, Shimura, & Norgren, 1998; Reilly, Grigson, & Norgren, 1993). A different pattern of impaired and spared functions follow lPBN lesions supports the conclusion that such damage diminishes sensitivity to gastrointestinal feedback, both negative and positive, which in turn precludes aversive and appetitive associative learning (Reilly & Trifunovic, 2000a, 2000b). Thus, the mPBN and lPBN have different, and specific, roles in CTA acquisition. To our knowledge, the effects of mPBN lesions on taste neophobia have not been investigated, except for examination of performance on the first CTA learning trial (i.e., taste consumption before malaise onset). Because rats with mPBN lesions respond in a concentration-dependent manner to taste stimuli (e.g., Flynn, Grill, Schwartz, & Norgren, 1991; Spector, Grill & Norgren, 1993; Spector, Scalera, Grill, & Norgren, 1995), the elevated intake on first exposure to the novel taste stimulus suggests an attenuation of taste neophobia in the lesioned animals. On the other hand, lesions of the lPBN are reported to have a selective deficit on taste neophobia (completely abolishing the neophobic reaction to alanine and saccharin but having no influence on the neophobic response to quinine; Reilly &Trifunovic, 2001). Can the taste neophobia deficits in rats with mPBN or lPBN lesions be explained in terms of the same disruptions responsible for the absence of CTA in those animals? Since the mPBN CTA deficit is attributed to a lesion-induced disruption of learning (to associate the taste with the malaise) and taste neophobia is an innate aversive response it is unlikely that the same disruption can account for both deficits. Although the absence of aversive gastrointestinal feedback is the basis for the attenuation of taste neophobia, the initial occurrence of taste neophobia is keyed off the novelty of the taste stimulus. So, again, it is unlikely that the deficits in rats with lPBN lesions can be attributed to disruption of the same function. We conclude that the mPBN and lPBN have different functions in CTA and taste neophobia processing.
Turning now to the target areas that showed no differential immunoreactivity to novel versus familiar saccharin. These null results are difficult to interpret for several reasons. For example, (i) the area may be involved in taste neophobia but during a different time window from that investigated herein (i.e., 15 min drinking period); (ii) the area may be involved but it has an inhibitory function (i.e., neural activity that cannot be detected by c-Fos immunoreactivity; Kovács, 2008; Stark, Davies, Williams & Luckman, 2006); (iii) neurons in the area may require more sustained activation than provided in the present procedure (i.e., activation occurred but was below threshold for c-Fos expression); (iv) the area may be involved when the taste is novel (trial 1) and also when the taste is familiar (trial 6), as perhaps may be the case for the PrC (Gutierrez et al., 2004; Morillas et al., 2017). Of course, the area may not be involved in taste neophobia.
Although the rNST, BNST, and LH are components of the central gustatory system, there appears to be no reports that have specifically investigated whether they contribute to taste neophobia (none of these structures appears to affect CTA acquisition consequent to bilateral lesions; e.g., Grigson, Shimura & Norgren, 1997; Roman, Nebieridze, Sastre, & Reilly, 2006; but see Dayawansa, Ruch, & Norgren, 2014). The NAc has been extensively investigated as part of the brain reward system and is involved in many processes, including, for example, addiction and feeding (e.g., Norgren, Hajnal, & Mungarndee, 2006; Volkow, Michaelides, & Baler, 2019). Pharmacological studies have implicated the NAc, particularly the shell, in the attenuation of taste neophobia (Grau-Perales et al., 2020; Ramírez-Lugo, Zavala-Vega, Pedroza-Llinas, Núñez-Jaramillo, & Bermúdez-Rattoni, 2015).
The NBM has a role in taste neophobia. That is, during exposure to a novel taste stimulus activity in the NBM modulates acetylcholine release in the GC, which, in turn, affects intake (Rodríguez-García & Miranda, 2016; for review see; Miranda et al., 2003; Osorio-Gómez, Guzmán-Ramos, & Bermúdez-Rattoni, 2018). In addition, Arieli, Gerbi, Shein-Idelson and Moran (2020) have proposed that a BLA-NBM-GC circuit is important for the processing of novel tastes. Thus, the absence of c-Fos in the present study was surprising. Neural manipulations of the PrC, which has reciprocal connections with the BLA and GC (Agster, Pereira, Saddoris, & Burwell, 2016; Pereira, Agster, & Burwell, 2016), indicate a role for the cortical area in the neophobic reaction and/or the attenuation of taste neophobia (De la Cruz, Rodriguez-Ortiz, Balderas, & Bermudez-Rattoni, 2008; Gutierrez, De la Cruz, Rodriguez-Ortiz, & Bermudez-Rattoni, 2004; Morillas et al., 2017; Ramos 2015, 2020).
Finally, the present study replicated the finding in our original report (Lin, Roman et al., 2012) that c-Fos expression in the MeA was not influenced by consumption of a novel taste stimulus. Because lesions of the MeA attenuate taste neophobia (Lin, Roman et al., 2009; see also Arthurs & Reilly, 2013) it was proposed in that earlier c-Fos article that the MeA may have an inhibitory function in taste neophobia. That speculation was confirmed by Arthurs, Lin and Reilly (2018) who found that that chemogenetic inhibition of MeA neurons enhanced the strength of the neophobic response to a novel saccharin solution. However, this involvement of the MeA in neophobia does not have a knock-on effect to the acquisition of CTA, which is not disrupted by MeA lesions (e.g., Rollins, Stines, McGuire & King, 2001; Yamamoto, Fujimoto, Shimura & Sakai, 1995; for a review see Reilly & Bornovalova, 2005).
The present study aimed to extend the analysis of neural circuits underpinning the detection of taste novelty and intake modulation of novel substances involved in taste neophobia. To this effect, we analysed the expression of c-Fos in fourteen brain structures, including those known to relay taste information within the central gustatory system and other related structures, after ingestion of a small volume of either a novel or familiar saccharin solution. The six structures that displayed increased c-Fos immunoreactivity after consuming a novel saccharin solution (BLA, GC, GT, CNA, mPBN, lPBN) confirm their involvement in the taste neophobia circuit; however, the sole absence of increased immunoreactivity does not disprove, of itself, the involvement of any given region. While undifferentiated immunoreactivity in a studied region could indicate a lack of its involvement, it could also, for example, result from the region being active within a different time window than tested, not reaching sufficiently sustained activation, or operating in an inhibitory role. The apparent simplicity of the behavioral response in the expression of taste neophobia (licking or not licking the solution) belies, as the present results show, the involvement of a complex network of neural structures from brainstem to cortex. We have used c-Fos to help identify some of the components of this network, but other components are also likely to be involved. These structures may be exposed with further c-Fos studies, and the engagement of other methods may yield interesting results. Indeed, the recruitment of techniques that allow for more sensitive and specific detection of neural activity will be essential if a system-level understanding of the neural structures and processes involved in taste neophobia is to be achieved.
4. Experimental procedures
4.1. Animals
Ten experimentally naive male Sprague-Dawley rats, weighing ~300 g, were obtained from Charles Rivers Laboratories (Wilmington, MA). On arrival in the laboratory, they were individually housed in polycarbonate cages (26.5 x 48 x 20 cm) in a vivarium maintained at ~22 °C with a 12:12 h light-dark cycle (lights on at 7.00 am) and given free access to food (Teklad 2018 diet; Envigo, Madison, WI) and water. Prior to the experimental procedures, water availability was gradually reduced to two 15-min access periods, one in the mornings and, 4-hr later, the second in the afternoon. The procedures used in this study were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago and comply with guidelines of the American Psychological Association (2012) and the National Institutes of Health (2011).
4.2. Procedure
When water intake stabilized on the deprivation schedule described above, the animals were counterbalanced according to their weight and assigned to one of two groups: Novel (n = 6) and Familiar (n = 4). All behavioral testing occurred in the home cages. Taste trials (0.3% saccharin) were spaced 3 days apart and separated by two water days to allow rehydration from any reduced fluid intake occasioned by the taste trials. Saccharin trials always occurred in the morning access periods. On Trial 1 all animals received 15-min access to saccharin, except Group Novel were capped at 3 ml; the rats in Group Novel were perfused 90 min later. Group Familiar rats were given 4 more saccharin trials with 15-min free access to saccharin. The sixth trial for Group Familiar rats was limited to 3 ml access to saccharin (to match the amount consumed by the Novel group on Trial 1); the rats in Group Familiar were perfused 90 min later.
4.3. Histology
Each rat was anesthetized with Fatal Plus (Vortech, Dearborn, MI) at a dose of 300 mg/kg and subsequently transcardially perfused with heparinized saline followed by ice cold 10% formalin. The brain of each rat was dissected immediately thereafter and was stored in the same formalin solution overnight, then transferred into a phosphate buffered saline (PBS) solution kept at 4*C for short term storage. Each brain was divided into 3 blocks which were sectioned at 50 μm on a vibratome (Leica VT1000S, Buffalo Grove, IL) in the coronal plane. Sections from each block were processed during one session to avoid inconsistencies of c-Fos staining. Table 2 provides information about the number of sections used for analysis of each target area, the anteroposterior range of those sections, and which sub nuclei were included in the analysis.
4.4. Immunohistology
The sections were transferred into 12-well tissue culture plates and treated with 1% H2O2 for 15 min, then washed and blocked in a PBS-TR solution (PBS + 0.2% Triton-X) (Sigma-Aldrich, St. Louis, MO) and 5% NGS (Normal Goat Serum; Vector Laboratories, Burlingame, CA) for 1 h. Next, the sections were transferred into a PBS-TR solution with the primary rabbit c-Fos antibody (Encore Biotechnology, Gainesville, FL) at a dilution of 1:20,000 with 1% NGS, and incubated for 48 h. The sections were then washed in PBS and transferred into a PBS-TR solution containing the secondary goat anti-rabbit biotinylated antibody (Vector Laboratories) at a dilution of 1:500 with 1% NGS and incubated overnight. The following day, sections were washed in PBS and transferred into a PBS solution containing the avidin-biotin reagent (Vector Laboratories) at a dilution of 1:500 and incubated overnight. Following another washing, the sections were visualized using a 3,3 ′-diaminobenzidine (DAB) kit (Vector Laboratories). Next, all sections were mounted on glass slides and dried, then cleared in Xylene and cover slipped using Permount mounting medium (Fisher Scientific, Waltham, MA). The sections were visualized and documented on a Leica microscope system (Olympus BX43, Olympus, Waltham, MA) and the resulting images were analyzed for the number of c-Fos expressing cells in each area of interest using a custom algorithm in ImageJ software.
4.5. Statistical Analysis
All analyses were conducted using GraphPad Prism version 9.3.1 for Windows (GraphPad Software, San Diego, CA). The across-trials analysis of Group Familiar intake employed a one-way analysis of variance followed by Fisher's Least significant difference (LSD) test for specific comparisons. Assessment of the taste-evoked c-Fos counts for the same target area in each group was achieved using separate independent t-tests. The α level was set at .05.
Research Highlights.
We examined c-Fos expression in the central gustatory system and related structures after either novel or familiar saccharin was consumed.
The novel taste induced higher levels of c-Fos expression than familiar taste in BLA, CNA, GT, GC, mPBN, and lPBN.
The potentiated c-Fos expression induced by novel tastes was not found in the BNST, MeA, rNST, LH, NAc, NAcS, NBM and the PrC
Acknowledgments
This research was supported by grant DC06456 from the National Institute of Deafness and Other Communication Disorders. Jan Wiaderkiewicz is now at the Department of Physiology and Biophysics, University of Illinois at Chicago, 1853 W Polk St, Chicago, IL 60612, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Jan Wiaderkiewicz: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization. Steve Reilly: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing - Original Draft, Writing - Review & Editing, Project administration, Funding acquisition.
REFERENCES
- Alden M, Besson JM, Bernard JF, 1994. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J. Comp. Neurol 341, 289–314. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Petrides M, Iversen SD, 1981. Differential effects of amygdaloid lesions on conditioned taste aversion learning by rats. Physiol. Behav 27, 397–400. [DOI] [PubMed] [Google Scholar]
- Agster KL, Pereira IT, Saddoris MP, Burwell RD, 2016. Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. II. Efferents. Hippocampus, 26, 1213–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association, 2012. Guidelines for ethical conduct in the care and use of nonhuman animals in research. Washington DC: American Psychological Association. [Google Scholar]
- Arieli E, Gerbi R, Shein-Idelson M, Moran A 2020. Temporally-precise basolateral amygdala activation is required for the formation of taste memories in gustatory cortex. J. Physiol 598, 5505–5522 [DOI] [PubMed] [Google Scholar]
- Arthurs J, Lin J-Y, Reilly S, 2018. Inhibiting gustatory thalamus or medial amygdala has opposing effects on taste neophobia. Neurobiol. Learn. Mem 156, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthurs J, Reilly S, 2013. Role of the gustatory thalamus in taste learning. Behav. Brain Res 250, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA, 1958. Experiments on “neophobia” in wild and laboratory rats. Brit. J. Psychol 49, 195–201. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM, 1993. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J. Comp. Neurol 329, 201–229. [DOI] [PubMed] [Google Scholar]
- Bester H, Bourgeais L, Villanueva L, Besson JM, Bernard JF, 1999. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHA-L study in the rat. J. Comp. Neurol 405, 421–449. [PubMed] [Google Scholar]
- Cho YK, Mao L, Li CS, 2008. Modulation of solitary taste neurons by electrical stimulation of the ventroposteromedial nucleus of the thalamus in the hamster. Brain Res. 1221, 67–79. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Beckstead RM, Norgren R, 1982. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J. Autonom. Nerv. Syst 6, 303–322. [DOI] [PubMed] [Google Scholar]
- Corson J, Aldridge A, Wilmoth K, Erisir A, 2012. A survey of oral cavity afferents to the rat nucleus tractus solitarii. J. Comp. Neurol 520, 495–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, 1989. The microstructure of ingestive behavior. Annals of the New York Academy of Sciences, 575, 106–121. [DOI] [PubMed] [Google Scholar]
- Dayawansa S, Ruch S, Norgren R, 2014. Parabrachial-hypothalamic interactions are required for normal conditioned taste aversions. Am. J. Physiol. Regul. Integr. Comp. Physiol 306, R190–R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz V, Rodriguez-Ortiz CJ, Balderas I, & Bermudez-Rattoni F, 2008. Medial temporal lobe structures participate differentially in consolidation of safe and aversive taste memories. Eur. J. Neurosci 28, 1377–1381. [DOI] [PubMed] [Google Scholar]
- Domjan M, 1977. Attenuation and enhancement of neophobia for edible substances. In: Barker LM, Best M, Domjan M (Eds.), Learning mechanisms in food selection. Baylor University Press: Waco, TX, pp. 151–179. [Google Scholar]
- Dovey TM, 2018. Avoidant/restrictive food intake disorder: An eating disorder on a spectrum with food neophobia. In: Reilly S (Ed.). Food neophobia: Behavioral and biological influences. Elsevier. pp. 329–349. [Google Scholar]
- Dwyer DM, 2012. Licking and liking: the assessment of hedonic responses in rodents. Q. J. Exp. Psychol 65, 371–394. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ, Schwartz GJ, Norgren R, 1991. Central gustatory lesions: I. Preference and taste reactivity tests. Behav. Neurosci 105, 933–943. [DOI] [PubMed] [Google Scholar]
- Grau-Perales AB, Expósito AN, Gómez-Chacón B, Morón I, Gallo M, 2020. Accumbens and amygdala in taste recognition memory: The role of d1 dopamine receptors. Neurobiol. Learn. Mem 174, 107277. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Reilly S, Scalera G, Norgren R, 1998. The parabrachial nucleus is essential for acquisition of a conditioned odor aversion in rats. Behav. Neurosci 112, 1104–1113. [PubMed] [Google Scholar]
- Grigson PS, Reilly S, Shimura T, Norgren R, 1998. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: Further evidence for an associative deficit. Behav. Neurosci 112, 160–171. [PubMed] [Google Scholar]
- Grigson PS, Shimura T, Norgren R, 1997. Brainstem lesions and gustatory function: II. The role of the nucleus of the solitary tract in Na+ appetite, conditioned taste aversion, and conditioned odor aversion in rats. Behav. Neurosci 111, 169–177. [PubMed] [Google Scholar]
- Grill HJ, Norgren R, 1978. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science, 201, 267–269. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, De la Cruz V, Rodriguez-Ortiz CJ, & Bermudez-Rattoni F, 2004. Perirhinal cortex muscarinic receptor blockade impairs taste recognition memory formation. Learn. Mem 11, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R, Téllez LA, Bermúdez-Rattoni F, 2003. Blockade of cortical muscarinic but not NMDA receptors prevents a novel taste from becoming familiar. Eur. J. Neurosci 17, 1556–1562. [DOI] [PubMed] [Google Scholar]
- Halsell CB, 1992. Organization of parabrachial nucleus efferents to the thalamus and amygdala in the golden hamster. J. Comp. Neurol 317, 57–78. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB, 1990. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol 293, 540–580. [DOI] [PubMed] [Google Scholar]
- Kang Y, Lundy RF, 2009. Terminal field specificity of forebrain efferent axons to brainstem gustatory nuclei. Brain Res. 1248, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Lundy RF, 2010. Amygdalofugal influence on processing of taste information in the nucleus of the solitary tract of the rat. J. Neurophysiol 104, 726–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB, 1998. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res. 813, 283–302. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Braun JJ, 1977. Absence of differential associative responses to novel and familiar taste stimuli in rats lacking gustatory neocortex. J. Comp. Physiol. Psychol 91, 498–507. [DOI] [PubMed] [Google Scholar]
- Koh MT, Wilkins EE, Bernstein IL, 2003. Novel tastes elevate c-fos expression in the central amygdala and insular cortex: implication for taste aversion learning. Behav. Neurosci 117, 1416–1422. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R, 1986. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 379, 342–352. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, 2008. Measurement of immediate-early geneactivation-c-fos and beyond. J. Neuroendocrinol 20, 665–672. [DOI] [PubMed] [Google Scholar]
- Lin J-Y, Amodeo LR, Arthurs J, Reilly S, 2012. Taste neophobia and palatability: the pleasure of drinking. Physiol. Behav 106, 515 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S, 2017. Conditioned taste aversions: From poisons to pain to drugs of abuse. Psychon. Bull. Rev 24, 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S, 2018. The effects of amygdala and cortical inactivation on taste neophobia. Neurobiol. Learn. Mem 155, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-Y, Reilly S, 2012. Amygdala-gustatory insular cortex connections and taste neophobia. Behav. Brain Res 235, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Roman C, Arthurs J, Reilly S, 2012. Taste neophobia and c-Fos expression in the rat brain. Brain Res. 1448, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-Y, Roman C, St. Andre J, Reilly S, 2009. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 1251, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, 1989. Latent Inhibition and Conditioned Attention Theory. Cambridge University Press, Cambridge. [Google Scholar]
- Lubow RE, 2009. Conditioned taste aversion and latent inhibition: A review. In Reilly S, Schachtman TR (Eds.), Conditioned taste aversion: Behavioral and neural processes (pp. 37–57). New York: Oxford University Press. [Google Scholar]
- Mao L, Cho YK, Li CS, 2008. Modulation of activity of gustatory neurons in the hamster parabrachial nuclei by electrical stimulation of the ventroposteromedial nucleus of the thalamus. Am. J. Physiol 294, R1461–R1473. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray T, Saper CB, 1990. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J. Comp. Neurol 295, 624–661. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Ferreira G, Ramırez-Lugo L, Bermudez-Rattoni F, 2003. Role of cholinergic system on the construction of memories: Taste memory encoding. Neurobiol. Learn. Mem 80, 211–222. [DOI] [PubMed] [Google Scholar]
- Miranda MI, LaLumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL, 2003. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. European J. Neurosci 18, 2605–2610. [DOI] [PubMed] [Google Scholar]
- Morillas E, Gómez-Chacón B Gallo M, 2017. Flavor and object recognition memory impairment induced by excitotoxic lesions of the perirhinal cortex. Neurobiol. Learn. Mem 144, 230–234. [DOI] [PubMed] [Google Scholar]
- Mungarndee SS, Lundy RF, Norgren R, 2006. Central gustatory lesions and learned taste aversions: Unconditioned stimuli. Physiol. Behav 87, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A, 2000. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci. Res 36, 297–309. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, 2011. Guide for the care and use of laboratory animals. Washington DC: National Academy Press. [Google Scholar]
- Nicklaus S, Monnery-Patris S, 2018. Food neophobia in children and its relationships with parental feeding practices/style. In: Reilly S (Ed.). Food neophobia: Behavioral and biological influences (pp. 255–286). Elsevier. [Google Scholar]
- Norgren R, 1976. Taste pathways to hypothalamus and amygdala. J. Comp. Neurol 166, 12–30. [DOI] [PubMed] [Google Scholar]
- Norgren R, Grigson PS, 1996. The role of the central gustatory system in learned taste aversion. In Ono T, McNaughton B, Molotchnikoff S, Rolls E, & Nishijo H (Eds.), Perception, memory, and emotion: Frontiers in neuroscience (pp. 479–497). Elmsford, NY: Pergamon Press. [Google Scholar]
- Norgren T, Hajnal A, Mungarndee SS, 2006. Gustatory reward and the nucleus accumbens. Physiol. Behav 89, 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, Leonard CM, 1973. Ascending central gustatory pathways. J. Comp. Neurol 150, 217–238. [DOI] [PubMed] [Google Scholar]
- Osorio-Gómez D, Guzmán-Ramos K, Bermúdez-Rattoni F, 2018. Neurobiology of neophobia and its attenuation: Neural substrates and palatability. In: Reilly S (Ed.). Food neophobia: Behavioral and biological influences. Elsevier, pp. 111–128. [Google Scholar]
- Ottersen OP, 1982. Connections of the amygdala complex of the rat. IV: corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J. Comp. Neurol 205, 30–48. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Ben-Ari Y, 1979. Afferent connections to the amygdaloid complex of the rat and cat. J. Comp. Neurol 187, 401–424. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rana SA, 2009. Conditioned disgust, but not taste avoidance, may reflect conditioned nausea in rats. In Reilly S & Schachtman TR (Eds.), Conditioned taste aversion: Behavioral and neural processes (pp. 92–113). New York, NY: Oxford University Press. [Google Scholar]
- Paxinos G, & Watson C, 2005. The rat brain in stereotaxic coordinates (5th ed.). San Diego, California: Academic Press. [Google Scholar]
- Pereira IT, Agster KL, Burwell RD, 2016. Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. I. Afferents. Hippocampus, 26, 1189–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, Le Doux JE, 1997. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 20, 517–523. [DOI] [PubMed] [Google Scholar]
- Ramos JMJ, 2015. Differential contribution of perirhinal cortex and hippocampus to taste neophobia: Effect of neurotoxic lesions. Behav. Brain Res 284, 94–102. [DOI] [PubMed] [Google Scholar]
- Ramos JMJ, 2020. Perirhinal cortex supports both taste neophobia and its attenuation. Neurobiol. Learn. Mem 173, 107264. [DOI] [PubMed] [Google Scholar]
- Ramírez-Lugo L, Nunez-Jaramillo L, Bermúdez-Rattoni F., 2007. Taste memory formation: role of nucleus accumbens. Chem. Senses, 32, 93–97. [DOI] [PubMed] [Google Scholar]
- Ramírez-Lugo L, Zavala-Vega S, Pedroza-Llinas R, Núñez-Jaramillo L, Bermúdez-Rattoni F, 2015. Effects of glutamate and its metabotropic receptors class 1 antagonist in appetitive taste memory formation. Behav. Brain Res 284, 213–217. [DOI] [PubMed] [Google Scholar]
- Reilly S, 1999. The parabrachial nucleus and conditioned taste aversion. Brain Res. Bull 48, 239–254. [DOI] [PubMed] [Google Scholar]
- Reilly S, 2009. Central gustatory system lesions and conditioned taste aversion. In Reilly S & Schachtman TR (Eds.), Conditioned taste aversion: Behavioral and neural processes (pp. 309–327). New York, NY: Oxford University Press. [Google Scholar]
- Reilly S (Ed.) 2018a. Food neophobia: Behavioral and biological influences. Elsevier. [Google Scholar]
- Reilly S, 2018b. Taste neophobia: Neural substrates and palatability. In: Reilly S (Ed.). Food neophobia: Behavioral and biological influences. Elsevier, pp. 77–109. [Google Scholar]
- Reilly S, Bornovalova M, 2005. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neurosci. Biobehav. Rev 29, 1067–1088. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova MA, Dengler C, Trifunovic R, 2003. Effects of excitotoxic lesions of the gustatory thalamus on latent inhibition and blocking of conditioned taste aversion in rats. Brain. Res. Bull 62, 117–128. [DOI] [PubMed] [Google Scholar]
- Reilly S, Grigson PS, Norgren R, 1993. Parabrachial nucleus lesions and conditioned taste aversion: Evidence supporting an associative deficit. Behav. Neurosci 107, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Reilly S, & Schachtman TR (Eds.). 2009. Conditioned taste aversion: Behavioral and neural processes. Oxford, UK: Oxford University Press. [Google Scholar]
- Reilly S, Trifunovic R, 2000a. Lateral parabrachial nucleus lesions in the rat: Long- and short-duration gustatory preference tests. Brain Res. Bull 51, 177–186. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R 2000b.Lateral parabrachial nucleus lesions in the rat: Aversive and appetitive gustatory conditioning. Brain Res. Bull 52, 269–278. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R, 2001. Lateral parabrachial nucleus lesions in the rat: Neophobia and conditioned taste aversion. Brain Res. Bull 55, 359–366. [DOI] [PubMed] [Google Scholar]
- Revusky S, Garcia J, 1970. Learned aversions over long delays. In: Bower GM, Spence JT (Eds). Psychology of learning and motivation: Advances in research and theory, 4. Academic Press: New York, pp. 1–84. [Google Scholar]
- Richter CP, 1953. Experimentally produced behavior reactions to food poisoning in wild and domesticated rats. Ann. N.Y. Acad. Sci 56, 225 239. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García G, Miranda MI, 2016. Opposing roles of cholinergic and GABAergic activity in the insular cortex and nucleus basalis magnocellularis during novel recognition and familiar taste memory retrieval. J. Neurosci 36, 1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BL, Stines SG, McGuire HB, King BM, 2001. Effects of amygdala lesions on body weight, conditioned taste aversion, and neophobia. Physiol. Behav 72, 735–742. [DOI] [PubMed] [Google Scholar]
- Roman C, Lin J-Y, Reilly S, 2009. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Res. 1259, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S, 2006. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav. Neurosci, 120, 1257–1267. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD, 1998. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J. Comp. Neurol 399, 440–468. [DOI] [PubMed] [Google Scholar]
- Shimai S, Hoshishima K, 1982. Effects of bilateral amygdala lesions on neophobia and conditioned taste aversion in mice. Percept. Motor Skills, 54, 127–130. [DOI] [PubMed] [Google Scholar]
- Spector AC, 1995. Gustatory function in the parabrachial nuclei: Implications from lesion studies in rats. Rev. Neurosci 6, 143–175. [DOI] [PubMed] [Google Scholar]
- Spector AC, Grill HJ, Norgren R, 1993. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol. Behav 53, 277–283. [DOI] [PubMed] [Google Scholar]
- Spector AC, Scalera G, Grill HJ, Norgren R, 1995. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav. Neurosci 109, 939–954. [PubMed] [Google Scholar]
- St. Andre J, Reilly S, 2007. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav. Neurosci 121, 90–99. Correction printed in Behav. Neurosci. 121,1363. [DOI] [PubMed] [Google Scholar]
- Stark JA, Davies KE, Williams SR, Luckman SM, 2006. Functional magnetic resonance imaging and c-Fos mapping in rats following an anorectic dose of m-chlorophenylpiperazine. Neuroimage, 31, 1228–1237. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Li S, Mukae S, Tanabe T, 2021. Genetic recombination in disgust-associated bitter taste-responsive neurons of the central nucleus of amygdala in male mice. Neurosci. Lett 742,135456. [DOI] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD, 2009. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neurosci. 161, 475–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Herkenham M, 1991. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J. Comp. Neurol 313, 295–325. [DOI] [PubMed] [Google Scholar]
- Veening JG, 1978. Subcortical afferents of the amygdaloid complex in the rat: an HRP study. Neurosci. Let 8, 197–202. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE, 1984. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 303, 337–357. [DOI] [PubMed] [Google Scholar]
- Volkow N,D, Michaelides M, & Baler R, 2019. The neuroscience of drug reward and addiction. Physiol. Rev 99, 2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC, Bergula A, Holliday K, 2000. Forebrain projections to the rostral nucleus of the solitary tract in the hamster. J. Comp. Neurol 422, 429–447. [PubMed] [Google Scholar]
- Williams KE, Seiverling LJ, 2018. Neophobia in children with special needs: Selective eating and its treatment. In: Reilly S (Ed.). Food neophobia: Behavioral and biological influences. Elsevier, pp. 351–371. [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N, 1995. Conditioned taste aversion in rats with excitotoxic brain lesions. Neurosci. Res 22, 31–49. [DOI] [PubMed] [Google Scholar]