Abstract
Magnetization transfer (MT) is a neuroimaging technique that is frequently used to characterize the biophysical abnormalities in both gray and white matter regions of the brain. In our study, we used MT to examine the integrity of key nodes in frontal-subcortical circuits in four subject groups: patients diagnosed with type 2 diabetes with and without major depression (MDD), a healthy control group, and a group diagnosed with MDD without diabetes. In the MDD group, MT studies demonstrated lower magnetization transfer ratios (MTR), a marker of abnormalities in the macromolecular protein pool, in the thalami when compared with the control groups. The group with diabetes and MDD showed lower MTR in the globus pallidus when compared with the group with MDD. Biophysical measures, in subcortical nuclei, correlated inversely with measures of glycemic control, cerebrovascular burden and depression scores. These findings have broad implications for the underlying neuronal circuitry and neurobiology of mood disorders.
INTRODUCTION
Diabetes is a common metabolic disorder and it is estimated that ~ 26 million individuals had diabetes in 2010 and an additional 79 million had pre-diabetes (1). Diabetes results in striking economic consequences and it is estimated that one in five health-care dollars is spent to support the care of patients with diabetes (1). Diabetes is associated with compromise to multiple organ systems including the brain, heart, and kidney, and is associated with several mental health comorbidities including depression, anxiety, and eating disorders (2–7). In addition, diabetes is an established risk factor for dementia of the Alzheimer type (5, 6).
Clinically significant mood disorders occur in 10–20% of patients diagnosed with type 2 diabetes (7). Mood disorders associated with diabetes result in poor compliance and poorer medical and psychosocial outcomes (2). These include a decrease in quality of life, poor glycemic control, and increased unemployment, disability, health care expenditures, and diabetic complications (8, 9). Despite the consistent clinical and economic observations, the biological underpinnings of depression in patients with type 2 diabetes remain rudimentary.
Cortical-subcortical circuits have been implicated in several behavioral disorders (10–13). Multiple neuroimaging modalities including structural, functional, and microstructural approaches have been used to characterize the integrity of specific circuits in psychiatric disorders (11–13). The neural substrates underlying mood disorders are widely distributed in the brain (14) and connectomics-based approaches, using diffusion tensor imaging, have identified the default mode network and the cortical-subcortical network as being particularly impaired in a sample of patients with unipolar depression when compared with controls (15–18).
Magnetization transfer (MT) is a noninvasive magnetic resonance-related approach that permits us to examine the biophysical status of macromolecular proteins in cortical and subcortical regions (19–23). MT has been used to characterize the status of macromolecular proteins in several clinical brain disorders including mood disorders, schizophrenia, and multiple sclerosis (12). In an earlier report using MT, we demonstrated that major depression (MDD) in patients with diabetes is associated with lower magnetization transfer ratios (MTR) in the head of the caudate nucleus (12), bilaterally, when compared with our two comparison groups, healthy and diabetic controls (DC). More recently, we used MT to study the biophysical integrity of critical nodes in three cortical-subcortical circuits – the lateral dorsolateral prefrontal, the orbitofrontal, and the anterior cingulate circuits in patients with type 2 diabetes without any mood disturbance and healthy controls (HC) (23). Biophysical abnormalities were identified in the anterior cingulate and the head of the caudate nucleus on the right side in patients with type 2 diabetes when compared with controls (23). Further, in a study of patients with unipolar MDD without diabetes and comparison subjects without depression, we identified significantly lower MTR in the right caudate nucleus in the MDD group (11).
A limitation of our earlier MT study was the lack of a control group with MDD without diabetes (12). This limitation precluded us from commenting on the biological underpinnings of depression associated with diabetes when compared with patients diagnosed with unipolar depression without diabetes. The purpose of our current study was to use MT imaging to study the integrity of the macromolecular protein pool in the anterior cingulate, lateral orbitofrontal, and dorsolateral prefrontal circuits – the three major cortical-subcortical circuits that have been implicated in the control and regulation of behavior. We proposed to examine distinct structures – cortical and subcortical nodes – associated with these circuits in four groups of patients: patients diagnosed with type 2 diabetes with and without MDD, a HC group, and a group diagnosed with MDD without diabetes. This design would permit us to examine the impact of depression and diabetes on regional MTR independently, and thereby comment on the differential involvement of brain regions and circuits in specific subtypes of MDD.
MATERIALS AND METHODS
Subjects
The subjects belonged to four primary groups: patients with type 2 diabetes with (Diabetic Depressed; DD) and without MDD (DC), a non-diabetic control group (HC), and a group of patients with unipolar depression without diabetes (depressed, MDD) (Table 1). The study was approved by the UIC Institutional Review Board and written informed consent was obtained from all participants. Established clinical criteria were used to diagnose MDD.
Table 1.
Clinical and Demographic Characteristics
| HC | DC | DD | MDD | |
|---|---|---|---|---|
| n | 38 | 21 | 22 | 32 |
| Age (years) | 62.6±12.1 | 65.1±11.6 | 56.1±10.0 | 58.5±12.6 |
| Male : Female | 16 : 22 | 12 : 9 | 10 : 12 | 9 : 23 |
| Education years | 15.4±2.5 | 14.8±2.2 | 15.3±2.2 | 15.4±2.5 |
| CIRS | 3.7±2.7 | 7.3±3.1 | 11.8±4.4 | 8.2±3.5 |
| MMSE | 29±1 | 28.43±1.2 | 29.0±1.1 | 28.8±1.4 |
| HbA1c | 5.7±0.3 | 7.4±1.7 | 8.6±2.5 | 5.8±0.4 |
Abbreviations: CIRS, Cumulative Illness Rating Scale; DC, diabetic control; DD, diabetic depressed; HbA1c, hemoglobin A1c level; HC, healthy control; MDD, depressed; MMSE, mini-mental state examination.
The diagnosis of type 2 diabetes mellitus in patients was made by their primary-care physicians and was confirmed using the American Diabetes Association guidelines (an elevated non-fasting hemoglobin A1c level [>6.5% (48 mmol mol−1)] or the use of antidiabetic medications (oral hypoglycemic and/or insulin) when enrolled for this study. Type 2 diabetes mellitus patients reported using oral hypoglycemic medications and/or insulin for glycemic control. HC subjects were free of diabetes and had hemoglobin A1c levels within normal limits. All clinical methods, including inclusion and exclusion criteria, have been previously reported (12, 23) and are available online (see Supplementary eMethods).
MT Image Data Acquisition
Magnetic resonance imaging was performed on a Philips Achieva 3T scanner (Philips Medical Systems, Best, the Netherlands). MT images were acquired using a three-dimensional spoiled gradient-echo sequence with multi-shot echo-planar imaging readout: TR/TE = 64/15 ms, flip angle = 9°, field of view = 24 cm, 67 axial slices, slice thickness/gap = 2.2 mm/no gap, echo-planar imaging factor = 7, voxel size = 0.83 × 0.83 × 2.2 mm3, with a nonselective five-lobed Sinc-Gauss off-resonance MT prepulse (B1/Δf/dur=10.5μT/1.5kHz/24.5ms) optimized for maximum white matter/gray matter contrast (24). Parallel imaging was utilized with a reduction factor of 2 (25). Before the MT scan, high-resolution three-dimensional T1-weighted magnetization prepared rapid acquisition gradient echo images and T2-weighted fluid-attenuated inversion recovery images were also acquired for image registration and delineation of hyperintense areas in the brain (Supplementary eMethods).
Image Processing
T1-weighted magnetization prepared rapid acquisition gradient echo image, T2-weighted fluid-attenuated inversion recovery image, and MT images (with and without the MT prepulse: Ms and M0) were co-registered. MTR values were calculated on a voxel-by-voxel basis using the formula MTR = (M0-Ms)/M0. Regions of interest (ROIs) were placed on the co-registered T1-weighted image at the nodes of fronto-striato-thalamic circuits (10), including four subcortical regions, that are, head of caudate nucleus, putamen, globus pallidus, and thalamus, and three cortical regions, that are, anterior cingulate cortex, dorsolateral prefrontal cortex, and lateral orbitofrontal cortex in both hemispheres (see Fig. 1). Care was taken to ensure consistent placement of the subcortical ROIs for the MTR analysis; see Supplementary eMethods. During the placement of the subcortical ROIs, the co-registered fluid-attenuated inversion recovery image was closely examined to ensure that the ROIs were not placed in hyperintense areas. Moreover, we used constant volumes of ROIs in all of the defined subcortical regions, that is, 73.3 mm3 for head of caudate nucleus, putamen and thalamus, and 55 mm3 for globus pallidus. These two volumes were selected so that the MTR calculation in each subcortical ROI could be devoid of any partial volume effects from adjoining brain regions on all the involved subjects. For the three cortical ROIs, that are, anterior cingulate cortex, dorsolateral prefrontal cortex, and lateral orbitofrontal cortex (see Figs. 1c, d, and e), we used the FreeSurfer package (https://surfer.nmr.mgh.harvard.edu/) to parcellate these structures and evaluated MTR in each region. Generation of ROI masks and calculation of MTR were performed using in-house developed programs.
Figure 1.
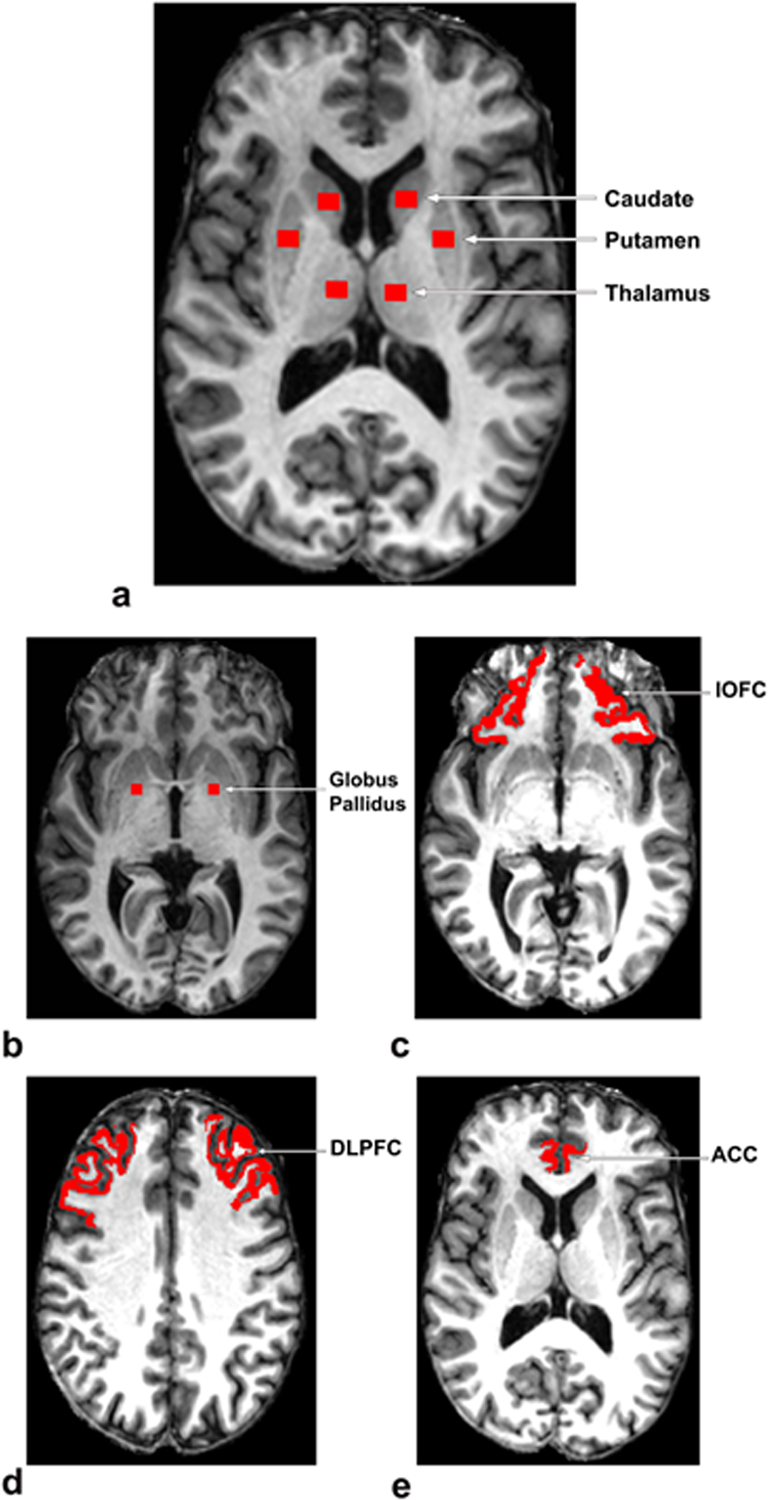
Regions of Interest (Head of Caudate Nucleus, Putamen, Thalamus, Globus Pallidus, lOFC, DLPFC, ACC) for MTR Analysis
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; lOFC, lateral orbitofrontal cortex.
Statistical Analysis
We used a mixed-effects linear model with random subject effects to incorporate within-subject correlations. Multiple MTR observations are nested within the same subject, hence it is expected that there will be within-subject correlations. Fixed covariates, age and sex, are added in the model because we see that age negatively impacts MTR (t = −4.45, P-value<0.001) and females have higher MTR (t = 2.23, P-value = 0.026) compared with males across all groups. Therefore, group comparisons are adjusted for age and sex. In addition, group comparisons are made using region-specific group means and standard deviations (s.d.) of MTR data. The random subject variance of our model is significant (z-value = 5.73, P-value < 0.0001), which justifies the effectiveness of our approach compared with analysis of covariance.
RESULTS
Model-based MTR adjusted mean values (with standard errors) for all regions are provided in Table 2. In Table 2, we find that estimated standard error remains the same in all regions within a group; however, it varies from one group to the other because of different sample sizes. Region-specific group comparisons in Table 2 reveal that mean MTR values adjusted for age and sex in the bilateral thalamus were significantly smaller (P-value ≤ 0.05) in the MDD group when compared with the HC and DC groups. In addition, adjusted mean MTR in the right thalamus of the DD group was lower than the DC group. Adjusted mean MTR in the right caudate was significantly lower in DD when compared with the HC group. Adjusted mean MTR was significantly lower in the DD when compared with the DC group in the bilateral pallidus and, in addition in the right pallidus, MTR was significantly lower in the DD when compared with the MDD group.
Table 2.
Regional Estimated MT Means and Standard Errors Adjusting for Age and Sex
| Region | HC (n=38) |
DC (n=21) |
DD (n=22) |
MDD (n=32) |
|---|---|---|---|---|
| L Caudate | 0.448 ± .011 | 0.439 ± .013 | 0.440 ± .012 | 0.446 ± .011 |
| R Caudate | 0.450 ± .011a | 0.431 ± .013 | 0.426 ± .012a | 0.438 ± .011 |
| L Thalamus | 0.524 ± .011b | 0.526 ± .013c | 0.512 ± .012 | 0.497 ± .011b,c |
| R Thalamus | 0.513 ± .011b | 0.528 ± .013c,d | 0.496 ± .012d | 0.490 ± .011b,c |
| L Pallidus | 0.473 ± .011 | 0.478 ± .013 | 0.457 ± .012e | 0.479 ± .011e |
| R Pallidus | 0.462 ± .011 | 0.470 ± .013d | 0.446 ± .012d,e | 0.475 ± .011e |
| L Putamen | 0.450 ± .011 | 0.460 ± .013 | 0.456 ± .012 | 0.444 ± .011 |
| R Putamen | 0.448 ± .011 | 0.454 ± .013 | 0.444 ± .012 | 0.445 ± .011 |
| L lOFC | 0.455 ± .011 | 0.450 ± .013 | 0.444 ± .012 | 0.443 ± .011 |
| R lOFC | 0.436 ± .011 | 0.431 ± .013 | 0.422 ± .012 | 0.426 ± .011 |
| L DLPFC | 0.404 ± .011 | 0.410 ± .013 | 0.409 ± .012 | 0.404 ± .011 |
| R DLPFC | 0.404 ± .011 | 0.406 ± .013 | 0.407 ± .012 | 0.400 ± .011 |
| L ACC | 0.439 ± .011 | 0.441 ± .013 | 0.434 ± .012 | 0.438 ± .011 |
| R ACC | 0.428 ± .011 | 0.429 ± .013 | 0.418 ± .012 | 0.422 ± .011 |
Abbreviations: ACC, anterior cingulate cortex; DC, diabetic control; DD, diabetic depressed; DLPFC, dorsolateral prefrontal cortex; HC, healthy control; lOFC, lateral orbitofrontal cortex; L, left; MDD, depressed; R, right.
Significant difference between HC and DD.
Significant difference between HC and MDD.
Significant difference between DC and MDD.
Significant difference between DC and DD.
Significant difference between MDD and DD.
Regions with significant group differences in MTR are highlighted in boldface.
We present region-specific comparisons in box plots (see Figure 2). The box plots are constructed using adjusted means and empirical Bayes estimates of random effects.
Figure 2.
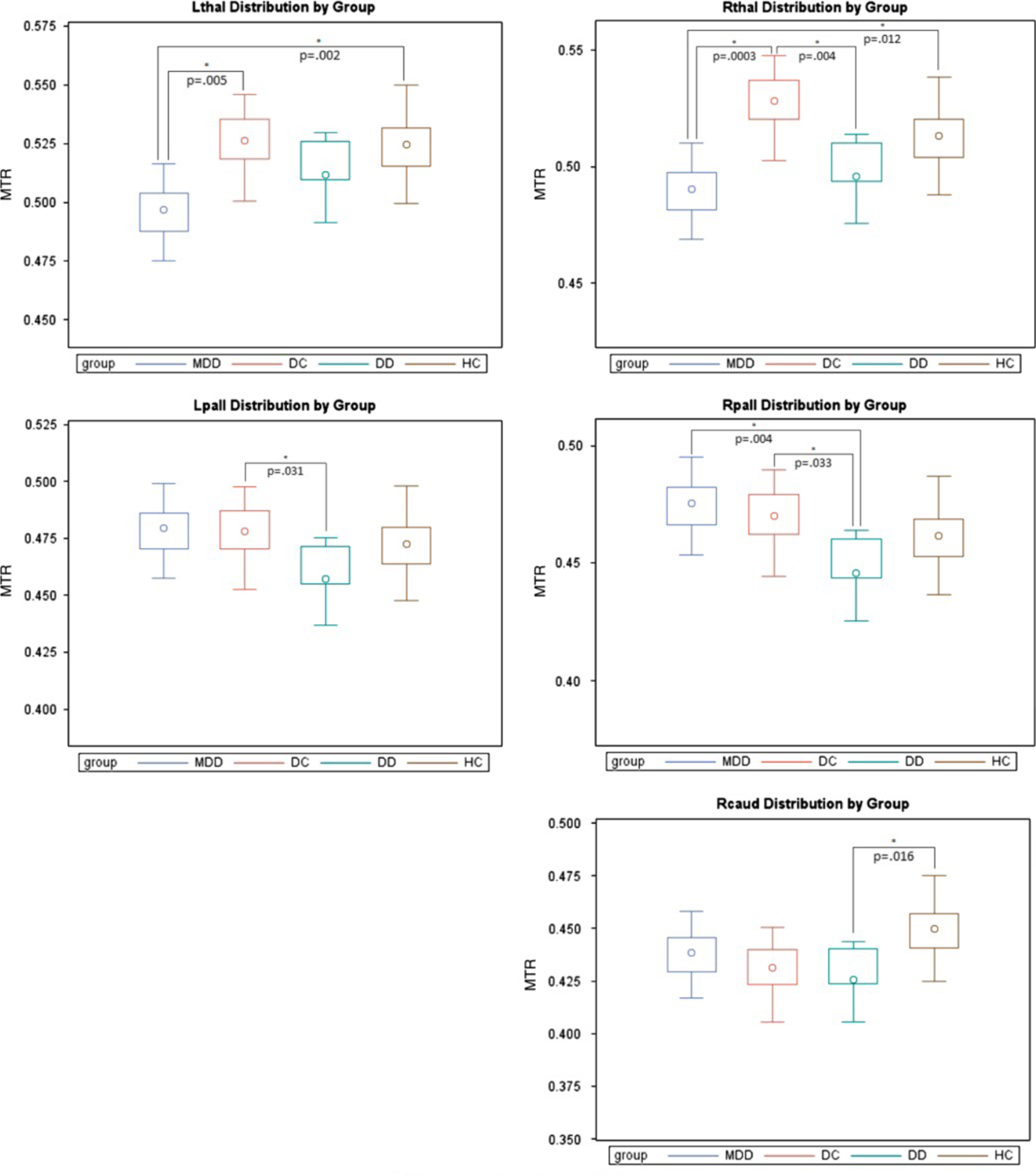
Regional MTR Distributions by Group
L, left; Lpall, left pallidus; Lthal, left thalamus; R, right; Rcaud, right caudate; Rpall, right pallidus; Rthal, right thalamus.
Pairwise comparisons were made for each region using both sided t-tests (level of significance = 0.05) to detect region-based group differences using raw, uncorrected data.
In Table 3, we provide region-specific adjusted (by age and sex) correlations of MTR in regions where there were statistically significant group differences with the Framingham Stroke Risk Profile total score (26)), hemoglobin A1c levels, and the Center for Epidemiological Study of Depression (27). All significant relationships were in biologically expected directions. Adjusted correlations between MTR and Framingham Stroke Risk Profile total score are statistically significant in the right caudate and the right globus pallidus and approach significance in the left globus pallidus. MTR in the right caudate correlates inversely with hemoglobin A1c levels, and MTR in the bilateral thalami show an inverse correlation with Center for Epidemiological Study of Depression scores across the entire sample.
Table 3.
Correlation Matrix for Significant Results Adjusting for Age and Sex [r(p)]
| Left Pallidus | Right Caudate | Right Pallidus | Left Thalamus | Right Thalamus | |
|---|---|---|---|---|---|
| FSRP TOTAL | −0.18 (0.0655) | −0.31 (0.0008) | −0.22 (0.0233) | 0.02 (0.8612) | −0.01 (0.9270) |
| HbA1c | −0.09 (0.3592) | −0.31 (0.0009) | −0.04 (0.6609) | −0.03 (0.7953) | −0.02 (0.8605) |
| CESD | 0.01 (0.9330) | −0.14 (0.1566) | 0.00 (0.9731) | −0.19 (0.0500) | −0.24 (0.0135) |
Abbreviations: CESD, Center for Epidemiological Study of Depression; FSRP, Framingham Stroke Risk Profile; HbA1c, hemoglobin A1c level.
DISCUSSION
Our study demonstrates biophysical compromise to several subcortical structures in the frontal-subcortical circuits in patients with MDD with and without type 2 diabetes when compared with non-depressed control subjects. Notably, patterns of regional biophysical compromise differed in the two subtypes of depression. In patients with unipolar MDD without diabetes, the thalamus, bilaterally, demonstrated biophysical changes with lower MTR when compared with the control groups. In the group with diabetes and MDD, the globus pallidus was compromised biophysically, bilaterally, when compared with the DC and additionally on the right side when compared with the group with unipolar depression. Further, we partially replicated our earlier observation of lower MTR in the right caudate in the group with type 2 diabetes mellitus and depression when compared with our HC. We did not detect any biophysical changes in cortical regions between any of the groups studied. Across the entire sample, MTR values in key subcortical nuclei declined with greater cerebrovascular risk, poor glycemic control, and higher depression scores.
Frontal-subcortical circuits have been consistently implicated in the pathophysiology of psychiatric disorders (11, 28). Three of the five circuits – the anterior cingulate, lateral orbitofrontal, and the dorsolateral prefrontal circuits – have an established role in the regulation of behavior and cognition and our findings underscore the importance of subcortical structures in the pathophysiology of MDD (11, 12, 28). In our earlier reports, we emphasized biophysical impairments in the caudate in both patients with type 2 diabetes and MDD and in patients diagnosed with unipolar depression without diabetes when compared with appropriate comparison groups (11, 12). In those studies, whereas MTR differences between MDD and control groups in the caudate were striking and statistically significant, lower MTR was also detected in other subcortical regions, though the patient-control differences did not reach statistical significance. In the earlier study comparing patients with unipolar depression and HC, MTR values in the left thalamus were lower in the MDD group, with a moderate effect size, though the differences were not statistically significant (11). In the study that compared patients with diabetes, with and without MDD, to controls, MTR values in the putamen were also lower in the group with MDD when compared with the control groups, though statistically insignificant (12). Our current study extends our earlier observations to include a broader range of subcortical structures in the pathophysiology of MDD. They demonstrate that multiple nodes in cortical-subcortical circuits might be impaired in patients with mood disorders, though the magnitude of compromise varies across regions. The inclusion of the group with unipolar depression without diabetes makes our current study a more complete one and permits us to compare and characterize the regional biophysical correlates of two subtypes of depression detected using MT.
Molecular imaging approaches help to identify alterations in key protein macromolecules in the gray and white matter (19–22). The primary outcome measure of MT imaging in vivo is the MTR, which reflects the underlying compromise to the protein compartment (19, 22). Post mortem MT and histopathology studies reveal that lower MTR in the white matter is associated with axonal loss and myelin compromise (29, 30). The origin of the MTR changes in the gray matter is more complex and heterogeneous and may reflect multiple neurobiological aberrations (31–34). In the gray matter cell membrane, proteins and phospholipids contribute to the macromolecular density that contributes to the signal (32). Injury to cell membranes, reductions in dendritic density and in neuronal size and numbers may, alone or in combination, be responsible for the decrease in MTR in the gray matter (32). Lower MTR has been reported across a spectrum of behavioral and neurological disorders as it represents underlying changes in neurochemistry and opens a window to the study of proteins and phospholipids in the brain.
Information from diffusion tensor imaging studies of specific white matter tracts has been used to examine the connectomics of the brain in mood disorders (15, 35, 36). A recent study using a connectomics-based, whole-brain analysis identified tracts associated with frontal-subcortical circuits and the default mode network as biophysically compromised in patients with unipolar depression when compared with controls (15). Zhang et al. (35) from our laboratory demonstrated lower FA and higher radial diffusivity in the anterior limb of the internal capsule in patients with MDD when compared with HC. The anterior limb of the internal capsule is the primary white matter bundle that connects the cortex with subcortical structures and nuclei. In that study, diabetes additionally contributed to the lower FA in our sample. Our current data indicate that frontal-subcortical circuit dysfunction is not limited to white matter tracts but includes biological dysfunction in specific subcortical structures including the thalamus, head of the caudate nucleus, and the globus pallidus.
All three of the frontal-subcortical circuits examined have anatomical connections from the globus pallidus/substantia nigra complex to specific nuclei in the thalamus (10, 37, 38). Efferents from the thalamus to specific cortical regions – the anterior cingulate, orbitofrontal, and the dorsal prefrontal regions – complete the frontal-striatal-pallidal-thalamic-cortical loops (10). Abnormalities in the thalamus have been demonstrated in neuroimaging and histological studies of patients with psychiatric disorders (39, 40). Increased thalamic functional activity has been demonstrated in the thalamus in patients with MDD (41) and increase thalamic metabolism pre-operatively predicted a positive response to cingulotomy in patients with refractory depression (42). Attenuated functional connectivity between the striatum and the ventromedial prefrontal cortex and stronger functional connectivity between the dorsal caudate and dorsoprefrontal cortex have been demonstrated in patients with MDD when compared with controls (13). Collectively, our findings are consistent with earlier reports that identify the thalamus and related subcortical structures as physiologically and biophysically compromised in patients with MDD during the acute episode (43). Abnormalities detected using functional magnetic resonance imaging studies should incorporate the underlying biophysical and related abnormalities for a more complete understanding of the biological integrity of these circuits in humans. Preferential connectivity, wherein some nuclei of the thalamus have stronger connectivity with key frontal regions, has been described anatomically (39). Improved resolution, with high-field magnets, will make it possible to obtain more precise information on specific nuclei in subcortical structures in the future using in vivo imaging.
The pathophysiology of diabetes includes both hyperglycemia and vascular compromise. Changes to the macro and microvasculature provide the basis for serious end-organ damage seen in patients with type 2 diabetes (44). This includes myocardial infarction, stroke, and renal and retinal damage. In our sample, the predicted correlations between regional MTR values in subcortical regions and measures of glycemic control and total cerebrovascular burden in the entire group are consistent with established pathophysiological findings and suggest that both mechanisms may be contributing to the mood changes observed in our patient groups. These observations are consistent with our finding that depression, when associated with diabetes, may impact different subcortical regions when compared with unipolar depression without diabetes. Striatal neurons are particularly vulnerable to biological insults including hypercortisolemia, excitatory amino acids, and neurodegeneration (45). The lower MTR in the striatum may, in part, be a reflection of the vulnerability of these neurons to metabolic injury associated with diabetes (45).
Depression is a heterogeneous behavioral syndrome that lacks etiologically specificity (46). There are many clinically recognized subtypes of depression such as post-stroke depression, depression associated with degenerative and medical disorders, and postpartum depression. The underlying neuronal abnormalities that lead to depression likely reside in several neuronal circuits with overlapping functions and may vary with the subtype of depression. The term ‘disconnection syndrome’ (47) has been broadly applied to neurobehavioral and psychiatric disorders on the premise that the primary abnormalities in psychiatric disorders reside in the connectivity between regions as opposed to the specific gray matter regions themselves. Voxel-based diffusion tensor imaging studies of patients with mood disorders and our findings demonstrating biological abnormalities in patients with MDD in key subcortical regions indicate that the biological abnormalities in frontal-subcortical circuits in patients with MDD reside in both subcortical nuclei and the white matter tracts that connect them.
Our data suggest that the differential involvement of the thalami and globus pallidus varies in our patients with MDD as opposed to patients with type 2 diabetes and MDD. The neurobiological mechanisms mediating depression in the context of diabetes may differ from the depression that is unassociated with diabetes. The differential involvement of subcortical nuclei in these two groups supports this preliminary assertion. Studies using larger samples of subtypes of MDD are needed to more directly address this question. Nonetheless, our data demonstrate significant biophysical compromise to key components of specific cortical-subcortical circuits. These findings have broad implications for the pathophysiology of mood disorders.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institute of Mental Health grants 5R01MH063764-09, 5R01MH073989-05, 5K23MH081175-04, and 1K01AG040192-01A1. We would like to thank Peter van Zijl and Joseph S. Gillen (Johns Hopkins University) for the MT sequence, which was developed by the support of the National Institute of Biomedical Imaging and Bioengineering resource grant P41 EB015909.
Footnotes
The authors declare no conflicts of interest.
Disclaimers: The authors have nothing to disclose.
Supplemental information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Seaquist E. Addressing the Burden of Diabetes. Journal of the American Medical Association. 2014;311(22):2267–8. [DOI] [PubMed] [Google Scholar]
- 2.Ducat L, Philipson L, Anderson B. The Mental Health Comorbidities of Diabetes. Journal of the American Medical Association. 2014;312(7):691–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaffe K, Falvey C, Hamilton N, Shchwartz A, Simonsick E, Satterfield S, et al. Diabetes, Glucose Control, and 9-Year Cognitive Decline Among Older Adults Without Dementia. Arch Neurol. 2012;69(9):1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan M, Katon W, Lovato L, Miller M, Murray A, Horowitz K, et al. Association of Depression With Accelerated Cognitive Decline Among Patients With Type 2 Diabetes in the ACCORD-MIND Trial. JAMA Psychiatry. 2013;70(10):1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawlings A, Sharrett A, Schneider A, Coresh J, Albert M, Couper D, et al. Diabetes in Midlife and Cognitive Change Over 20 Years. Ann Intern Med. 2014;161:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biessels G, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 7.Gavard J, Lustman P, Clouse R. Prevalence of depression in adults with diabetes: an epidemiological evaluation. Diabetes Care. 1993;16(8):1167–78. [DOI] [PubMed] [Google Scholar]
- 8.Egede L, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25(3):464–70. [DOI] [PubMed] [Google Scholar]
- 9.Katon W, Feltz-Cornelis C. Treatment of depression in patients with diabetes: efficacy, effectiveness and maintenance trials and new service models. In: Katon W, Maj M, Sartorius N, editors. Depression and Diabetes. Hoboken, NJ: John Wiley & Sons; 2010. p. 81–107. [Google Scholar]
- 10.Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–81. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Yang S, Ajilore O, Wu M, Charlton R, Lamar M. Subcortical biophysical abnormalities in patients with mood disorders. Molecular Psychiatry. 2014;19:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Gupta R, Thomas A, Ajilore O, Hellemann G. Focal Subcortical Biophysical Abnormalities in Patients Diagnosed with Type 2 Diabetes and Depression. Arch Gen Psychiatry. 2009;66:324–30. [DOI] [PubMed] [Google Scholar]
- 13.Furman D, Hamilton J, Gotlib I. Frontostriatal functional connectivity in major depressive disorder. Biology of Mood & Anxiety Disorders. 2011;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandya M, Altinay M, Malone D Jr., Anand A. Where in the Brain is Depression? Curr Psychiatry Rep. 2012;14:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korgaonkar M, Fornito A, Williams L, Grieve S. Abnormal Structural Networks Characterize Major Depressive Disorder: A Connectome Analysis. Biol Psychiatry. 2014;76(7):567–74. [DOI] [PubMed] [Google Scholar]
- 16.Bi Y, He Y. Connectomics Reveal Faulty Wiring Patterns for Depressed Brain. Biol Psychiatry. 2014;76(7):515–6. [DOI] [PubMed] [Google Scholar]
- 17.Ajilore O, Zhan L, GadElkarim J, Zhang A, Feusner J, Yang S, et al. Constructing the resting state structural connectome. Frontiers in Neuroinformatics. 2013;7(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlton R, Leow A, GadElkarim J, Zhang A, Ajilore O, Yang S, et al. Brain Connectivity in Late-Life Depression and Aging Revealed by Network Analysis. American Journal of Geriatric Psychiatry. 2015; 23(6): 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng J, Ceckler TL, Balaban RS. Quantitative 1H magnetization transfer imaging in vivo. Magnetic Resonance in Medicine. 1991;17(2):304–14. [DOI] [PubMed] [Google Scholar]
- 20.Balaban RS, Ceckler TL. Magnetization transfer contrast in magnetic resonance imaging. Magn Reson Q. 1992; 8(2):116–37. [PubMed] [Google Scholar]
- 21.Grossman RI. Magnetization transfer in multiple sclerosis. Annals of Neurology. 1994;36(S1):S97–S99. [DOI] [PubMed] [Google Scholar]
- 22.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001; 14(2):57–64. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Ajilore O, Wu M, Lamar M, Kumar A. Impaired Macromolecular Protein Pools in Fronto-Straito-Thalamic Circuits in Type 2 Diabetes Revealed by Magnetization Transfer Imaging. Diabetes. 2015;64(1):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith S, Farrell J, Jones C, Reich D, Calabresi P, van Zijl P. Pulsed magnetization transfer imaging with body coil transmission at 3 Tesla: feasibility and application. Magn Reson Med. 2006;56(4):866–875. [DOI] [PubMed] [Google Scholar]
- 25.Pruessmann K, Weiger M, Scheidegger M, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952–962. [PubMed] [Google Scholar]
- 26.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–8. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- 28.Price J, Drevets W. Neurocircuitry of Mood Disorders. Neuropsychopharmacology Reviews. 2010;35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Waesberghe JHTM, Kamphorst W, De Groot CJA, Van Walderveen MAA, Castelijns JA, Ravid R, et al. Axonal loss in multiple sclerosis lesions: Magnetic resonance imaging insights into substrates of disability. Annals of Neurology. 1999;46(5):747–54. [DOI] [PubMed] [Google Scholar]
- 30.Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. Journal of Magnetic Resonance Imaging. 2007;26(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaleeli Z, Altmann DR, Cercignani M, Ciccarelli O, Miller DH, Thompson AJ. Magnetization transfer ratio in gray matter: a potential surrogate marker for progression in early primary progressive multiple sclerosis. Arch Neurol. 2008. Nov;65(11):1454–9. [DOI] [PubMed] [Google Scholar]
- 32.Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003. Aug;60(8):779–88. [DOI] [PubMed] [Google Scholar]
- 33.Audoin B, Davies G, Rashid W, Fisniku L, Thompson AJ, Miller DH. Voxel-based analysis of grey matter magnetization transfer ratio maps in early relapsing remitting multiple sclerosis. Multiple Sclerosis. 2007;13(4):483–9. [DOI] [PubMed] [Google Scholar]
- 34.Steens SC, Bosma GP, Steup-Beekman GM, le Cessie S, Huizinga TW, & van Buchem MA. Association between microscopic brain damage as indicated by magnetization transfer imaging and anticardiolipin antibodies in neuropsychiatric lupus. Arthritis Research & Therapy. 2006;8(2):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang A, Ajilore O, Zhan L, GadElkarim J, Korthauer L, Yang S, et al. White Matter Tract Integrity of Anterior Limb of Internal Capsule in Major Depression and Type 2 Diabetes. Neuropsychopharmacology. 2013;38:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadayonnejad R, Yang S, Kumar A, Ajilore O. Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS One. 2014;9(4):e96033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLong M. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13(7):281–5. [DOI] [PubMed] [Google Scholar]
- 38.Price J. Prefrontal cortical networks related to visceral function and mood. Ann NY Acad Sci. 1999;877:383–96. [DOI] [PubMed] [Google Scholar]
- 39.Metzger C, van der Werf Y, Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging--from animal anatomy to in vivo imaging in humans. Frontiers in Neuroscience. 2013;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cullen T, Walker M, Parkinson N, Craven R, Crow T, Esiri M, et al. A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophr Res. 2003;60:157–66. [DOI] [PubMed] [Google Scholar]
- 41.Greicius M, Flores B, Menon V, Glover G, Solvason H, Kenna H, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2006;62:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dougherty D, Weiss A, Cosgrove G, Alpert N, Cassem E, Nierenberg A, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99(6):1010–7. [DOI] [PubMed] [Google Scholar]
- 43.Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. Journal of Affective Disorders. 2015;172:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harati Y. Diabetes and the nervous system. Endocrinol Metab Clin North Am. 1996;25(2):325–59. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell I, Cooper A, Griffiths M. The selective vulnerability of striatopallidal neurons. Progress in Neurobiology. 1999;59(6):691–719. [DOI] [PubMed] [Google Scholar]
- 46.Levinson D, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray N, et al. Genetic Studies of Major Depressive Disorder: Why Are There No Genome-wide Association Study Findings and What Can We Do About It? Biol Psychiatry. 2014;76(7):510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


