Abstract
Genome-wide association studies have identified many loci associated with Alzheimer’s dementia. However, these variants only explain part of the heritability of Alzheimer’s disease (AD). As genetic epistasis can be a major contributor to the “missing heritability” of AD, we conducted genome-wide epistasis screening for AD pathologies in two independent cohorts. First, we performed a genome-wide epistasis study of AD-related brain pathologies (Nmax = 1,318) in ROS/MAP. Candidate interactions were validated using cerebrospinal fluid biomarkers of AD in ADNI (Nmax = 1,128). Further functional analysis tested the association of candidate interactions with neuroimaging phenotypes. For tau and amyloid-β (Aβ) pathology, we identified 2,803 and 464 candidate SNP-SNP interactions, respectively. Associations of candidate SNP-SNP interactions with brain volume and white matter changes from neuroimages provides additional insights into their molecular functions. Transcriptional analysis supported possible gene-gene interactions identified by statistical screening through their co-expression in the brain. In summary, we outlined an exhaustive epistasis analysis to identify novel genetic interactions with potential roles in AD pathologies. We further delved into the functional relevance of candidate interactions by association with neuroimaging phenotypes and analysis of co-expression between corresponding gene pairs.
Keywords: Alzheimer’s disease, Tau, Amyloid-β, Epistasis
1. Introduction
The genetic architecture underlying Alzheimer’s disease (AD) is complex. For the familial form of AD, which only accounts for 1%~5% of AD, mutations in APP, PSEN1, and PSEN2 usually guarantee early onset of AD (Tanzi, 2012). By contrast, large-scale genome-wide association studies (GWAS) identified common variants with small effects for Alzheimer’s dementia (Lambert et al., 2013). Yet, the complete genetic architecture for late-onset AD remains elusive. It is estimated that about a quarter of the phenotypic variance for late-onset AD can be explained by APOE combined with common variants (Lee et al., 2013; Ridge et al., 2013); this value is considerably lower than the estimated two-thirds of heritability from twin studies (Gatz et al., 2006). It has been proposed that epistasis, which refers to the departure from “independence” of the effects of different genetic loci in the way that they combine to cause disease, can help explain some of the “missing heritability” (Raghavan and Tosto, 2017). Therefore, in this study, we investigated the genetic interactions for Alzheimer’s pathology to unravel novel genetic mechanisms missed by traditional GWAS.
Previous work on epistasis in AD mainly focused on specific gene pair interactions supported by prior biological evidence. For example, proinflammatory interleukin-6 (IL-6) level is relatively higher in AD patients and the anti-inflammatory interleukin-10 (IL-10) level is relatively lower in AD patients (Remarque et al., 2001). Subsequently, the genetic interactions between IL-6 and IL-10 were reported in a recent study by measuring the synergy factor (SF = 1.69, P = 0.01) (Combarros et al., 2009b). Moreover, elevated iron levels in AD patients spurred an interest in the discovery of potential interactions in iron metabolism. As a result, the interaction between hemochromatosis gene (HFE) and transferrin gene (TF) was identified and replicated by subsequent studies (Kauwe et al., 2010; Robson et al., 2004).
To identify novel genes and genetic interactions for AD susceptibility, it is necessary to screen epistasis on a genome-wide scale. However, there are major challenges to screening statistical epistasis through the genome. First, statistical epistasis appearing in one dataset usually cannot be replicated in another dataset. For example, one study of more than 100 possible epistasis pairs reported in previous studies could only replicate 27 pairs in their own data (Combarros et al., 2009a). Second, the stringent P value required for significance resulting from adjusting for multiple hypothesis tests often cannot be attained (Murk and DeWan, 2016). In addition, exhaustive screening of all possible epistasis is computationally intensive.
Even for fast screening methods, such as “BOolean Operation-based Screening and Testing” (BOOST), nearly 60hrs are required to evaluate 6.5 × 1010 interactions between 360,000 SNPs on a standard 3.0 GHz computer with 4G memory (Wan et al., 2010). On a genome-wide scale, there has been only two replicated genetic interactions reported to be associated with Alzheimer’s dementia. One is the interaction between rs6455128 (KHDRBS2) and rs7989332 (CRYL1) which was discovered in a French cohort and replicated in another cohort from Germany (Gusareva et al., 2014). In a recent study, the interaction between rs3733980 (WWC1) and rs7175766 (TLN2) that was associated with AD in males was identified and validated by Drosophila eye experiments (Gusareva et al., 2018).
To solve the aforementioned problems in the genome-wide interaction analysis (Gusareva et al., 2014), we conducted epistasis screening in two stages and validated epistasis in another independent dataset. More importantly, we leveraged neuropathologic endophenotypes which have greater power for genetic associations (Bennett et al., 2009). During the first stage, possible SNP-SNP interactions for AD-related neuropathology in The Religious Orders Study and Rush Memory and Aging Project (ROS/MAP) were filtered using a simple linear regression model. We reported all candidate SNP-SNP interactions that passed a less conservative correction accounting for the total number of tests performed in second stage. After the two-stage analysis, validation of candidate SNP-SNP interactions was evaluated on a subset of samples from The Alzheimer’s Disease Neuroimaging Initiative (ADNI) with cerebrospinal fluid (CSF) biomarkers of AD measured. Furthermore, candidate gene-gene interactions mapped by the SNPs were ranked by their co-expression in the brain. As a result, credible interactions contributing to AD pathology were discovered and validated by multiple sources.
2. Methods
2.1. Study subjects
ROS/MAP are longitudinal clinical-pathologic cohort studies of aging and dementia (Bennett et al., 2018). The diagnosis of Alzheimer’s dementia for each subject was obtained from a neurologist by reviewing all available clinical data at the time of death, blinded to post-mortem data. Amyloid-β (Aβ) and tau neuropathology were measured using immunohistochemistry and automated image processing for total amyloid and paired helical filament tau (PHF-tau), and a modified Bielschowsky silver staining technique for neuritic plaques, diffuse plaques and neurofibrillary tangles (see Supplementary Method). A total of 2,090 individuals in ROS/MAP have been genotyped in two batches, of which 1,374 individuals received clinical consensus diagnosis of cognitive status (no/mild cognitive impairment or Alzheimer’s dementia). Of 1,579 autopsied persons at the time of these analyses, 1,326 had genotype data, of which 1,318 individuals had either one of five pathological measurements that passed quality control. Of the 1,318 individuals, 1,316 had neurofibrillary tangles, neuritic plaques and diffuse plaques measured, 1,285 had total PHF-tau measured, and 1276 had total amyloid measured.
ADNI (including phases1, GO, and 2) is an international cooperative study to investigate the biomarkers of AD and develop treatment to slow or stop Alzheimer’s dementia progression (Petersen et al., 2010). All subjects were administered clinical evaluations at the time of enrollment by expert physicians. CSF total tau (T-tau), phosphorylated tau (P-tau) and β-amyloid (1–42) (Aβ1–42) levels were measured by the electrochemiluminescence immunoassays (see Supplementary Method). There are 1,550 genotyped individuals with a diagnosis of Alzheimer’s dementia in ADNI, of which 1,128 individuals had either one of the three CSF biomarkers that passed quality control. Of the 1,128 individuals, 1,127 had CSF T-tau and P-tau measured, 1,128 had CSF Aβ1–42 measured. Characteristics of the study participants for ROS/MAP (N=2,090) and ADNI (N=1,550) are summarized in Table 1.
Table 1.
Characteristics of study participants from ROS/MAP (N=2090) and ADNI1/GO/2 (N=1550).
| ROS/MAP | AD (n=568) | Non-AD (n=1522) | Diff (P)* |
|---|---|---|---|
| Sex (F/M) | 392 F, 176 M | 1067 F, 455M | 0.63 |
| Age at death, y (SD) | 90.92 (5.89) | 88.09 (6.72) | < 0.0001 |
| Education, y (SD) | 16.22 (3.66) | 16.34 (3.51) | 0.49 |
| MMSE (SD) | 12.69 (8.55) | 26.33 (4.39) | < 0.0001 |
| APOE ε4 status (−/+) | −382, +186 (0.33) | −1232, +290 (0.19) | < 0.0001 |
|
| |||
| ADNI | AD (n=599) | Non-AD (n=951) | Diff (P)* |
|
| |||
| Sex (F/M) | 253 F, 346 M | 423 F, 528M | 0.4 |
| Age at AD, y (SD) | 74.71 (8.12) | 77.22 (7.40) | < 0.0001 |
| Education, y (SD) | 14.79 (4.64) | 15.99 (3.26) | < 0.0001 |
| MMSE (SD) | 22.19 (5.63) | 28.35 (2.30) | < 0.0001 |
| APOE ε4 status (−/+) | −220, +379 (0.63) | −629, +322 (0.34) | < 0.0001 |
ROS/MAP, The Religious Orders Study/the Rush Memory and Aging Project; ADNI, Alzheimer’s Disease Neuroimaging Initiative; AD, Alzheimer’s disease; Diff, statistical difference between AD and non-AD; F, female; M, male; SD, standard deviation; MMSE, Mini-Mental Status Examination score; Age at AD, age when developed AD for ADs or age at last valid record for non-ADs; APOE ε4 status (−/+), presence of ε4 allele.
P values are calculated by Fisher’s exact tests (for sex and APOE ε4 status) or two-sample t-tests (for age at death, age at AD, education and MMSE)
2.2. Molecular and structural neuroimaging
For ADNI, molecular and structural neuroimaging data were generated. A total of 855 individuals underwent structural magnetic resonance imaging (MRI) protocols to generate estimates of entorhinal cortex and hippocampal volume. To generate estimates of fractional anisotropy (FA) for five bilateral fronto-temporal-occipital and interhemispheric white matter tracts (sagittal stratum, hippocampal segment of cingulum bundle, splenium of corpus callosum, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus) implicated in AD, diffusion tensor volumes were first obtained from diffusion-weighted images. Based on diffusion tensor volumes, estimates of FA across the five brain regions were generated for 216 subjects.
2.3. Genotyping and imputation
A total of 1,708 individuals in ROS/MAP were genotyped using the Affymetrix GeneChip 6.0 (Affymetrix, Inc., Santa Clara, CA, USA) at the Broad Institute’s Center for Genotype or the Translational Genomics Research Institute. An additional batch in ROS/MAP was generated on 382 individuals using the Illumina HumanOmniExpress (Illumina, Inc, San Diego, CA, USA) at the Children’s Hospital of Philadelphia. In total, there are 2,090 genotyped individuals in ROS/MAP. A total of 757 ADNI1 subjects were genotyped using the Illumina Human610-Quad BeadChip (Illumina, Inc., San Diego, CA, USA). A total of 793 ADNIGO/2 subjects were genotyped using the HumanOmniExpress BeadChip (Illumina Inc., San Diego, CA, USA). In total, there are 1,550 genotyped individuals in ADNI.
To merge the two batches in ROS/MAP, genotype data for 382 individuals from the second batches were phased using Eagle (v2.4.1) (Loh et al., 2016) and imputed using Minimac3 (v2.0.1) (Das et al., 2016). To validate the SNP-SNP interactions identified by ROS/MAP, genotype data from ADNI1 and ADNIGO/2 subjects were merged after being phased and imputed by the same pipeline. First, the genotyping data were aligned to the human assembly GRCh37/hg19 using UCSC’s liftOver tool (Casper et al., 2017). Next, the alleles were checked to match with the GRCh37 reference sequence. Imputation were carried out as described in a previous study (Van Leeuwen et al., 2015), with1000 Genomes Phase3 integrated haplotypes being the reference panel (Consortium et al., 2015). Imputed variants with an imputation quality statistic (R2) below 0.3 were discarded. Furthermore, calls with uncertainty greater than 0.2 or import dosage certainty smaller than 0.8 were treated as missing.
Using subjects in ROS/MAP as the discovery cohort, SNPs were first filtered by Hardy-Weinberg Equilibrium test with a Bonferroni based nominal significance threshold of 0.05/714014 SNPs = 7.0 × 10−8. Then, SNPs with minor allele frequency < 0.05, or missing calling rate > 0.10 were removed. Additionally, SNPs were linkage disequilibrium (LD) pruned by a window size of 50 bp, window increment of 1 bp and LD threshold of r2 0.75. After these steps, 279409 of 714014 variants passed filters and quality control. All aforementioned quality control steps were performed using PLINK (v1.90b4.10) (Chang et al., 2015).
2.4. Epistasis screening methods
For ROS/MAP discovery cohort, epistasis was screened against neurofibrillary tangles, total PHF-tau, neuritic plaques, diffuse plaques and total amyloid. We removed those SNP-SNP pairs for which there were less than 3 observations in the lowest SNP × SNP contingency table cell, which could lead to spurious results. More specifically, only SNP-SNP pairs with a cell size either more than 3 or equal to 0 in each cell of the 3 × 3 genotype matrix were kept for further analysis, resulting in 12,547,471,105 independent SNP-SNP pairs to be evaluated. Therefore, when applying the Bonferroni procedure, our threshold for statistical significance was set to α = 3.98 × 10−12. Simple linear regression was used to construct a pool of possible SNP-SNP interactions in the first stage. SNP-SNP interactions with nominal P values < 1 × 10−5 were selected for further analysis. Epistasis screening were performed using PLINK (Chang et al., 2015) in the first stage analysis. In the second stage, the genotypic model (see Supplementary Method) implemented in INTERSNP (Herold et al., 2009), which contains both additive and dominant effects for each SNP and all four possible epistasis models, was applied. Rather than the usual 0, 1, 2 coding which represents the number of minor alleles, Cordell’s dominant coding was used to represent the genotype of each SNP in this model (Cordell, 2002). The effects of sex, age (determined by the date of death in ROS/MAP or date of examination in ADNI) and APOE genotype (number of ε4 alleles) were examined in the model as covariates. Finally, we report all SNP-SNP interactions as “suggestive” that passed a less conservative correction accounting for the total number of pairs analyzed in the second stage. These interaction results may warrant future investigation and are reported along with significant interactions in the supplementary tables.
SNP-SNP interactions that were identified in the discovery cohort were validated using the CSF biomarkers of AD in ADNI by the genotypic model described above. The union of candidate interactions for tau related pathologies (including neurofibrillary tangles and total PHF-tau) were furtherer tested for their association with CSF T-tau and P-tau levels in ADNI. While the union of candidate interactions for Aβ related pathologies (including neuritic plaques, diffuse plaques and total amyloid) were further tested for their association with CSF Aβ1–42 levels in ADNI. P values were Bonferroni corrected. To avoid spurious interactions caused by long-range LD, SNP-SNP interactions on the same chromosome with an R2 > 0.2 were filtered out. In addition, to deduce biological epistasis from the statistically significant epistasis, interactions were visualized by plotting the mean value of each cell in a continency table of 3 × 3 genotype combinations.
2.5. Gene expression analysis
RNA was extracted from the gray matter of the dorsolateral prefrontal cortex of 724 subjects from the ROS/MAP cohorts. These samples were quantified by Nanodrop, and quality was evaluated by Agilent Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). A total of 582 RNA-Seq samples met quality (Bioanalyzer RNA integrity (RIN) score >5) and quantity (5ug) thresholds. Then RNA-Seq data were processed by a parallelized and automatic pipeline, which includes trimming the beginning and ending bases from each read, identifying and trimming adapter sequences from reads, detecting and removing rRNA reads and aligning reads to the reference genome. Non-gapped aligner Bowtie was used to align reads to transcriptome reference, and then RSEM was applied to estimate expression levels for all transcripts. The FPKM were quantile normalized, and the potential batch effects were removed by the combat package in R (see Supplementary Method).
Co-expression between two interacting genes was calculated using Pearson correlation. Gene pairs were obtained by mapping SNPs in the SNP-SNP interactions to the nearest genes within a distance of 10 kb. Genes which were detected (FRKM > 0) in less than 100 samples were excluded from analysis. After co-expression analysis, gene-gene interactions were ranked by their co-expression P value.
3. Results
3.1. Genome-wide epistasis screening and validation
Genome-wide epistasis screening for neurofibrillary tangles, total PHF-tau, neuritic plaques, diffuse plaques and total amyloid were carried out in ROS/MAP. First, the Pearson correlation coefficients of five measurements were examined (Fig. 1). As expected, measurements of tau neuropathology, neurofibrillary tangles and total PHF-tau, were highly correlated. Measurements of Aβ neuropathology (neuritic plaques, diffuse plaques and total amyloid) showed moderate correlations. For neurofibrillary tangles and total PHF-tau, a total of 435,358 and 526,204 SNP-SNP interactions met the criterion of a cell size either more than 3 or equal to 0 and a nominal P threshold of 1 × 10−5 by linear regression in the first stage. In the second stage, 32,960 (neurofibrillary tangles) and 65,263 (total PHF-tau) suggestive SNP-SNP interactions were reported after adjusting for age, sex and APOE status using a genotypic model that includes both additive and dominant effects, as well as four different interaction terms. Among them, 2,700 (neurofibrillary tangles) and 9,005 (total PHF-tau) SNP-SNP interactions were significant under a Bonferroni based nominal significance threshold of 3.98 × 10−12. For validation, candidate SNP-SNP interactions discovered for neurofibrillary tangles and total PHF-tau in ROS/MAP were tested for their association with CSF T-tau and P-tau levels in ADNI. 2,286 and 2,330 SNP-SNP interactions were validated by T-tau and P-tau, respectively (Table 2). Among them, 270 and 294 SNP-SNP interactions which were significant under Bonferroni correction in ROS/MAP were validated by T-tau and P-tau, respectively (Supplemental Table S1, S2). There are 1,813 SNP-SNP interactions validated by both T-tau and P-tau and 2,803 SNP-SNP interactions validated by either T-tau or P-tau (Table 2), conforming with the high correlation between T-tau and P-tau levels (R2 = 0.98, see Supplementary Fig. S1).
Fig. 1.
Pair-wise Pearson correlation coefficients of neurofibrillary tangles, total PHF-tau, neuritic plaques, diffuse plaques and total amyloid in ROS/MAP.
Table 2.
ROS/MAP Stage1 and Stage2, as well as ADNI1/GO/2 replicated SNP-SNP interactions.
| ROS/MAP | ADNI | |||
|---|---|---|---|---|
|
| ||||
| Phenotype | First stage | Second stage | Phenotype | Validation |
| Neurofibrillary tangles | 435,358 | 32,960 | CSF T-tau/P-tau | 2286/2330 (2803) |
| Total PHF-tau | 526,204 | 65,263 | ||
|
| ||||
| Neuritic plaques | 305,415 | 3,980 | CSF Aβ1–42 | 464 |
| Diffuse plaques | 337,902 | 10,931 | ||
| Total amyloid | 305,302 | 3,816 | ||
ROS/MAP, The Religious Orders Study/the Rush Memory and Aging Project; ADNI, Alzheimer’s Disease Neuroimaging Initiative; AD, Alzheimer’s disease.
For neuritic plaques, diffuse plaques and total amyloid, a total of 305,415, 337,902 and 305,302 SNP-SNP interactions met the criterion of a cell size either more than 3 or equal to 0 and a nominal P threshold of 1 × 10−5 in the first stage. 3,980 (neuritic plaques), 10,931 (diffuse plaques) and 3,816 (total amyloid) suggestive SNP-SNP interactions were reported by genotypic model. Among them, 47 (neuritic plaques), 212 (diffuse plaques) and 20 (total amyloid) SNP-SNP interactions were significant under a Bonferroni based nominal threshold of 3.98 × 10−12. For validation, candidate SNP-SNP interactions discovered for neuritic plaques, diffuse plaques and total amyloid in ROS/MAP were tested for their association with CSF Aβ1–42 levels in ADNI, resulting in 464 SNP-SNP interactions being validated after filtering out spurious interactions caused by long range LD (Table 2). Among them, 11 SNP-SNP interactions which were significant under Bonferroni correction in ROS/MAP were validated (Supplemental Table S3).
Overall, we obtained 2,803 and 464 validated SNP-SNP interactions for tau and Aβ neuropathology, respectively (Supplementary Table S1–S3). Interestingly and unexpected, there was no overlap between identified SNP-SNP interactions for the two AD hallmarks.
3.2. Functional annotations for candidate interactions
To gain an overview of the functional consequences of the candidate SNP-SNP interactions, we mapped the identified SNPs onto the genome. We found that most SNPs fall on the distal intergenic regions or introns (Fig. S2), which highlights the possible function of ‘junk DNA’ in genetic regulation. As reported in a previous study, the brain has distinct expression and epigenetic patterns, including a greater extent of noncoding transcription than other tissues(Gandal et al., 2018).
Moreover, the enriched gene ontology (GO) terms, KEGG pathways and Reactome (Yu et al., 2012; Yu and He, 2016) for genes mapped by these SNPs were examined. First, we examined the genes related to tau pathology, seven of the top 10 enriched biological processes in GOs were related to the nervous system, such as, negative regulation of neuron differentiation, axon development and axonogenesis (Fig. 2A). Pathway analysis shows enrichment of genes in neuroactive ligand-receptor interaction, cAMP signaling pathway and various other pathways (Fig. S3). The most significantly enriched pathway was neuroactive ligand-receptor interaction, which was already found to be related to analgesic, anticonvulsant, and repair reduction of cholinergic neurons and the secretion of neurotoxic inflammatory cytokine related to AD (Liu et al., 2019). As for cAMP signaling pathway, it has important roles in the long-term potentiation and is a promising drug targetable pathways for AD (Fiorito et al., 2018; Vitolo et al., 2002).
Fig. 2. Functional annotation of SNPs identified by Tau related pathology and Aβ related pathology.
A. For Tau related pathology, top 10 biological processes identified by gene ontology enrichment analysis for genes mapped by the identified SNPs. B. For Aβ related pathology, top 10 biological processes identified by gene ontology enrichment analysis for genes mapped by the identified SNPs.
Next, we examined the genes mapped by interacting SNPs for Aβ pathology. GO analysis showed that various biological processes including positive regulation of nervous system development, glutamate receptor signaling pathway and regulation of cation transmembrane transport were enriched (Fig. 2B). Pathway analysis shows the enrichment of two pathways: neuronal system and deleted in colorectal carcinoma (DCC) mediated attractive signaling. Interestingly, (amyloid precursor protein) APP functionally acts as a co-receptor for DCC to mediate axon guidance (Rama et al., 2012). More specifically, APP interacts with DCC in the presence of netrin-1 and enhances netrin-1-mediated DCC intracellular signaling, such as MAPK activation (Rama et al., 2012). In summary, the functional consequences of the candidate interactions for tau and Aβ neuropathology are tightly connected to the relevant GO terms or pathways, thereby providing robust support for their involvement in AD.
3.3. Transcriptome analysis of candidate interactions
The expression levels of two interacting genes are frequently co-altered (Wang et al., 2014). Therefore, we performed a co-expression analysis of interacting gene pairs. There are 2803 validated SNP-SNP interactions identified using tau pathology. After SNPs were mapped to the corresponding genes, there are 1195 gene pairs retained for co-expression analysis. Of 1195 interacting gene pairs, 522 gene pairs showed significant co-expression under a Bonferroni corrected P value of 0.05 (793 gene pairs were significant under a nominal P value of 0.05) (Supplementary Table S4). MAPK9 which was previously associated with T-tau, P-tau and neurofibrillary AD pathology (Kim et al., 2015) and OPCML which showed significant differential expression in AD patients (Zelaya et al., 2015), was the most significantly co-expressed gene pair (R2 = 0.93, Pnominal = 6.33 × 10−241). Particularly, MAPK9 also showed interaction and high correlation with CAMKK1 (R2 = 0.83, Pnominal = 6.66 × 10−140), which was involved in the phosphorylation of tau protein by activation of calcium/calmodulin dependent protein kinase IV (Ye et al., 2017). Therefore, the role of MAPK9 in Tau related AD pathology is worth a further experimental validation. Moreover, the interaction between GRIN2A and EPHA4 (R2 = 0.88, Pnominal = 1.67 ×10−175) was ranked in second place by co-expression, providing promising drug targets for the treatment of AD. GRIN2A encodes a subunit of N-methyl-D-aspartate receptor (NMDAR), which is inhibited by several FDA approved drugs for treatment of AD (Olivares et al., 2012). Meanwhile, EphA4 protein has been suggested as potential drug target for AD (Gu et al., 2018), and blockade of EphA4 signaling ameliorates synaptic dysfunctions in mouse models of AD (Fu et al., 2014). TENM3 and TLN2 (R2 = 0.88, Pnominal = 3.73 × 10−175), whose interaction was ranked in third place by co-expression, have both been associated with AD in a sex specific manner (Deming et al., 2018; Gusareva et al., 2018), indicating the complex interplay between sex and genetic background regarding AD predisposition.
Regarding Aβ neuropathology, of 179 gene pairs meeting the selection criterion, 81 gene pairs were co-expressed in the brain with a Bofferroni corrected P smaller than 0.05 (111 gene pairs with Pnominal < 0.05) (Supplementary Table S5). INPP4B and RBFOX1 was the most significantly co-expressed gene pair (R2 = 0.83, Pnominal = 7.10 × 10−140). RBFOX1 encodes proteins that can regulate alternative splicing events. Downregulation of RBFOX1 leads to destabilization of mRNAs encoding for synaptic transmission proteins, which contribute to the loss of synaptic function in AD (Alkallas et al., 2017). Interestingly, INPP4B was 5-fold lower expressed in lymphoblastoid cells lines exhibiting high Aβ sensitivity (Hadar et al., 2016), conforming with the downregulation of RBFOX1 in AD. CSMD1 and SLC16A14 (R2 = 0.74, Pnominal = 2.20 × 10−94), whose interaction was ranked in second place by co-expression, were both associated to cognitive functions (Athanasiu et al., 2017; Fisel et al., 2018). Gene-gene interactions that were validated by co-expression with a Pnominal < 0.05 for Aβ and tau are displayed in Supplementary Table S4 and S5, respectively.
3.4. Candidate interactions and in vivo neuroimaging
To discovery possible implications of the AD pathology on brain damage, we tested the association of candidate interactions with brain atrophy and white matter injury. Specifically, the SNP-SNP interactions of tau and Aβ neuropathology were analyzed for their association with entorhinal cortex volume, hippocampal volume and estimates of fractional anisotropy (FA) across 5 regions using data derived from in vivo neuroimaging. There are no significant SNP-SNP interactions for entorhinal cortex volume and hippocampal volume after adjusting for multiple tests.
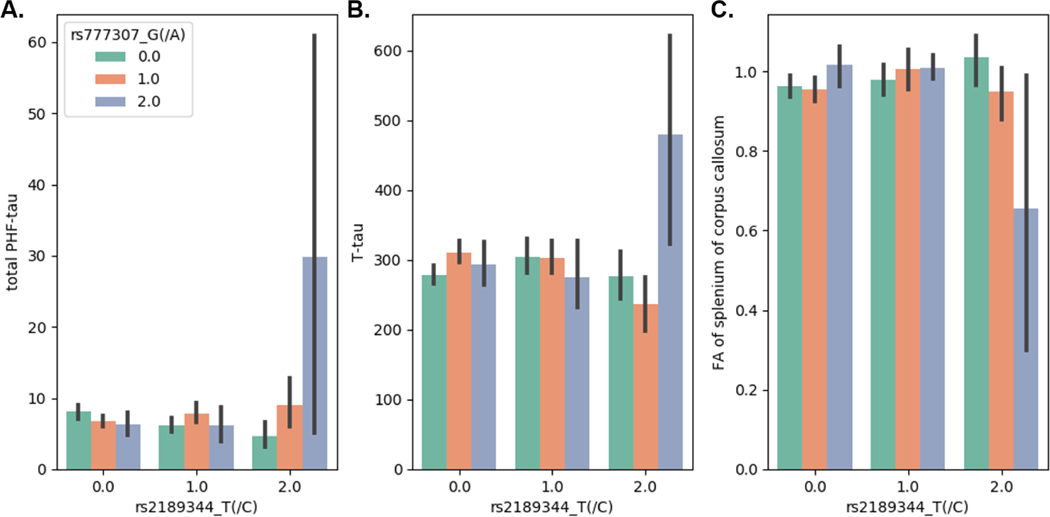
However, one SNP-SNP interaction associated with tau neuropathology in ROS/MAP and ANDI was also associated with FA estimates after adjusting for multiple tests. Specifically, the interaction between rs2189344 and rs777307 interaction was associated with FA estimates in splenium of corpus callosum (Left, Pnominal = 1.40 × 10−4; Right Pnominal = 3.37 × 10−6). Individuals with TT genotype in rs2189344 and GG genotype in rs777307 showed higher total PHF-tau in the brain, higher CSF T-tau levels and lower FA estimates in splenium of corpus callosum (Fig. 3).
Fig. 3.
The interaction effects of rs2189344 and rs777307 on total PHF-tau, CSF T-tau and fractional anisotropy (FA) estimates of splenium of corpus callosum. The mean value of each measurement is plotted against each pairwise genotype combination of rs2189344 and rs777307. The error bar indicates standard deviation.
The rs2189344 is in the intron of AC004538.3, which encodes several antisense RNAs. The rs777307 is in the intron of AC072062.1, which encodes long non-coding RNAs. Leveraging expression QTLs (eQTLs) in the genotype tissue expression project database (Lonsdale et al., 2013), we found that rs2189344 was a significant eQTL of AC004538.3 (Pnominal = 3.1 × 10−5) and rs777307 was also a significant eQTL of AC072062.1 (Pnominal = 1.6 × 10−8). Together, the interaction indicates the important role of non-coding RNAs in AD pathology and supports the association between tau pathology and white matter damage (Amlien and Fjell, 2014). Moreover, this result further shows the involvement of white matter in AD, which has been traditionally considered as a gray matter disease.
4. Discussion
Tau and Aβ neuropathology are the two neuropathological hallmarks of AD [39]. Therefore, we conducted a genome-wide epistasis analysis of these two pathologies in post-mortem brain in ROS/MAP, and validated the discovery using CSF biomarkers of AD in ADNI. As a result, we identified 2803 and 464 candidate interactions for tau and Aβ neuropathology, respectively. Next, the functional consequences of the candidate SNP-SNP interactions were examined. We found that most SNPs are on the distal intergenic regions or introns, which is in accordance with the assumed regulatory function of epistasis. GO terms and pathways related to the nervous system were enriched by the genes mapped by the SNPs, providing additional validation of candidate interactions.
Here, we mapped a SNP to genes only by its genomic position. Idealy, regulatory information such as eQTLs and 3D proximity obtained by chromosome conformation capture-based approaches can be employed to map a SNP to its corresponding genes. However, one SNP could be mapped to dozens of genes after incorporating eQTL and 3D proximity. As a result, for one SNP-SNP interaction, it could be mapped to hundreds of gene pairs, which would lead to confusions in downstream analysis. Consequently, in this study only the genomic position was considered when mapping a SNP to genes.
After SNP-SNP interactions were mapped into gene-gene interactions, we ranked gene pairs according to their co-expression in the brain, therefore providing a ranked list for further examination. For tau neuropathology, 793 of 1195 gene pairs were supported by their significant co-expression. For Aβ neuropathology, 111 of 179 gene pairs were supported by their significant co-expression. These gene-gene interactions provide a valuable resource for further exploration of disease mechanisms and discovery of new drugs. For example, several interactions including MAPK9-CAMKK1 and GRIN2A-EPHA4 are involved in Ca2+-mediated signaling function (Corrigan et al., 2005), providing new evidences for the disruption of cellular calcium homeostasis in AD. Furthermore, these gene-gene interactions can be promising drug targets after functional validation, as partially inhibition of several targets can be more efficient than complete inhibition of a single target (Csermely et al., 2005).
Additionally, SNP-SNP interactions identified were tested for their association with brain atrophy and white matter injury. However, no significant SNP-SNP interaction was found for entorhinal cortex volume and hippocampal volume after adjusting for multiple tests, which may be caused by the lack of statistical power due to small sample sizes. One SNP-SNP interaction, rs2189344 and rs777307, was associated with FA estimates in splenium of corpus callosum, suggesting the association between tau neuropathology and white matter damage. The biological mechanisms of the SNP-SNP interaction warrant further experimental validation.
We also tested the association of candidate SNP-SNP interactions with diagnosis of Alzheimer’s dementia. Interestingly, only 4 of the 464 interaction for Aβ neuropathology and 49 of 2803 interactions for tau neuropathology are significantly associated with Alzheimer’s dementia in both ROS/MAP and ADNI, conforming to the fact that tau and Aβ neuropathology may be found in individuals with or without a diagnosis of Alzheimer’s dementia. It also indicates that tau and Aβ neuropathology alone may be insufficient for the development of Alzheimer’s dementia.
Overconservative P value threshold caused by Bonferroni correction can lead to high false negative rate (high Type II error) (White et al., 2019). To alleviate this problem, we also reported SNP-SNP interactions as “suggestive” that passed a less conservative correction accounting for the number of tests performed in the second stage of our analysis. To reduce possible false positives, independent replication cohorts were used to validate the SNP-SNP interactions identified by the two-stage analysis. And further investigations are required for interactions that are suggestive of (but not reaching) significance.
Statistically significant epistasis is not necessarily a biologically meaningful interaction. Statistical epistasis represents the departure from a specific linear model describing the relationship between the alleles at different loci with respect to their contribution to a phenotype (Cordell, 2002). However, biological inferences from statistical epistasis require prior knowledge of the specific alleles involved. Here, we attempted to partially interpret statistical epistasis by visualizing each cell in the 3 × 3 two-locus genotype combination. Another limitation of statistical epistasis is that statistical models can be incredible when there are cells with very few samples in the 3 × 3 two-locus genotype table, which often leads to misleading significant interactions by regression methods. We alleviated this problem by excluding pairs with a cell size smaller than 3 in any cell of the 3 × 3 continency table.
Supplementary Material
Genetic interactions help explain the “missing heritability” in Alzheimer’s disease.
We conducted a genome-wide epistasis analysis for AD pathologies.
The epistasis signals were replicated in independent data.
Transcriptome analysis supported the interactions (such as INPP4B/RBFOX1 interaction).
Association with neuroimages provides insights into molecular functions of interactions.
Acknowledgments
1. All authors must disclose:
(a) Any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) their work. Examples of potential conflicts of interest which should be disclosed include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. If there are no actual or potential conflicts of interest, please state this. Should a significant conflict of interest be present, the Editors reserve the right to reject the article on that basis.
(b)Whether any author’s institution has contracts relating to this research through which it or any other organization may stand to gain financially now or in the future.
(c) Any other agreements of authors or their institutions that could be seen as involving a financial interest in this work.
All authors confirm that:
(a) No actual or potential conflicts of interest.
(b) No author’s institution has contracts relating to this research through which it or any other organization may stand to gain financially now or in the future.
(c) No other agreements of authors or their institutions that could be seen as involving a financial interest in this work.
2. Please disclose sources of financial support related to the manuscript being submitted.
This work was supported by the Fundamental Research Funds for the Central Universities (Grant 2662017PY115).
Work from Rush was supported in part by grants P30AG10161, R01AG15819, R01AG17917, U01AG61356, R01AG30146, the Illinois Department of Public Health, and the Translational Genomics Research Institute (Kronos Science). We are indebted to the participants in the Religious and the Orders Study and the Rush Memory and Aging Project.
Data obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database were funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). The investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
3. Please verify that the data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
We confirm that the data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
4. When applicable, provide statements verifying that appropriate approval and procedures were used concerning human subjects and animals.
We confirm that appropriate approval and procedures were used concerning human subjects and animals.
5. Please verify that all authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data
We confirm that all authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
The above statements were confirmed by all authors including:
Hui Wang, Jingyun Yang, Julie A. Schneider, Philip L De Jager, David A. Bennett and Hong-Yu Zhang
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alkallas R, Fish L, Goodarzi H, Najafabadi HS, 2017. Inference of RNA decay rate from transcriptional profiling highlights the regulatory programs of Alzheimer’s disease. Nature communications 8, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlien IK, Fjell AM, 2014. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience 276, 206–215. [DOI] [PubMed] [Google Scholar]
- Athanasiu L, Giddaluru S, Fernandes C, Christoforou A, Reinvang I, Lundervold AJ, Nilsson L-G, Kauppi K, Adolfsson R, Eriksson E, 2017. A genetic association study of CSMD1 and CSMD2 with cognitive function. Brain, behavior, and immunity 61, 209–216. [DOI] [PubMed] [Google Scholar]
- Bennett DA., Buchman AS., Boyle PA., Barnes LL., Wilson RS., Schneider JA., 2018. Religious Orders Study and Rush Memory and Aging Project. J. Alzheimers Dis. 64, S161–S189. 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, De Jager PL, Leurgans SE, Schneider JA, 2009. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurology 72, 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper J, Zweig AS, Villarreal C, Tyner C, Speir ML, Rosenbloom KR, Raney BJ, Lee CM, Lee BT, Karolchik D, 2017. The UCSC genome browser database: 2018 update. Nucleic acids research 46, D762–D769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ, 2015. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combarros O, Cortina-Borja M, Smith AD, Lehmann DJ, 2009a. Epistasis in sporadic Alzheimer’s disease. Neurobiology of aging 30, 1333–1349. [DOI] [PubMed] [Google Scholar]
- Combarros O, van Duijn CM, Hammond N, Belbin O, Arias-Vásquez A, Cortina-Borja M, Lehmann MG, Aulchenko YS, Schuur M, Kölsch H, 2009b. Replication by the Epistasis Project of the interaction between the genes for IL-6 and IL-10 in the risk of Alzheimer’s disease. Journal of neuroinflammation 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium GP, Auton A, Brooks LD, 2015. A global reference for human genetic variation. Nature 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ, 2002. Epistasis: what it means, what it doesn’t mean, and statistical methods to detect it in humans. Human molecular genetics 11, 2463–2468. [DOI] [PubMed] [Google Scholar]
- Corrigan C, Subramanian R, Miller MA, 2005. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development 132, 5225–5237. [DOI] [PubMed] [Google Scholar]
- Csermely P, Agoston V, Pongor S, 2005. The efficiency of multi-target drugs: the network approach might help drug design. Trends in pharmacological sciences 26, 178–182. [DOI] [PubMed] [Google Scholar]
- Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, 2016. Next-generation genotype imputation service and methods. Nature genetics 48, 1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming Y, Dumitrescu L, Barnes LL, Thambisetty M, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, 2018. Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta neuropathologica 136, 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito J, Deng S-X, Landry DW, Arancio O, 2018. Targeting the NO/cGMP/CREB Phosphorylation Signaling Pathway in Alzheimer’s Disease, in: Neurochemistry. IntechOpen. [Google Scholar]
- Fisel P, Schaeffeler E, Schwab M, 2018. Clinical and functional relevance of the monocarboxylate transporter family in disease pathophysiology and drug therapy. Clinical and translational science 11, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu AK, Hung K-W, Huang H, Gu S, Shen Y, Cheng EY, Ip FC, Huang X, Fu W-Y, Ip NY, 2014. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proceedings of the National Academy of Sciences 111, 9959–9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Won H, van Bakel H, Varghese M, Wang Y, 2018. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362, eaat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M., Reynolds CA., Fratiglioni L., Johansson B., Mortimer JA., Berg S., Fiske A., Pedersen NL., 2006. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63, 168–174. 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- Gu S, Fu W-Y, Fu AK, Tong EPS, Ip FC, Huang X, Ip NY, 2018. Identification of new EphA4 inhibitors by virtual screening of FDA-approved drugs. Scientific reports 8, 7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusareva ES, Carrasquillo MM, Bellenguez C, Cuyvers E, Colon S, Graff-Radford NR, Petersen RC, Dickson DW, John JMM, Bessonov K, 2014. Genome-wide association interaction analysis for Alzheimer’s disease. Neurobiology of aging 35, 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusareva ES, Twizere J-C, Sleegers K, Dourlen P, Abisambra JF, Meier S, Cloyd R, Weiss B, Dermaut B, Bessonov K, 2018. Male-specific epistasis between WWC1 and TLN2 genes is associated with Alzheimer’s disease. Neurobiology of aging 72, 188–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar A, Milanesi E, Squassina A, Niola P, Chillotti C, Pasmanik-Chor M, Yaron O, Martásek P, Rehavi M, Weissglas-Volkov D, 2016. RGS2 expression predicts amyloid-β sensitivity, MCI and Alzheimer’s disease: genome-wide transcriptomic profiling and bioinformatics data mining. Translational psychiatry 6, e909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold C, Steffens M, Brockschmidt FF, Baur MP, Becker T, 2009. INTERSNP: genome-wide interaction analysis guided by a priori information. Bioinformatics 25, 3275–3281. [DOI] [PubMed] [Google Scholar]
- Kauwe JSK, Bertelsen S, Mayo K, Cruchaga C, Abraham R, Hollingworth P, Harold D, Owen MJ, Williams J, Lovestone S, 2010. Suggestive synergy between genetic variants in TF and HFE as risk factors for Alzheimer’s disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 153, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Nho K, Risacher SL, Shen L, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ, 2015. Mapre2 as a novel Alzheimer’s disease target gene from gwas of CSF amyloid beta 1–42, tau and hyperphosphorylated tau in the ADNI cohort. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11, P767–P768. [Google Scholar]
- Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, DeStefano AL, Bis JC, Beecham GW, 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics 45, 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Harold D, Nyholt DR, ANZGene Consortium, International Endogene Consortium, Genetic and Environmental Risk for Alzheimer’s disease Consortium, Goddard ME, Zondervan KT, Williams J, Montgomery GW, Wray NR, Visscher PM, 2013. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum. Mol. Genet. 22, 832–841. 10.1093/hmg/dds491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu A, Zan J, Wang P, You Q, Tan A, 2019. Network Pharmacology Deciphering Mechanisms of Volatiles of Wendan Granule for the Treatment of Alzheimer’s Disease. Evidence-Based Complementary and Alternative Medicine 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P-R, Danecek P, Palamara PF, Fuchsberger C, Reshef YA, Finucane HK, Schoenherr S, Forer L, McCarthy S, Abecasis GR, 2016. Reference-based phasing using the Haplotype Reference Consortium panel. Nature genetics 48, 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, 2013. The genotype-tissue expression (GTEx) project. Nature genetics 45, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murk W, DeWan AT, 2016. Exhaustive genome-wide search for SNP-SNP interactions across 10 human diseases. G3: Genes, Genomes, Genetics 6, 2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares D, K Deshpande V, Shi Y, K Lahiri D, H Greig N, T Rogers J, Huang X, 2012. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Current Alzheimer Research 9, 746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jagust WJ, Shaw LM, Toga AW, 2010. Alzheimer’s disease neuroimaging initiative (ADNI): clinical characterization. Neurology 74, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan N, Tosto G, 2017. Genetics of Alzheimer’s disease: the importance of polygenic and epistatic components. Current neurology and neuroscience reports 17, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama N, Goldschneider D, Corset V, Lambert J, Pays L, Mehlen P, 2012. Amyloid precursor protein regulates netrin-1-mediated commissural axon outgrowth. Journal of Biological Chemistry 287, 30014–30023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remarque EJ, Bollen E, Weverling-Rijnsburger AWE, Laterveer JC, Blauw GJ, Westendorp RGJ, 2001. Patients with Alzheimer’s disease display a pro-inflammatory phenotype. Experimental Gerontology 36, 171–176. [DOI] [PubMed] [Google Scholar]
- Ridge PG, Mukherjee S, Crane PK, Kauwe JSK, 2013. Alzheimer’s Disease: Analyzing the Missing Heritability. PLoS One 8. 10.1371/journal.pone.0079771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson KJH., Lehmann DJ., Wimhurst VLC., Livesey KJ., Combrinck M., Merryweather-Clarke AT., Warden DR., Smith AD., 2004. Synergy between the C2 allele of transferrin and the C282Y allele of the haemochromatosis gene (HFE) as risk factors for developing Alzheimer’s disease. Journal of medical genetics 41, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, 2012. The genetics of Alzheimer disease. Cold Spring Harbor perspectives in medicine 2, a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka S-K, Parkkinen L, Hartikainen P, Soininen H, Pirttilä T, 2009. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of neurology 66, 382–389. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen EM, Kanterakis A, Deelen P, Kattenberg MV, Abdellaoui A, Hofman A, Schönhuth A, Menelaou A, de Craen AJ, van Schaik BD, 2015. Population-specific genotype imputations using minimac or IMPUTE2. Nature protocols 10, 1285. [DOI] [PubMed] [Google Scholar]
- Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M, 2002. Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proceedings of the National Academy of Sciences 99, 13217–13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Yang C, Yang Q, Xue H, Fan X, Tang NL, Yu W, 2010. BOOST: A fast approach to detecting gene-gene interactions in genome-wide case-control studies. The American Journal of Human Genetics 87, 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fu AQ, McNerney ME, White KP, 2014. Widespread genetic epistasis among cancer genes. Nature communications 5, 4828. [DOI] [PubMed] [Google Scholar]
- White T, van der Ende J, Nichols TE, 2019. Beyond bonferroni revisited: concerns over inflated false positive research findings in the fields of conservation genetics, biology, and medicine. Conservation Genetics 1–11. [Google Scholar]
- Ye S, Wang T, Cai B, Wang Y, Li J, Zhan J, Shen G, 2017. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer’s disease model rats. Neural regeneration research 12, 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, He Q-Y, 2016. ReactomePA: an R/Bioconductor package for reactome 650 pathway analysis and visualization. Molecular BioSystems 12, 477–479. [DOI] [PubMed] [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y, 2012. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics: a journal of integrative biology 16, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya MV, Pérez-Valderrama E, de Morentin XM, Tuñon T, Ferrer I, Luquin MR, Fernandez-Irigoyen J, Santamaría E, 2015. Olfactory bulb proteome dynamics during the progression of sporadic Alzheimer’s disease: identification of common and distinct olfactory targets across Alzheimer-related co-pathologies. Oncotarget 6, 39437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.