Abstract
Background
Pelvic floor symptoms (PFS), such as lower urinary tract symptoms, defecation disorders, sexual problems, and genital‐pelvic pain, are prevalent in men. Thorough physical assessments of the external anal sphincter (EAS) and the puborectal muscle (PRM) are the keys to unraveling the role of muscle dysfunction.
Objectives
To explore associations within and between the EAS and PRM and between muscle (dys‐) function and the number of male PFS.
Methods
This cross‐sectional study purposively enrolled men aged ≥21 years with 0–4 symptoms from a larger study. After extensive external and internal digital pelvic floor assessment, we explored (1) agreement between muscle function of the EAS versus PRM (using cross tabulation), (2) associations within and between the EAS and PRM (using heatmaps), and (3) associations between muscle function and number of PFS (using a visual presentation [heatmaps] and χ 2 tests).
Results
Overall, 42 out of 199 men (21%) had completely normal muscle function. Sixty‐six (33.2%) had no symptoms, of which 53 (80%) had some degree of muscle dysfunction. No clear dose–response relationship existed between muscle (dys‐) function and the number of symptoms. The PRM showed both more dysfunction and severer dysfunction than the EAS.
Conclusions
No clear association exists between muscle dysfunction and the number of symptoms, and the absence of PFS does not indicate normal muscle function for all men. Dysfunction levels are highest for the PRM. Further pelvic floor muscle research is warranted in men with PFS.
Keywords: digital assessment, heatmap, male pelvic floor musculature, male pelvic floor symptoms
1. INTRODUCTION
Dysfunction of the pelvic floor musculature in males has mainly been studied in isolation for pain, defecation, and sexual conditions. 1 To date, no study has explored the relationship between pelvic floor muscle function and multiple pelvic floor symptoms (PFS) in males, contrasting starkly with research into female muscle function. 2 Equally, no prevalence study has focused on the relationship within and between male pelvic floor muscle function. This is surprising given that the male pelvic floor musculature forms an essential component of the urogenital and bowel mechanism, having complex, coordinated, and bidirectional interplay with the prostate, bladder, and intestines for continence, urination, defecation, and sexual intercourse. 3 Indeed, clear scope exists for an association between the pelvic floor musculature and PFS. We, therefore, started a large prospective cohort study to explore the presence of concomitant PFS in a general population, applying diagnostics that are generally available in daily care for men with different PFS, especially physical examination.
In the current substudy, we describe the outcomes of the physical assessment of the external anal sphincter (EAS) and puborectal muscle (PRM). Although the EAS and PRM are anatomically close to each other, outcomes by separate assessment may have implications for pelvic floor muscle treatment. The physical assessment involved assessing the complexity of muscle function by testing the hypothesis that (A) EAS dysfunction coincides with PRM dysfunction, and (B) male pelvic floor muscle dysfunction is associated with the number of PFS.
2. MATERIALS AND METHODS
2.1. Study design, setting, and participants
This cross‐sectional study is part of a larger observational cohort study that will be detailed in a separate article. In short, through general practitioners (GPs), we invited people aged ≥16 years living in a Dutch municipal area and asked them to complete an initial questionnaire study and to take part in subsequent substudies. People with terminal illnesses, cognitive impairment (e.g., due to dementia) precluding informed consent, current psychological condition precluding informed consent, or not suitable or too ill to participate based on the judgment of the GP, were excluded. The current substudy, conducted between July 2019 and January 2020, used purposive sampling from the total group participants to enroll men aged ≥21 years, with and without PFS, who had completed the initial questionnaire study. With this sampling method, we aimed to include a broad range of PFS (100 men with one or more PFS), allowing us to compare groups with men without PFS (n = 100). As such, outcomes cannot be generalized to the overall study population.
2.2. Pelvic floor symptoms
We were interested in lower urinary tract symptoms (LUTS), bowel symptoms, sexual disorders, and pain defined according to the terminology of the International Continence Society (ICS). 4 , 5 Responses to the following questionnaires were used to determine the presence or absence of these symptoms: LUTS were identified using the International Consultation on Incontinence Questionnaire‐Male LUTS Module (ICIQ‐mLUTS) 6 ; bowel symptoms, using the Groningen Defecation and Fecal Continence (DeFeC) questionnaire 7 ; sexual symptoms, using the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire, IUGA‐Revised (PISQ‐IR), 8 item M1 of the Sexual Health in the Netherlands questionnaire, 9 and the ICIQ‐Male Sexual Matters Associated with LUTS Module for sexual dysfunction (ICIQ‐MLUTS‐5sex) 6 ; and pain, using a questionnaire constructed for the parent study that included items on pain in specific areas. Although we aimed to use the symptom scores of these questionnaires to define the presence or absence of a given symptom, most lacked established cut‐off values. Therefore, we used upper quartiles when interpreting the presence of lower urinary tract and bowel symptoms. To define the absence of lower urinary tract and bowel symptoms, the lowest quartile was used. Presence or absence was used when interpreting sexual dysfunction and pain. Participants were grouped by the number of affected domains, from zero to four, and age, aiming to achieve an equal age distribution in each category. Supporting Information: File 1 details the questionnaires and sampling procedure.
2.3. Pelvic floor assessment
Digital pelvic floor musculature assessment was performed by a pelvic floor physical therapist with ample experience, according to an ICS protocol for pelvic floor physical therapy, using digital, external (per perineum), and internal (per rectum) assessments. 4 , 5 , 10 The assessment included external visual inspection of the anal region and internal digital palpation of the EAS and the PRM (Supporting Information: File 2). No data are available on the intertester and intratester reliability of this assessment in men, as previous research was limited to vaginal assessment in women. To reduce the risk of observer bias (especially confirmation bias), the pelvic floor physical therapist was blinded to the PFS status of the participant.
In the absence of clear definitions, we categorized the different assessment items according to our definitions of normal or abnormal pelvic floor muscle function, ICS standards, and established protocols. 4 , 5 , 10 Table 1 summarizes the definitions of normal function, the items used in the digital assessment of the EAS and PRM, and the assessment outcome codes.
Table 1.
Digital pelvic floor muscle assessment items, definitions of normal function, and pelvic floor muscle assessment outcome codes for heatmaps
| EAS | PRM | |||
|---|---|---|---|---|
| Item | Description | Normal function (heatmap) | Description | Normal function (heatmap) |
| Tone |
Normal |
0 = normal tone |
Normal |
0 = normal tone |
|
Decreased |
1 = decreased/increased tone |
Decreased |
1 = decreased/increased tone | |
|
Increased |
Increased |
|||
| Voluntary contraction |
Yes, circular closing and contraction in cephalad ventral direction |
0 = yes |
Yes, in cephalad‐ventral direction |
0 = yes |
|
Straining |
0.5 = straining |
Straining |
0.5 = straining | |
|
No, no movement |
1 = no, no movement |
No, no movement |
1 = no, no movement | |
| Voluntary relaxation (after contraction) |
Yes: relaxation felt after instruction; normal finding |
0 = complete (delayed) relaxation |
Yes: relaxation felt after instruction: normal finding |
0 = complete relaxation |
|
Partial or delayed relaxation |
0.5 = partial or delayed relaxation |
Partial relaxation |
0.5 = partial relaxation | |
|
No: Absent = nonrelaxation PFM |
1 = no relaxation |
No: Absent = nonrelaxation PFM |
1 = no relaxation | |
| Strength: maximum voluntary contraction |
Strong |
0 = strong/normal (moderate) |
Strong |
0 = strong/normal (moderate) |
|
Normal (moderate) |
0.5 = weak |
Normal (moderate) |
0.5 = weak | |
|
Weak |
1 = absent |
Weak |
1 = absent | |
|
Absent |
Absent |
|||
| Repeatability of contraction (Frequency of maximum voluntary contraction (1 s) |
10 times |
0 = 7–10 times |
10 times |
0 = 7–10 times |
|
7–9 times |
0.33 = 4–6 times |
7–9 times |
0.33 = 4–6 times | |
|
4–6 times |
0.66 =1–3 times |
4–6 times |
0.66 = 1–3 times | |
|
1–3 times |
1 = 0 times |
1–3 times |
1 = 0 times | |
|
0 times |
0 times |
|||
| Endurance |
7–10 s |
0 = 7–10 s |
7–10 s |
0 = 7–10 s |
|
3–7 s |
0.33 = 3–6.99 s |
3–7 s |
0.33 = 3–6.99 s | |
|
1–3 s |
0.66 = 1–2.99 s |
1–3 s |
0.66 = 1–2.99 s | |
|
0–1 s |
1 = 0–0.99 s |
0–1 s |
1 = 0‐0.99 s | |
| Anorectal angle |
Yes, increased at contraction |
0 = yes | ||
|
No, no increase at contraction |
1 = no | |||
| Sphincter closed |
Yes, closed |
0 = yes | ||
| (at rest) |
No, not closed |
1 = no | ||
Note: Description is based on Frawley et al. 5
Normal function is shown in bold and the endurance item is the mean average of three endurance contractions (10 s each).
Abbreviations: EAS, external anal sphincter; PFM, pelvic floor musculature; PRM, puborectal muscle.
As we did not focus on LUTS as a single PFS, but on the co‐occurrence of different PFS, we did not assess urethral sphincter (dys‐) function, as for this, invasive techniques are needed.
2.4. Statistical analysis
Patient characteristics are presented as absolute numbers and percentages or as means and standard deviations, as appropriate. Analysis was then conducted as follows. First, to identify possible agreement between (dys‐) function of the EAS and PRM and to detect if any disagreements showed directionality, we cross‐tabulated the different elements of each pelvic floor muscle assessment. Second, we constructed two heatmaps to visualize possible relationships within and between the EAS and PRM, as well as between the function items of both muscles. Third, we constructed four heatmaps to visualize the relationship between the items of the EAS and the PRM (dys‐) function and the presence of symptoms according to the number of domains affected (0–4). The heatmaps created graphical representations of the data in different colors to help identify patterns or associations. 11 It helps to give an overview of patterns in the complexity of data. Finally, using our definitions of normal pelvic floor muscle function, we performed χ 2 tests to assess the percentage of men with completely normal function by assessment item in relation to the number of PFS, as compared to abnormal function of the EAS and PRM. Due to the exploratory nature of this study, we refrained from further statistical testing.
3. RESULTS
3.1. Participants and descriptive statistics
Of the 400 men invited, 199 took part in the full assessment of pelvic floor muscle function (mean age, 63.0 ± 12.5 years). Among these, the mean body mass index was 27.3 ± 3.7 kg/m2, 22 (11.1%) smoked, and 49 (24.6%) did heavy work. Furthermore, 7 (3.5%) had undergone bladder surgery, 19 (9.5%) bowel surgery, 11 (5.5%) anal or perineal surgery, and 21 (10.6%) prostate surgery. Sixty‐six men (33.2%) reported no PFS (Figure 1).
Figure 1.
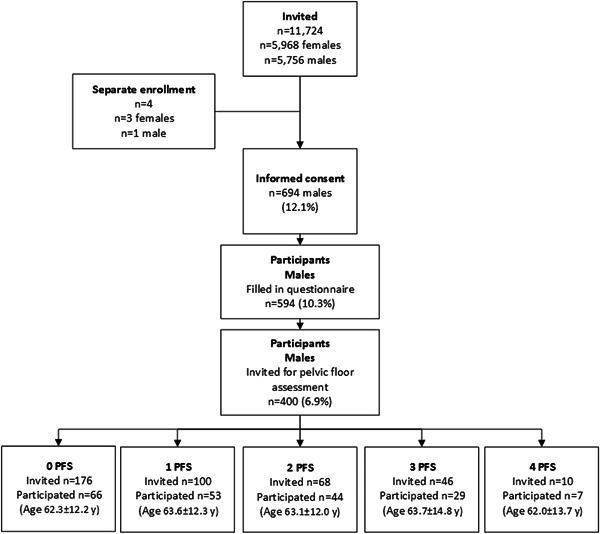
Participant flow chart. PFS, pelvic floor symptoms.
3.2. Agreement of EAS and PRM items
We found that two function items, “voluntary contraction” (182 men; 91.4%) and “frequency of maximum voluntary” contractions (180 men; 90.5%), exhibited the best agreement between the EAS and PRM. We observed complete “voluntary relaxation” of both the EAS and PRM in 70 men (35.2%), partial “voluntary relaxation” in 28 (14.1%), and no “voluntary relaxation” in 8 (4.0%). In 53 men (26.6%), complete “voluntary relaxation” of the EAS coincided with partial “voluntary relaxation” of the PRM. In 52 men (26.1%), normal EAS “tone” coincided with increased PRM “tone.” For “maximum voluntary contraction,” the normal function of one muscle coincided with strong and weak contraction of the other muscle in 21 (10.6%) and 43 (21.6%) men, respectively (Supporting Information: File 3).
3.3. Association within and between pelvic floor muscles
Forty‐two men (21%) had a completely normal EAS and PRM function. No clear pattern of association was evident within the EAS (Figure 2A), but there appeared to be an association between decreased or increased “tone” and dysfunctional “voluntary relaxation,” “maximum voluntary contraction,” and “anorectal angle” within the PRM. However, there was no clear pattern of association between the EAS and PRM.
Figure 2.
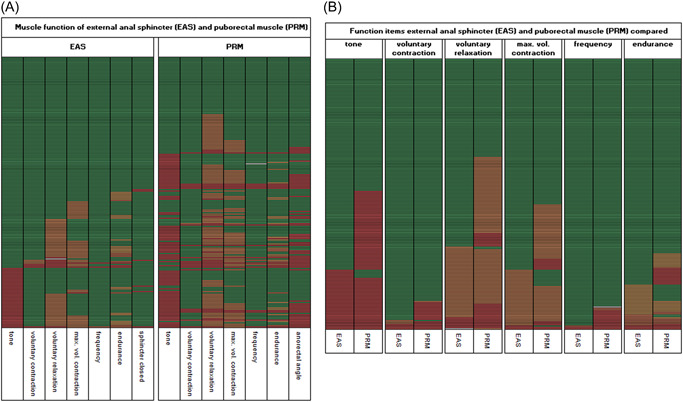
Comparison of EAS and PRM function. (A) EAS and PRM function. (B) Comparison of EAS and PRM function items. Data were sorted according to EAS dysfunction in the first columns with all other items for EAS and PRM; the green cells indicate normal function (at the top) and the red cells indicate dysfunction (at the bottom). Each line (row) includes the outcomes of an individual participant. Colors of cells: white (missing data), green (normal function), light orange (slight function decrease), orange (moderate function decrease), dark orange (strong function decrease), and red (very strong function decrease). For tone (both EAS and PRM), red represents an increase or decrease of tone; other red cells represent “no closure of EAS” and “no increase of anorectal angle,” as appropriate. EAS, external anal sphincter; max. vol., maximum voluntary; PRM, puborectal muscle.
3.4. Association between the items of the pelvic floor muscles
We observed possible dysfunction at the item level for both muscle groups in the “voluntary relaxation,” “tone,” and “maximum voluntary contraction” domains (Figure 2B). These patterns appeared to be one‐directional, with dysfunction of the EAS corresponding to dysfunction of the PRM, but not the other way around. Generally, the PRM showed both more dysfunction and more severe dysfunction compared to the EAS.
3.5. Association between muscle (dys‐) function and the number of PFS
The PRM showed more dysfunction compared to the EAS in all groups. There was no clear dose–response relationship between the number of PFS and the presence of pelvic floor muscle dysfunction. Of the 66 men without PFS, 53 (80%) had some degree of muscle dysfunction (Figure 3).
Figure 3.
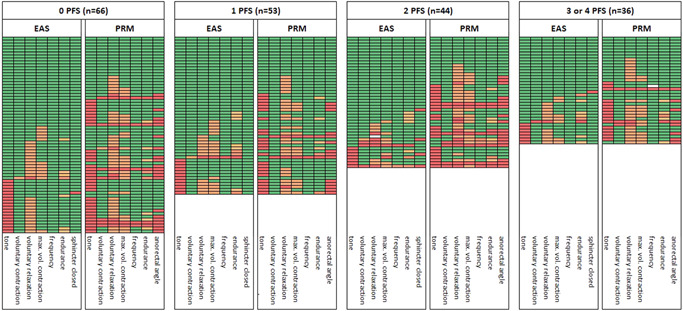
Heatmap of EAS and PRM function items by the number of pelvic floor symptoms. Data were sorted according to EAS dysfunction in the first columns with all other items for EAS and PRM; the green cells indicate normal function (at the top) and the red cells indicate dysfunction (at the bottom). Each line (row) includes the outcomes of an individual participant. Colors of cells: white (missing data), green (normal function), light orange (slight function decrease), orange (moderate function decrease), dark orange (strong function decrease), and red (very strong function decrease). For tone (both EAS and PRM), red represents an increase or decrease of tone; other red cells represent “no closure of EAS” and “no increase of anorectal angle,” as appropriate. EAS, external anal sphincter; max. vol., maximum voluntary; PRM, puborectal muscle.
Table 2 shows an overview of the percentages of men with EAS and PRM dysfunction by assessment item. Percentages of EAS “tone” and EAS “voluntary relaxation” dysfunction were higher in asymptomatic men, while EAS “voluntary contraction” and “EAS closed” dysfunction was highest in men with two symptoms, and EAS “maximum voluntary contraction” was highest in men with one symptom. Concerning the PRM, approximately half had dysfunctional “tone” (either increased or decreased) and more than half had dysfunctional “voluntary relaxation,” which was highest in the group with two symptoms.
Table 2.
Overview of percentage with muscle dysfunction by number of pelvic floor symptoms
| Number of pelvic floor symptomsa | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3–4 | |
| Number of participants (n) | 66 | 53 | 44 | 36 |
| EAS (%) | ||||
|
Tone |
27.3 | 22.6 | 15.9 | 19.4 |
|
Voluntary contraction |
1.5 | 1.9 | 9.1 | 2.8 |
|
Voluntary relaxation |
37.9 | 24.5 | 23.3 | 33.3 |
|
Maximum voluntary contraction |
18.2 | 30.2 | 20.5 | 19.4 |
|
Frequency of maximum voluntary contraction |
0 | 1.9 | 4.5 | 0 |
|
Endurance in categories |
10.6 | 18.9 | 18.2 | 22.2 |
|
Closed |
1.5 | 0 | 9.1 | 2.8 |
| PRM (%) | ||||
|
Tone |
51.5 | 45.3 | 50.0 | 44.4 |
|
Voluntary contraction |
12.1 | 3.8 | 15.9 | 8.3 |
|
Voluntary relaxation |
62.1 | 54.7 | 72.7 | 63.9 |
|
Maximum voluntary contraction |
39.4 | 30.2 | 52.3 | 38.9 |
|
Frequency of maximum voluntary contractions |
7.6 | 3.8 | 18.2 | 2.9 |
|
Endurance |
19.7 | 15.1 | 31.8 | 19.4 |
|
Anorectal angle during contraction |
33.3 | 26.4 | 40.9 | 22.2 |
| Normal function of EAS and PRM (%) | 19.7 | 24.5 | 20.5 | 19.4 |
Abbreviations: EAS, external anal sphincter; PRM, puborectal muscle.
Lower urinary tract symptoms, defecation disorders, sexual problems, and genital‐pelvic pain.
Finally, we found no significant difference between the number of symptom domains (0–4) and either normal or abnormal muscle function (EAS and PRM) (χ² test, p = 0.88).
4. DISCUSSION
In this exploratory study of men with and without PFS, we hypothesized that EAS and PRM dysfunction would coincide and that pelvic floor muscle dysfunction would be associated with the number of symptom domains affected. Our data add to knowledge about male pelvic floor muscle (dys‐) function in relation to PFS.
Overall, no clear dose–response relationship existed between the number of PFS and pelvic floor muscle (dys‐) function. Our findings suggest that associations may be present within the PRM between tone dysfunction and “voluntary relaxation,” “maximum voluntary contraction,” and “anorectal angle.” A possible one‐sided dysfunction was seen at the item level for the EAS and the PRM in the “voluntary relaxation,” “tone,” and “maximum voluntary contraction” domains. In general, the PRM showed not only more dysfunction but also more severe dysfunction compared with the EAS, typically in the presence of two PFS. So, despite EAS and PRM being anatomically close to each other, the separate assessment revealed notable differences, that could have implications for pelvic floor muscle treatment.
Micturition, sexual, and defecation processes may be affected by the suboptimal function of the pelvic floor muscles and the pelvic organs and by factors such as higher age, prostate surgery, obesity, chronic obstipation, and stress reactions. 12 , 13 The complexity of male pelvic floor musculature anatomy and function and the lack of a gold standard for the assessment and diagnosis of male pelvic floor musculature (dys‐) function makes research a challenge. 14 Cross‐tabulation showed that in 26.1% a normal “tone” of the EAS coincided with an increased “tone” of the PRM. Although the pain, reported by 22.1% may partly explain this result, it does not fully explain the difference in tone. Hetrick et al. showed that men with chronic pelvic pain syndrome had a higher resting tone than controls, but found no difference between the different male pelvic floor muscles. 15 Pelvic floor muscle tone assessment is considered difficult due to the influence of neurological and neuromuscular processes, emotion, pain, and contractile and viscoelastic tissue functionality, among which palpation alone cannot discriminate. 5 , 16 To the best of our knowledge, no other study has described the differences in tone within and between male pelvic floor muscles by digital assessment. Cross‐tabulation showed normal “voluntary relaxation” of the EAS and partial “voluntary relaxation” of the PRM in 26.6% of men. This is consistent with the more dysfunctional relaxation of the PRM that we found, compared to the EAS in men (Figures 2 and 3). Partial or absent voluntary relaxation may result from increased pelvic floor muscle tone due to pain or discomfort in the pelvic region. 4 , 17 Cross‐tabulation also showed impaired “maximum voluntary contraction” of the PRM compared to the EAS in 24.1%. This could result from prolonged overactivity of the PRM and reduced blood flow in the internal pudendal artery and venous systems causing muscle fatigue. 5 , 18 The difference in function between muscles could be explained by the difficulty to contract the PRM due to its location within the pelvis. Indeed, some studies have shown that females were either unable or had difficulty performing correct deep pelvic floor musculature contraction during the assessment. 19
The heatmaps revealed comparable results for the EAS and the PRM at the item level for “voluntary relaxation,” “tone,” and “maximum voluntary contraction.” The literature reveals no strict consensus about the relationship between the EAS and the PRM, though several studies have found connections between the two muscles. This supports the general approach that they act as a functional unit for holding and passing urine and feces. 3 , 14 , 20 However, the PRM and EAS have also been reported to have phylogenetically different innervations, indicating some dichotomy between the muscles. 21
Contrary to our expectations, no linear relationship existed between the number of PFS and the presence of pelvic floor muscle dysfunction. Our choices of cut‐off values for lower urinary tract and defecation symptoms, and the fact that we only assessed the number of PFS, could account for these results. Equally, the overrepresentation of some symptom combinations and no discrimination of symptoms within a domain might have influenced the results. Moreover, our findings suggest that pelvic floor muscle function might be influenced by a participant's sensitivity and awareness of the pelvic floor muscles, their concentration during the muscle assessment, the influence of other muscles for motor control of the pelvic ring and lower spine, the urge for bowel movement or flatus during the assessment, as well as the severity and degree of PFS, the intimacy of the assessment, and the presence of bladder, prostate, or intestinal conditions. 22 Pelvic floor function could also have been influenced by experiences in earlier pelvic floor musculature assessment or medical assessments, sexuality, and history of sexual abuse. 23
This exploratory study benefited from focusing on the associations within and between EAS and PRM function and their relationship to the number of pelvic floor symptom domains. Furthermore, we included men of all ages with and without PFS, and we used validated questionnaires with clear cut‐off values. We identified differences in muscle function by thorough pelvic floor musculature assessment that prevented undesirable activity in other muscles, with the same well‐experienced pelvic floor physical therapist performing all assessments. 24 As such, inter‐rater reliability issues were not present, but intra‐rater reliability might limit the outcomes of our study. We are unaware of previous studies on the inter‐rater and intrarater reliability in male pelvic floor assessment, reflecting a lack of studies on the male pelvic floor. To support health care professionals, we applied methods that are easily available, especially digital assessment. Other methods, like ultrasound or magnetic resonance imaging, could reveal different outcomes but are less feasible to apply in general care. Despite these strengths, important limitations should be considered. Most men were aged 55–75 years, skewing the age distribution and precluding extrapolation to general male populations. Furthermore, we only focused on functional aspects of the pelvic floor musculature and on the number of PFS domains. Analysis of specific male PFS within the four studied domains, coupled with analysis of symptom severity, could have affected the results and is warranted for future studies. Further evaluation of pelvic floor musculature in association with key symptoms could be valuable, as well as the analysis of electromyographic data that were collected in this study as well.
In the context of objective measurement, we could have reported timing, speed of contraction, and coordination, but since these outcomes can be more precisely verified by electromyography, we decided not to perform these assessments. However, these data may give additional insight, but as such data are not readily available in general practice, and to keep an overview of all data, we kept this aside in the current study.
Despite these limitations, we think this study will help to unravel the complexity of pelvic floor musculature function in relation to PFS in men.
5. CONCLUSION
We found no clear dose–response relationship between the number of PFS and the presence of pelvic floor musculature dysfunction. Interestingly, one in three had no symptoms but only one in five of all men had a complete normal muscle function. Of the men without PFS, most had some degree of muscle dysfunction. This could indicate either that these men had not yet noticed the PFS or that the pelvic floor muscle dysfunction was situational and influenced by other factors than the PFS during the assessment. In general, we found no clear pattern of association between the EAS and the PRM, and PRM showed more dysfunction than the EAS. We now plan to analyze pelvic floor muscle function by the severity of specific PFS in males. Hopefully, our studies will stimulate further research into male pelvic floor muscle function, seeking to unravel the complex relationship with PFS.
AUTHOR CONTRIBUTIONS
Study conception and design: Françoise J. M. Notenboom‐Nas, Grietje E. Knol‐de Vries, Marijke C. Ph. Slieker‐ten Hove, Janny H. Dekker, Gommert A. van Koeveringe, Marco H. Blanker. Data collection: Françoise J. M. Notenboom‐Nas and Lotte Beijer, Yme Tolsma. Data analysis: Françoise J. M. Notenboom‐Nas, Grietje E. Knol‐de Vries, Marijke C. Ph. Slieker‐ten Hove, Marco H. Blanker. Interpretation of results: Françoise J. M. Notenboom‐Nas, Grietje E. Knol‐de Vries, Marijke C. Ph. Slieker‐ten Hove, Janny H. Dekker, Gommert A. van Koeveringe, Marco H. Blanker. Writing of the paper: All authors.
CONFLICTS OF INTEREST
MarijkeC.Ph. Slieker‐ten Hove: KOL Indiba (Indiba. com). She has an advising role for pelvic floor physical therapists who want to use Tecar therapy in pelvic floor dysfunction. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The Medical Ethical Committee of the University Medical Center of Groningen approved the study with no. NL67503.042.18. Eligible participants received written information about the muscle function assessment and confirmed their willingness to take part in the current substudy. The study was approved by the medical ethical committee. All participants provided written informed consent and received a €20 gift card for participating in this substudy. Registered at ClinicalTrials. gov no. NCT03558802.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
We want to thank Dr. Robert Sykes (www.doctored.org.uk) who provided technical editing services for the final drafts of this manuscript. This study was funded by ZonMw (Gender and Health 849200004).
Notenboom‐Nas FJM, Knol‐de Vries GE, Beijer L, et al. Exploring pelvic floor muscle function in men with and without pelvic floor symptoms: a population‐based study. Neurourol Urodyn. 2022;41:1739‐1748. 10.1002/nau.24996
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (FJMNN), upon reasonable request.
REFERENCES
- 1. Smith CP. Male chronic pelvic pain: an update. Indian J Urol. 2016;32(1):34‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lakhoo J, Khatri G, Elsayed RF, et al. MRI of the male pelvic floor. Radiographics. 2019;39(7):2003‐2022. [DOI] [PubMed] [Google Scholar]
- 3. Rocca Rossetti S. Functional anatomy of pelvic floor. Arch Ital Urol Androl. 2016;88(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 4. D'Ancona C, Haylen B, Oelke M, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38(2):433‐477. [DOI] [PubMed] [Google Scholar]
- 5. Frawley H, Shelly B, Morin M, et al. An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol Urodyn. 2021;40(5):1217‐1260. [DOI] [PubMed] [Google Scholar]
- 6. Huang W, Wang Q, Chen J, Wu P. Development and validation of the International Consultation on Incontinence Modular Questionnaire for Male Lower Urinary Tract Symptoms (ICIQ‐MLUTS) and the ICIQ‐MLUTS Long Form in Chinese population. Low Urin Tract Symptoms. 2019;11(4):189‐194. [DOI] [PubMed] [Google Scholar]
- 7. Meinds RJ, Timmerman MEW, van Meegdenburg MM, Trzpis M, Broens PMA. Reproducibility, feasibility and validity of the Groningen defecation and fecal continence questionnaires. Scand J Gastroenterol. 2018;53(7):790‐796. [DOI] [PubMed] [Google Scholar]
- 8. van Dongen H, van der Vaart H, Kluivers KB, Elzevier H, Roovers JP, Milani AL. Dutch translation and validation of the pelvic organ prolapse/incontinence sexual questionnaire‐IUGA revised (PISQ‐IR). Int Urogynecol J. 2019;30(1):107‐114. [DOI] [PubMed] [Google Scholar]
- 9. Seksuele gezondheid in Nederland . 2017. Accessed November 15, 2021. https://rutgers.nl/wp-content/uploads/2021/03/Seksuele-Gezondheid-in-Nederland-2017.pdf
- 10. Slieker‐ten Hove MC, Pool‐Goudzwaard AL, Eijkemans MJ, Steegers‐Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200(2):184.e1‐184.e7. [DOI] [PubMed] [Google Scholar]
- 11. Oike H, Ogawa Y, Oishi K. Simple and quick visualization of periodical data using microsoft excel. Methods Protoc. 2019;2(4):81. 10.3390/mps2040081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voorham‐van der Zalm PJ, Lycklama À, Nijeholt GAB, Elzevier HW, Putter H, Pelger RCM. “Diagnostic investigation of the pelvic floor”: a helpful tool in the approach in patients with complaints of micturition, defecation, and/or sexual dysfunction. J Sex Med. 2008;5(4):864‐871. [DOI] [PubMed] [Google Scholar]
- 13. Hodges PW, Stafford RE, Hall L, et al. Reconsideration of pelvic floor muscle training to prevent and treat incontinence after radical prostatectomy. Urol Oncol . 2020;(5):354‐371. [DOI] [PubMed]
- 14. Stoker J. Anorectal and pelvic floor anatomy. Best Pract Res Clin Gastroenterol. 2009;23(4):463‐475. [DOI] [PubMed] [Google Scholar]
- 15. Hetrick DC, Glazer H, Liu YW, Turner JA, Frest M, Berger RE. Pelvic floor electromyography in men with chronic pelvic pain syndrome: a case‐control study. Neurourol Urodyn. 2006;25(1):46‐49. [DOI] [PubMed] [Google Scholar]
- 16. Quaghebeur J, Petros P, Wyndaele JJ, De Wachter S. The innervation of the bladder, the pelvic floor, and emotion: a review. Auton Neurosci. 2021;2(35):102868. [DOI] [PubMed] [Google Scholar]
- 17. Butrick CW. Pathophysiology of pelvic floor hypertonic disorders. Obstet Gynecol Clin North Am. 2009;36(3):699‐705. [DOI] [PubMed] [Google Scholar]
- 18. Cohen D, Gonzalez J, Goldstein I. The role of pelvic floor muscles in male sexual dysfunction and pelvic pain. Sex Med Rev. 2016;4(1):53‐62. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong AA, Nguyen MM, Wieslander CK, Tarnay CM. All levels of providers can effectively and efficiently teach pelvic floor strength assessment at time of pelvic examination. Female Pelvic Med Reconstr Surg. 2019;25(2):154‐156. [DOI] [PubMed] [Google Scholar]
- 20. Rociu E, Stoker J, Eijkemans MJ, Laméris JS. Normal anal sphincter anatomy and age‐ and sex‐related variations at high‐spatial‐resolution endoanal MR imaging. Radiology. 2000;217(2):395‐401. [DOI] [PubMed] [Google Scholar]
- 21. Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18(7):507‐519. [DOI] [PubMed] [Google Scholar]
- 22. Mateus‐Vasconcelos ECL, Ribeiro AM, Antônio FI, Brito LGO, Ferreira CHJ. Physiotherapy methods to facilitate pelvic floor muscle contraction: a systematic review. Physiother Theory Pract. 2018;34(6):420‐432. [DOI] [PubMed] [Google Scholar]
- 23. Davis KA, Knight RA. Childhood maltreatment experiences and problematic sexual outcomes in adult males who have sexually offended: further evidence of the potency of male caregiver psychological abuse. Child Abuse Negl. 2019;96:104097. [DOI] [PubMed] [Google Scholar]
- 24. Pena CC, Bø K, Ossa AMP, et al. Are visual inspection and digital palpation reliable methods to assess ability to perform a pelvic floor muscle contraction? An intra‐rater study. Neurourol Urodyn. 2021;40(2):680‐687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (FJMNN), upon reasonable request.


