Abstract
We have earlier shown that galectin-3, a lactose-binding mammalian lectin that is secreted from activated macrophages, basophils, and mast cells, induces activation of the NADPH oxidase in exudated but not in peripheral blood neutrophils (A. Karlsson, P. Follin, H. Leffler, and C. Dahlgren, Blood 91:3430–3438, 1998). The alteration in responsiveness occurring during extravasation correlated with mobilization of the gelatinase and/or specific granules to the cell surface, indicating a role for mobilizable galectin-3 receptors. In this study we have investigated galectin-3-induced NADPH oxidase activation, measured as superoxide production, in lipopolysaccharide (LPS)-primed neutrophils. Upon galectin-3 challenge, the LPS-primed cells produced superoxide, both extracellularly and intracellularly. A primed extracellular response to formylmethionyl-Leu-Phe (fMLF) was also achieved. The exposure of complement receptors 1 and 3 as well as the formyl peptide receptor on the cell surface was markedly increased after LPS treatment, indicating that granule fusion with the plasma membrane had occurred. Further assessment of specific markers for neutrophil granules showed that the LPS treatment had mobilized the gelatinase granules but only a minor fraction of the specific granules. We thus suggest that the mechanism behind LPS priming lies at the level of granule (receptor) mobilization for galectin-3 as well as for fMLF.
The innate immune defense toward microorganisms is largely dependent on neutrophil granulocytes. The neutrophil effector functions include the production of oxygen radicals that have bactericidal functions as well as being potentially tissue destructive (3). Hence, tight regulation of the radical-producing enzyme system, the NADPH oxidase, is critical for mounting an effective defense against infection without destroying surrounding tissues.
Most studies of neutrophil activation use cells isolated from peripheral blood. However, neutrophils act in vivo mainly after exudation from the bloodstream. Concomitant with extravasation, the cells become primed (i.e., hyperresponsive) with respect to the NADPH oxidase activity induced by inflammatory mediators, e.g., the chemoattractant formylmethionyl-Leu-Phe (fMLF) (17). Similarly, galectin-3, an endogenous, lactose-binding lectin, activates the NADPH oxidase in exudated but not peripheral blood neutrophils (22). In addition to having stimulating effects on immune cells, galectin-3 is released in large amounts from activated macrophages and mast cells (27, 28, 39), supporting its participation in inflammatory processes, potentially as an inflammatory mediator. For a review on galectin-3 and its biological functions, see reference 26.
The priming phenomenon has been described for many settings in neutrophil activation processes, prominent examples being the effect of bacterial lipopolysaccharide (LPS) and the adhesion-related priming of tumor necrosis factor-induced responses (35). Many priming mechanisms have been suggested, and it is reasonable to believe that different mechanisms, alone or in combination, may be the cause of different priming events (9, 14, 20). Neutrophils having encountered bacterial LPS are primed with respect to oxidative response; i.e., LPS per se does not activate the NADPH oxidase but induces hyperresponsiveness to other stimuli, such as fMLF (13, 19, 43, 45). The prevailing views propose that the mechanism for this LPS priming involves alterations of intracellular signaling (e.g., changed levels of various second messengers) and/or direct effects on the NADPH oxidase (19). We have recently proposed that one important mechanism behind the primed state induced during neutrophil extravasation is the mobilization of intracellular granules, endowing the plasma membrane with granule membrane proteins (22). Such mobilization of specific receptors could explain priming vis-à-vis fMLF and galectin-3, respectively.
To shed further light on this issue with regard to LPS priming, we have investigated the effect of LPS on the neutrophil response to galectin-3 and the concomitant mobilization of granules. The data suggest that LPS priming is sufficient to induce galectin-3 responsiveness and that gelatinase granule mobilization (accompanied by receptor upregulation) is a major cause for the galectin-3 responding state. We also show that receptor mobilization is a plausible explanation for the LPS-induced priming of the neutrophil response to fMLF.
MATERIALS AND METHODS
Isolation of human neutrophils.
Neutrophils were isolated from buffy coats from healthy blood donors by using dextran sedimentation and Ficoll-Paque gradient centrifugation (8). The cells were washed and resuspended (107/ml) in Krebs-Ringer phosphate buffer containing glucose (10 mM), Ca2+ (1 mM), and Mg2+ (1.5 mM) (KRG; pH 7.3). This procedure allows for cells to be isolated with minimal mobilization effects (1).
Neutrophil cytoplasts were prepared according to the method of Roos et al. (37) as described earlier (10).
Priming with LPS.
LPS from Escherichia coli serotype O111:B4 was dissolved in KRG to 1 mg/ml and sonicated to prepare a homogeneous solution. Cells (107/ml) were incubated in the presence or absence of LPS (10 μg/ml [a relatively high concentration required to induce priming in the absence of serum]) at 4 or 37°C for 30 min and were then directly used for NADPH oxidase activation studies or marker analysis.
Preparation of galectin-3.
Recombinant human galectin-3 was produced in E. coli and purified as previously described (31). The lectin was stored at 4°C in phosphate-buffered saline (pH 7.2) containing lactose (150 mM). When used, the lectin preparation was applied to a gel filtration column (PD10; Pharmacia, Uppsala, Sweden) in order to remove lactose and was diluted to 400 μg/ml in KRG.
Neutrophil NADPH oxidase activity.
Superoxide anion production by the NADPH oxidase was determined using a luminol/isoluminol enhanced chemiluminescence (CL) system (12). CL activity was measured in a six-channel Biolumat LB 9505 (Berthold Co., Wildbad, Germany), using disposable 4-ml polypropylene tubes with a 0.90-ml reaction mixture containing 106 neutrophils. The tubes were equilibrated in the Biolumat for 5 min at 37°C, after which the stimulus (0.1 ml) was added. The light emission was recorded continuously. To quantify intracellularly and extracellularly generated superoxide, two different reaction mixtures were used. Tubes used for measurement of extracellular release of superoxide anion contained neutrophils, horseradish peroxidase (HRP; a cell-impermeable peroxidase; 4 U), and isoluminol (a cell-impermeable CL substrate; 6 × 10−5 M) (30). By a direct comparison of the superoxide dismutase (SOD)-inhibitable reduction of cytochrome c and SOD-inhibitable CL, 7.2 × 107 cpm was found to correspond to a production of 1 nmol of superoxide (a millimolar extinction coefficient for cytochrome c of 21.1 was used). Tubes used for measurement of intracellular generation of reactive oxygen species contained neutrophils, SOD (a cell-impermeable scavenger for O2−; 50 U), catalase (a cell-impermeable scavenger for H2O2; 2,000 U) and luminol (a cell-permeable CL substrate; 6 × 10−5 M). Results are presented as means and standard deviations (SD).
Marker analysis.
The mobilization of subcellular organelles was determined by measuring the exposure of complement receptors 1 and 3 (CR1 and CR3, respectively) and the formyl peptide receptor (FPR) on the neutrophil surface as well as determining the release of gelatinase and vitamin B12-binding protein into the supernatant.
Exposure of CR1 was measured by labeling the cells with mouse anti-human CD35 (Dakopatts M0710; 10 μl to a cell pellet of 106 cells) and subsequent binding of fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (DAKO F0479; 1/2,000). To measure CR3 exposure, the cells were labeled with phycoerythrin-conjugated monoclonal antibodies specific for CD11b (DAKO M741; 10 μl to a cell pellet of 106 cells). The cells were examined by FACScan (Becton Dickinson, Mountain View, Calif.) (29).
The amount of FPR on the cell surface was determined by incubating the neutrophils with radiolabeled fMLF in the presence or absence of excess unlabeled fMLF as described earlier (1). In short, 100 μl of dibutylphthalate mixed with dinonylphthalate (10:3, vol/vol) was layered on top of 10 μl of urea (6 M) in Eppendorf tubes; 50 μl of [3H]fMLF (8 × 10−8 M) together with 50 μl of KRG or unlabeled fMLF (4 × 10−5 M) in KRG was layered on top of the oil. Neutrophils (2 × 106; 100 μl) were added to the fMLF solution, and the tubes were incubated on melting ice for 1 h. The tubes were centrifuged at 9,000 × g for 15 s in a Beckman microcentrifuge (Beckman Instruments, Fullerton, Calif.) to remove unbound peptide. The bottom of the centrifuge tubes (containing the pelleted cells) was excised and collected for determination of cell-bound radioactivity. The background (the measured value in the presence of excess unlabeled fMLF) was subtracted from all values.
Vitamin B12-binding protein was determined with the cyanocobalamin technique as described by Gottlieb et al. (18). Gelatinase was measured using an enzyme-linked immunosorbent assay (ELISA) (24).
Reagents.
LPS (E. coli serotype O111:B4; L-2630), fMLF, phorbol myristate acetate (PMA), isoluminol, and luminol were obtained from Sigma Chemical Co. (St. Louis, Mo.). [3H]fMLF was supplied by Du Pont NEN (Boston, Mass.). Dibutylphthalate and dinonylphthalate was obtained from Merck (Whitehouse Station, N.J.). Catalase, SOD, and HRP were purchased from Boehringer (Mannheim, Germany). Dextran and Ficoll-Paque were from Pharmacia. [57Co]vitamin B12 was supplied by Amersham Laboratories (Amersham, Buckinghamshire, England). Antibodies for the gelatinase ELISA were a kind gift from Lars Kjeldsen and Niels Borregaard, Copenhagen, Denmark. All other antibodies were from DAKO (Glostrup, Denmark).
RESULTS
LPS-induced priming of neutrophil NADPH oxidase activity.
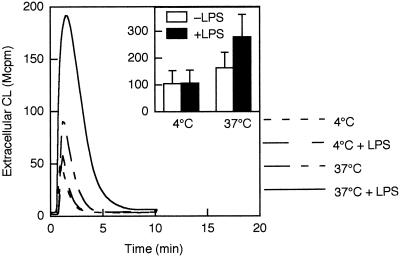
Neutrophil priming induced by bacterial LPS is a time- and temperature-dependent phenomenon. To establish a reference for comparison with the literature (13, 19, 43, 45), we first measured fMLF-induced extracellular release of oxygen radicals from LPS-primed and unprimed neutrophils (Fig. 1). In agreement with previous studies, the response to fMLF was slightly increased in the cell population incubated at 37°C in the absence of LPS (1), while pretreatment of the cells with LPS for 30 min at 37°C showed a markedly enhanced response to fMLF (10−7 M) (Fig. 1) (13, 19). No increase in response was detected in cells incubated with LPS at 4°C. There was no difference in superoxide production between LPS-primed and nonprimed neutrophils stimulated with the protein kinase C activator PMA (data not shown).
FIG. 1.
fMLF-induced extracellular release of oxygen radicals in LPS-primed and unprimed neutrophils. Cells were preincubated for 30 min at 4 or 37°C in the presence or absence of LPS (10 μg/ml), after which they were stimulated with fMLF (10−7 M). The extracellular release of superoxide anion was measured by isoluminol-amplified CL in the presence of HRP, and the CL responses are given as 106 cpm (Mcpm). Kinetics of a representative experiment as well as the mean peak value (+SD) of three experiments (inset) are shown.
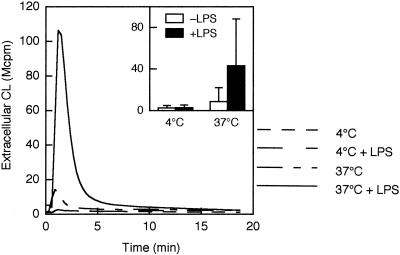
Next, we performed the same set of experiments but with fMLF instead of galectin-3 as the activator. Neutrophils pretreated with LPS at 37°C exhibited a pronounced extracellular release of oxygen radicals in response to galectin-3 (Fig. 2). In contrast, neutrophils pretreated with LPS at 4°C or incubated at 37°C in the absence of LPS showed little or no response to galectin-3.
FIG. 2.
Galectin-3-induced extracellular release of oxygen radicals in LPS-primed and unprimed neutrophils. Cells were preincubated for 30 min at 4 or 37°C in the presence or absence of LPS (10 μg/ml), after which they were stimulated with galectin-3 (40 μg/ml). The extracellular release of superoxide anion was measured by isoluminol-amplified CL in the presence of HRP, and CL responses are given as 106 cpm (Mcpm). Kinetics of a representative experiment as well as the mean peak value (+SD) of three experiments (inset) are shown.
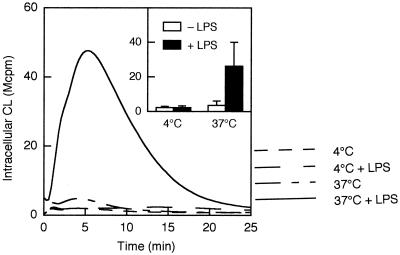
Galectin-3, in contrast to fMLF, also induces production of intracellular oxygen radicals (22), presumably produced by NADPH oxidase present in the specific granules (for a review, see reference 12). In analogy to the extracellular response, galectin-3 induced an intracellular response in cells primed with LPS at 37°C but only a barely detectable or no response in the other cell populations (Fig. 3).
FIG. 3.
Galectin-3-induced intracellular production of oxygen radicals in LPS-primed and unprimed neutrophils. Cells were preincubated for 30 min at 4 or 37°C in the presence or absence of LPS (10 μg/ml), after which they were stimulated with galectin-3 (20 μg/ml). The intracellular production of superoxide anion was measured by luminol-amplified CL in the presence of SOD and catalase, and CL responses are given as 106 cpm (Mcpm). Kinetics of a representative experiment as well as the mean peak value (+SD) of three experiments (inset) are shown.
NADPH oxidase activity in enucleated neutrophil cytoplasts.
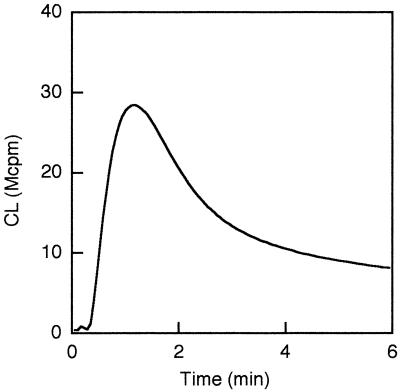
Cytoplasts, consisting of an organelle-free cytoplasm surrounded by plasma membrane, have successfully been used to assess the role of neutrophil granules in specific cellular responses (11, 15). We found that galectin-3 activated the NADPH oxidase in nonprimed neutrophil cytoplasts, with a similar time course and about 30% magnitude (Fig. 4) of an equivalent number of primed neutrophils (Fig. 2). The NADPH oxidase activity was not further increased in cytoplasts pretreated with LPS (the ratio between the CL activity in LPS-treated and nontreated cytoplasts being 0.95 ± 0.21 [n = 3]). Likewise, LPS failed to prime cytoplasts when they were stimulated by fMLF (data not shown).
FIG. 4.
Galectin-3-induced production of oxygen radicals in neutrophil cytoplasts. Cytoplasts resuspended to a concentration of 106/ml were stimulated with galectin-3 (20 μg/ml). The extracellular release of superoxide anion was measured by isoluminol-amplified CL in the presence of HRP, and the CL response is given as 106 cpm (Mcpm). Kinetics of a representative experiment are shown. The mean peak value (±SD) of three experiments was 20.4 ± 7.2 Mcpm.
LPS-induced mobilization of subcellular organelles.
Receptors for galectin-3 are stored in the gelatinase and specific granules in peripheral blood neutrophils (16). We have suggested that mobilization of these receptors to the cell surface is what renders the cells responsive to galectin-3 after in vitro priming with fMLF or in vivo extravasation (22). Similarly, the FPR mediating the neutrophil response induced by fMLF is distributed among the plasma membrane, the secretory vesicles, and the gelatinase and specific granules (40). To assess the possibility that granule mobilization and increased receptor expression on the cell surface is the cause of the priming effect induced by LPS, we examined the cell surface exposure of known membrane components (Fig. 5) and extracellular release of granule contents (Fig. 6) in the various cell populations described above.
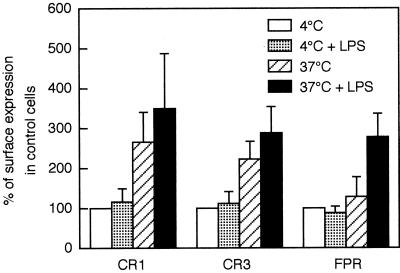
FIG. 5.
Cell surface exposure of CRs and FPR in LPS-primed and unprimed neutrophils. Neutrophils were preincubated for 30 min in the presence or absence of LPS (10 μg/ml) at 4 or 37°C. Cell surface exposure of CR1 and CR3 as measured by fluorescence-activated cell sorting analysis and cell surface exposure of FPR as measured by binding of radiolabeled fMLF are shown. The data for each cell population, expressed as percentage of the value obtained with control cells (4°C; open bars), are given as mean + SD, n = 6 (CR1 and CR3) or n = 3 (FPR). The control value (100%) for the specific (total minus nonspecific) fMLF binding to FPR was 13.2 fmol/106 cells. Nonspecific binding, measured in the presence of excess unlabeled fMLF, was 10 fmol/106 under all conditions tested. CR1 is a marker for the plasma membrane and secretory vesicles; CR3 and FPR are present in the secretory vesicles as well as in the gelatinase and specific granules.
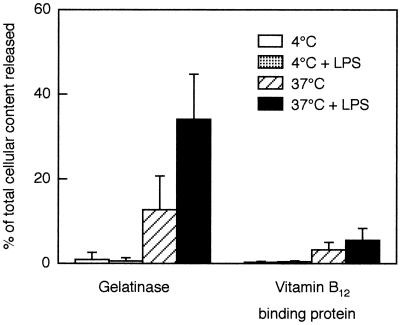
FIG. 6.
Extracellular release of gelatinase and vitamin B12-binding protein from LPS-primed and unprimed neutrophils. Neutrophils were preincubated for 30 min in presence or absence of LPS (10 μg/ml) at 4 or 37°C. Bars represent release into the medium of gelatinase (marker for the gelatinase and specific granules) and vitamin B12-binding protein (marker for the specific granules), as a percentage (mean + SD, n = 5) of the total amount in control cells.
Cells incubated at 4°C, with or without LPS, showed baseline surface expression of CR1, CR3, and FPR (Fig. 5), as seen in peripheral blood leukocytes, and no release of gelatinase or vitamin B12-binding protein (Fig. 6). Thus, there was no evidence for mobilization of markers from intracellular stores at 4°C, which was expected since vesicle transport is blocked at low temperatures. Cells incubated at 37°C in the absence of LPS for 30 min showed a substantially increased cell surface exposure of CR1 but released only 13% of their gelatinase and 3% of their vitamin B12-binding protein. In resting cells, intracellular CR1 is localized mainly in the secretory vesicles (42). Hence, the data indicate that these, the most easily mobilized neutrophil organelles, were extensively mobilized merely by incubation at 37°C as described previously (40). The low release of gelatinase and vitamin B12-binding protein indicates mobilization of only a small fraction of the gelatinase and specific granules, respectively. CR3 and FPR are both localized, to different degrees, in all three mobilizable organelles, i.e., the secretory vesicles, gelatinase granules, and specific granules (6, 40, 41). Thus, the moderate mobilization of CR3 and very slight mobilization (to 130% of control) of FPR after incubation at 37°C is in accordance with degranulation of the secretory vesicles.
The cells incubated at 37°C with LPS showed only a slight additional increase (over cells incubated at 37°C without LPS) in the surface exposure of CR1 and CR3 but a dramatic increase in the release of gelatinase (from 13 to 35%) as well as in the exposure of FPR (from 130 to 280%). The release of vitamin B12-binding protein from specific granules, however, increased only from 3 to 5%. Hence, the major effect of LPS was the mobilization of gelatinase granules, whereas it had only a slight effect on mobilization of specific granules. Since gelatinase is about equally distributed in gelatinase granules and specific granules, the percent mobilization of gelatinase granules can be estimated as 2 × (percent release of gelatinase − percent release of vitamin B12-binding protein). With this calculation, about 60% of gelatinase granules were mobilized in the presence of LPS, whereas about 20% were mobilized at 37°C without LPS.
DISCUSSION
We show that galectin-3 is a potent agonist of LPS-primed neutrophils but not of unprimed peripheral blood cells and that degranulation of gelatinase granules is a major event induced by LPS in neutrophils. These results raise two questions. The first, how do the data relate to previously proposed mechanisms for neutrophil priming by LPS and other agents, we have begun to address. The second, what role does galectin-3 play in relation to the pathophysiological effects of LPS, is a question that at the present we can only speculate upon.
The major degranulation effect specifically related to LPS is, interestingly, mobilization of gelatinase granules with little mobilization of specific granules. As noted in Results, about 60% of the gelatinase granules but only 5% of the specific granules were mobilized in the cells treated with LPS at 37°C. Mere incubation of the cells at 37°C resulted in 20% mobilization of gelatinase granules but contributed 3% of the 5% of mobilized specific granules. The specific signaling inducing this exclusive degranulation of the gelatinase granules will be further investigated.
The molecular mechanisms behind LPS-dependent priming of the response to fMLF, the most widely studied agonist in this context, have been extensively discussed. The suggested mechanisms include alterations of intracellular signaling pathways (increased protein phosphorylation, phospholipase activity, intracellular Ca2+ changes, and cross talk between Ca2+ increase and tyrosine phosphorylation), altered assembly of the NADPH oxidase, and proteolytic processing of cell surface proteins (13, 19, 20, 43, 44, 47). In addition, there are studies reporting an increased amount of FPR on LPS-primed neutrophils (32, 45). This has not been regarded as a major mechanism behind the induction of the primed response, most probably due to the findings in a paper published in 1984 by Guthrie et al. (19), which reported that LPS priming of the oxidative response was not accompanied by an increased number of cell surface FPR, at that time believed to be mobilized from a storage pool identified as the specific granules. However, since then, secretory vesicles and gelatinase granules, both of which store FPR (40), have been identified (7, 25). Based on our findings that LPS priming is accompanied by increased exposure of the FPR on the cell surface, we challenge the prevailing view and suggest that receptor upregulation from granule stores indeed makes an important contribution to the LPS-induced priming of the neutrophil response to fMLF. In fact, in cells incubated at 4 or 37°C, without or with LPS, the relative levels of fMLF cell surface binding (100, 130, and 280, respectively [Fig. 5]) agree very well with the relative levels of fMLF-induced activation (100, 160, and 280, respectively [Fig. 1]).
To further investigate the role of intracellular storage granules in LPS priming, we examined enucleated cytoplasts which lack such granules and have successfully been used for similar purposes (11, 15). The galectin-3-induced NADPH oxidase response present in nontreated cytoplasts was not enhanced by LPS, supporting a role for granule mobilization in the priming process. The presence of galectin-3 responsiveness in nonprimed cytoplasts is not surprising since it is well known that the cytoplast preparation procedure (involving an ultracentrifugation step at 37°C in the presence of the actin-disrupting drug cytochalasin B) results in some granule mobilization (36). It is difficult to evaluate the exact magnitude of the activation in cytoplasts (about 30% of an equivalent number of neutrophils) since plasma membrane is lost during cytoplast preparation (37).
Additional effects related to gelatinase granule mobilization may also be important for the priming phenomenon. Proteases released from the gelatinase granules have been proposed to be involved in remodeling of the cell surface, resulting in shedding of interleukin-8 receptors, FcγRIII, L-selectin, and CR1 (5, 23, 33, 38). Such proteolytic reorganization of the cell surface may result in increased accessibility or activation of other receptors. Mobilization of secretory vesicle or gelatinase granules also results in transfer of the b cytochrome (a membrane component of the NADPH oxidase) to the plasma membrane, which has been indicated to be a decisive effect of LPS priming resulting in enhanced assembly of the NADPH oxidase (13).
Galectin-3 differs from fMLF in that it did not activate nonprimed peripheral blood neutrophils (Fig. 2). It only slightly activated cells incubated at 37°C without LPS, whereas with LPS strong activation was seen. Hence, LPS converts the neutrophils from galectin-3 nonresponsive to galectin-3 responsive. This applies both to the extracellular (Fig. 2) and intracellular (Fig. 3) production of oxygen radicals.
A reasonable explanation for the LPS priming of neutrophils vis-à-vis activation by galectin-3 is the transfer of galectin-3 receptors to the plasma membrane when gelatinase granules are mobilized. Previously we have shown that CD66a and CD66b (CEACAM1 and CEACAM8, respectively [4]) are possible receptors for galectin-3 and reside in gelatinase granules of unprimed neutrophils (16, 22). However, as with fMLF, we cannot exclude that other rearrangements of the cell surface accompanying gelatinase granule mobilization are also involved in the induction of galectin-3 responsiveness. Since LPS treatment did not affect the NADPH oxidase response to the protein kinase C agonist PMA, we conclude that direct effects on the NADPH oxidase assembly or activity are not plausible explanations of the priming effect.
The difference between fMLF and galectin-3 regarding responsiveness or priming is probably dependent primarily on receptor localization. The fMLF receptors are localized not only in gelatinase granules but also on the cell surface in resting cells as well as in the easily mobilized secretory vesicles. In contrast, very few of the galectin-3 receptors (CD66a and CD66b) are present on the surface of resting cells or in secretory vesicles but reside mainly in the gelatinase and specific granules. Thus, the increased responsiveness to fMLF in cells incubated only at 37°C is probably due primarily to mobilization of fMLF receptors from secretory vesicles, while galectin-3 still cannot bind and activate the cells. However, after mobilization also of the gelatinase granules, the response to fMLF increases further, and the response to galectin-3 appears.
Although the gelatinase granule release in cells incubated at 37°C without LPS is around 20% there is only a slight (much less than 20%) galectin-3-induced response. Since galectin-3-induced activation of neutrophils is dependent on the N-terminal, aggregating domain of the molecule (22), a plausible explanation to the discrepancy is that the neutrophil surface has to expose a threshold amount of receptor molecules for an aggregation of galectin-3 to occur, thereby cross-linking receptors on the cell surface.
Infections involving gram-negative bacteria are accompanied by accumulation of LPS in the vicinity of the infection site. The involvement of inflammatory cells in this event is evident, and LPS induces the production of various inflammatory cytokines in monocytes and macrophages, enhancing the inflammatory process (34). The activation of monocytes/macrophages by LPS also leads to an increased production and release of galectin-3 (2, 28). The results we present here show that LPS in addition primes neutrophils to further activation by galectin-3. This suggests that galectin-3 may play an active role at the site of infection, exhibiting proinflammatory functions. Furthermore, the need for priming of the neutrophils to achieve a responding cell (e.g., to galectin-3) may per se be a regulatory mechanism, inducing a cellular response (such as release of toxic oxygen radicals) only at sites where it is functional and necessary, e.g., in inflamed or infected areas.
If released in sufficient amounts and systemically, LPS may cause the pathophysiological state known as bacterial sepsis or endotoxic shock, with subsequent failure of various organs. These frequently fatal syndromes appear to require neutrophil leukocytes. In cases of sepsis, LPS is probably the sole or major factor priming the neutrophils in the bloodstream. This results in a distinctive functional profile of the neutrophils in which they have decreased chemotactic responsiveness but increased adhesiveness and hypersensitivity to various activators of oxygen radical and protease release (46, 48). Many host factors, including tumor necrosis factor alpha and other cytokines, have been implicated as such activators and thus as important cofactors to LPS in propagating the neutrophil-induced tissue injury seen in septic shock and organ failure; antagonists of them have been tried therapeutically without much success (21). The present results suggest that neutrophils in this endotoxic state (46) have mobilized a major part of their gelatinase granules, and, moreover, have become hyperresponsive to galectin-3. This adds gelatinase granule contents, e.g., gelatinase, and galectin-3, to the host factors that might be important for development of septic shock and organ failure in conjunction with endotoxinemia.
ACKNOWLEDGMENTS
The skillful technical assistance of Lisbeth Björck and Marie Samuelsson is gratefully acknowledged.
This work was supported by the Fredrik and Ingrid Thuring Foundation, Swedish Rheumatism Association, Swedish Society for Medicine, Anna-Greta Crafoord Foundation for Rheumatological Research, Swedish Medical Research Council, and Swedish network and graduate school for inflammation research.
REFERENCES
- 1.Andersson T, Dahlgren C, Lew P D, Stendahl O. Cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Correlation to changes in the cytosolic free Ca2+ level and action of phorbol myristate acetate. J Clin Investig. 1987;79:1226–1233. doi: 10.1172/JCI112941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askew D, Yurochko A D, Burger C J, Elgert K D. Normal and tumor-bearing host macrophage responses: variability in accessory function, surface markers, and cell-cycle kinetics. Immunol Lett. 1990;24:21–29. doi: 10.1016/0165-2478(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 3.Babior B M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 4.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes K V, Karlsson A, Kuroki M, Lin S H, Lucka L, Najjar S M, Neumaier M, Öbring B, Shively J E, Skubitz K M, Stanners C P, Thomas P, Thompson J A, Virji M, vonKleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 5.Bennett T A, Lynam E B, Sklar L A, Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J Immunol. 1996;156:3093–3097. [PubMed] [Google Scholar]
- 6.Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 7.Borregaard N, Heiple J M, Simons E R, Clark R A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 9.Condliffe A M, Kitchen E, Chilvers E R. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Colch) 1998;94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 10.Dahlgren C, Johansson A, Lundqvist H, Bjerrum O W, Borregaard N. Activation of the oxygen-radical-generating system in granules of intact human neutrophils by a calcium ionophore (ionomycin) Biochim Biophys Acta. 1992;1137:182–188. doi: 10.1016/0167-4889(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 11.Dahlgren C, Johansson A, Orselius K. Difference in hydrogen peroxide release between human neutrophils and neutrophil cytoplasts following calcium ionophore activation. A role of the subcellular granule in activation of the NADPH-oxidase in human neutrophils? Biochim Biophys Acta. 1989;1010:41–48. doi: 10.1016/0167-4889(89)90182-1. [DOI] [PubMed] [Google Scholar]
- 12.Dahlgren C, Karlsson A. The neutrophil respiratory burst oxidase. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo F R, Renee J, McCormick S, Nakamura M, Apicella M, Weiss J P, Nauseef W M. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Investig. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downey G P, Fukushima T, Fialkow L, Waddell T K. Intracellular signaling in neutrophil priming and activation. Semin Cell Biol. 1995;6:345–356. doi: 10.1016/s1043-4682(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 15.English D, Gabig T G. Differentiation of cellular processes involved in the induction and maintenance of stimulated neutrophil adherence. Blood. 1986;67:1314–1322. [PubMed] [Google Scholar]
- 16.Feuk-Lagerstedt E, Jordan E T, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592–5598. [PubMed] [Google Scholar]
- 17.Follin P, Briheim G, Dahlgren C. Mechanisms in neutrophil priming: characterization of the oxidative response induced by formylmethionyl-leucyl-phenylalanine in human exudated cells. Scand J Immunol. 1991;34:317–322. doi: 10.1111/j.1365-3083.1991.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb C, Lau K, Wasserman L R, Herbert V. Rapid charcoal assay for intrinsic factor (IF), gastric juice unsaturated B12 binding capacity, antibody to IF, and serum unsaturated B12 binding capacity. J Hematol. 1965;25:875–883. [PubMed] [Google Scholar]
- 19.Guthrie L A, McPhail L C, Henson P M, Johnston R B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharides. J Exp Med. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallett M B, Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 21.Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5:123–132. doi: 10.1016/s1357-4310(98)01430-0. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 23.Khandaker M H, Mitchell G, Xu L, Andrews J D, Singh R, Leung H, Madrenas J, Ferguson S S, Feldman R D, Kelvin D J. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood. 1999;93:2173–2185. [PubMed] [Google Scholar]
- 24.Kjeldsen L, Bjerrum O W, Hovgaard D, Johnsen A H, Sehested M, Borregaard N. Human neutrophil gelatinase: a marker for circulating blood neutrophils. Purification and quantitation by enzyme linked immunosorbent assay. Eur J Haematol. 1992;49:180–191. doi: 10.1111/j.1600-0609.1992.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Kjeldsen L, Sengeløv H, Lollike K, Nielsen M H, Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83:1640–1649. [PubMed] [Google Scholar]
- 26.Leffler H. Introduction to galectins. Trends Glycosci Glycotechnol. 1997;9:9–19. [Google Scholar]
- 27.Lindstedt R, Apodaca G, Barondes S H, Mostov K E, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993;268:11750–11757. [PubMed] [Google Scholar]
- 28.Liu F T, Hsu D K, Zuberi R I, Kuwabara I, Chi E Y, Henderson W R., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 29.Lundahl J, Dahlgren C, Eklund A, Hed J, Hernbrand R, Tornling G. Quartz selectively down-regulates CR1 on activated human granulocytes. J Leukoc Biol. 1993;53:99–103. doi: 10.1002/jlb.53.1.99. [DOI] [PubMed] [Google Scholar]
- 30.Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radical Biol Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 31.Massa S M, Cooper D N, Leffler H, Barondes S H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- 32.McLeish K R, Klein J B, Lederer E D, Head K Z, Ward R A. Azothemia, TNF alpha, and LPS prime the neutrophil oxidative burst by distinct mechanisms. Kidney Int. 1996;50:407–416. doi: 10.1038/ki.1996.330. [DOI] [PubMed] [Google Scholar]
- 33.Middelhoven P J, Ager A, Roos D, Verhoeven A J. Involvement of a metalloprotease in the shedding of human neutrophil Fcγ RIIIB. FEBS Lett. 1997;414:14–18. doi: 10.1016/s0014-5793(97)00959-9. [DOI] [PubMed] [Google Scholar]
- 34.Murphy K, Haudek S B, Thompson M, Giroir B P. Molecular biology of septic shock. New Horiz. 1998;6:181–193. [PubMed] [Google Scholar]
- 35.Nathan C F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Investig. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrequin P R, Todd III R F, Smolen J E, Boxer L A. Expression of specific granule markers on the cell surface of neutrophil cytoplasts. Blood. 1986;67:1119–1125. [PubMed] [Google Scholar]
- 37.Roos D, Voetman A A, Meerhof L J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983;97:368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadallah S, Hess C, Miot S, Spertini O, Lutz H, Schifferli J A. Elastase and metalloproteinase activities regulate soluble complement receptor 1 release. Eur J Immunol. 1999;29:3754–3761. doi: 10.1002/(SICI)1521-4141(199911)29:11<3754::AID-IMMU3754>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, Hughes R C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- 40.Sengeløv H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J. 1994;299:473–479. doi: 10.1042/bj2990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengeløv H, Kjeldsen L, Diamond M S, Springer T A, Borregaard N. Subcellular localization and dynamics of Mac-1 (αmβ2) in human neutrophils. J Clin Investig. 1993;92:1467–1476. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sengeløv H, Kjeldsen L, Kroeze W, Berger M, Borregaard N. Secretory vesicles are the intracellular reservoir of complement receptor 1 in human neutrophils. J Immunol. 1994;153:804–810. [PubMed] [Google Scholar]
- 43.Surette M E, Dallaire N, Jean N, Picard S, Borgeat P. Mechanisms of the priming effect of lipopolysaccharides on the biosynthesis of leukotriene B4 in chemotactic peptide-stimulated human neutrophils. FASEB J. 1998;12:1521–1531. doi: 10.1096/fasebj.12.14.1521. [DOI] [PubMed] [Google Scholar]
- 44.Tauber A I, Karnad A B, Hartshorn K L, Myers J B, Schwartz J H. Biochemistry of the acute allergic reactions: 5th International Symposium. New York, N.Y: Alan R. Liss, Inc.; 1989. pp. 297–309. [Google Scholar]
- 45.Vosbeck K, Tobias P, Mueller H, Allen R A, Arfors K E, Ulevitch R J, Sklar L A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990;47:97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- 46.Wagner J G, Roth R A. Neutrophil migration during endotoxemia. J Leukoc Biol. 1999;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- 47.Watson F, Edwards S W. Stimulation of primed neutrophils by soluble immune complexes: priming leads to enhanced intracellular Ca2+ elevations, activation of phospholipase D, and activation of the NADPH oxidase. Biochem Biophys Res Commun. 1998;29:819–826. doi: 10.1006/bbrc.1998.8524. [DOI] [PubMed] [Google Scholar]
- 48.Welbourn C R, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]