Abstract
Background and objective
Asthma and chronic obstructive pulmonary disease (COPD) are two prevalent and complex diseases that require personalized management. Although a strategy based on treatable traits (TTs) has been proposed, the prevalence and relationship of TTs to the diagnostic label and disease severity established by the attending physician in a real‐world setting are unknown. We assessed how the presence/absence of specific TTs relate to the diagnosis and severity of ‘asthma’, ‘COPD’ or ‘asthma + COPD’.
Methods
The authors selected 30 frequently occurring TTs from the NOVELTY study cohort (NOVEL observational longiTudinal studY; NCT02760329), a large (n = 11,226), global study that systematically collects data in a real‐world setting, both in primary care clinics and specialized centres, for patients with ‘asthma’ (n = 5932, 52.8%), ‘COPD’ (n = 3898, 34.7%) or both (‘asthma + COPD’; n = 1396, 12.4%).
Results
The results indicate that (1) the prevalence of the 30 TTs evaluated varied widely, with a mean ± SD of 4.6 ± 2.6, 5.4 ± 2.6 and 6.4 ± 2.8 TTs/patient in those with ‘asthma’, ‘COPD’ and ‘asthma + COPD’, respectively (p < 0.0001); (2) there were no large global geographical variations, but the prevalence of TTs was different in primary versus specialized clinics; (3) several TTs were specific to the diagnosis and severity of disease, but many were not; and (4) both the presence and absence of TTs formed a pattern that is recognized by clinicians to establish a diagnosis and grade its severity.
Conclusion
These results provide the largest and most granular characterization of TTs in patients with airway diseases in a real‐world setting to date.
Keywords: airways, allergy, asthma, bronchitis, chronic obstructive pulmonary disease, COPD, emphysema, smoking
Short abstract
This study shows which treatable traits are present and/or absent in patients diagnosed with ‘asthma’, ‘COPD’ (chronic obstructive pulmonary disease) and ‘asthma + COPD’ in a global, observational study of more than 11,000 patients (NOVELTY), and how their prevalence changes with disease severity.
See related Editorial
INTRODUCTION
Asthma and chronic obstructive pulmonary disease (COPD) are two prevalent and heterogeneous chronic airway diseases that may overlap, 1 , 2 thus requiring personalized clinical management. 3 To this end, a strategy based on so‐called treatable traits (TTs), which is agnostic to the traditional diagnostic labels of asthma or COPD, has been proposed. 4 , 5 , 6 , 7 TTs can be identified by their observable clinical characteristics (i.e., phenotypes) and/or through validated biomarkers that indicate the presence/absence of distinct molecular mechanisms (i.e., endotypes) in the pulmonary (e.g., airflow limitation, chronic bronchitis, emphysema, among others), extra‐pulmonary (e.g., obesity, cardiovascular disease or gastroesophageal reflux, among others) and behavioural/environmental domains (e.g., smoking, treatment compliance, familiar/social support among others). 4 , 5 , 6 TTs can coexist, interact and change with time in the same patient. 4 , 5 , 6 Recent clinical trials have shown that management of patients with chronic airway diseases guided by TTs improves clinical outcomes. 7 , 8 , 9 , 10 , 11 , 12 , 13
TTs have been explored in several previous small studies in patients with asthma or COPD, 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 but their prevalence and association with disease label and severity in a large, global, real‐world setting that encompasses a broad spectrum of patients with chronic airway diseases are unknown. We hypothesized that the prevalence and association with disease label and severity vary by trait. We explored this hypothesis in the NOVELTY cohort (NCT02760329), a large, 3‐year, real‐world, prospective observational study in patients diagnosed with ‘asthma’, ‘COPD’ or ‘asthma + COPD’ in primary care and specialized centres around the globe. 15 , 16
METHODS
The methodology of NOVEL observational longiTudinal studY (NOVELTY) has been published elsewhere 15 , 16 and is summarized briefly.
Study design and patients
NOVELTY is an ongoing prospective study that comprised patients diagnosed with ‘asthma’, ‘COPD’ or ‘asthma + COPD’ by their attending physician in primary care or pulmonary or allergy clinics from 19 countries in the Americas, Asia, Australia and Europe (first patient enrolled in July 2016). 15 , 16 Patients were excluded only if their primary respiratory diagnosis was not asthma or COPD, they had either participated in a respiratory interventional trial during the previous 12 months or were considered unlikely to complete 3 years of follow‐up. Data from patients in China and one site in Mexico were excluded from the present analysis due to new data transfer regulations and eligibility criteria, respectively. The severity of the disease (mild, moderate and severe) was established by the attending physicians based on their clinical judgement. 16 Recruitment was stratified by diagnostic label and disease severity.
Measurements
Patients had yearly visits with follow‐up data collected by their healthcare practitioner for 3 years or until study discontinuation, whichever comes first. Patients also completed questionnaires every 3 months using either a web‐based platform or by telephone. As detailed elsewhere, 15 demographics and clinical data were assessed using standardized questionnaires. Spirometry was determined following international recommendations and results were expressed as % predicted and proportion below the lower limit of normal using the Global Lung Function Initiative multi‐ethnic reference equations. 17 Peripheral venous blood was collected from consenting patients for cell counts. 15 Thirty specific TTs (Table 1) were selected by co‐authors based on previous literature, personal experience and availability in NOVELTY.
TABLE 1.
List and definitions of TTs used in this study
| TT | Definition | References |
|---|---|---|
| Pulmonary | ||
| Non‐reversible airflow limitation | Post‐bronchodilator FEV1/FVC ratio <LLN | [4] |
| Bronchodilator reversibility | FEV1 reversibility ≥12% + ≥200 ml | [4] |
| PRISm | FEV1 <80% predicted + FEV1/FVC ≥LLN | [18] |
| Frequent productive cough | SGRQ item 1 (cough) and item 2 (phlegm) | [4] |
| Non‐EOS frequent productive cough | <100 blood eosinophils/μl + frequent productive cough | [19] |
| Exacerbation prone | ≥1 Antibiotics and/or OCS‐treated exacerbations in the last 12 months | [10, 20] |
| Emphysema | Medical history + historical chest x‐ray or chest CT or DLCO <LLN | [4] |
| Bronchiectasis | Medical history or historical chest CT | [6, 21] |
| Obstructive sleep apnoea | Medical history | [4] |
| Nasal/sinus polyps | Medical history | [22] |
| Allergic rhinosinusitis | Medical history | [6] |
| Non‐allergic rhinosinusitis | Medical history | [23] |
| Other respiratory allergies | Medical history (conjunctivitis or mould allergy or animal allergy)) | [24] |
| Extra‐pulmonary | ||
| Non‐respiratory allergies | Current/past total IgE or positive skin‐prick (latex, drug, food), atopic eczema or anaphylaxis | [24] |
| Gastroesophageal reflux | Medical history | [5] |
| Obesity | BMI ≥30 kg/m2 or medical history (obesity) if BMI was missing | [6] |
| Cachexia | BMI <18.5 kg/m2 | [4] |
| Anaemia | Hb <130 g/L (men) or <110 g/L (women) | [6] |
| Osteoporosis | Medical history | [10] |
| Coronary heart disease | Medical history | [6] |
| Heart failure | Medical history | [6] |
| Cerebrovascular disease | Medical history | [25] |
| Depression or anxiety | Medical history | [4] |
| Systemic inflammation | Blood neutrophils ≥6 × 109/L | [10, 26] |
| Th2 inflammation low | <100 blood eosinophils/μl + FeNO <20 ppb | Newly proposed |
| Th2 inflammation high | ≥300 blood eosinophils/μl ± FeNO ≥50 ppb | [6] |
| Behavioural/environmental | ||
| Smoking | Current smoker | [4] |
| Occupational exposures | Medical history | [4] |
| Indoor use of biomass/coal | Medical history | [4] |
| Frequent reliever use | Reliever used ≥3–6 times/week | Newly proposed |
Abbreviations: CT, computed tomography; DLCO, diffusing capacity of the lungs for carbon monoxide; EOS, eosinophilic; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LLN, lower limit of normal; OCS, oral corticosteroids; ppb, parts per billion; PRISm, preserved ratio impaired spirometry; SGRQ, St George's Respiratory Questionnaire; Th2, type‐2 airway inflammation; TT, treatable trait.
Statistical analysis
Differences across disease and severity levels were assessed using analysis of variance (followed by post hoc contrasts where necessary) or the chi‐square test. Analyses were carried out using R version 3.5.1. 27 Because the very large sample of the study population greatly facilitated the finding of many statistically significant differences (p < 0.05) to identify clinically meaningful differences in prevalence, we used an arbitrary threshold of a ≥2‐ or ≤0.5‐fold change difference. To identify associations between TTs, we computed their co‐occurrence (i.e., the proportion of patients presenting two given TTs) per diagnostic label. The results of this co‐occurrence are presented graphically in the form of a network analysis, where TT pairs co‐occurring in ≥5% of the individuals per diagnostic label are linked. 28 Finally, to investigate the association between TTs and diagnostic labels, TTs were dichotomized as binary variables (present/absent) and their associations with diagnostic labels were explored using chi‐square tests.
RESULTS
Patient characteristics
The analysis included 11,226 patients, 5932 (52.8%) of whom were diagnosed with ‘asthma’, 3898 (34.7%) with ‘COPD’ and 1396 (12.4%) with ‘asthma + COPD’ (Table 2). Patients with ‘asthma’ were approximately a decade younger and included a higher proportion of females and never‐smokers. Patients with ‘COPD’ or ‘asthma + COPD’ were more symptomatic and reported a history of pneumonia more frequently than patients with asthma. Exacerbations were most frequently reported by patients with ‘asthma + COPD’. Non‐reversible airflow limitation was more prevalent and more severe in patients with ‘COPD’ or ‘asthma + COPD’ than in those with ‘asthma’. Inhaled oral corticosteroids, leukotrienes and biologic treatments were used more frequently by patients with ‘asthma’ or ‘asthma + COPD’ than those with ‘COPD’. Type‐2 airway inflammation (Th2) biomarkers, such as circulating eosinophils and fractional exhaled nitric oxide, were higher in patients with ‘asthma’ and lower in patients with ‘COPD’. However, differences in eosinophil levels were of small magnitude and likely statistically significant due to the large sample size. Patients with COPD showed higher levels of circulating leucocytes and neutrophils. Figure S1 in the Supporting Information shows the distribution of disease severity across disease labels. Although there were statistically significant differences, these are likely the result of recruiting a large number of patients, but this large population of mild, moderate and severe disease in turn allowed the investigation of TTs in the different severity strata.
TABLE 2.
Main characteristics (mean ± SD or %) of patients studied
| Demographics and exposures | ‘Asthma’ (n = 5932) | ‘Asthma + COPD’ (n = 1396) | ‘COPD’ (n = 3898) | p‐value a |
|---|---|---|---|---|
| Age, mean, years ± SD | 52.0 ± 17.1 | 64.7 ± 10.3 | 66.6 ± 9.6 | <0.0001 |
| Male, % | 37.5 | 53.1 | 61.5 | <0.0001 |
| BMI, mean, kg/m2 ± SD | 28.0 ± 6.6 | 28.5 ± 6.4 | 27.6 ± 6.3 | <0.0001 |
| Smoking status, % | <0.0001 | |||
| Current smoker | 8.1 | 24.5 | 29.6 | |
| Former smoker | 30.2 | 63.5 | 64.0 | |
| Never smoked | 61.7 | 12.0 | 6.3 | |
| Cumulative smoking exposure, mean, pack‐years ± SD | 16.7 ± 23.3 | 36.5 ± 28.9 | 47.8 ± 40.2 | <0.0001 |
| Symptoms and clinical history | ||||
| Dyspnoea (mMRC ≥Grade 2), % | 20.8 | 43.6 | 53.2 | <0.0001 |
| Frequent productive cough, % | 2.2 | 8.5 | 6.5 | <0.0001 |
| CAAT total score, mean ± SD | 14.0 ± 8.5 | 17.2 ± 8.5 | 17.0 ± 8.3 | <0.0001 |
| SGRQ total score, mean ± SD | 29.8 ± 20.9 | 39.9 ± 22.1 | 41.5 ± 21.8 | <0.0001 |
| Physician‐reported exacerbations in the past 12 months, mean ± SD | 0.7 ± 1.5 | 1.0 ± 1.8 | 0.6 ± 1.3 | <0.0001 |
| ≥1 exacerbation, % | 34.1 | 47.0 | 35.0 | <0.0001 |
| ≥1 hospital admission, % | 3.7 | 9.0 | 10.4 | <0.0001 |
| History of pneumonia, % | 3.5 | 7.2 | 7.4 | <0.0001 |
| Lung function | ||||
| Post‐bronchodilator FEV1/FVC, mean ± SD | 74.4 ± 11.8 | 59.6 ± 14.7 | 56.7 ± 16.2 | <0.0001 |
| Post‐bronchodilator FEV1/FVC <0.70, % | 28.3 | 73.5 | 74.9 | <0.0001 |
| Post‐bronchodilator FEV1% predicted, mean ± SD | 86.3 ± 20.3 | 68.3 ± 21.5 | 61.6 ± 23.0 | <0.0001 |
| Treatments | ||||
| Short‐acting bronchodilator only (±add‐on), % | 8.5 | 10.8 | 23.2 | <0.0001 |
| Inhaled steroids maintenance, any combination, % | 88.6 | 83.8 | 55.9 | <0.0001 |
| Triple therapy (ICS + LAMA + LABA), % | 13.7 | 48.7 | 36.2 | <0.0001 |
| Leukotrienes or methylxanthine, % | 30.7 | 26.4 | 9.2 | <0.0001 |
| Maintenance oral corticosteroids, % | 3.9 | 3.9 | 1.9 | <0.0001 |
| Biologic treatment, % | 9.8 | 4.2 | 0.1 | <0.0001 |
| Biomarkers | ||||
| Blood leucocytes ×109/L, mean ± SD | 7.1 ± 2.1 | 7.6 ± 2.2 | 7.6 ± 2.1 | <0.0001 |
| Blood neutrophils ×109/L, mean ± SD | 4.4 ± 1.8 | 4.9 ± 2.0 | 5.0 ± 1.9 | <0.0001 |
| Blood lymphocytes ×109/L, mean ± SD | 2.0 ± 0.6 | 2.0 ± 0.7 | 2.0 ± 0.7 | 0.8206 |
| Blood eosinophils/μl, mean ± SD | 222.8 ± 189.8 | 212.4 ± 179.2 | 182.9 ± 127.1 | <0.0001 |
| Platelets ×109/L, mean ± SD | 261.4 ± 72.4 | 264.6 ± 78.9 | 250.3 ± 80.2 | <0.0001 |
| FeNO, ppb, mean ± SD | 32.3 ± 30.7 | 24.1 ± 23.8 | 19.9 ± 17.9 | <0.0001 |
| Th2, % | <0.0001 | |||
| Low | 10.1 | 14.1 | 15.0 | |
| Medium | 57.4 | 60.0 | 67.4 | |
| High | 32.5 | 25.9 | 17.6 | |
Abbreviations: ANOVA, analysis of variance; CAAT, Chronic Airways Assessment Test; COPD, chronic obstructive pulmonary disease; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroids; LABA, long‐acting β2‐agonist; LAMA, long‐acting muscarinic antagonist; mMRC, modified Medical Research Council; ppb, parts per billion; SGRQ, St George's Respiratory Questionnaire; Th2, type‐2 airway inflammation; Th2‐high, ≥300 blood eosinophils/μl ± FeNO ≥50 ppb; Th2‐low, <100 blood eosinophils/μl + FeNO <20 ppb; Th2‐medium, neither Th2‐low nor Th2‐high.
p‐values are based on the chi‐square test for categorical variables and ANOVA for continuous variables.
Prevalence of TTs in the entire study population
The prevalence of the 30 TTs studied here: (1) varied from almost 50% (non‐reversible airflow limitation) to <10% in several other TTs (Figure 1A); (2) did not change substantially after adjusting for age (Table S1 in the Supporting Information); (3) was different in patients diagnosed in primary versus specialized care clinics (Table S2 in the Supporting Information); (4) the mean number of TTs per patient was 5.1 ± 2.7, indicating that most TTs do not present in isolation; (5) although there were many statistically significant regional differences in the prevalence of TTs (Tables [Link], [Link] in the Supporting Information), the TT distribution pattern was similar in United States–Canada, Latin America, Korea–Japan, Europe and Australia (Figure 1B); and (6) the presence (OR > 1) or absence (OR < 1) of 20 (66.6%) TTs was significantly associated with severe disease (p‐values: 0.05 to <10140 as indicated by the edge thickness), whereas the remaining 10 (33.4%) TTs were not (Figure S2 in the Supporting Information).
FIGURE 1.
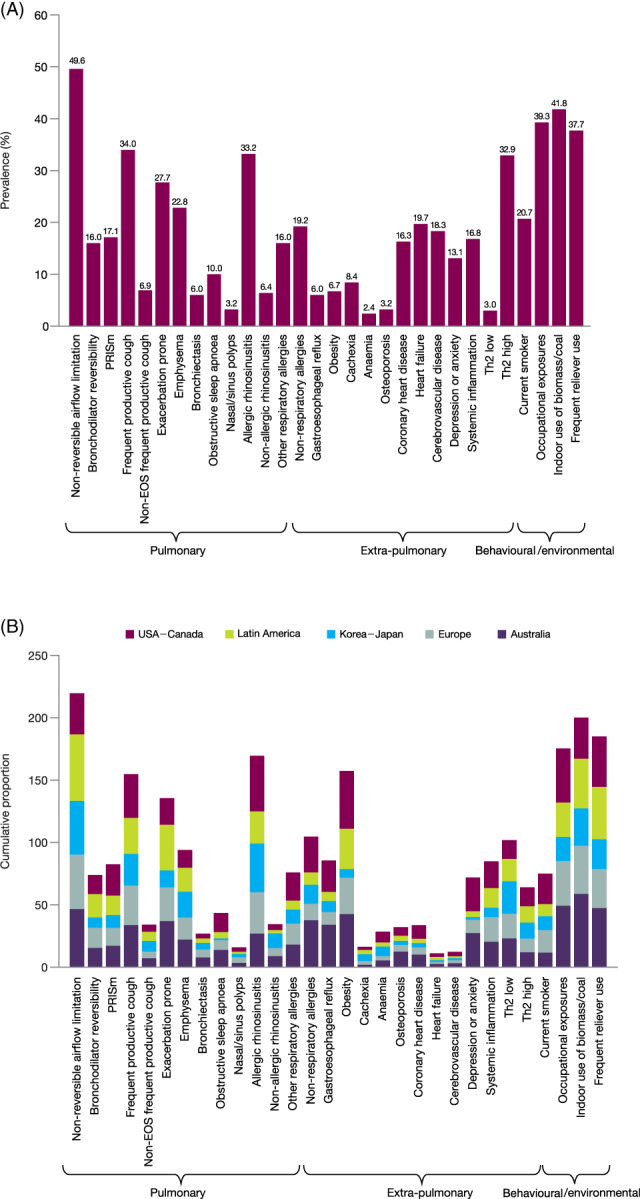
Prevalence of the investigated TTs in the entire study population (A) and by geographical region (B). Non‐evaluable traits due to missing data were adjusted by weighting for the proportion of positive traits among those evaluable in a patient. EOS, eosinophilic; PRISm, preserved ratio impaired spirometry; Th2, type‐2 airway inflammation; TT, treatable trait
Relationship of TTs with diagnostic label
Differences in the prevalence of specific TTs across diagnostic labels achieved statistical significance in all 30 TTs (Table 3), likely due to the large sample size of the studied population. To address this, Figure 2A presents a fold change‐based heat map for the three potential comparisons across the three diagnostic labels. It shows that the prevalence of 12 (40%) TTs changed with disease label. Furthermore, the pattern of TTs in patients with ‘asthma’ was opposite in patients with ‘COPD’, with a mixed pattern in those with ‘asthma + COPD’. As these particular TTs are used in clinical practice to establish these diagnoses, we labelled them as ‘diagnostic TTs’. Importantly, however, the prevalence of the remaining 18 (60%) TTs was similar across disease labels, including some highly clinically relevant TTs such as bronchodilator reversibility, frequent productive cough and exacerbation prone (Figure 2A). Figure S3 in the Supporting Information shows that, on average, patients with ‘asthma’ had the lowest number of TTs (4.6 ± 2.6), followed by those with ‘COPD’ (5.4 ± 2.6) and those with ‘asthma +COPD’ (6.4 ± 2.8; all p < 0.0001).
TABLE 3.
Prevalence of the 30 analyzed TTs in patients with ‘asthma’, ‘asthma + COPD’ or ‘COPD’
| ‘Asthma’ | ‘Asthma + COPD’ | ‘COPD’ | p‐value | |
|---|---|---|---|---|
| Pulmonary | ||||
| Non‐reversible airflow limitation | 23.2 | 61.8 | 63.9 | <0.0001 |
| Bronchodilator reversibility | 15.8 | 19.1 | 13.1 | <0.0001 |
| PRISm | 15.6 | 17.0 | 18.8 | 0.0008 |
| Frequent productive cough | 25.0 | 38.9 | 38.1 | <0.0001 |
| Non‐EOS frequent productive cough | 4.6 | 8.8 | 7.4 | 0.0002 |
| Exacerbation prone | 24.0 | 35.1 | 24.1 | <0.0001 |
| Emphysema | 1.7 | 29.2 | 37.6 | <0.0001 |
| Bronchiectasis | 4.7 | 7.7 | 5.7 | <0.0001 |
| Obstructive sleep apnoea | 7.7 | 11.7 | 10.7 | <0.0001 |
| Nasal/sinus polyps | 5.6 | 3.2 | 0.8 | <0.0001 |
| Allergic rhinosinusitis | 51.5 | 36.3 | 11.9 | <0.0001 |
| Non‐allergic rhinosinusitis | 8.6 | 8.0 | 2.6 | <0.0001 |
| Other respiratory allergies | 24.8 | 19.2 | 3.9 | <0.0001 |
| Extra‐pulmonary | ||||
| Non‐respiratory allergies | 22.1 | 21.6 | 13.9 | <0.0001 |
| Gastroesophageal reflux | 14.5 | 20.4 | 15.5 | <0.0001 |
| Obesity | 31.9 | 35.7 | 31.2 | 0.0085 |
| Cachexia | 2.6 | 2.4 | 4.2 | <0.0001 |
| Anaemia | 3.1 | 7.8 | 7.3 | <0.0001 |
| Osteoporosis | 4.9 | 8.9 | 6.3 | <0.0001 |
| Coronary heart disease | 2.8 | 9.9 | 12.5 | <0.0001 |
| Heart failure | 0.6 | 2.5 | 4.2 | <0.0001 |
| Cerebrovascular disease | 1.6 | 4.2 | 3.7 | <0.0001 |
| Depression or anxiety | 13.1 | 20.5 | 15.4 | <0.0001 |
| Systemic inflammation | 15.0 | 21.5 | 22.7 | <0.0001 |
| Th2‐low | 10.1 | 14.1 | 15.0 | <0.0001 |
| Th2‐high | 24.2 | 18.7 | 11.9 | <0.0001 |
| Behavioural/environmental | ||||
| Current smoker | 8.1 | 24.5 | 29.6 | <0.0001 |
| Occupational exposures | 29.2 | 45.3 | 43.3 | <0.0001 |
| Indoor use of biomass/coal | 30.7 | 51.8 | 42.9 | <0.0001 |
| Frequent reliever use | 30.5 | 45.6 | 37.1 | <0.0001 |
Note: Data show percentage of patients with available data.
Abbreviations: COPD, chronic obstructive pulmonary disease; EOS, eosinophilic; PRISm, preserved ratio impaired spirometry; Th2, type‐2 airway inflammation; TT, treatable trait.
FIGURE 2.
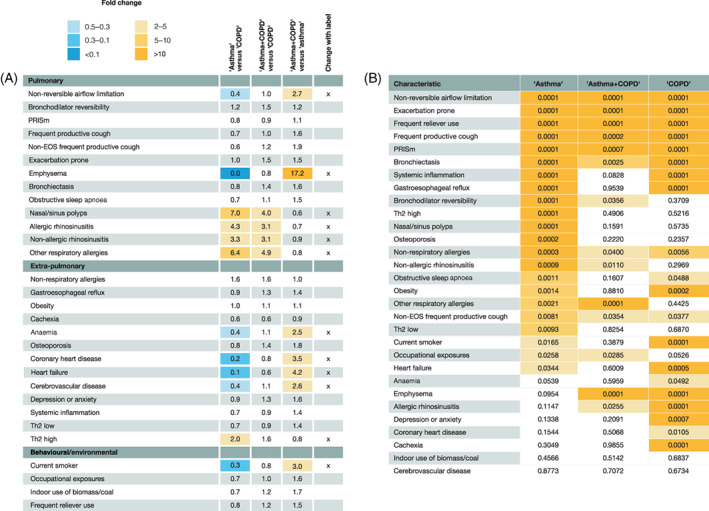
(A) Heat map of fold changes in prevalence of TTs across the three diagnostic labels considered (columns from left to right: ‘asthma’ vs. ‘COPD’, ‘asthma + COPD’ vs. ‘COPD’ and ‘asthma + COPD’ vs. ‘asthma’). The final column highlights (x) those TTs with a ≥2‐ (orange cells) or ≤0.5‐ (blue cells) fold change between diagnostic labels. (B) Heat map of statistically significant p‐values (ANOVA) associated with differences in prevalence of a given TT by disease severity in patients with ‘asthma’, ‘asthma + COPD’ and ‘COPD’. TTs are ordered by p‐value in patients with ‘asthma’. Darkest shading, p ≤ 0.001; mid‐shading, p ≤ 0.01; light shading, p ≤ 0.05; no shading, p > 0.05. ANOVA, analysis of variance; COPD, chronic obstructive pulmonary disease; EOS, eosinophilic; PRISm, preserved ratio impaired spirometry; Th2, type‐2 airway inflammation; TT, treatable trait
Relationship of TTs with disease severity
The presence (OR > 1) or absence (OR < 1) of 20 (66.6%) TTs was significantly associated with severe disease (p‐values: 0.05 to <10140; Figure S2 in the Supporting Information). However, these associations varied by disease label (Table S6 in the Supporting Information). Figure 2B shows a heat map of the TT where prevalence significantly varied by disease severity within each disease label. The number (and type) of TTs where prevalence changed significantly in relation to disease severity was higher in ‘asthma’ than in ‘COPD’ or ‘asthma + COPD’. In contrast, the prevalence of non‐reversible airflow limitation, exacerbation prone, frequent reliever use, preserved ratio impaired spirometry and bronchiectasis was significantly related, with higher disease severity in all three diseases; the prevalence of other TTs was independent of the level of disease severity.
TT co‐occurrence
Figure 3 presents a network of TT co‐occurrence by disease label. In ‘asthma’, the most frequent TT pairs included rhinosinusitis, respiratory and non‐respiratory allergies, Th2‐high markers, obesity, occupational exposures, indoor use of biomass/coal and frequent reliever use. In ‘COPD’, the most prevalent TT pairs included non‐reversible airflow limitation, emphysema, frequent productive cough, environmental exposures (including smoking), exacerbation prone and frequent reliever use (Figure 3). Finally, the co‐occurrence network was most complex in patients diagnosed with ‘asthma + COPD’ (albeit slightly weaker, where seven TTs were involved in more than seven highly prevalent TT pairs, including allergic rhinosinusitis [as in the ‘asthma’ network], some TTs with high connectivity in the ‘COPD’ network [such as airflow limitation and occupational exposures], as well as exacerbation prone and obesity that also emerged in the ‘asthma + COPD’ network). Importantly, this co‐occurrence analysis sought to describe relationships between TTs, not to infer causal associations.
FIGURE 3.
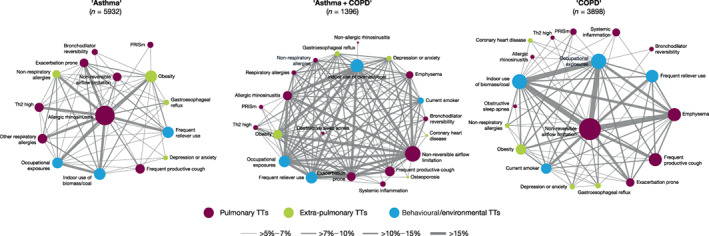
Network of TT co‐occurrence (i.e., the proportion of patients presenting two given TTs) by disease label. Colour node indicates their pulmonary, extra‐pulmonary or behavioural/environmental origin. Node size is proportional to its prevalence, and the width of the edge (links) indicates the proportion of patients in whom a given TT pair co‐occur. COPD, chronic obstructive pulmonary disease; PRISm, preserved ratio impaired spirometry; Th2, type‐2 airway inflammation; TT, treatable trait
Pattern recognition
In clinical practice, physicians establish a given diagnosis by pattern recognition (i.e., by identifying a number of present and absent traits). 29 Figure 4 shows that ‘asthma’ was significantly associated with the presence of 10 TTs and the absence of 13 other TTs, ‘COPD’ with the presence of 17 other TTs and the absence of six TTs and ‘asthma + COPD’ with the presence of 21 TTs (which included most of those already identified in patients diagnosed with ‘asthma’ or ‘COPD’) but with no absent TTs. Figure 4 also shows that the TT pattern changes in relation to disease severity, but in opposite directions in severe ‘asthma’ and severe ‘COPD’ as the former was characterized by an increase in the number of present TTs and a reduction in absent TTs, whereas the opposite occurred in ‘COPD’. By contrast, the pattern of severe ‘asthma + COPD’ did not change substantially with disease severity, except for the consideration of systemic inflammation and the non‐association with cerebrovascular disease, obesity and non‐allergic rhinosinusitis.
FIGURE 4.
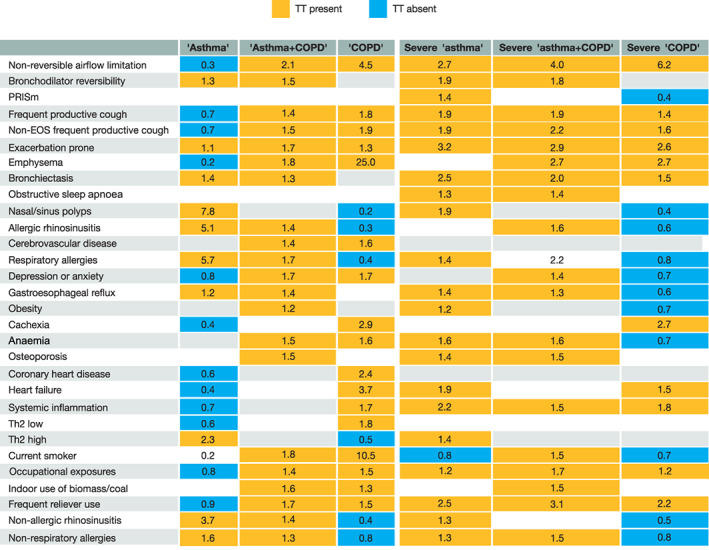
Pattern recognition heat map in relation to diagnostic label (left three columns) and severity assessment (right three columns). For this analysis, TTs were dichotomized as binary variables (present/absent) and their associations with diagnostic labels and severity of disease were explored using chi‐square tests. Coloured cells indicate statistically significant associations (p < 0.05), where cell colour (orange or blue) corresponds to an OR > 1 (i.e., presence of the TT) or an OR < 1 (i.e., absence of the TT), respectively. COPD, chronic obstructive pulmonary disease; EOS, eosinophilic; PRISm, preserved ratio impaired spirometry; Th2, type‐2 airway inflammation; TT, treatable trait
DISCUSSION
This study shows that: (1) the prevalence of the 30 TTs investigated here varies widely within the NOVELTY cohort, without clear geographical variations, but differs by care setting; (2) six (pulmonary) TTs were significantly associated with a diagnosis of ‘asthma’ or ‘COPD’ (‘diagnostic TTs’), but the prevalence of the remaining pulmonary, and most of the extra‐pulmonary and behavioural/environmental TTs was independent of the disease label; (3) the diagnosis of ‘asthma + COPD’ was associated with all TTs identified in ‘asthma’ or ‘COPD’ separately; (4) some, but not all, TTs can co‐occur and their prevalence varies with disease severity; (5) the pattern of present/absent TTs relates to disease label and severity assessment. Collectively, these results provide a comprehensive repository of information on TTs in chronic airway diseases, contribute to better delineate their heterogeneity and help to better understand how the presence/absence of different TTs contribute to a specific diagnostic label and the assessment of disease severity by the attending physician.
Several previous smaller studies have investigated TTs in patients with asthma, COPD or both. 8 , 9 , 10 , 11 , 12 , 13 , 14 An analysis of data at recruitment in the NOVELTY cohort demonstrated marked heterogeneity within, and considerable overlap between, physician‐assigned diagnoses and the assessment of severity in patients with ‘asthma’ and/or ‘COPD’. 16 The current analysis of TTs is the largest to date and the first to compare their prevalence, relationship with the diagnostic label, disease severity and pattern of co‐occurrence in different real‐life healthcare settings.
In this study we identified six pulmonary TTs, including nasal sinus polyps and several allergies in ‘asthma’ and non‐reversible airflow limitation and emphysema in ‘COPD’ (plus smoking and comorbidities), that were distinctly associated with the diagnostic labels of ‘asthma’ or ‘COPD’. This is not surprising as these pulmonary ‘diagnostic TTs’ are most often used in practice to precisely establish these clinical diagnoses. However, importantly for clinical practice, the prevalence of 18 pulmonary, extra‐pulmonary and/or behavioural/environmental TTs was similar, irrespective of the diagnostic label used, including some TTs traditionally considered almost exclusive to ‘asthma’ or ‘COPD’, such as bronchodilator reversibility, Th2 and frequent productive cough. This has clinical relevance because it indicates a high degree of heterogeneity, and that these 18 TTs should be considered in any patient with chronic airway disease, irrespective of the diagnostic label.
We also found that the prevalence of some, but not all, TTs increased with disease severity and that the number of TTs typically associated with a perception of a more severe disease varied between disease labels, whereas the prevalence of other TTs did not change at all. This suggests that the individual consideration of TTs, irrespective of label and severity assessment, can facilitate more personalized and precise management and, eventually, a better outcome. Moreover, we also found that a given clinical label was significantly associated with a pattern of both present and absent TTs (Figures 3 and 4), in keeping with the concept that, in clinical practice, physicians establish a diagnosis (and start a treatment) based on pattern recognition; 29 when a precise clinical diagnosis of ‘asthma’ or ‘COPD’ is difficult, ‘asthma + COPD’ appears to be chosen instead.
Our study has several strengths and limitations. The large sample size, global participation and real‐world setting, which allowed for the inclusion of patients with chronic airway diseases who are typically excluded from randomized clinical trials, 30 , 31 , 32 , 33 are strengths. Indeed, the generalizability of NOVELTY data has been presented previously. 16 However, we acknowledge several potential limitations. First, we studied 30 TTs selected by co‐authors based on previous literature, personal experience and availability in the NOVELTY database (Table 1); yet the list of potential TTs of interest is much larger 4 , 5 , 6 , 7 and other TTs may need to be considered in future studies. In particular, some potentially relevant TTs in the behavioural/environmental domain, such as adherence, inhaler technique and/or inhaler polypharmacy, were not included in NOVELTY 15 and could not, therefore, be analyzed here. Second, the diagnostic label and assessment of disease severity were established in each patient by the attending physician, according to their own clinical experience and judgement 16 and possibly, but not certainly, according to the current guideline recommendations. Although, at first glance, this may be seen as a limitation, we think that it actually allows for a better understanding of what drives disease labelling and severity scoring (hence, likely treatment too) by the attending physician in real‐world clinical practice. 16 Accordingly, the higher number of TTs in patients recruited in specialty clinics can imply either a better diagnostic and therapeutic workup or a greater disease complexity. In addition, the lack of mandatory computed tomography scans for the identification of specific TTs (e.g., emphysema, bronchiectasis or other comorbid conditions) and differences in the definitions used versus those proposed in clinical trials 34 , 35 , 36 may lead to an inaccurate (underestimation) of the true prevalence of these conditions. Finally, the cross‐sectional nature of this analysis does not allow for the investigation of the stability of TTs over time, their relationship with specific therapeutic interventions and/or with relevant clinical outcomes.
In conclusion, this analysis provides the largest and most granular characterization of TTs in patients with airway diseases in a real‐world setting to date. It shows that a few TTs are tightly linked with the disease label of ‘asthma’ (allergic and non‐allergic rhinosinusitis, nasal sinus polyps and several allergies) or ‘COPD’ (non‐reversible airflow limitation and emphysema), whereas many others occur independent of the diagnostic label. Likewise, the prevalence of some, but not all, TTs changes with the assessment of disease severity by the attending physician.
AUTHOR CONTRIBUTION
Alvar Agustí: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (lead); writing – review and editing (equal). Eleni Rapsomaniki: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Richard Beasley: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Rod Hughes: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Hana Müllerová: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Alberto Papi: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Ian D. Pavord: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Maarten van den Berge: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Rosa Faner: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (lead); writing – review and editing (equal).
CONFLICTS OF INTEREST
Alvar Agustí has received payments in his role as a member of the scientific committee of NOVELTY; received research grants from AstraZeneca, Chiesi, GSK, Menarini, MSD and Zambon; consulting fees from AstraZeneca, Chiesi, GSK, Menarini, MSD and Zambon; and payment or honoraria from AstraZeneca, Chiesi, GSK, Menarini, MSD and Zambon. Eleni Rapsomaniki and Rod Hughes are employees of AstraZeneca. Hana Müllerová is an employee and shareholder of AstraZeneca. Richard Beasley received research grants from AstraZeneca, Cure Kids (NZ), Genentech, GSK and HRC (NZ); consulting fees from AstraZeneca, Avillion and Theravance; payment or honoraria from AstraZeneca, Cipla and Asthma and Respiratory Foundation NZ; support for attending meetings from AstraZeneca and Theravance; and is the Chair of the adult asthma guidelines group for the Asthma and Respiratory Foundation of NZ. Alberto Papi received research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Pfizer, Sanofi and Teva; consulting fees from AstraZeneca, Avillion, Chiesi, Elpen Pharmaceuticals, GSK, IQVIA, Novartis and Sanofi; and payment or honoraria from AstraZeneca, Avillion, Boehringer Ingelheim, Chiesi, Edmond Pharma, Elpen Pharmaceuticals, GSK, IQVIA, Menarini, MSD, Mundipharma, Novartis, Sanofi, Teva and Zambon. Ian D. Pavord received research grants from Chiesi; consulting fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Dey Pharma, Genentech, GSK, Knopp Biosciences, Merck, MSD, Napp Pharmaceuticals, Novartis, Regeneron Pharmaceuticals Inc., Respivert, Schering‐Plough and Teva; payment or honoraria from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Regeneron Pharmaceuticals Inc., Sanofi and Teva; support for attending meetings from AstraZeneca, Chiesi, GSK, Regeneron Pharmaceuticals Inc., Sanofi, Teva and Napp Pharmaceuticals; and other financial or non‐financial interests from AstraZeneca, Boehringer Ingelheim, GSK, Regeneron Pharmaceuticals Inc., Sanofi and Teva. Maarten van den Berge received research grants from Genentech, GSK, Novartis, Roche and Sanofi. Rosa Faner received research grants from AstraZeneca, GSK, Instituto de Salud Carlos III—Spanish National Health Service and Menarini; consulting fees from GSK; and payment or honoraria from Chiesi.
HUMAN ETHICS APPROVAL DECLARATION
The study was approved by the relevant Institutional Review Boards, and all patients provided written informed consent.
Supporting information
Appendix S1. Collaborators (NOVELTY Study Investigators).
Figure S1. Distribution of physician‐assessed severity by disease label.
Figure S2. Association of severe disease across all diagnoses with the presence (continuous lines) or absence (dashed lines) of certain TTs in the entire study population.
Figure S3. Frequency distribution of the number of TTs present per patient in ‘asthma’, ‘asthma + COPD’ and ‘COPD’ in the entire study population.
Table S1. Prevalence of TTs by disease label, unadjusted and adjusted for age, ordered by age‐adjusted asthma.
Table S2. Prevalence of TTs by the attending physician.
Table S3. Prevalence of TTs in patients with ‘asthma’ by region.
Table S4. Prevalence of TTs in patients with ‘COPD’ by region.
Table S5. Prevalence of TTs in patients with ‘asthma + COPD’ by region.
Table S6. Prevalence of TTs by disease label and level of severity.
Video Abstract Summary of the research findings of treatable traits in the NOVELTY Study presented by Prof Alvar Agusti and Dr Rosa Faner.
ACKNOWLEDGEMENTS
The authors thank participants in the NOVELTY study for their willingness to contribute to medical research, and all field investigators for the quality of their work. Editorial support, under the direction of the authors, was provided by Richard Knight, PhD, CMC Connect, McCann Health Medical Communications, and was funded by AstraZeneca, Cambridge, UK, in accordance with Good Publication Practice (GPP3) guidelines. The NOVELTY study is funded by AstraZeneca.
A list of collaborators (NOVELTY Study Investigators) is available in Appendix S1 in the Supporting Information.
Agustí A, Rapsomaniki E, Beasley R, Hughes R, Müllerová H, Papi A, et al. Treatable traits in the NOVELTY study. Respirology. 2022;27(11):929–940. 10.1111/resp.14325
Associate Editor: Sanjay H. Chotirmall; Senior Editor: Fanny W. S. Ko
Funding information AstraZeneca
See related Editorial
Contributor Information
Rosa Faner, Email: rfaner@ub.edu.
for the NOVELTY Study Investigators:
Gabriel Benhabib, Xavier Bocca Ruiz, Ricardo del Olmo, Raul Eduardo Lisanti, Gustavo Marino, Walter Mattarucco, Juan Nogueira, Maria Parody, Pablo Pascale, Pablo Rodriguez, Damian Silva, Graciela Svetliza, Carlos F. Victorio, Roxana Willigs Rolon, Anahi Yañez, Gary Anderson, Stuart Baines, Simon Bowler, Peter Bremner, Sheetal Bull, Patrick Carroll, Mariam Chaalan, Claude Farah, Gary Hammerschlag, Kerry Hancock, Zinta Harrington, Gregory Katsoulotos, Joshua Kim, David Langton, Donald Lee, Matthew Peters, Lakshman Prassad, Helen Reddel, Dimitar Sajkov, Francis Santiago, Frederick Graham Simpson, Sze Tai, Paul Thomas, Peter Wark, José Eduardo Delfini Cançado, Thúlio Cunha, Marina Lima, Alexandre Pinto Cardoso, Marcelo Rabahi, Syed Anees, John Bertley, Alan Bell, Amarjit Cheema, Guy Chouinard, Michael Csanadi, Anil Dhar, Ripple Dhillon, J. Mark FitzGerald, David Kanawaty, Allan Kelly, William Killorn, Daniel Landry, Robert Luton, Piushkumar Mandhane, Andrew McIvor, Bonavuth Pek, Robert Petrella, Mohsen Sadatsafavi, Daniel Stollery, Meihua Chen, Yan Chen, Wei Gu, Kim Ming Christopher Hui, Manxiang Li, Shiyue Li, Ma Lijun, Guangyue Qin, Weidong Song, Wei Tan, Yijun Tang, Chen Wang, Tan Wang, Fuqiang Wen, Feng Wu, Ping Chao Xiang, Zuke Xiao, Shengdao Xiong, Jinghua Yang, Jingping Yang, Caiqing Zhang, Min Zhang, Ping Zhang, Wei Zhang, Xiaohe Zheng, Dan Zhu, Fabio Bolivar Grimaldos, Alejandra Cañas Arboleda, Carlos Matiz Bueno, Dora Molina de Salazar, Elisabeth Bendstrup, Ole Hilberg, Carsten Kjellerup, Ulla Weinreich, Philippe Bonniaud, Olivier Brun, Pierre‐Régis Burgel, Christos Chouaid, Francis Couturaud, Jacques de Blic, Didier Debieuvre, Dominique Delsart, Axelle Demaegdt, Pascal Demoly, Antoine Deschildre, Gilles Devouassoux, Carole Egron, Lionel Falchero, François Goupil, Romain Kessler, Pascal Le Roux, Pascal Mabire, Guillaume Mahay, Stéphanie Martinez, Boris Melloni, Laurent Moreau, Chantal Raherison, Emilie Riviere, Pauline Roux‐Claudé, Michel Soulier, Guillaume Vignal, Azzedine Yaici, Sven Philip Aries, Robert Bals, Ekkehard Beck, Andreas Deimling, Jan Feimer, Vera Grimm‐Sachs, Gesine Groth, Felix Herth, Gerhard Hoheisel, Frank Kanniess, Thomas Lienert, Silke Mronga, Jörg Reinhardt, Christian Schlenska, Christoph Stolpe, Ishak Teber, Hartmut Timmermann, Thomas Ulrich, Peter Velling, Sabina Wehgartner‐Winkler, Juergen Welling, Ernst‐Joachim Winkelmann, Carlo Barbetta, Fulvio Braido, Vittorio Cardaci, Enrico Maria Clini, Maria Teresa Costantino, Giuseppina Cuttitta, Mario di Gioacchino, Alessandro Fois, Maria Pia Foschino‐Barbaro, Enrico Gammeri, Riccardo Inchingolo, Federico Lavorini, Antonio Molino, Eleonora Nucera, Alberto Papi, Vincenzo Patella, Alberto Pesci, Fabio Ricciardolo, Paola Rogliani, Riccardo Sarzani, Carlo Vancheri, Rigoletta Vincenti, Takeo Endo, Masaki Fujita, Yu Hara, Takahiko Horiguchi, Keita Hosoi, Yumiko Ide, Minehiko Inomata, Hiromasa Inoue, Koji Inoue, Sumito Inoue, Motokazu Kato, Masayuki Kawasaki, Tomotaka Kawayama, Toshiyuki Kita, Kanako Kobayashi, Hiroshi Koto, Koichi Nishi, Junpei Saito, Yasuo Shimizu, Toshihiro Shirai, Naruhiko Sugihara, Ken‐ichi Takahashi, Hiroyuki Tashimo, Keisuke Tomii, Takashi Yamada, Masaru Yanai, Ruth Cerino Javier, Alfredo Domínguez Peregrina, Marco Fernández Corzo, Efraín Montano Gonzalez, Alejandra Ramírez‐Venegas, Adrian Rendon, Willem Boersma, R. S. Djamin, Michiel Eijsvogel, Frits Franssen, Martijn Goosens, Lidwien Graat‐Verboom, Johannes Veen, Rob Janssen, Kim Kuppens, Maarten van den Berge, Mario van de Ven, Richard Beasley, Ole Petter Brunstad, Gunnar Einvik, Kristian Jong Høines, Alamdar Khusrawi, Torbjorn Oien, Yoon‐Seok Chang, Young Joo Cho, Yong Il Hwang, Woo Jin Kim, Young‐Il Koh, Byung‐Jae Lee, Kwan‐Ho Lee, Sang‐Pyo Lee, Yong Chul Lee, Seong Yong Lim, Kyung Hun Min, Yeon‐Mok Oh, Choon‐Sik Park, Hae‐Sim Park, Heung‐Woo Park, Chin Kook Rhee, Ho Joo Yoon, Hyoung‐Kyu Yoon, Alvar Agusti García‐Navarro, Rubén Andújar, Laura Anoro, María Buendía García, Paloma Campo Mozo, Sergio Campos, Francisco Casas Maldonado, Manuel Castilla Martínez, Carolina Cisneros Serrano, Lorena Comeche Casanova, Dolores Corbacho, Felix Del Campo Matías, Jose Echave‐Sustaeta, Gloria Francisco Corral, Pedro Gamboa Setién, Marta García Clemente, Ignacio García Núñez, Jose García Robaina, Mercedes García Salmones, Jose Maria Marín Trigo, Marta Nuñez Fernandez, Sara Nuñez Palomo, José Olaguibel Rivera, Luis Pérez de Llano, Ana Pueyo Bastida, Ana Rañó, José Rodríguez González‐Moro, Albert Roger Reig, José Velasco Garrido, Dan Curiac, Christer Janson, Cornelia Lif‐Tiberg, Anders Luts, Lennart Råhlen, Stefan Rustscheff, Frances Adams, Drew Bradman, Emma Broughton, John Cosgrove, Patrick Flood‐Page, Liz Fuller, Timothy Harrison, David Hartley, Keith Hattotuwa, Gareth Jones, Keir Lewis, Lorcan McGarvey, Alyn Morice, Preeti Pandya, Manish Patel, David Price, Kay Roy, Ramamurthy Sathyamurthy, Swaminathan Thiagarajan, Alice Turner, Jørgen Vestbo, Wisia Wedzicha, Tom Wilkinson, Pete Wilson, Lo'Ay Al‐Asadi, James Anholm, Frank Averill, Sandeep Bansal, Alan Baptist, Colin Campbell, Michael A. Campos, Bradley Chipps, Gretchen Crook, Samuel DeLeon, Alain Eid, Ellen Epstein, Stephen Fritz, Hoadley Harris, Mitzie Hewitt, Fernando Holguin, Golda Hudes, Richard Jackson, Alan Kaufman, David Kaufman, Ari Klapholz, Harshavardhan Krishna, Daria Lee, Robert Lin, Barry Make, Diego Maselli‐Caceres, Vinay Mehta, James N. Moy, Ugo Nwokoro, Purvi Parikh, Sudhir Parikh, Frank Perrino, James Ruhlmann, Catherine Sassoon, Russell A. Settipane, Daniel Sousa, Peruvemba Sriram, and Richard Wachs
DATA AVAILABILITY STATEMENT
This manuscript has associated data in a repository. Data underlying the findings described in this manuscript, including individual de‐identified participant data, protocols and clinical trial documents, may be obtained in accordance with AstraZeneca's data‐sharing policy (described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure) through Vivli (https://vivli.org/).
REFERENCES
- 1. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. J Allergy Clin Immunol Pract. 2022;10(1S):S1–18. 10.1016/j.jaip.2021.10.001. Epub 2021 Oct 28. [DOI] [PubMed]
- 2. Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. the 2020 gold science committee report on covid‐19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. 10.1164/rccm.202009-3533SO. [DOI] [PMC free article] [PubMed]
- 3. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma—redefining airways diseases. Lancet. 2017;391:350–400. [DOI] [PubMed] [Google Scholar]
- 4. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate ST, et al. Treatable traits: toward precision medicine of airway diseases. Eur Respir J. 2016;47:410–9. [DOI] [PubMed] [Google Scholar]
- 5. Agustí A, Bafadhel M, Beasley R, Bel EH, Faner R, Gibson PG, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J. 2017;50:1–13. [DOI] [PubMed] [Google Scholar]
- 6. McDonald VM, Fingleton J, Agusti A, Hiles SA, Clark VL, Holland AE, et al. Treatable traits: a new paradigm for 21(st) century management of chronic airway diseases. Eur Respir J. 2019;53:1802058. [DOI] [PubMed] [Google Scholar]
- 7. Agusti A, Barnes N, Cruz AA, Gibson PG, Heaney LG, Inoue H, et al. Moving towards a treatable traits model of care for the management of obstructive airways diseases. Respir Med. 2021;187:106572. [DOI] [PubMed] [Google Scholar]
- 8. Irwin RS, Curley FJ, French CL. Difficult‐to‐control asthma. Contributing factors and outcome of a systematic management protocol. Chest. 1993;103:1662–9. [DOI] [PubMed] [Google Scholar]
- 9. Heaney LG, Conway E, Kelly C, Johnston BT, English C, Stevenson M, et al. Predictors of therapy resistant asthma: outcome of a systematic evaluation protocol. Thorax. 2003;58:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald VM, Hiles SA, Godbout K, Harvey ES, Marks GB, Hew M, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24:37–47. [DOI] [PubMed] [Google Scholar]
- 11. McDonald VM, Clark VL, Cordova‐Rivera L, Wark PAB, Baines KJ, Gibson PG. Targeting treatable traits in severe asthma: a randomised controlled trial. Eur Respir J. 2020;55:1901509. [DOI] [PubMed] [Google Scholar]
- 12. Heaney LG, Busby J, Hanratty CE, Djukanovic R, Woodcock A, Walker SM, et al. Composite type‐2 biomarker strategy versus a symptom‐risk‐based algorithm to adjust corticosteroid dose in patients with severe asthma: a multicentre, single‐blind, parallel group, randomised controlled trial. Lancet Respir Med. 2020;9:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullerova H, Cockle SM, Gunsoy NB, Nelsen LM, Albers FC. Clinical characteristics and burden of illness among adolescent and adult patients with severe asthma by asthma control: the IDEAL study. J Asthma. 2020;58:459–70. [DOI] [PubMed] [Google Scholar]
- 14. Hew M, Denton E. Prioritizing treatable traits in airways disease. J Allergy Clin Immunol Pract. 2021;9:1265–6. [DOI] [PubMed] [Google Scholar]
- 15. Reddel HK, Gerhardsson de Verdier M, Agusti A, Anderson G, Beasley R, Bel EH, et al. Prospective observational study in patients with obstructive lung disease: NOVELTY design. ERJ Open Res. 2019;5:00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reddel HK, Vestbo J, Agustí A, Anderson GP, Bansal AT, Beasley R, et al. Heterogeneity within and between physician‐diagnosed asthma and/or COPD: NOVELTY cohort. Eur Respir J. 2021;58:2003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez‐Garcia MA, Faner R, Oscullo G, Ddl R, Soler‐Cataluña J‐J, Ballester M, et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease. A network analysis. Am J Respir Crit Care Med. 2020;201:1078–85. [DOI] [PubMed] [Google Scholar]
- 20. Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal‐Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. [DOI] [PubMed] [Google Scholar]
- 21. Martinez‐Garcia MA, de la Rosa D, Soler‐Cataluna JJ, Donat‐Sanz Y, Catalan SP, Agramunt LM, et al. Prognostic value of bronchiectasis in patients with moderate‐to‐severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–31. [DOI] [PubMed] [Google Scholar]
- 22. Håkansson K, Konge L, Thomsen SF, Backer V, von Buchwald C. Sinonasal inflammation in COPD: a systematic review. Eur Respir J. 2013;42:1402–11. [DOI] [PubMed] [Google Scholar]
- 23. Håkansson K, von Buchwald C, Thomsen SF, Thyssen JP, Backer V, Linneberg A. Nonallergic rhinitis and its association with smoking and lower airway disease: a general population study. Am J Rhinol Allergy. 2011;25:25–9. [DOI] [PubMed] [Google Scholar]
- 24. Simpson A, Tan VY, Winn J, Svensén M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–6. [DOI] [PubMed] [Google Scholar]
- 25. Lahousse L, Tiemeier H, Ikram MA, Brusselle GG. Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med. 2015;109:1371–80. [DOI] [PubMed] [Google Scholar]
- 26. Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–61. [DOI] [PubMed] [Google Scholar]
- 27. R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 28. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scadding JG. Health and disease: what can medicine do for philosophy? J Med Ethics. 1988;14:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herland K, Akselsen JP, Skjonsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger "real life" population of patients with obstructive lung disease? Respir Med. 2005;99:11–9. [DOI] [PubMed] [Google Scholar]
- 31. Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Travers J, Marsh S, Caldwell B, Williams M, Aldington S, Weatherall M, et al. External validity of randomized controlled trials in COPD. Respir Med. 2007;101:1313–20. [DOI] [PubMed] [Google Scholar]
- 33. Roche N, Reddel HK, Agusti A, Bateman ED, Krishnan JA, Martin RJ, et al. Integrating real‐life studies in the global therapeutic research framework. Lancet Respir Med. 2013;1:e29–30. [DOI] [PubMed] [Google Scholar]
- 34. Ezponda A, Casanova C, Divo M, Marin‐Oto M, Cabrera C, Marin JM, et al. Chest CT‐assessed comorbidities and all‐cause mortality risk in COPD patients in the BODE cohort. Respirology. 2022;27:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agusti A, Faner R. CT in COPD: to be or not to be. Respirology. 2022;27:258–9. [DOI] [PubMed] [Google Scholar]
- 36. Aliberti S, Goeminne PC, O'Donnell AE, Aksamit TR, Al‐Jahdali H, Barker AF, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10:298–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Collaborators (NOVELTY Study Investigators).
Figure S1. Distribution of physician‐assessed severity by disease label.
Figure S2. Association of severe disease across all diagnoses with the presence (continuous lines) or absence (dashed lines) of certain TTs in the entire study population.
Figure S3. Frequency distribution of the number of TTs present per patient in ‘asthma’, ‘asthma + COPD’ and ‘COPD’ in the entire study population.
Table S1. Prevalence of TTs by disease label, unadjusted and adjusted for age, ordered by age‐adjusted asthma.
Table S2. Prevalence of TTs by the attending physician.
Table S3. Prevalence of TTs in patients with ‘asthma’ by region.
Table S4. Prevalence of TTs in patients with ‘COPD’ by region.
Table S5. Prevalence of TTs in patients with ‘asthma + COPD’ by region.
Table S6. Prevalence of TTs by disease label and level of severity.
Video Abstract Summary of the research findings of treatable traits in the NOVELTY Study presented by Prof Alvar Agusti and Dr Rosa Faner.
Data Availability Statement
This manuscript has associated data in a repository. Data underlying the findings described in this manuscript, including individual de‐identified participant data, protocols and clinical trial documents, may be obtained in accordance with AstraZeneca's data‐sharing policy (described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure) through Vivli (https://vivli.org/).


