Abstract
Aims
To confirm the reno‐protective effects of sodium‐glucose cotransporter‐2 (SGLT2) inhibitors compared with dipeptidyl peptidase‐4 (DPP‐4) inhibitors on the onset and progression of chronic kidney disease (CKD) in routine clinical practice.
Materials and Methods
We conducted a retrospective cohort study using the Clinical Practice Research Datalink Aurum database linked to Hospital Episode Statistics. The primary outcome was risk of the composite CKD endpoint based on the recent consensus guidelines for kidney disease: >40% decline in estimated glomerular filtration rate (eGFR), kidney death or end‐stage kidney disease (ESKD; a composite of kidney transplantation, maintenance of dialysis, sustained low eGFR <15 ml/min/1.73m² or diagnosis of ESKD). Secondary outcomes were components of the composite CKD endpoint, analysed separately. Patients were propensity‐score‐matched 1:1 for SGLT2 inhibitor versus DPP‐4 inhibitor use.
Results
A total of 131 824 people with type 2 diabetes (T2D) were identified; 79.0% had no known history of CKD. During a median follow‐up of 2.1 years, SGLT2 inhibitor initiation was associated with lower risk of progression to composite kidney endpoints than DPP‐4 inhibitor initiation (7.48 vs. 11.77 events per 1000 patient‐years, respectively). Compared with DPP‐4 inhibitor initiation, SGLT2 inhibitor initiation was associated with reductions in the primary composite CKD endpoint (hazard ratio [HR] 0.64, 95% confidence interval [CI] 0.56‐0.74), all‐cause mortality (HR 0.74, 95% CI 0.64‐0.86) and ESKD (HR 0.37, 95% CI 0.25‐0.55), reduced the rate of sustained low eGFR (HR 0.33, 95% CI 0.19‐0.57), and reduced diagnoses of ESKD in primary care (HR 0.04, 95% CI 0.01‐0.18). Results were consistent across subgroup and sensitivity analyses.
Conclusions
In adults with T2D, initiation of an SGLT2 inhibitor was associated with a significantly reduced risk of CKD progression and death compared with initiation of a DPP‐4 inhibitor.
Keywords: diabetes complications, DPP4 inhibitor, observational study, population study, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Heart failure (HF), chronic kidney disease (CKD), or both, are common among people with type 2 diabetes (T2D). 1 , 2 , 3 , 4 , 5 A previous cross‐sectional study reported that 58% of 32 208 people with T2D without known albuminuria had comorbid CKD, which is associated with higher cardiovascular and all‐cause mortality. 2 , 6 In a further multinational study of 772 336 patients with T2D who were free from cardio‐renal disease, 18% developed a first manifestation of cardio‐renal disease, represented by CKD (36% of those with cardio‐renal disease), HF (24%), stroke (16%), myocardial infarction (14%) and peripheral arterial disease (10%), during a mean follow‐up of 4.5 years. HF or CKD alone doubled cardiovascular (hazard ratio [HR] 2.02, 95% confidence interval [CI] 1.75‐2.33) and all‐cause mortality (HR 2.05, 95% CI 1.82‐2.32). In combination, HF and CKD further increased the risk of cardiovascular (HR 3.91; 95% CI 3.02‐5.07) and all‐cause mortality (HR 3.14, 95% CI 2.90‐3.40) more than threefold. 6 , 7
Randomized clinical trials (RCTs) and retrospective observational studies reported that sodium‐glucose cotransporter‐2 inhibitors (SGLT2) inhibitors improve cardio‐renal outcomes compared with other glucose‐lowering therapies. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Available RCTs show that glucagon‐like peptide‐1 (GLP‐1) receptor agonists (GLP‐1RAs) and SGLT2 inhibitors reduce the risk of major cardiovascular events (ie, myocardial infarction, stroke, and cardiovascular death), but only SGLT2 inhibitors reduce the incidence of severe kidney disease (ie, a composite of worsening estimated glomerular filtration rate [eGFR], end‐stage kidney disease [ESKD] or kidney death). 15 More recently, the DAPA‐CKD trial reported that dapagliflozin, an SGLT2 inhibitor, reduced the relative risk of kidney failure in a broad population of patients with CKD, with and without T2D. 18
In contrast to SGLT2 inhibitors, available clinical trials do not suggest that dipeptidyl peptidase‐4 (DPP‐4) inhibitors have beneficial effects on major adverse cardiovascular events, kidney disease or mortality. 19 , 20 , 21 , 22 However, DPP‐4 inhibitors have been shown to be associated with reduced mortality in retrospective observational studies in some patients with T2D. 23 , 24 , 25 We previously showed that SGLT2 inhibitors were associated with reductions in all‐cause mortality, cardiovascular mortality, HF hospitalization and CKD hospitalization compared with DPP‐4 inhibitors in T2D patients initially free from cardio‐renal disease. 26 In a subsequent retrospective observational study, designed to mimic the DECLARE‐TIMI 58 population, one of the largest studies assessing people with T2D who had or were at risk for atherosclerotic cardiovascular disease, 27 we have also shown that SGLT2 inhibitors were again associated with reductions in all‐cause mortality, cardiovascular mortality, HF, stroke and CKD hospitalizations compared with DPP‐4 inhibitors. Results were consistent across subgroups and sensitivity analyses. 17
The management guidelines of both the American Diabetes Association and the European Association for the Study of Diabetes recommend prescribing SGLT2 inhibitors for patients with T2D and atherosclerotic CVD in whom HF, CKD or both coexist or are of important relevance. 28 , 29 Despite this, DPP‐4 inhibitors and sulphonylureas remain the most commonly prescribed antidiabetic drugs after metformin. 30 Head‐to‐head randomized studies comparing kidney outcomes between SGLT2 inhibitors and DPP‐4 inhibitors, however, remain scarce and network meta‐analysis (undertaken due to limited head‐to‐head studies between the two drug classes) were inconclusive. 31 More recently, Rhee et al reported that SGLT2 inhibitors were associated with lower risks of hospitalization for HF, but not nonfatal myocardial infarction or stroke, compared with DPP‐4 inhibitors across different stages of CKD. 32 This study, as well as other recent retrospective observational studies, however, did not compare the effectiveness of SGLT2 inhibitors with that of DPP‐4 inhibitors with regard to kidney outcomes. Against this background, we hypothesized that SGLT2 inhibitors will reduce the risk of adverse kidney outcomes to a greater extent than DPP‐4 inhibitors in people with T2D, with or without established cardio‐renal disease.
Despite kidney failure being an important outcome for diabetes research, no harmonized international consensus definitions of kidney failure or key surrogates of kidney failure existed for use in clinical studies. Previous studies therefore used a variety of definitions of kidney failure, which contributed to controversy and confusion. 33 In view of this, the International Society of Nephrology convened an international multi‐stakeholder group meeting, comprising participants from 18 countries, to develop a standardized consensus definition for kidney failure outcomes in clinical trials and markers that predict progression to kidney failure. 33
The aim of this retrospective observational study was therefore to conduct a comparative effectiveness analysis of new users of SGLT2 inhibitors versus DPP‐4 inhibitors, focusing on the risk of renal outcomes using the international consensus definitions of kidney outcomes 33 in patients with T2D.
2. MATERIALS AND METHODS
This retrospective cohort study used the Clinical Practice Research Datalink (CPRD) Aurum database, which contains data collected routinely from primary care practices in England and shows high levels (>90%) of correctness and completeness for data on T2D. 34 , 35 CPRD Aurum data were linked to Hospital Episode Statistics (HES), which include details of hospital admissions in England, and death registration (Office for National Statistics [ONS]) data, which provide information on all‐cause and cause‐specific mortality. Patients with T2D were identified by diagnostic codes and confirmed by records of prescriptions of glucose‐lowering drugs, as defined previously in Birkeland et al. 26
The new‐user index date refers to the initial prescription for an SGLT2 inhibitor or DPP‐4 inhibitor. Patients initiating an SGLT2 inhibitor and a DPP‐4 inhibitor on the same date and those with a previous prescription of the same drug class 12 months before initiation were excluded. The analysis followed patients with T2D aged at least 18 years from the day after the index date (study start date January 1, 2013) until the earliest study outcome, death, move out of the practice or study end date (November 30, 2018). People with type 1 diabetes or gestational diabetes were excluded.
The primary outcome was risk of the composite CKD endpoint based on the consensus guidelines for kidney disease 33 as defined in Table 1: ≥40% eGFR decline (confirmed by a second measurement over 28 days) or ESKD or kidney death. ESKD was a composite of kidney transplantation, maintenance of dialysis, sustained low eGFR<15 ml/min/1.73m² or diagnosis of ESKD (eGFR <15 ml/min/1.73m², confirmed at a subsequent measurement). The components of the composite CKD endpoint were analysed separately (online supplementary material). Time to event was taken at the first measurement, but excluded if not confirmed by a second measurement. Creatinine was used to estimate eGFR when a direct eGFR value was not available (3% of all patients).
TABLE 1.
Summary of primary composite chronic kidney disease endpoint
| Components of primary composite CKD outcome | Definition | ||
|---|---|---|---|
| Sustained percent decline in eGFR | Percent decline in eGFR of ≥40% from baseline and sustained over at least 4 weeks | ||
| ESKD | Kidney transplantation | Receipt of a kidney transplant | |
| Maintenance dialysis | Dialysis performed for at least 4 weeks | ||
| Sustained low eGFR | eGFR < 15 mL/min per 1.73 m2 sustained over at least 4 weeks | ||
| Diagnosis at primary care | Diagnosis of CKD stage 5: eGFR<15 mL/min per 1.73 m2 | ||
| Kidney death | Death with chronic kidney disease as the underlying cause of death | ||
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESKD, end‐stage kidney disease.
Diagnostic history was defined using primary care (CPRD) and secondary care (HES) diagnoses. Comorbidities, diagnosis, laboratory measurements and prescriptions in primary care were defined from the presence of relevant SNOMED‐CT codes obtained from clinical codes repositories (CALIBER, UCL Institute of Health Informatics and Data Compass, London School of Hygiene and Tropical Medicine). Comorbidities in secondary care, as well as outcomes of specific hospitalizations, were defined by the presence of International Classification of Diseases‐10 codes, as outlined previously in Birkeland et al. 26
Each patient receiving an SGLT2 inhibitor was propensity‐score‐matched 1:1 to a patient using a DPP‐4 inhibitor. The propensity score included age, sex, presence of microvascular complications, frailty (defined as 3 or more consecutive days in hospital within the year before the index date as in previous studies 14 , 17 , 26 , 36 ) and cardiovascular comorbidities (online supplementary material), following the method described by Birkeland et al, 26 and was based on intention to treat. To avoid immortal time bias, only the first episode of either SGLT2 or DPP‐4 inhibitor treatment during the inclusion period was eligible.
Baseline characteristics are described using standard statistical measures including mean and standard deviations for numerical variables and frequencies and percentages for categorical variables. An imbalance in baseline characteristics was considered when a standardized difference of more than 10% occurred between the two groups. Time to first event was compared between groups using Cox proportional hazards models. When an imbalance occurred, the treatment effect was estimated using Cox regression that contained only those covariates considered to be unbalanced. 37
Results are presented as relative risk reductions or hazard ratio (HRs) and 95% confidence interval (CIs). Sensitivity analyses were performed in order to evaluate the stability of the findings: data for the primary analysis were additionally adjusted for multiple covariates: age, gender, frailty, baseline glycated haemoglobin (HbA1c) and eGFR, history of HF, myocardial infarction, stroke, unstable angina, CKD, cancer, dialysis and use of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), β‐blockers, aldosterone antagonists, calcium channel blockers, statins, insulins, sulphonylureas, loop diuretics and thiazide diuretics. Sensitivity analyses were performed without alogliptin or saxagliptin or vildagliptin, which are associated with either increased or inconclusive risk of hospitalization for HF compared with sitagliptin or other comparators respectively, 38 , 39 as well as assessed using on‐treatment analysis, instead of intention‐to‐treat analysis, where a patient is considered as on‐treatment as long as there is no gap longer than twice the length of a dispense.
Analyses for each outcome were also stratified according to the presence of prior CVD, patient age and sex, history of HF, CKD, or cancer; baseline HbA1c, eGFR, urine albumin‐to‐creatinine ratio and baseline use of ACE inhibitors or ARBs, β‐blockers, loop diuretics, GLP‐1RAs, insulin, sulphonylureas, and statins. Subgroup‐specific propensity scores were not developed to prevent introducing instability into the point estimates, especially for subgroups with low numbers. Instead, confounders were adjusted as in the sensitivity analyses in multivariate Cox proportional hazard models. To determine whether there were significantly different treatment effects for each subgroup, each variable was included in a separate adjusted Cox proportional hazard model, along with an interaction between subgroup and treatment group.
3. RESULTS
Overall, 131 824 people with T2D were identified (Figure 1 and online supplementary material). Of these, 27 664 (21.0%) had a documented history of CKD, 117 089 (88.8%) had a history of using or were taking metformin, 59 300 (45.0%) had used sulphonylureas and 16 964 (12.9%) were using insulin. Before propensity‐score matching, patients initiated on an SGLT2 inhibitor were younger, less frail, had a lower burden of HF and CKD, had a higher HbA1c and eGFR, and were more frequently using glucose‐lowering drugs except for sulphonylureas (Table 2). Loop diuretics were less frequently prescribed to SGLT2 inhibitor initiators, while the prescription levels of antihypertensives were almost equal between the two groups.
FIGURE 1.
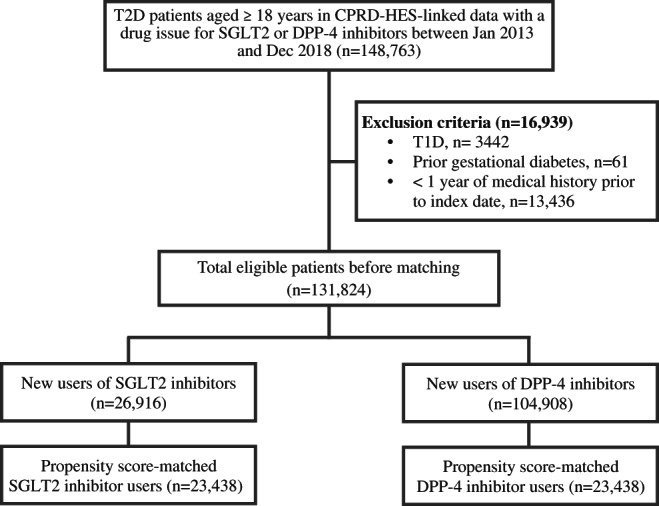
Patient disposition. Eligibility criteria included patients with a diagnosis of type 2 diabetes (T2D) in clinical practices in England. It included individuals who were alive and actively contributing data to clinical practice at new treatment initiation. CPRD, Clinical Practice Research Datalink; HES, Hospital Episode Statistics; T1D, type 1 diabetes mellitus; DPP‐4, dipeptidyl‐peptidase‐4; SGLT2, sodium‐glucose‐cotransporter‐2
TABLE 2.
Baseline characteristics, before and after propensity‐score matching
| Pre‐matching | Post‐matching | |||||
|---|---|---|---|---|---|---|
| Variables | SGLT2 inhibitors | DPP‐4 inhibitors | Std D (%) | SGLT2 inhibitors | DPP‐4 inhibitors | Std D (%) a |
| Number of patients | 26 916 | 104 908 | 23 438 | 23 438 | ||
| Age, years (SD) | 56.4 (10.93) | 64.4 (13.46) | 64.8% | 56.8 (10.96) | 56.4 (12.37) | −3.5% |
| Females, n (%) | 11 558 (42.9%) | 44 775 (42.7%) | −0.5% | 9972 (42.5%) | 9869 (42.1%) | −0.9% |
| Microvascular complications, n (%) | 6275 (23.3%) | 26 505 (25.3%) | 4.6% | 5380 (23.0%) | 5265 (22.5%) | −1.2% |
| Frailty b , n (%) | 6062 (22.5%) | 16 317 (15.6%) | −17.8% | 5140 (21.9%) | 5144 (21.9%) | 0.0% |
| HbA1c, % (SD) | 9.2 (1.67) | 8.8 (1.60) | −27.3% | 9.2 (1.66) | 9.1 (1.71) | −0.9% |
| eGFR, mL/min/1.73m2 (SD) | 80.0 (13.64) | 70.5 (20.18) | −54.9% | 79.7 (13.56) | 80.4 (16.16) | 4.6% |
| CKD, n (%) | 1916 (7.1%) | 25 748 (24.5%) | 49.2% | 1773 (7.6%) | 1487 (6.3%) | −4.8% |
| HF, n (%) | 1043 (3.9%) | 9295 (8.9%) | 20.5% | 930 (4.0%) | 818 (3.5%) | −2.5% |
| CVD prevention | ||||||
| Statins, n (%) | 20 237 (75.2%) | 82 065 (78.2%) | 7.2% | 17 508 (74.7%) | 17 424 (74.3%) | −0.8% |
| Antihypertensives, (%) | 17 437 (64.8%) | 72 974 (69.6%) | 10.2% | 15 055 (64.2%) | 14 879 (63.5%) | −1.6% |
| ACE inhibitors, (%) | 12 236 (45.5%) | 47 924 (45.7%) | 0.4% | 10 530 (44.9%) | 10 499 (44.8%) | −0.3% |
| ARBs, n (%) | 4379 (16.3%) | 19 481 (18.6%) | 6.1% | 3768 (16.1%) | 3567 (15.2%) | −2.4% |
| Beta blockers, n (%) | 5435 (20.2%) | 28 052 (26.7%) | 15.5% | 4718 (20.1%) | 4633 (19.8%) | −0.9% |
| Loop diuretics, n (%) | 1818 (6.8%) | 14 663 (14.0%) | 23.9% | 1594 (6.8%) | 1479 (6.3%) | −2.0% |
| Aldosterone antagonists, n (%) | 520 (1.9%) | 3579 (3.4%) | 9.2% | 449 (1.9%) | 435 (1.9%) | −0.4% |
| Glucose‐lowering drugs | ||||||
| Metformin, n (%) | 25 113 (93.3%) | 91 976 (87.7%) | −19.3% | 21 939 (93.6%) | 22 070 (94.2%) | 2.3% |
| Sulphonylureas, n (%) | 10 697 (39.7%) | 48 603 (46.3%) | 13.3% | 9201 (39.3%) | 9210 (39.3%) | 0.1% |
| GLP‐1RAs, n (%) | 4339 (16.1%) | 1764 (1.7%) | −52.4% | 1920 (8.2%) | 1383 (5.9%) | −9.0% |
| Thiazolidinediones, n (%) | 1976 (7.3%) | 6414 (6.1%) | −4.9% | 1665 (7.1%) | 1613 (6.9%) | −0.9% |
| Insulin, n (%) | 7589 (28.2%) | 9375 (8.9%) | −51.1% | 4833 (20.6%) | 4190 (17.9%) | −7.0% |
| Index year | ||||||
| 2013 | 678 (2.5%) | 15 636 (14.9%) | 45.0% | 642 (2.7%) | 642 (2.7%) | 0.0% |
| 2014 | 2898 (10.8%) | 15 675 (14.9%) | 12.5% | 2425 (10.3%) | 2425 (10.3%) | 0.0% |
| 2015 | 5052 (18.8%) | 17 254 (16.4%) | −6.1% | 4178 (17.8%) | 4178 (17.8%) | 0.0% |
| 2016 | 5277 (19.6%) | 19 183 (18.3%) | −3.4% | 4550 (19.4%) | 4550 (19.4%) | 0.0% |
| 2017 | 6024 (22.4%) | 19 169 (18.3%) | −10.2% | 5296 (22.6%) | 5296 (22.6%) | 0.0% |
| 2018 | 6987 (26.0%) | 17 991 (17.1%) | −21.5% | 6347 (27.1%) | 6347 (27.1%) | 0.0% |
An imbalance in baseline characteristics was considered when standardized difference > 10%. All numbers in parenthesis are percentage if not stated otherwise.
Three or more consecutive days in hospital within the year prior to index.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP‐4, dipeptidyl‐peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; HF, heart failure; SD, standard deviation; Std D, standardized difference; SGLT2, sodium‐glucose cotransporter‐2.
Propensity‐score‐matched baseline characteristics (n = 23 438 in each group) were: proportion of women 42.5% and 42.1% in the SGLT‐2 inhibitor and DPP‐4 inhibitor groups, respectively; mean age 56.8 and 56.4 years, respectively; mean HbA1c 77 mmol/mol (9.2%) and 76 mmol/mol (9.1%), respectively; and mean eGFR 79.7 and 80.4 mL/min per 1.73 m2, respectively (Table 2). Of the propensity‐score‐matched population, 97% had a direct eGFR measurement. In the other 3%, eGFR was calculated using the Modification of Diet in Renal Disease Study equation with ethnic conversion. 40 The median (interquartile range [IQR]) period between the first and second eGFR measurement for sustained 40% decline was 126 (56, 300) days. The median (IQR) period between first and second eGFR measurement for sustained low eGFR (<15 ml/min/1.73m²) was 113 (53, 219) days. The rate of missing data for eGFR and HbA1c was low, even before matching (2.6% and 2.7%, respectively). The rate of missing eGFR data at follow‐up was also low at 0.9% and therefore no imputations were carried out.
During a median follow‐up of 2.1 years, SGLT2 inhibitor initiation was associated with lower risk of progression to composite kidney endpoints: 359 primary composite kidney outcomes among initiators of SGLT2 inhibitors (7.48 events per 1000 patient‐years) compared with 568 among initiators of DPP‐4 inhibitors (11.77 events per 1000 patient‐years).
Initiation of SGLT2 inhibitors was associated with reductions in the primary composite endpoint (HR 0.64, 95% CI 0.56‐0.74; P < 0.001) compared with initiation of DPP‐4 inhibitors. SGLT2 inhibitor initiation was associated with reductions in all‐cause mortality (HR 0.74, 95% CI 0.64‐0.86; P < 0.001) and ESKD (HR 0.37, 95% CI 0.25‐0.55; P < 0.001). SGLT2 inhibitor initiation also attenuated the eGFR decline (≥40% decline in eGFR: HR 0.66, 95% CI 0.57‐0.76; P < 0.001) and was associated with a lower rate of sustained low eGFR (HR 0.33, 95% CI 0.19‐0.57; P < 0.001) compared with DPP‐4 inhibitor initiation. In addition, SGLT2 inhibitor initiation was associated with reduced diagnoses of ESKD (stage 5 CKD, eGFR <15 ml/min/1.73m²) in primary care compared with DPP‐4 inhibitor initiation (HR 0.04, 0.01‐0.18; P < 0.001 [Figure 2]). Post hoc analysis of the eGFR values over time showed a stable eGFR or rise in eGFR level among patients on SGLT2 inhibitors compared with declining eGFR values for patients receiving DPP‐4 inhibitors (Figures S7 and S8).
FIGURE 2.
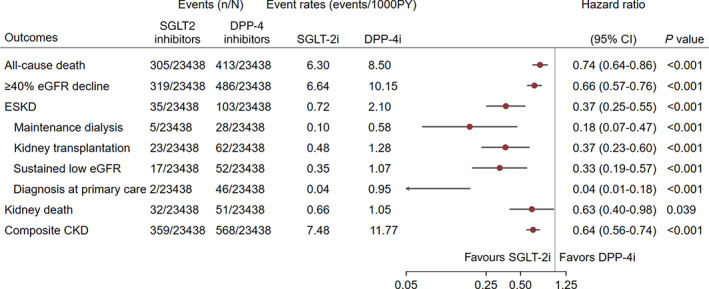
Risk of kidney events in type 2 diabetes. Composite chronic kidney disease (CKD) endpoint: ≥40% estimated glomerular filtration rate (eGFR) decline or end‐stage kidney disease (ESKD) or kidney death. P value derived from the test statistic for testing for difference in sodium‐glucose‐cotransporter‐2 (SGLT2) inhibitor initiation versus dipeptidyl‐peptidase‐4 (DPP‐4) inhibitor initiation
For many components of the endpoints, we observed that the results were largely consistent across a variety of subgroups, including ethnicity, concomitant use of ACE inhibitors or ARBs, use of diuretics, statins, insulin or GLP‐1 analogue use. In addition, subgroup analysis showed that the relative risk reduction in the primary composite endpoint and all‐cause mortality with SGLT2 inhibitors was independent of baseline eGFR and urine albumin‐to‐creatinine ratio (online supplementary material). The renal benefits of SGLT2 inhibitors were seen regardless of prior CKD or prior HF, but were more pronounced in those with prior CKD and HF (Figure S4).
Crucially, we also observed that differences in mortality were evident only in patients with a lower baseline HbA1c, whereas differences in renal outcomes appeared to be independent of glucose control: SGLT2 inhibitors versus DPP‐4 inhibitors significantly lowered all‐cause mortality to a greater extent in those with better glucose control (<53mmol/mol: HR 0.47, 95% CI 0.24‐0.91; 53‐86 mmol/mol: HR 0.67, 95% CI 0.55‐0.82; >10%: HR 0.93, 95% CI 0.73‐1.19; P‐interaction = 0.039), while the relative risk reduction in the primary composite renal endpoint was independent of baseline HbA1c (<53mmol/mol: HR 0.49, 95% CI 0.27‐0.87; 53‐86 mmol/mol: HR 0.61, 95% CI 0.51‐0.74; >86mmol/mol: HR 0.59, 95% CI 0.48‐0.73; P‐interaction = 0.324). Interestingly also, SGLT2 inhibitors reduced composite CKD endpoints in patients with no previous myocardial infarction (HR 0.56, 95% CI 0.48‐0.65), but not among patients with previous myocardial infarction (HR 0.93, 95% CI 0.65‐1.32; P‐interaction = 0.036).
Similar results were obtained when the effect of SGLT2 inhibitors versus DPP‐4 inhibitors was assessed in the on‐treatment population, as well as in sensitivity analysis excluding alogliptin, saxagliptin or vildagliptin (Figures S5 and S6). Initiation of SGLT2 inhibitors was associated with a 76% lower relative risk of the primary composite outcome (HR 0.24, 95% CI 0.16‐0.36; P < 0.001) in the on‐treatment analyses and a 38% lower relative risk when comparing to sitagliptin only (HR 0.62, 95% CI 0.54‐0.70; P < 0.001).
4. DISCUSSION
This study adds to a growing body of evidence showing that SGLT2 inhibitors reduce the relative risk of renal events in a broad population of patients with CKD with and without T2D. 13 , 18 , 27 , 41 , 42 Suggestions that SGLT2 inhibitors may show reno‐protective effects were first reported in trials designed for cardiovascular endpoints (EMPA‐REG OUTCOME, 43 DECLARE‐TIMI 53, 27 CANVAS 44 ), then confirmed in trials specifically designed for renal outcomes (CREDENCE 45 ). Subsequently, the results were confirmed in trials designed for renal outcomes and including nondiabetic patients (DAPA‐CKD18). However, the evidence for the relative efficacy or effectiveness of SGLT2 inhibitors compared with DPP‐4 inhibitors—the latter being one of the most widely prescribed second‐line agents in the United Kingdom—remains unclarified for renal outcomes in clinical practice. Previous studies have also used a variety and inconsistent definitions of adverse renal outcomes.
This retrospective observational study showed that, in adults with T2D, initiation of SGLT2 inhibitors was associated with a reduced risk of kidney disease progression and death compared with DPP‐4 inhibitor initiation, using recent consensus definitions of outcomes for kidney disease. 33 Furthermore, in contrast to use of DPP‐4 inhibitors, where we observed eGFR decline over time, use of SGLT2 inhibitors was associated with stable eGFR or rise in eGFR values over time.
All components of the primary outcome were significantly lower with SGLT2 inhibitors compared to DPP‐4 inhibitors. Results were consistent across a variety of subgroup and sensitivity analyses (online supplementary material). In particular, subgroup analysis showed that the relative risk reduction in the primary composite endpoint was independent of baseline eGFR, urine albumin‐to‐creatinine ratio and baseline HbA1c. However, the reduction in all‐cause mortality appeared to be dependent on baseline HbA1c—significant reduction was seen among patients with optimal glycaemic control, HbA1c <53mmol/mol, whereas differences in kidney outcomes appear to be independent of glycaemic control. We speculate that individuals with higher HbA1c levels may reflect a patient group (in routine clinical practice) who are more likely to be nonadherent to lifestyle measures and cardio‐protective pharmacological agents and to have greater insulin resistance, and hence higher mortality risk, irrespective of SGLT2 inhibitor use. This is somewhat analogous to the post hoc analysis of the ACCORD study, where excess mortality occurred especially among individuals who attempted the intensive strategy but failed to reduce HbA1c much from their baseline levels and continued to have HbA1c levels higher than 53mmol/mol while using this strategy. 46 Interestingly, no significant interactions between DPP‐4 inhibitors and SGLT2 inhibitors were observed for mortality outcomes for prior insulin or prior GLP‐1RA use. Nonetheless, these observations confirm the hypothesis that the cardiovascular benefits of SGLT2 inhibitor use are independent of the glucose‐lowering effects. Further studies need to exclude the possibility of a type 1 statistical error (false positive). The subgroup analyses also did not correct for multiple comparisons. Conversely, the reno‐protective effects of SGLT2 inhibitors were independent of HbA1c levels and reflect the multi‐factorial mechanism of renal disease progression targeted by SGLT2 inhibitors, which is independent of glucose control.
Our findings complement and are consistent with RCTs and observational studies investigating the effectiveness of SGLT inhibitors compared with other glucose‐lowering therapies to reduce cardio‐renal endpoints. Furthermore, the evidence from this retrospective observational study is in line with, and expands, the results of clinical trials that reported that SGLT2 inhibitors slow the rate of eGFR decline and reduce the composite renal endpoints (≥40% eGFR decline, confirmed by a second measurement over 28 days or development of ESKD or kidney death) as recommended by the recent international consensus definitions of clinical trial outcomes for kidney failure. 33 The use of consensus definitions of renal endpoints will help to standardize future studies investigating renal endpoints. This is important because, although previous studies have shown benefits of SGLT2 inhibitors on renal outcomes, a variety of renal endpoints have been used. The DAPA‐CKD trial reported that, during a median follow‐up of 2.3 years, dapagliflozin significantly reduced composite renal outcomes of sustained decline in eGFR of at least 50%, ESKD or death from renal or cardiovascular causes. Dapagliflozin also slowed the long‐term decline in eGFR in CKD patients by 0.95 mL/min per 1.73 m2 per year (95% CI 0.63‐1.27) compared with placebo, and did so to a greater extent in people with T2D (1.18 mL/min per 1.73 m2 per year; 95% CI 0.79‐1.56) than in those without T2D (0.46 mL/min per 1.73 m2 per year; 95% CI −0.10‐1.03; P‐interaction = 0.040). The decline in eGFR slope was also most marked in patients with higher baseline HbA1c and greater urine albumin‐to‐creatinine ratio. 18 In DECLARE‐TIMI 58, during a median follow‐up of 4.2 years, dapagliflozin reduced the risk of kidney events (≥40% decrease in eGFR to <60 mL/min per 1.73 m2, new ESKD, or death from renal or cardiovascular causes) by 24% compared with placebo. 27 In the CREDENCE trial, canagliflozin reduced the risk of composite kidney outcomes (ESKD, doubling of serum creatinine, or renal or cardiovascular death) across a range of baseline HbA1c values. 41 The EMPA‐REG OUTCOME trial reported that the adjusted mean eGFR slope did not decline during long‐term empagliflozin treatment, but increased with mean eGFR returning toward baseline. 42 CVD‐REAL 3 propensity‐score matched 35 561 patients with T2D who were starting SGLT2 inhibitors with the same number of people taking other glucose‐lowering drugs. SGLT2 inhibitors were associated with an attenuated reduced decline in eGFR compared with other glucose‐lowering drugs. During a mean follow‐up of 14.9 months, SGLT2 inhibitor use was associated with a significant reduction in achieving the composite renal endpoint of >50% eGFR decline or developing ESKD. 13
Although previous studies have investigated the efficacy and effectiveness of SGLT2 inhibitors to improve renal endpoints, none of the previous studies have directly compared the effectiveness of SGLT2 inhibitors against DPP‐4 inhibitors for clinically important renal endpoints. The only other study that compared the same two classes of drugs in the same patient group was one by Rhee et al, who compared SGLT2 inhibitors with DPP‐4 inhibitors across different stages of CKD in T2D, 32 however, renal endpoints were not among the outcomes explored. Interestingly, in that study, the cardiovascular benefits of SGLT2 inhibitors were only seen in patients with T2D with no CKD or those with CKD stages 1 or 2 (ie, eGFR >60 mL/min per 1.73 m2). A similar trend was observed in this study, where SGLT2 inhibitor use was associated with lower risks of all‐cause death and composite renal outcomes in T2D with baseline eGFR >60 mL/min per 1.73 m2 in the subgroup analyses, although between‐group differences were not statistically significant.
The findings of the present study, using the new consensus definitions for kidney outcomes,33 build on those of our previous retrospective observational study that used more crude hospitalization definitions, which also reported improved kidney outcomes in patients receiving SGLT2 inhibitors. In patients with no history of cardio‐renal disease (HR 0.75, 95% CI 0.63‐0.88; P < 0.001), and those with established or high risk of CVD (HR 0.49, 95% CI 0.43‐0.54; P < 0.001), SGLT2 inhibitors were associated with reductions in hospitalization for CKD. 17
In the EMPEROR‐Reduced trial, empagliflozin reduced the risks of the primary outcome (cardiovascular death or hospitalization for HF), total hospitalizations for HF, and kidney outcomes, independently of baseline severity of diabetes, and was effective across HbA1c levels compared with placebo in patients with class II to IV HF and an ejection fraction of 40% or less. 47 In a similar cohort to that of EMPEROR‐Reduced, the DAPA‐HF study showed that dapagliflozin reduced the risk of worsening HF or cardiovascular death, regardless of the presence or absence of T2D, compared with placebo. 48 These studies enrolled patients predominately based on HF and, therefore, a different cohort from that in the present analysis. Despite this, the results for cardiovascular mortality are broadly consistent with our results.
Our study has some limitations, including that fact that it was a retrospective cohort study, which cannot establish causality. The use of propensity‐score matching meant that the analysis included 61% of the full dataset. Selection bias resulting from matching or exclusion of patients also cannot be ruled out. The groups were well matched, although the proportion of patients with a history of using metformin (94%) was relatively high and patients generally had preserved renal function (mean eGFR 80 mL/min per 1.73 m2), possibly reflecting the younger age cohorts receiving SGLT2 inhibitors in contemporary clinical practice. We cannot exclude the possibility that residual confounding by covariates not included in the propensity scores may have influenced the results. The consistent results from subgroup of individuals with a record of albumin creatine ratio measure and sensitivity analyses suggest, however, that the findings are robust and clinically relevant. Creatinine measures will be more readily available in those with severe renal disease compared with mild CKD. However, only 3% of the population had their eGFR calculated with creatinine using the Modification of Diet in Renal Disease Study equation with ethnic conversion, 40 the other 97% of the propensity‐score‐matched population had eGFR measures readily available, therefore, this is unlikely to markedly alter the results.
The analysis was not able to distinguish potential differences between drugs within the same pharmacological class. In addition, numbers of propensity‐score‐matched patients in certain subgroups were relatively small. Most patients were White (online supplementary material). All‐cause mortality statistically favours SGLT2 inhibitors except for people of Asian and African ethnic origin. The small numbers make deriving definitive conclusions problematic and should be investigated further.
Further studies should elucidate the mechanisms underlying the reno‐protective benefits offered by SGLT2 inhibitors. Additional analyses are required to understand the different benefits resulting from SGLT2 inhibitor use and DPP‐4 inhibitor use in Black patients, those with baseline eGFR between 30 and 59 mL/min/1.73m2 and those younger than 50 years. Further analyses should also explore the impact on healthcare resources and the overall cost benefit arising from the renal actions of SGLT2 inhibitors, continue the study with a longer follow‐up to observe any additional findings, and assess the urine‐to‐plasma urea ratio to determine progression and regression. We could not analyse the urine‐to‐plasma urea ratio in detail because of the small numbers in each group and database limitations. In addition, loop diuretics have been the cornerstone of HF treatment, but affect kidney urodynamics. 49
The benefits of SGLT2 inhibitors are explicit in the current National Health Service (NHS) of England and Wales without any changes to the current patient pathways. Additional research should focus on benefits that may be afforded with an optimized patient pathway and to see if these benefits are applicable to other international healthcare systems.
In conclusion, in adults with T2D, initiation of SGLT2 inhibitor therapy was associated with significantly reduced risk of kidney disease progression and death compared with initiation of DPP‐4 inhibitor use. These results demonstrate that the benefits of SGLT2 inhibitors seen in kidney outcomes trials are also evident in routine clinical practice. This retrospective observational study adds to the evidence base supporting the beneficial clinical outcomes associated with SGLT2 inhibitors compared with DPP‐4 inhibitors.
AUTHOR CONTRIBUTIONS
All authors participated in the research design. Ruiqi Zhang performed the data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and contributed to the scientific discussion. Kamlesh Khunti is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST
Kamlesh Khunti has acted as a consultant, speaker or received grants for investigator‐initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin‐Chemie AG/Menarini Group, Janssen and Napp. Iskandar Idris has acted as an advisory board member, speaker or received grants for Eli Lilly, Novo Nordisk, Merck Sharp & Dohme, AstraZeneca, Abbot Diabetes Care, Sanofi and Boehringer. Ruiqi Zhang, Jil B. Mamza, Mike Ford and Tamsin Morris are employed by AstraZeneca UK Ltd, a biopharmaceutical company that develops, manufactures, and markets medicines in the cardiovascular, kidney and metabolic disease area. Amitava Banerjee has received research grants from AstraZeneca.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14799.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
Medical writing and manuscript support was received from Jonathan Tulip and Mark Greener (Omega Scientific UK Ltd). AstraZeneca funded all medical writing and manuscript support. Kamlesh Khunti is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS or the Department of Health and Social Care. Iskandar Idris is supported by the NIHR Nottingham Biomedical Research Centre and carried out work at/was supported by the NIHR Nottingham Clinical Research Facilities. Amitava Banerjee is supported by research funding from NIHR, the British Medical Association, AstraZeneca, UK Research and Innovation, and the Innovative Medicines Initiative‐2 (BigData@Heart Consortium, under grant agreement no. 116074, supported by the European Union's Horizon 2020 research and innovation programme and EFPIA; chaired by D.E. Grobbee and S.D. Anker, partnering with 20 academic and industry partners and the european society of cardiology). This study is based in part on data from the CPRD obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The ONS is also acknowledged as the provider of the ONS data contained within the CPRD data. The interpretation and conclusions contained in this study are those of the author/s alone. Copyright © (2020), reused with the permission of NHS Digital. All rights reserved. The study protocol was approved by the Independent Scientific Advisory Committee (ISAC) of CPRD; protocol reference number: 19_231. This research was funded by AstraZeneca.
Idris I, Zhang R, Mamza JB, et al. Significant reduction in chronic kidney disease progression with sodium‐glucose cotransporter‐2 inhibitors compared to dipeptidyl peptidase‐4 inhibitors in adults with type 2 diabetes in a UK clinical setting: An observational outcomes study based on international guidelines for kidney disease. Diabetes Obes Metab. 2022;24(11):2138‐2147. doi: 10.1111/dom.14799
Funding information Medical writing and manuscript support: Jonathan Tulip and Mark Greener (Omega Scientific UK Ltd). AstraZeneca funded all medical writing and manuscript support.
DATA AVAILABILITY STATEMENT
This study is based in part on data from the CPRD obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The ONS is also acknowledged as the provider of the ONS Data contained within the CPRD Data. The interpretation and conclusions contained in this study are those of the author/s alone. Copyright (2020), reused with the permission of NHS Digital. All rights reserved
REFERENCES
- 1. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879‐1884. [DOI] [PubMed] [Google Scholar]
- 2. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057‐2063. [DOI] [PubMed] [Google Scholar]
- 3. Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population‐based Reykjavik study. Diabetes Care. 2005;28:612‐616. [DOI] [PubMed] [Google Scholar]
- 4. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62:298‐302. [DOI] [PubMed] [Google Scholar]
- 5. Taylor CJ, Ordóñez‐Mena JM, Roalfe AK, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000‐2017: population based cohort study. BMJ. 2019;364:l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22:1607‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawson CA, Seidu S, Zaccardi F, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UKover twenty years. EClinicalMedicine. 2021;32:100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31‐39. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413‐1424. [DOI] [PubMed] [Google Scholar]
- 10. Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA‐HF according to background heart failure therapy. Eur Heart J. 2020;41:2379‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Docherty KF, Jhund PS, Anand I, et al. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA‐HF. Circulation. 2020;142:1623‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709‐717. [DOI] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice (CVD‐REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27‐35. [DOI] [PubMed] [Google Scholar]
- 14. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71:2628‐2639. [DOI] [PubMed] [Google Scholar]
- 15. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 16. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs. Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Idris I, Zhang R, Mamza JB, et al. Lower risk of hospitalisation for heart failure, kidney disease and death with sodium glucose co‐transporter‐2 compared to dipeptidyl peptidase‐4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: a retrospective cohort study in UKprimary care. Diabetes Obes Metab. 2021;23:2207‐2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Jongs N, Chertow GM, et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA‐CKD trial. Lancet Diabetes Endocrinol. 2021;9:743‐754. doi: 10.1016/s2213-8587(1021)00242-00244 [DOI] [PubMed] [Google Scholar]
- 19. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327‐1335. [DOI] [PubMed] [Google Scholar]
- 20. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232‐242. [DOI] [PubMed] [Google Scholar]
- 21. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317‐1326. [DOI] [PubMed] [Google Scholar]
- 22. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Y, Cai Z, Zhang J. DPP‐4 inhibitors may improve the mortality of coronavirus disease 2019: a meta‐analysis. PLoS One. 2021;16:e0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rakhmat II, Kusmala YY, Handayani DR, et al. Dipeptidyl peptidase‐4 (DPP‐4) inhibitor and mortality in coronavirus disease 2019 (COVID‐19)—a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2021;15:777‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yen F‐S, Chiang J‐H, Hwu C‐M, et al. All‐cause mortality of insulin plus dipeptidyl peptidase‐4 inhibitors in persons with type 2 diabetes. BMC Endocr Disord. 2019;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birkeland KI, Bodegard J, Banerjee A, et al. Lower cardiorenal risk with sodium‐glucose cotransporter‐2 inhibitors versus dipeptidyl peptidase‐4 inhibitors in type 2 diabetes patients without cardiovascular and renal diseases: a large multinational observational study. Diabetes Obes Metab. 2021;23:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347‐357. [DOI] [PubMed] [Google Scholar]
- 28. Davies MJ, D'Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;2018(41):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Diabetes Association . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2020;44:S111‐S124. [DOI] [PubMed] [Google Scholar]
- 30. Nicolucci A, Charbonnel B, Gomes MB, et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second‐line therapy: results from the global DISCOVER study programme. Diabetes Obes Metab. 2019;21:2474‐2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bae JH, Park EG, Kim S, Kim SG, Hahn S, Kim NH. Comparative renal effects of dipeptidyl peptidase‐4 inhibitors and sodium‐glucose cotransporter 2 inhibitors on individual outcomes in patients with type 2 diabetes: a systematic review and network meta‐analysis. Endocrinol Metab. 2021;36:388‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rhee JJ, Han J, Montez‐Rath ME, et al. Cardiovascular outcomes associated with prescription of sodium‐glucose co‐transporter‐2 inhibitors versus dipeptidyl peptidase‐4 inhibitors in patients with diabetes and chronic kidney disease. Diabetes Obes Metab. 2022;24:928‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levin A, Agarwal R, Herrington WG, et al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int. 2020;98:849‐859. [DOI] [PubMed] [Google Scholar]
- 34. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) aurum. Int J Epidemiol. 2019;48:1740‐1740g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Persson R, Vasilakis‐Scaramozza C, Hagberg KW, et al. CPRD aurum database: assessment of data quality and completeness of three important comorbidities. Pharmacoepidemiol Drug Saf. 2020;29:1456‐1464. [DOI] [PubMed] [Google Scholar]
- 36. Khunti K, Kosiborod M, Kim DJ, et al. Cardiovascular outcomes with sodium‐glucose cotransporter‐2 inhibitors vs other glucose‐lowering drugs in 13 countries across three continents: analysis of CVD‐REAL data. Cardiovasc Diabetol. 2021;20:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen T‐L, Collins GS, Spence J, et al. Double‐adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palazzuoli A, Ceccarelli E, Ruocco G, Nuti R. Clinical impact of oral antidiabetic medications in heart failure patients. Heart Fail Rev. 2018;23:325‐335. [DOI] [PubMed] [Google Scholar]
- 39. McInnes G, Evans M, Del Prato S, et al. Cardiovascular and heart failure safety profile of vildagliptin: a meta‐analysis of 17000 patients. Diabetes Obes Metab. 2015;17:1085‐1092. [DOI] [PubMed] [Google Scholar]
- 40. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247‐254. [DOI] [PubMed] [Google Scholar]
- 41. Cannon CP, Perkovic V, Agarwal R, et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE trial. Circulation. 2020;141:407‐410. [DOI] [PubMed] [Google Scholar]
- 42. Wanner C, Heerspink HJL, Zinman B, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA‐REG OUTCOME trial. J Am Soc Nephrol. 2018;29:2755‐2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 44. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 45. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 46. Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the action to control cardiovascular risk in diabetes (ACCORD) trial. Circulation. 2010;24(122):844‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR‐reduced trial. Circulation. 2021;143:337‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995‐2008. [DOI] [PubMed] [Google Scholar]
- 49. Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75:1178‐1195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
This study is based in part on data from the CPRD obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The ONS is also acknowledged as the provider of the ONS Data contained within the CPRD Data. The interpretation and conclusions contained in this study are those of the author/s alone. Copyright (2020), reused with the permission of NHS Digital. All rights reserved


