Abstract
Background and Aim
Symptoms of small intestinal bacterial overgrowth (SIBO) and celiac disease (CeD) often overlap, and studies suggest a link between SIBO and CeD. We thus conducted a systematic review and meta‐analysis to compare SIBO prevalence in CeD patients and controls and assessed effects of antimicrobial therapy on gastrointestinal symptoms in SIBO positive CeD patients.
Methods
Electronic databases were searched until February 2022 for studies reporting SIBO prevalence in CeD. Prevalence rates, odds ratio (OR), and 95% confidence intervals (CI) of SIBO in CeD and controls were calculated.
Results
We included 14 studies, with 742 CeD patients and 178 controls. The pooled prevalence of SIBO in CeD was 18.3% (95% CI: 11.4–28.1), with substantial heterogeneity. Including case–control studies with healthy controls, SIBO prevalence in CeD patients was significantly increased (OR 5.1, 95% CI: 2.1–12.4, P = 0.0001), with minimal heterogeneity. Utilizing breath tests, SIBO prevalence in CeD patients was 20.8% (95% CI: 11.9–33.7), almost two‐fold higher compared with culture‐based methods at 12.6% (95% CI: 5.1–28.0), with substantial heterogeneity in both analyses. SIBO prevalence in CeD patients nonresponsive to a gluten free diet (GFD) was not statistically higher as compared with those responsive to GFD (OR 1.5, 95% CI: 0.4–5.0, P = 0.511). Antibiotic therapy of SIBO positive CeD patients resulted in improvement in gastrointestinal symptoms in 95.6% (95% CI: 78.0–99.9) and normalization of breath tests.
Conclusions
This study suggests a link between SIBO and CeD. While SIBO could explain nonresponse to a GFD in CeD, SIBO prevalence is not statistically higher in CeD patients non‐responsive to GFD. The overall quality of the evidence is low, mainly due to substantial “clinical heterogeneity” and the limited sensitivity/specificity of the available diagnostic tests.
Keywords: bacterial overgrowth, breath tests, celiac disease, gluten sensitive enteropathy, prevalence, small intestinal bacterial overgrowth
Introduction
Celiac disease is an immune‐mediated enteropathy that is triggered by the ingestion of gluten in genetically susceptible individuals. Clinically celiac disease manifests with symptoms of malabsorption, including diarrhea, abdominal pain, abdominal distention, and weight loss. A gluten free diet (GFD) is the only effective treatment currently available. 1 However, 7–30% of the patients continue to have symptoms of malabsorption despite adherence to the GFD and require further evaluation. 2 , 3
Nonresponsive (unresponsive) celiac disease can be described as failure to respond to a GFD, or the recurrence of gastrointestinal symptoms despite adherence to a GFD in a patient who responded initially to GFD. 2 The most common causes of unresponsiveness or nonresponsive to GFD is continued gluten exposure, either deliberately or by accidental ingestion. Coexistence of other conditions, such as small intestinal bacterial overgrowth (SIBO), pancreatic insufficiency, giardiasis, lymphocytic colitis, ulcerative jejunitis, and refractory celiac disease, may also be involved. 4
Gastrointestinal symptoms such as bloating, distension, flatulence, abdominal discomfort, diarrhea, and weight loss are frequently associated with SIBO 5 and may cause structural changes such as atrophy of small intestinal villi 6 with subsequent alterations of small intestinal absorption. SIBO overlaps with other gastrointestinal disorders, often making it unclear if it is the cause, consequence, or an epiphenomenon in relation to the other disorder. 7 , 8 The presence of ≥ 105 colony forming units per milliliter (CFU/mL) of colonic‐type bacteria in culture of jejunal aspirate has traditionally been considered the “gold standard” for establishing diagnosis of SIBO. 9 However, this technique is invasive, prone to cross contamination from oropharyngeal and luminal microbes, and there is controversy regarding the best cut off values for SIBO diagnosis 10 ; hence, it is rarely used in routine clinical settings. These limitations have led to the development of breath tests, which when compared with the “gold standard,” have sub‐optimal sensitivity and specificity for SIBO diagnosis. Furthermore, breath tests have several methodological problems including use of different substrates and doses of substrates, length of the test, sampling intervals, and definition of a normal and abnormal breath test, which may question their validity as diagnostic tests in clinical practice. 11 Thus, one of the main limitations in diagnosing SIBO is the lack of sensitive and specific diagnostic tests. 12
Several studies have reported an increased prevalence of SIBO in celiac disease, even considering SIBO as a potential cause for poor response to GFD 4 , 13 , 14 , 15 ; however, the results are conflicting. Furthermore, studies have shown that antibiotic treatment of SIBO in patients with poorly responsive celiac disease is successful in improving symptoms. 8 , 14 , 15 We thus performed a systematic review and meta‐analysis to determine and compare (i) the prevalence of SIBO (including methane positive SIBO) in control subjects and in patients with celiac disease, and specifically in patients with celiac disease who are nonresponsive to GFD; (ii) explore the link between diagnostic modality and variations in SIBO prevalence across different geographic regions; (ii) assess the risk factors for SIBO in patients with celiac disease and; (iv) assess the effect of antibiotic treatment on symptom improvement in SIBO positive patients with celiac disease.
Methods
Protocol and registration
This systematic review and meta‐analysis meets the preferred reporting items for systematic reviews and meta‐analysis statement requirements (PRISMA). 16 , 17 The protocol for this systematic review was prospectively registered with PROSPERO (CRD42021274197).
Search strategy
Electronic databases, including PUBMED, MEDLINE (OvidSP) and EMBASE, were searched from initiation (1966) up to February 2022 for all studies assessing prevalence of SIBO in patients with celiac disease. The detailed literature search strategy is outlined in the PRISMA flow diagram (Fig. 1) and was conducted with the expert assistance of our librarian. The search strategy for MEDLINE has been outlined in Figure S1. For further details, see the supporting information.
Figure 1.
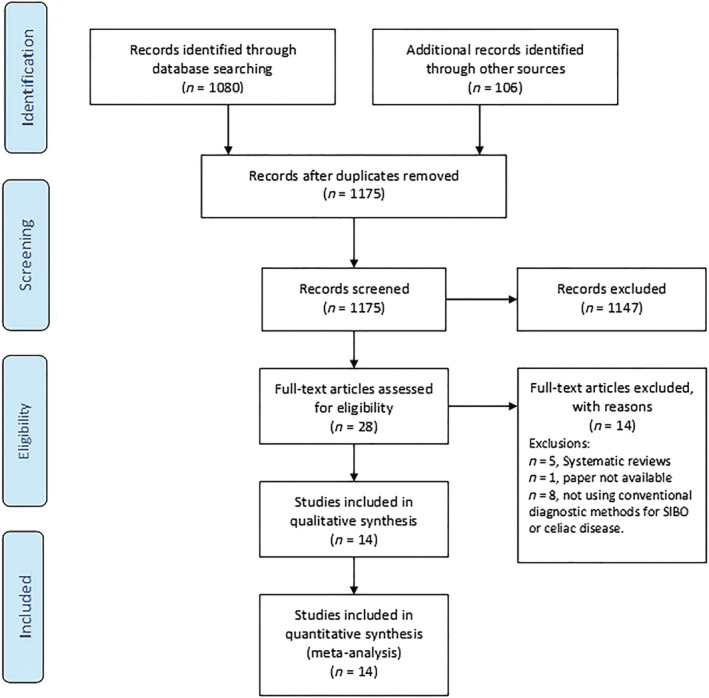
PRISMA flow diagram.
Selection of studies
Two authors (P. T. and T. H.) independently conducted an initial screen of abstracts and titles. Abstracts were eliminated if the study did not investigate the association between SIBO and celiac disease or celiac sprue or gluten‐sensitive enteropathy. Full texts of the remaining articles were retrieved and reviewed. Eligibility criteria for study inclusion are provided in Table 1. The studies that were excluded are outlined in Table S1. For further details, see the supporting information.
Table 1.
Eligibility criteria for the studies included in systematic review and meta‐analysis
| Eligibility criteria |
| • Cohort and case–control studies, published as full papers in peer reviewed journals. |
| • Adults and children with a presumed diagnosis of celiac disease based on meeting specific diagnostic criteria † |
| • Non celiac control group, referred to as ‘controls’ included ‘healthy asymptomatic controls’ as well as ‘patient controls’ including subjects undergoing evaluation for unexplained gastrointestinal symptoms (e.g. suspected SIBO) |
| • Studies reporting on efficacy data after antibiotic treatment of SIBO positive patients with celiac disease were also included. |
| • Clinically validated methods to diagnose SIBO ‡ |
| • Participants not specially selected. |
American College of Gastroenterology (ACG) Clinical Guidelines: Diagnosis and Management of Celiac Disease. 2 European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) New Guidelines for the Diagnosis of Pediatric Coeliac Disease (2020). 38
Lactulose breath test, glucose breath test, or small bowel aspirate and culture (or any combination of these).
Data extraction and quality assessment
All data were extracted independently by two authors into a Microsoft Excel spreadsheet (2010 Professional edition; Microsoft Corp, Redmond, Washington, USA). The variables extracted are detailed in the supporting information. The quality of the prevalence studies included was assessed by using the Joanna Briggs Institute (JBI) critical appraisal tools. 18 In addition, the quality of the case–control included studies were assessed using the Newcastle‐Ottawa scale (NOS) 19 ; details are outlined in the supporting information.
Data analysis
In an initial step, case numbers of patients with celiac disease and controls (using various diagnostic modalities) in the respective cohorts were determined. We calculated pooled prevalence rates and 95% confidence intervals (CI) for SIBO in celiac disease. In a second step, the pooled odds ratio (OR) and 95% CI for the prevalence of SIBO in patients with celiac disease and their respective controls were calculated. Subgroup analysis stratified by diagnostic modalities, geographic prevalence, response to GFD, effect of PPI, and methane positive SIBO in patients with celiac disease was also performed. Lastly, we compared the proportion of patients responding to antibiotic therapy regarding normalization of breath tests and assessed the symptom response after antibiotic treatment in SIBO positive celiac disease patients and controls.
Analyses for the association between SIBO and celiac disease were carried out utilizing Comprehensive Meta‐analysis Software (CMS) Version 3.3.070. NJ, USA, and further details are outlined in the supporting information.
Results
Selection outcome
The literature search (outlined in Fig. 1) revealed 14 studies eligible for inclusion in this systematic review and meta‐analysis. Of the 14 studies retained, five were case–control studies, 15 , 20 , 21 , 22 , 23 and the remaining nine were cohort studies. 3 , 4 , 13 , 14 , 24 , 25 , 26 , 27 , 28 The characteristics of all the studies included in this meta‐analysis including the methodology pertaining to diagnosis of SIBO and patient characteristics are outlined in Table 2 and Tables S2 and S3.
Table 2.
Characteristics of studies showing mode of diagnosis and prevalence of SIBO in celiac disease
| No. | Author | Study year | Region | Type of study | Patients with CeD, n | Criteria for CeD diagnosis | Controls, n | Type of control | Mode of diagnosis of SIBO | SIBO in CeD, n (%) | SIBO in controls, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dewar D et al. 24 | 2012 | UK | Cohort | 100 | Duodenal histology | NA | NA | LBT | 9 (9) | NA |
| 2 | Abdulkarim A et al. 4 , ‡ | 2002 | USA | Cohort | 49 | Duodenal histology | NA | NA | Small bowel aspirate and culture | 7 (14.3) | NA |
| 3 | Tursi A et al. 14 | 2003 | Italy | Cohort | 15 | Duodenal histology + response to GFD | NA | NA | LBT | 10 (66.7) | 0 |
| 4 | Prizont R et al. 25 | 1970 | USA | Cohort | 6 | Duodenal histology | NA | NA | Jejunal aspirate and culture | 3 (50) | NA |
| 5 | Miele L et al. 20 | 2009 | Italy | Case–control | 27 | Duodenal histology | 24 | Healthy controls | GBT | 15 (55.6) | 5 (20.8) |
| 6 | Rubio‐Tapia A et al. 13 | 2009 | USA | Cohort | 149 | Duodenal histology + serology | NA | NA | Duodenal aspirate and culture | 14 (9.4) | NA |
| 7 | Leffler D et al. 3 | 2007 | USA | Cohort | 99 | Duodenal histology | NA | NA | GBT or LBT | 6 (6.1) | NA |
| 8 | Choung R et al. 26 | 2011 | USA | Cohort | 51 | NA | NA | NA | Duodenal aspirate and culture | 1 (2) | NA |
| 9 | Safi M et al. 21 , ‡ | 2019 | Saudi Arabia | Case–control | 32 | Duodenal histology + serology | 52 | Healthy controls | LBT | 10 (31.3) | 4 (7.7) |
| 10 | Lasa J et al. 22 , † | 2015 | Argentina | Case–control | 15 | Duodenal histology + serology | 15 | Healthy controls | LBT | 3 (20) | 2 (13.2) |
| 11 | Chang M et al. 27 | 2011 | USA | Cohort | 49 | Duodenal histology | NA | NA | LBT | 4 (8.2) | NA |
| 12 | Rana S et al. 15 | 2007 | India | Case–control | 87 | Duodenal histology + serology | 87 | Healthy controls | GBT | 18 (20.7) | 0 |
| 13 | Ghoshal UC et al. 28 | 2004 | India | Cohort | 12 | Duodenal histology | NA | NA | GBT | 1 (8.3) | NA |
| 14 | Mooney P et al. 23 , † | 2014 | UK | Case–control | 51 | NA | 125 | Patient controls | GBT | 11 (21.6) | 39 (31.2) |
SIBO, small intestinal bacterial overgrowth; CeD, celiac disease; GBT, glucose breath test; LBT, lactulose breath test; CFU/mL, colony forming unit/mL; NA, not available; n, number.
Studies which both hydrogen and methane were measured during breath test.
In this study, 24/32 patients with celiac disease and 32/52 controls were between 0 and 18 years old.
Prevalence of small intestinal bacterial overgrowth in celiac disease
In total, the 14 studies reported the prevalence of SIBO in 742 adults with celiac disease. Overall, 112/742 (pooled prevalence 18.3%, 95% CI 11.4–28.1, P = 0.0001) patients with celiac disease also tested positive for SIBO (Fig. 2 and Figure S2). However, there was substantial heterogeneity in the overall analysis (I 2 = 82.6, P = 0.0001) and visual inspection of the funnel plot revealed overall asymmetry, suggesting the potential for publication bias (Fig. S3). This is consistent with the results of Egger's test.
Figure 2.
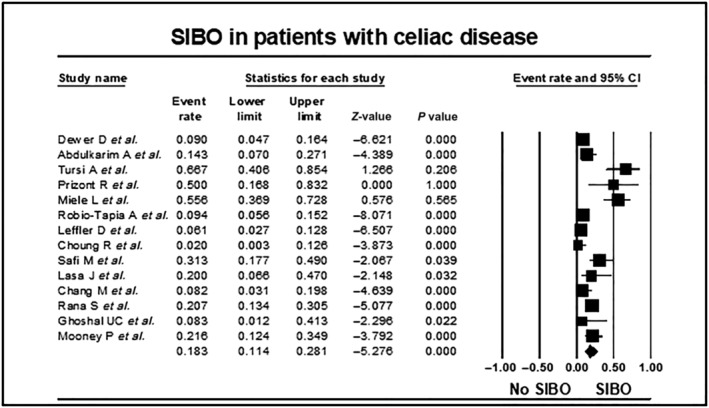
Forest plot of studies showing prevalence of SIBO in patients with celiac disease, stratified according to mode of diagnosis of SIBO (18.3%, 95% CI 11.4–28.1, P = 0.0001, I 2 = 82.6, P = 0.0001).
Influence of selection criteria for controls, and risk of bias on the small intestinal bacterial overgrowth prevalence in patients with celiac disease and controls
Healthy controls
Four out of five studies 15 , 20 , 21 , 22 included healthy controls (161 patients with celiac disease and 178 controls) while one study 23 included 51 patients with celiac disease and 125 control patients with nonspecific gastrointestinal symptoms. The pooled OR for SIBO in patients with celiac disease as compared with controls was 3.2, 95% CI 0.8–12.0, P = 0.087 (Fig. S4), but there was also substantial heterogeneity in the analysis (I 2 = 77.9, P = 0.001). Including the four case–control studies with healthy controls, SIBO prevalence in patients with celiac disease was approximately 5‐fold higher at 28.6% (95% CI 21.7–36.2) compared with 6.2% in healthy controls (95% CI 3.1–10.8, Table S4). Importantly, the pooled OR for SIBO in patients with celiac disease as compared with healthy controls was 5.1 (95% CI 2.1–12.4, P = 0.0001, Fig. 3) and with minimal heterogeneity in this analysis (I 2 = 18.7, P = 0.297).
Figure 3.
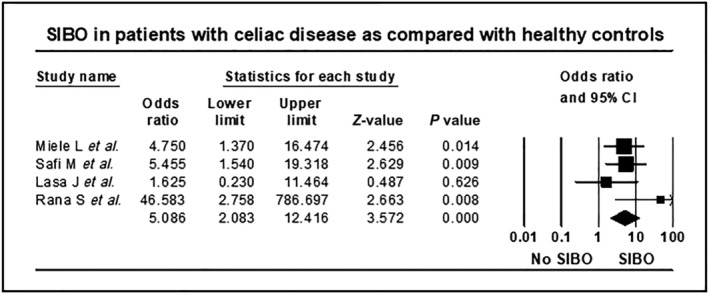
Forest plot of studies showing prevalence of SIBO in patients with celiac disease and heathy controls, stratified according to mode of diagnosis of SIBO (OR = 5.1, 95% CI 2.1–12.4, P = 0.0001, I 2 = 18.7, P = 0.297).
High‐quality studies with low risk of bias
The quality of the included studies based on the NOS and the JBI critical appraisal is shown in Table S5 and S6. The majority (3/5, 60%) of the case–control studies were of high‐quality, defined as a score of ≥ 6 using the NOS. Utilizing the JBI critical appraisal tool 2/5 case–control studies presented a low risk of bias, 1 presented a moderate risk, and 2 presented a high risk. Similarly, out of nine cohort studies, two presented with either low or moderate risk, and five presented with high risk of bias. When only the high‐quality studies were examined (n = 7), the SIBO prevalence in patients with celiac disease remained unchanged (18.7%, 95% CI 10.2–31.9, P < 0.0001, Fig. S5) and with substantial heterogeneity in the analysis (I 2 = 84.9, P = 0.0001).
Prevalence of small intestinal bacterial overgrowth in patients with celiac disease unresponsive to a gluten free diet as compared with those responsive to gluten free diet
Nine studies assessed the prevalence of SIBO in patients with celiac disease who had persistent symptoms and were nonresponsive to GFD, Table S9. The pooled prevalence of SIBO in patients with nonresponsive celiac disease while on a GFD was 17.1% (95% CI 9.5–28.7, P = 0.0001), with substantial heterogeneity in the analysis (I 2 = 79.2, P = 0.0001), Figure S6. Three studies 13 , 23 , 28 reported on SIBO prevalence in patients with celiac disease, stratified according to their response to GFD. The odds of SIBO in patients with celiac disease nonresponsive to GFD was not significantly higher as compared with patients with celiac disease and responsive to GFD (1.5, 95% CI 0.4–5.0, P = 0.511, Fig. S7). There was moderate heterogeneity in the analysis (I 2 = 37.5, P = 0.201).
Comparison of prevalence of small intestinal bacterial overgrowth in celiac disease diagnosed with breath‐tests versus small bowel aspirate and culture
Overall, four studies utilized small bowel aspirate and culture and 10 studies utilized breath test [five utilized lactulose breath test (LBT), four utilized glucose breath test (GBT), and one utilized LBT or GBT (unspecified) for SIBO diagnosis], Table 2.
Utilizing breath tests, the prevalence of SIBO in patients with celiac disease was 20.8% (95% CI 11.9–33.7, P = 0.0001); however, there was substantial heterogeneity in this analysis (I2 = 84.0, P = 0.0001). Visual inspection of the funnel plot revealed overall asymmetry, suggesting the potential for publication bias, consistent with the results of Egger's test. There was no significant difference in SIBO prevalence in patients with celiac disease utilizing LBT (22.1%, 95% CI 13.1–46.3, P = 0.022) as compared with GBT (26.5%, 95% CI 13.1–46.3, 0.022, Fig. S2), but there was substantial heterogeneity among the studies using either GBT (I2 = 78.8, P = 0.003) or LBT (I2 = 85.6, P = 0.0001). Importantly, the studies using jejunal aspirate and culture with a diagnostic threshold of ≥105 CFU/ml of bacteria determined that SIBO prevalence in patients with celiac disease was significantly lower at 12.6% (95%CI 5.1–28.0, P = 0.0001) compared with SIBO diagnosis by breath test, and substantial heterogeneity was also apparent for the studies using this approach (I2 = 71.9 P = 0.013). Out of the 4 case–control studies, 2 utilized GBT and 2 utilized LBT for SIBO diagnosis, hence subgroup analysis according to type of test for SIBO diagnosis were not conducted.
Prevalence of small intestinal bacterial overgrowth in patients with celiac disease and controls in different geographic regions
The overall prevalence of SIBO in patients with celiac disease was lowest in the studies from the USA (8.7%, 95% CI 6.1–11.9) while prevalence rates were substantially higher in the studies from Asia (22.1, 95% CI 15.4–30.2) and Europe (23.3%, 95% CI 17.5–29.9), Table S8.
Effect of antibiotic treatment on symptoms in celiac disease with small intestinal bacterial overgrowth
Four studies reported on 23 SIBO positive patients with celiac disease, who underwent antibiotic treatment for variable duration, Table S7. Significant symptom improvement was reported for 22/23, (95.6%, 95%CI 78.0–99.9) patients post antibiotic therapy. Two studies 14 , 28 which repeated breath test after completion of antibiotic therapy, found normalization of breath test in all SIBO positive patients with celiac disease. None of the studies reported any adverse events.
Association of small intestinal bacterial overgrowth with histology, celiac serology, and markers of malabsorption in patients with celiac disease
Three studies 13 , 21 , 23 failed to reveal any significant differences between the degree of intestinal damage on histology or celiac serology among celiac disease patients with or without SIBO. Furthermore, 2 studies 21 , 23 found no difference in patient characteristics and biochemistry among SIBO positive and SIBO negative patients with celiac disease, whereas 1 study 13 found patients with celiac disease and SIBO to be older, and had signs of malabsorption (lower level of hemoglobin, b‐carotene, albumin, and higher level of fecal fat), when compared with patients with celiac disease without SIBO.
Discussion
This systematic review and meta‐analysis identified 14 published peer‐reviewed, (4 case–control and 10 cohort) studies from 6 different countries and includes 742 patients with celiac disease and 178 controls. Thus far, this is the largest pooled analysis of case–control and cohort studies exploring the link between SIBO in patients with celiac disease, controls, and predicting the risk factors for SIBO in celiac disease. Overall, the data suggest a significant increase of SIBO prevalence in patients with celiac disease compared with healthy controls (OR = 5.1, 95% CI 2.1–12.4). Furthermore, SIBO prevalence rates in patients with celiac disease nonresponsive to GFD was numerically, (but not statistically) greater when compared with patients with celiac disease responsive to GFD. There were considerable variations in SIBO prevalence in patients with celiac disease and controls across different geographic regions, with lowest SIBO prevalence in patients with celiac disease seen in studies conducted in the USA, while prevalence rates were substantially higher in the studies from Asia and Europe. This is likely explained by the diagnostic modality used for SIBO diagnosis.
While the exact prevalence of nonresponsive celiac disease is unknown, between 7 and 30% of patients with celiac disease have persistent gastrointestinal symptoms despite a GFD and require further evaluation. 2 Although numerically higher, we did not find a statistically significant increase (P = 0.511) in the SIBO prevalence rates of patients with celiac disease nonresponsive to GFD as compared with those responsive to GFD. Thus, while SIBO remains an important cause for otherwise unexplained gastrointestinal symptoms in patients with celiac disease nonresponsive to GFD, other co‐existent conditions like unintentional gluten ingestion, refractory sprue, and microcytic colitis need to be excluded.
In patients with celiac disease, SIBO could be the consequence of damage to the intestinal epithelium and/or intestinal dysmotility associated with active celiac disease, 29 rather than SIBO being the cause of intestinal epithelial damage and subsequent gastrointestinal manifestations, including malabsorption. While the available data were limited, we found no association from the studies included here between SIBO and the degree of intestinal damage or celiac serology in patients with celiac disease. In addition, the association between SIBO in patients with celiac disease and markers of malabsorption/malnutrition (hemoglobin, beta‐carotene, albumin, and level of fecal fat) were inconclusive. Thus, it cannot be ruled out that SIBO could worsen malabsorption in patients with celiac disease.
Our meta‐analysis showed almost all (95.6%) SIBO positive patients with celiac disease who underwent short courses of antibiotic treatment reported improvement and/or normalization of their symptoms, which was accompanied with the normalization of their breath test. Antibiotic therapy was well tolerated and none of the studies reported any adverse events. Thus, the small intestinal dysbiosis is potentially the cause of unexplained gastrointestinal symptoms in at least a proportion of patients with celiac disease. Thus, testing and treating SIBO in patients with celiac disease with unexplained gastrointestinal symptom could potentially improve symptoms and reduce the likelihood of malnutrition.
The meta‐analysis reveals substantial heterogeneity and a high risk of publication bias across the studies included in the primary and most subgroup analyses. For this reason, we also conducted subgroup analysis according to the type of study. Once again, substantial heterogeneity was noted when subgroup analysis included only cohort studies or only case–control studies (including both healthy controls and patient controls). However, conducting subgroup analysis including case–control studies with only heathy controls revealed significantly increased prevalence rates of SIBO in patients with celiac disease as compared with healthy controls with only minimal heterogeneity in this analysis.
Next, we conducted subgroup analysis according to type of diagnostic test used in cohort studies. The prevalence of SIBO was significantly higher in patients with celiac disease when breath test was utilized for SIBO diagnosis as compared with studies utilizing culture‐based methods, with at least moderate heterogeneity seen in all analyses. Moreover, there was no difference in SIBO prevalence rates in studies utilizing LBT as compared with GBT with substantial heterogeneity in both analyses. Similar, subgroup analysis was not possible for cases‐control studies due to the small number of studies utilizing different diagnostic modalities for SIBO diagnosis.
To further address the heterogeneity seen in the primary analysis, we conducted sensitivity analysis, by separately restricting the analysis only to those studies with ‘high‐quality’ NOS‐assessment scores (i.e., with a relatively low risk of bias). However, conducting sensitivity analysis did not reduce the heterogeneity or risk of bias. Thus, the high heterogeneity scores and high risk of bias in the primary analysis could at least partially be explained by the inherent limitations of the cohort studies, and ‘patient controls’ in the case control studies. The cohort studies either had very small sample sizes, were retrospective audits of insufficiently defined study cohorts with limited information regarding the recruitment process or did not account for effects of confounders (e.g. PPI, antibiotic, probiotic use or prior surgery). Furthermore, 4/5 case control studies included healthy asymptomatic subjects in the control group, minimizing the risk of bias. As such, the other contributing factors could be the poor sensitivity and specificity and the lack of a uniform test for SIBO diagnosis.
The influence of the diagnostic modality on the SIBO prevalence in celiac disease is further exemplified by the variability seen in studies from different geographic regions. The lowest SIBO prevalence rates were reported from the USA, where small bowel aspirate and culture was used for the majority of the studies, while substantially higher SIBO prevalence rates in celiac disease were reported by studies from Asia and Europe, where breath tests were used for SIBO diagnosis.
One of the limitations of this systematic review and meta‐analysis is the failure to systematically assesses methane positivity on breath test in patients with celiac disease. None of the studies included in this systematic review and meta‐analysis, reported on prevalence of methane positive SIBO in celiac disease. Methane positivity has been found to be associated with irritable bowel syndrome (IBS), constipation subtype 30 and inversely associated with inflammatory bowel disease (IBD). 30 , 31 To emphasize the significance of measuring methane in patients with suspected intestinal dysbiosis, the most recent American College Guidelines for SIBO 32 have coined the term, intestinal methanogen overgrowth, to indicate methane production by methanogens (archaea) on breath test rather than SIBO (bacteria). Thus, by not measuring methane, the prevalence of SIBO in celiac disease could have been underestimated.
A recent population‐based 33 case–control study found antisecretory medication (both PPI and histamine‐2 receptor antagonists), to be a risk factor for celiac disease (OR 5.96; 95% CI 3.58–9.91). Recent studies show that PPI induced acid suppression potentially promotes colonization of the distal small intestine by colonic flora. 34 Thus, treatment with antisecretory drugs could be a risk factor for SIBO in celiac disease. Indeed, PPI use was associated with higher duodenal mucosal bacterial load than non‐users 35 and in a recent meta‐analysis by Su et al. 36 reported that PPI therapy was associated with a moderately increased risk (OR = 1.7, 95% CI 1.2–2.4) of SIBO in various gastrointestinal diseases. Thus, one of the important limitations of this meta‐analysis is that none of the studies have controlled for effects of PPI use on SIBO prevalence in celiac disease.
The previous meta‐analysis examining SIBO in celiac disease 37 included only 10 studies as compared with 14 studies included in primary analysis in the current meta‐analysis. More importantly, this provided the opportunity to conduct meaningful subgroup analyses of subsets of these studies and examine the magnitude of heterogeneity inherent to them. We also were able to analyze other important predictors or risk factors for SIBO in patients with celiac disease, assess the effect of antibiotic therapy on SIBO eradication in celiac disease, and environmental factors like geographic variation. Furthermore, we assessed the impact of SIBO on non‐responsive celiac disease and on small intestinal histology, serology, and markers of malnutrition in celiac disease. However, this systematic review and meta‐analysis is not without limitations. The diagnosis of SIBO is hampered by the lack of a valid and universally accepted diagnostic test. Also, some case control studies included patients with a variety of diseases or unexplained gastrointestinal symptoms as controls. It is also worth noting the small sample size with < 50 participants per arm in some studies and some sub‐group analyses are based upon a small number of studies.
In conclusion, our systematic review and meta‐analysis suggests that SIBO prevalence is increased in patients with celiac disease compared to healthy controls. However, SIBO prevalence in patients with celiac disease unresponsive to a GFD was not significantly different compared with patients responsive to a GFD. While the data are limited, SIBO in celiac disease was not associated with more severe changes in small intestinal histology or celiac serology, while effects on markers of malnutrition remain inconclusive. Treatment of SIBO positive patients with celiac disease with antibiotic therapy is associated with a statistically significant symptom improvement and normalization of a positive breath test. It needs to be noted that most of the comparative analysis revealed moderate heterogeneity and risk of bias and there is substantial ‘clinical heterogeneity’ most likely due to lack of uniform selection criteria for cases and controls, failure to assess influence of potential confounders like PPI therapy and lack of validated tests for SIBO diagnosis. Thus, the overall quality of evidence is low, and the results need to be interpreted with caution.
Supporting information
Figure S1: Search strategy for MEDLINE.
Figure S2: Forest plot of studies showing prevalence of SIBO in patients with celiac disease, stratified according to mode of diagnosis of SIBO, (18.3%, 95%CI 11.4–28.1, P = 0.0001), (I2 = 82.6, P = 0.0001). SIBO in patients with celiac disease utilizing small bowel aspirate and culture is 12.6% (95% CI 5.1–28.0, P = 0.0001), (I2 = 71.9, P = 0.013) utilizing LBT is 22.1% (95%CI 8.7–45.6, P = 0.022), (I2 = 85.6, P = 0.0001) is and utilizing GBT is 26.5% (95%CI 13.1–46.3, P = 0.022), (I2 = 78.8, P = 0.003).
Figure S3: Funnel plot of SIBO in patients with celiac disease.
Figure S4: Forest plot of studies showing prevalence of SIBO in patients with celiac disease and controls, (OR = 3.2, 95%CI 0.8–12.0, P = 0.087), (I2 = 77.9, P = 0.001).
Figure S5: Forest plot of studies showing prevalence of SIBO in patients with celiac disease, including only high‐quality studies, (18.7% (95%CI 10.2–31.9, P < 0.0001), (I2 = 84.9, P = 0.0001).
Figure S6: Forest plot of studies showing prevalence of SIBO in patients with celiac disease nonresponsive to a gluten free diet, (17.1%, 95%CI 9.5–28,7, P = 0.0001), (I2 = 79.2, P = 0.0001).
Figure S7: Forest plot of studies showing prevalence of SIBO in patients with celiac disease nonresponsive to a gluten free diet (GFD) as compared to patients with celiac disease who respond to a GFD, (OR = 1.5, 95%CI 0.5–5.0, P = 0.511), (I2 = 37.6, P = 0.201).
Table S1: Studies excluded from the systematic review and meta‐analysis.
Table S2: Assessment of risk factors for SIBO in celiac disease in studies included in the systematic review and meta‐analysis.
Table S3: Assessment of cut off criteria for diagnosing SIBO in patients with celiac disease and controls.
Table S4: Summary of findings reported in the systematic review and meta‐analysis.
Table S5: Newcastle‐Ottawa scale for assessment of quality of case control studies assessing the prevalence of SIBO in patients with celiac disease included in the systematic review and meta‐analysis.
Table S6: Joanna Briggs Institute (JBI) Critical Appraisal Tools for quality assessment of cohort studies and the case group of the case–control studies assessing SIBO in patients with celiac disease included in the systematic review and meta‐analysis.
Table S7: Studies evaluating the effect of antibiotic treatment in celiac disease patients with SIBO.
Table S8: Studies assessing the prevalence of SIBO in patients with celiac disease and controls according to geographic distribution.
Table S9: Studies assessing the prevalence of SIBO in patient with celiac disease, not responding to gluten free diet.
Acknowledgments
The authors would like to acknowledge our Librarian, Mr Marcos Riba, who has assisted with the literature search. Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Shah, A. , Thite, P. , Hansen, T. , Kendall, B. J. , Sanders, D. S. , Morrison, M. , Jones, M. P. , and Holtmann, G. (2022) Links between celiac disease and small intestinal bacterial overgrowth: A systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology, 37: 1844–1852. 10.1111/jgh.15920.
Declaration of conflict of interest: GH report to be on the advisory boards Australian Biotherapeutics, Glutagen, Bayer and received research support from Bayer, Abbott, Pfizer, Janssen, Takeda, Allergan. He serves on the Boards of the West Moreton Hospital and Health Service, Queensland, UQ Healthcare, Brisbane and the Gastro‐Liga, Germany. He has a patent for the Brisbane aseptic biopsy device and serves as Editor of the Gastro‐Liga Newsletter. MM serves on the scientific advisory board (honorary) for Genie Biome (Hong Kong) and has received research support from Bayer and Soho Flordis International (SFI) Health. Other co‐authors have no conflict of interest to declare.
Author contribution: Ayesha Shah, Parag Thite, Teressa Hansen and Gerald Holtmann: study idea, concept and design, data extraction and interpretation of data, drafting of the manuscript. Ayesha Shah and Parag Thite share equal first co‐authorship. Bradley J Kendall: drafting of the manuscript and review of final manuscript. Mark Morrison: drafting of the manuscript and review of final manuscript. David S Sanders: drafting of the manuscript and review of final manuscript. Michael Jones: data analysis, drafting of the manuscript and review of final manuscript.
Financial support: National Health and Medical Research Council (APP1084544) and Centre for Research Excellence (APP170993).
Guarantor of the article: Prof Gerald Holtmann.
References
- 1. Green PH, Cellier C. Celiac disease. N. Engl. J. Med. 2007; 357: 1731–1743. [DOI] [PubMed] [Google Scholar]
- 2. Rubio‐Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA, American College of Gastroenterology . ACG clinical guidelines: diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013; 108: 656–676 quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leffler DA, Dennis M, Hyett B et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin. Gastroenterol. Hepatol. 2007; 5: 445–450. [DOI] [PubMed] [Google Scholar]
- 4. Abdulkarim AS, Burgart LJ, See J et al. Etiology of nonresponsive celiac disease: results of a systematic approach. Am. J. Gastroenterol. 2002; 97: 2016–2021. [DOI] [PubMed] [Google Scholar]
- 5. Grace E, Shaw C, Whelan K, Andreyev HJN. Review article: small intestinal bacterial overgrowth‐‐prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment. Pharmacol. Ther. 2013; 38: 674–688. [DOI] [PubMed] [Google Scholar]
- 6. Riordan SM, McIver CJ, Wakefield D, Duncombe VM, Thomas MC, Bolin TD. Small intestinal mucosal immunity and morphometry in luminal overgrowth of indigenous gut flora. Am. J. Gastroenterol. 2001; 96: 494–500. [DOI] [PubMed] [Google Scholar]
- 7. Quigley EM, Abu‐Shanab A. Small intestinal bacterial overgrowth. Infect. Dis. Clin. North Am. 2010; 24: 943–959 viii‐ix. [DOI] [PubMed] [Google Scholar]
- 8. Ghoshal U, Ghoshal UC, Ranjan P et al. Spectrum and antibiotic sensitivity of bacteria contaminating the upper gut in patients with malabsorption syndrome from the tropics. BMC Gastroenterol. 2003; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paik CN, Choi MG, Lim CH et al. The role of small intestinal bacterial overgrowth in postgastrectomy patients. Neurogastroenterol. Motil. 2011; 23: e191–e196. [DOI] [PubMed] [Google Scholar]
- 10. Khoshini R, Dai SC, Lezcano S et al. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig. Dis. Sci. 2008; 53: 1443–1454. [DOI] [PubMed] [Google Scholar]
- 11. Shah A, Holtmann G. Clinical Conditions Associated with Small Intestinal Bacterial Overgrowth. Gastrointestinal Diseases and their Associated Infections. Elsevier Inc., 2019; 67–83. [Google Scholar]
- 12. Shah A, Morrison M, Holtmann GJ. Gastroduodenal "Dysbiosis": a New Clinical Entity. Curr. Treat. Options Gastroenterol. 2018; 16: 591–604. [DOI] [PubMed] [Google Scholar]
- 13. Rubio‐Tapia A, Barton SH, Rosenblatt JE et al. Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J. Clin. Gastroenterol. 2009; 43: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am. J. Gastroenterol. 2003; 98: 839–843. [DOI] [PubMed] [Google Scholar]
- 15. Rana SV, Sinha SK, Lal S et al. Small intestinal bacterial overgrowth in North Indian patients with celiac disease. Trop. Gastroenterol. 2007; 28: 159–161. [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah A, Jones MP, Holtmann GJ. Basics of meta‐analysis. Indian J. Gastroenterol. 2020; 39: 503–513. [DOI] [PubMed] [Google Scholar]
- 18. Munn Z, Moola S, Lisy K et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015; 13: 147–153. [DOI] [PubMed] [Google Scholar]
- 19. Wells GA, Shea B, O'Connell D et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Oxford, 2000. [Google Scholar]
- 20. Miele L, Valenza V, La Torre G et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–1887. [DOI] [PubMed] [Google Scholar]
- 21. Safi MA, Jiman‐Fatani AA, Saadah OI. Small intestinal bacterial overgrowth among patients with celiac disease unresponsive to a gluten free diet. Turk. J. Gastroenterol. 2020; 31: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lasa JS, Zubiaurre I, Fanjul I et al. Small intestinal bacterial overgrowth prevalence in celiac disease patients is similar in healthy subjects and lower in irritable bowel syndrome patients. Rev. Gastroenterol. Mex. 2015; 80: 171–174. [DOI] [PubMed] [Google Scholar]
- 23. Mooney PD, Evans KE, Sanders DS. Letter: coeliac disease and small intestinal bacterial overgrowth‐‐is dysmotility the missing link? Aliment. Pharmacol. Ther. 2014; 39: 902–903. [DOI] [PubMed] [Google Scholar]
- 24. Dewar DH, Donnelly SC, McLaughlin SD et al. Celiac disease: management of persistent symptoms in patients on a gluten‐free diet. World J. Gastroenterol. 2012; 18: 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prizont R, Hersh T, Floch MH. Jejunal bacterial flora in chronic small bowel disease. I. Celiac disease. II. Regional enteritis. Am. J. Clin. Nutr. 1970; 23: 1602–1607. [DOI] [PubMed] [Google Scholar]
- 26. Choung RS, Ruff KC, Malhotra A et al. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment. Pharmacol. Ther. 2011; 33: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 27. Chang MS, Minaya MT, Cheng J et al. Double‐blind randomized controlled trial of rifaximin for persistent symptoms in patients with celiac disease. Dig. Dis. Sci. 2011; 56: 2939–2946. [DOI] [PubMed] [Google Scholar]
- 28. Ghoshal UC, Ghoshal U, Misra A et al. Partially responsive celiac disease resulting from small intestinal bacterial overgrowth and lactose intolerance. BMC Gastroenterol. 2004; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sadik R, Abrahamsson H, Kilander A et al. Gut transit in celiac disease: delay of small bowel transit and acceleration after dietary treatment. Am. J. Gastroenterol. 2004; 99: 2429–2436. [DOI] [PubMed] [Google Scholar]
- 30. Gandhi A, Shah A, Jones MP et al. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: A systematic review and meta‐analysis. Gut Microb. 2021; 13: 1933313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah A, Morrison M, Burger D et al. Systematic review with meta‐analysis: the prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019; 49: 624–635. [DOI] [PubMed] [Google Scholar]
- 32. Pimentel M, Saad RJ, Long MD, Rao SSC. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020; 115: 165–178. [DOI] [PubMed] [Google Scholar]
- 33. Lebwohl B, Spechler SJ, Wang TC et al. Use of proton pump inhibitors and subsequent risk of celiac disease. Dig. Liver Dis. 2014; 46: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laine L, Ahnen D, Mcclain, Solcia, Walsh. Review article: potential gastrointestinal effects of long‐term acid suppression with proton pump inhibitors. Aliment. Pharmacol. Ther. 2000; 14: 651–668. [DOI] [PubMed] [Google Scholar]
- 35. Shah A, Shanahan E, Berendsen E et al. Quantitative PCR as a novel approach to determine influence of density of bacterial colonization on health and disease. J. Gastroenterol. Hepatol. 2018; 33: 49–50. [Google Scholar]
- 36. Su T, Lai S, Lee A et al. Meta‐analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J. Gastroenterol. 2018; 53: 27–36. [DOI] [PubMed] [Google Scholar]
- 37. Losurdo G, Marra A, Shahini E et al. Small intestinal bacterial overgrowth and celiac disease: A systematic review with pooled‐data analysis. Neurogastroenterol. Motil. 2017; 29. [DOI] [PubMed] [Google Scholar]
- 38. Husby S, Koletzko S, Korponay‐Szabó I et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020; 70: 141–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Search strategy for MEDLINE.
Figure S2: Forest plot of studies showing prevalence of SIBO in patients with celiac disease, stratified according to mode of diagnosis of SIBO, (18.3%, 95%CI 11.4–28.1, P = 0.0001), (I2 = 82.6, P = 0.0001). SIBO in patients with celiac disease utilizing small bowel aspirate and culture is 12.6% (95% CI 5.1–28.0, P = 0.0001), (I2 = 71.9, P = 0.013) utilizing LBT is 22.1% (95%CI 8.7–45.6, P = 0.022), (I2 = 85.6, P = 0.0001) is and utilizing GBT is 26.5% (95%CI 13.1–46.3, P = 0.022), (I2 = 78.8, P = 0.003).
Figure S3: Funnel plot of SIBO in patients with celiac disease.
Figure S4: Forest plot of studies showing prevalence of SIBO in patients with celiac disease and controls, (OR = 3.2, 95%CI 0.8–12.0, P = 0.087), (I2 = 77.9, P = 0.001).
Figure S5: Forest plot of studies showing prevalence of SIBO in patients with celiac disease, including only high‐quality studies, (18.7% (95%CI 10.2–31.9, P < 0.0001), (I2 = 84.9, P = 0.0001).
Figure S6: Forest plot of studies showing prevalence of SIBO in patients with celiac disease nonresponsive to a gluten free diet, (17.1%, 95%CI 9.5–28,7, P = 0.0001), (I2 = 79.2, P = 0.0001).
Figure S7: Forest plot of studies showing prevalence of SIBO in patients with celiac disease nonresponsive to a gluten free diet (GFD) as compared to patients with celiac disease who respond to a GFD, (OR = 1.5, 95%CI 0.5–5.0, P = 0.511), (I2 = 37.6, P = 0.201).
Table S1: Studies excluded from the systematic review and meta‐analysis.
Table S2: Assessment of risk factors for SIBO in celiac disease in studies included in the systematic review and meta‐analysis.
Table S3: Assessment of cut off criteria for diagnosing SIBO in patients with celiac disease and controls.
Table S4: Summary of findings reported in the systematic review and meta‐analysis.
Table S5: Newcastle‐Ottawa scale for assessment of quality of case control studies assessing the prevalence of SIBO in patients with celiac disease included in the systematic review and meta‐analysis.
Table S6: Joanna Briggs Institute (JBI) Critical Appraisal Tools for quality assessment of cohort studies and the case group of the case–control studies assessing SIBO in patients with celiac disease included in the systematic review and meta‐analysis.
Table S7: Studies evaluating the effect of antibiotic treatment in celiac disease patients with SIBO.
Table S8: Studies assessing the prevalence of SIBO in patients with celiac disease and controls according to geographic distribution.
Table S9: Studies assessing the prevalence of SIBO in patient with celiac disease, not responding to gluten free diet.


