Abstract
Aim
“Host modulatory therapy” (HMT) with ω‐3 fatty acids aims at reducing inflammation. With HMT as an adjunct, a better result of periodontal therapy is expected. The aim of this systematic review and meta‐analysis (MA) was to examine the additional effect of ω‐3 fatty acids to non‐surgical periodontal therapy (SRP) on the probing pocket depth (PPD) and the clinical attachment level (CAL).
Materials and Methods
MEDLINE‐PubMed and Cochrane‐CENTRAL libraries were searched up to January 2021 for randomized controlled trials in patients with chronic periodontitis, treated with SRP/placebo as controls and SRP/ω‐3 fatty acids as the test group.
Results
The search identified 173 unique abstracts, and screening resulted in 10 eligible publications. Descriptive analysis showed a significant effect on the PPD and CAL in favour of the groups with ω‐3 fatty acids in the majority of comparisons. MA revealed that adjunctive use of ω‐3 fatty acids to SRP resulted in 0.39 mm more PPD reduction (95% CI: −0.58; −0.21) and 0.41 mm more CAL gain (95% CI: −0.63; −0.19) than SRP alone.
Conclusions
In patients with periodontitis, dietary supplementation with ω‐3 fatty acids as an adjunct to SRP is more effective in reducing the PPD and improving the CAL than SRP alone. If SRP is indicated, the use of ω‐3 fatty acids can be considered for a moderate extra added effect on PPD reduction and CAL gain. The strength of this recommendation is moderate.
Keywords: DHA, EPA, omega‐3, periodontal therapy, periodontal treatment
Clinical Relevance.
Scientific rationale for study: The biomechanism of ω‐3 fatty acids has a potential to halt the progression of periodontitis. A systematic review and meta‐analysis may image the effect of this treatment as an adjunct to non‐surgical periodontal therapy (SRP).
Principal findings: Significant improvement in the probing pocket depth (PPD) and the clinical attachment level (CAL) was observed when ω‐3 fatty acids were added as an adjunct to SRP.
Practical implications: In patients with periodontitis, SRP with ω‐3 fatty acids (≥2 g/day) is a moderately effective adjunctive treatment in reducing the PPD and improving the CAL. In fact, it is more effective than SRP alone.
1. INTRODUCTION
Periodontal disease is a multi‐factorial inflammatory disease caused by the interaction between pathogens and the immune response of the host, causing breakdown of connective tissues and bones that support the teeth (Offenbacher, 1996). Traditional treatment for periodontitis mostly concentrates on diminishing the bacterial infection by means of mechanical disruption of the biofilm, plaque control, antibiotics, and possibly surgery. Subgingival debridement forms the basis of periodontal treatment.
Besides this traditional approach, additional therapy can be used in the management of periodontal disease. One of these additional measures is a treatment that intervenes with the inflammatory response of the host, called the “host modulatory therapy” (HMT), which was first introduced in dentistry by Williams (1990). HMT is administered systemically or locally as part of a patient's periodontal treatment and used as an adjunct to conventional periodontal therapy. The patients' treatment could be more predictable in this way (Salvi & Lang, 2005). Different types of medicines have already been evaluated as a complementary therapy, such as bisphosphonates, certain non‐steroidal anti‐inflammatory drugs, and tetracycline (Kornman, 1999). These medicines proved to be effective in treating periodontal disease, but all of them had their limitations and side effects.
Omega‐3 polyunsaturated fatty acids (ω‐3 fatty acids), such as docosahexaenoic acid (DHA; C22: 6, n‐3) and eicosapentaenoic acid (EPA; C20: 5, n‐3), were pointed out by Simopoulos (2008) to have anti‐inflammatory, protective, and therapeutic effects on rheumatoid arthritis, ulcerative colitis, atherosclerosis, cancer, heart and vascular disease, and periodontitis. To date, it is known that ω‐3 fatty acids provide a decrease in eicosanoids (prostaglandin E2 and inflammatory cytokines). These prostaglandins are inflammatory mediators (Calder, 2006). Serhan et al. (2008) showed that a new set of lipid mediators, resolvin and protectin, are enzymatically converted by ω‐3 fatty acids. Resolvins and protectins both reduce neutrophil infiltration and increase the recruitment of monocytes.
Because the stock of ω‐3 fatty acids in the body is very limited, its presence in the tissue is mostly determined by nutrition (Simopoulos, 2008). ω‐3 fatty acids can be transformed into resolvin D, resolving E, protectins, and neuroprotectins. These are named specialized pro‐resolving mediators (SPM), which are important for controlling and eliminating inflammation (Bannenberg & Serhan, 2010). There are indications that aspirin (acetylsalicylic acid [ASA]), in the presence of ω‐3 fatty acids, produces 18R‐Resolvin, which is a strong anti‐inflammatory lipid mediator. This linkage limits excessive polymorphonuclear neutrophil (PMN) infiltration and prevents inflammation. ASA seems essential for an increased activity of stereoisomers (18R‐Resolvin) by inhibiting the COX2 activity.
With HMT as an adjunct to non‐surgical periodontal treatment, a better result could be expected because HMT with ω‐3 fatty acids also aims at reducing inflammation by decreasing the production of inflammatory eicosanoids, cytokines, and reactive oxygen species (ROS) (Calder, 2009). The biomechanism of ω‐3 fatty acids has the theoretical potential to halt the progression of periodontitis. Using ω‐3 fatty acids as an adjunct to SRP is an innovative method of treatment, which has been researched multiple times. The clinical practice guideline of the European Federation of Periodontology on the treatment of stage I–III periodontitis (Sanz et al., 2020) recommended “not to use omega‐3 Polyunsaturated fatty acids (PUFAs) as an adjunct to subgingival instrumentation”. This was a Grade A recommendation based on a systematic review (Donos et al., 2020) that evaluated three placebo‐controlled randomized controlled clinical trials (RCTs) with 6‐month administration of ω‐3 PUFAs. In four recent systematic reviews (Kruse et al., 2020; Corbella et al., 2021; Castro Dos Santos et al., 2022; Chatterjee et al., 2022) published in non‐dental and dental journals, ω‐3 fatty acids as an adjunct to SRP were evaluated. All four systematic reviews included studies that also evaluated diabetes patients. As diabetes and periodontal disease have a bidirectional relationship (Sanz et al., 2018), including diabetes patients may influence the results. Therefore, in order to evaluate as a proof of principle, there is a need for this systematic review focused on clinical trials considering supplementation of ω‐3 (or ω‐3 in combination with ASA) as an adjunct to non‐surgical periodontal therapy (SRP) for the effect on clinical parameters PPD and CAL in systemically healthy patients.
2. MATERIALS AND METHODS
This systematic review and meta‐analysis (MA) were prepared as described, in accordance with the Cochrane Handbook for Systematic Reviews of Intervention and the Transparent Reporting of Systematic Reviews and Meta‐analyses (PRISMA‐statement), which provides guidance for the preparations and the guidelines of the PRISMA‐statement (Moher et al., 2009). The protocol detailing the review method was developed “a priori”, following an initial discussion between the members of the research team and registered at PROSPERO as CRD42022136457.
2.1. Focused question
What is in systematically healthy patients with periodontitis who were treated with scaling and root planing (SRP) + ω‐3 fatty acids in the test group and SRP + placebo in the control group. What is the effect on the PPD and CAL?
2.2. Search strategy
A structured search strategy was designed to retrieve all relevant studies that evaluated the effectiveness on the PPD and CAL of using ω‐3 fatty acids during non‐SRP compared to placebo. The National Library of Medicine, Washington, DC (MEDLINE‐PubMed) and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched from their initiation up to January 2021 for appropriate papers that answered the focused question. In addition, the reference lists of the included studies were hand‐searched by the two reviewers (M.R. and E.B.) to identify additional potentially relevant studies. For details regarding the search terms used, see Table 1.
TABLE 1.
Search terms used for PubMed‐MEDLINE, Cochrane‐CENTRAL and EMBASE. The search strategy was customized according to the database being searched.
|
The following strategy was used in the search: {<subject> AND <intervention>} {<subject> {<Subject: (<periodontal disease> OR <periodontitis>) OR (periodontal disease [MeSH] OR (<periodontal therapy>) OR (periodontitis [MeSH] [textword]}> AND <intervention> (<omega‐3 fatty acids OR EPA OR DHA OR fish oil>)} |
Note: The asterisk (*) was used as a truncation symbol.
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
2.3. Screening and selection
The titles and abstracts from the obtained studies were independently screened by two reviewers (M.R. and E.B.) to select the studies that potentially met the inclusion criteria. No language restrictions were imposed. Based on the title and abstract, the full‐text versions of potentially relevant papers were obtained. These were categorized as definitely eligible, definitely not eligible, or questionable. Disagreements concerning eligibility were resolved by consensus, or if any disagreement persisted, the judgement of a third reviewer (M.T.) was decisive. The papers that fulfilled all the inclusion criteria were processed for data extraction.
The inclusion criteria were as follows:
RCTs or controlled clinical trials (CCTs)
- Conducted in humans:
- ≥18 years of age
- In good general health (no systemic disorder)
- Diagnosed with periodontitis
- Received non‐surgical periodontal treatment
Intervention: ω‐3 fatty acids
Comparison: SRP + placebo
Outcome parameters evaluated: Primary outcome: PPD and CAL
2.4. Methodological quality assessment
Two reviewers (M.R. and E.B.) independently scored the individual methodological quality of the included studies using the checklist presented in Online appendix S2. A study was classified as having a “low risk of bias” when random allocation, defined inclusion/exclusion criteria, blinding to the patient and the examiner, balanced experimental groups, identical treatment between groups (except for the intervention), and reporting of follow‐up were present. Studies that met six of these seven criteria were considered to have a potentially moderate risk of bias. If two or more of these seven criteria were absent, the study was considered to have a high risk of bias (Van der Weijden et al., 2009).
2.5. Data extraction
The characteristics of the population, intervention, comparison, and outcomes were extracted independently from all studies by two reviewers (M.R. and E.B.). Disagreements between the reviewers were resolved through discussion and consensus. If a disagreement persisted, the judgement of a third reviewer (M.T.) was decisive; however, during the whole process, it was not necessary to consult the third reviewer. Some of the studies provided standard error (SE) of the mean. Where needed and possible, the authors calculated standard deviation based on the sample size (SE = SD/√N). For those papers that provided insufficient data to be included in the analysis, the first or corresponding author was contacted to request additional data.
2.6. Data analysis
As a summary, a descriptive data presentation was used for all studies, summarized using vote counting. As not all studies could be included into the quantitative analysis, subsequently, where feasible, MA was performed for the two outcome parameters. The difference of means (DiffM) between the test and control groups was calculated using a “random or fixed effects” model where appropriate (Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020, p. 4). If there were four or more comparisons to be analysed, the “random‐effects” model was chosen to calculate the weighted average of the treatment effects across the studies (Higgins Green Handbook). If there were fewer than four studies, the “fixed effects” model was used. The heterogeneity was tested using the chi‐square test and the I 2 statistic. When studies were considered similar enough to allow for comparison, heterogeneity was examined statistically. Heterogeneity was tested using the chi‐square test and the I 2 statistic (Higgins & Green, 2012). The chi‐square test resulting in a p‐value <.1 was considered to be an indication of significant statistical heterogeneity. As an approximate guide for assessing the degree of inconsistency across studies, an I 2 statistic of 0%–40% was interpreted as potentially not important, a statistic of 30%–60% was taken to represent moderate heterogeneity, 50%–90% was taken to represent substantial heterogeneity, and 75%–100% was taken to represent considerable heterogeneity (Ryan, 2016). If possible, testing for publication bias was performed as proposed by Egger et al. (1997). A sub‐analysis was performed for ω‐3 with and without ASA because of a possible adjunctive effect of ASA on treatment outcomes.
2.7. Assessment of clinical and methodological heterogeneity
The factors used to evaluate the heterogeneity of the outcomes of the different studies were as follows:
Study design and evaluation period
Characteristics of subjects
Study groups
Side effects
Industry funding
2.8. Grading the “body of evidence”
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to rank the evidence and certainty (Guyatt et al., 2008). Two reviewers (M.T. and D.E.S.) rated the quality of the evidence and the strength and direction (Smiley et al., 2015) of the recommendations according to the following aspects: risk of bias, consistency of results, directness of evidence, precision and publication bias, and magnitude of the effect. There was no disagreement between the two reviewers.
3. RESULTS
3.1. Search and selection results
Figure 1 and online appendix S2 describe the flow of the search process. A total of 173 unique papers were identified, from which 11 full‐text articles were obtained (El‐Sharkawy et al., 2010; Elkhouli, 2011; Martinez et al., 2013; Deore et al., 2014; Martinez et al., 2014; Salman et al., 2014; Keskiner et al., 2017; Umrania et al., 2017; Elgendy & Kazem, 2018; Kujur et al., 2020; Stańdo et al., 2020) and screened to confirm eligibility. The manual searching of the reference lists of the selected papers provided no additional suitable publications. Two publications provided data on the same experiment (Martinez et al., 2013, 2014). Consequently, 10 eligible experiments were included in this systematic review.
FIGURE 1.
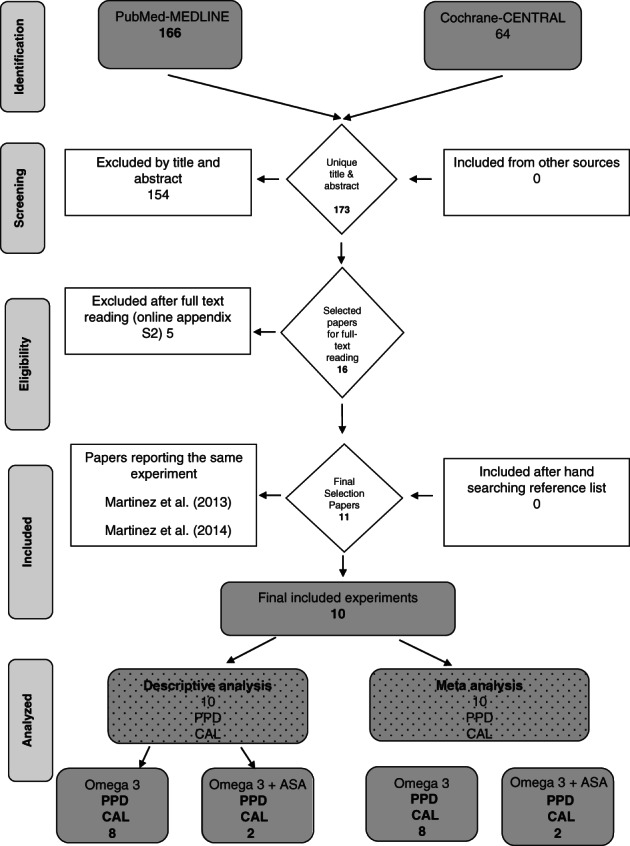
Search and selection results.
3.2. Assessment of heterogeneity
Some heterogeneity was observed in the eight experiments with respect to the evaluation period. Information regarding the study outline and characteristics is shown in detail in Table 1. All studies included participants in good general health. Regarding periodontal health, various criteria and diagnoses were used as parameters for inclusion. Eight of the 10 experiments did not include smokers (El‐Sharkawy et al., 2010; Deore et al., 2014; Keskiner et al., 2017; Umrania et al., 2017; Elgendy & Kazem, 2018; Kujur et al., 2020; Stańdo et al., 2020) while one study (Elkhouli, 2011) did exclude heavy smokers (>10 cigarettes), and the other study (Martinez et al., 2014) did not exclude smokers, but only one person at the placebo group was a smoker. Further details on heterogeneity aspects are described in Table 2 and Online appendix S3.
TABLE 2.
Overview of the studies processed for data extraction
| Author publication year | Design blind follow up #participants (end) | Participants characteristics diagnosis gender age, mean (SD) | Treatment groups | Original authors' conclusion |
|---|---|---|---|---|
| El‐Sharkawy et al. (2010) | RCT, parallel, double blind, 6 months | Untreated advanced chronic periodontitis | TG: SRP + ω‐3 fatty acids (1000 mg) + aspirin 3 t.i.d. + 81 mg aspirin | The results of this preliminary clinical study suggest that dietary supplementation with ω‐3 fatty acids and 81 mg aspirin may provide a sustainable, low‐cost intervention to augment periodontal therapy |
| 80 (80) | ?♂/?♀ No statistic difference between gender | CG: SRP + placebo | ||
| Mean age: 45.15 (8.0) | ||||
| Elkhouli (2011) | RCT, parallel, double blind, 6 months | Moderate advanced chronic periodontitis | TG: SRP + ω‐3 (1 g) 3 t.i.d. + 75 mg aspirin | The findings suggest that the combination therapy demonstrated successful reduction of gingival inflammation, reduction of pocket depth and attachment level gain, accompanied by a trend for modulation of the cytokines profile in gingival crevicular fluid |
| 40 (40) | 25♂/15♀ | CG: SRP + placebo (300 g) 3 t.i.d. | ||
| Mean age: 42.6 (9.7) | ||||
| Deore et al. (2014) | RCT, parallel, double blind, 3 months | Generalized chronic periodontitis | TG: SRP + ω‐3 fatty acids (180 mg EPA 120 mg DHA) | The findings suggest that ω‐3 fatty acids can successfully reduce gingival inflammation, pocket depth, and attachment level gain. Dietary supplementation with ω‐3 fatty acids may have potential benefits as a host modulatory agent in the prevention and/or adjunctive management of chronic periodontitis |
| 60 (58) | ?♂/?♀ No statistic difference between gender | CG: SRP + placebo (300 mg liquid paraffin) | ||
| Mean age: 44.9 (5.05) | ||||
| Salman et al. (2014) | RCT, parallel, double blind, 3 months | Chronic periodontitis | TG: SRP + ω‐3 fatty acids (1000 mg) | The results of this study suggest that dietary supplementation with ω‐3 may provide a sustainable, low‐cost intervention to augment periodontal therapy |
| 50 (50) | 50♂/0♀ | CG: SRP + placebo | ||
| Mean age: ? | ||||
| Age range: 30–60 | ||||
| Martinez et al. (2014) | RCT, parallel, double blind, 12 months | Generalized chronic periodontitis | TG: SRP + ω‐3 fatty acids (120 mg EPA 180 mg DHA) 3 t.i.d. | There was no effect on the clinical outcome of periodontal therapy with ω‐3 supplementation observed |
| 15 (15) | 6♂/9♀◊ | CG: SRP + placebo 3 t.i.d. | ||
| Mean age: 44.6 (17.6) | ||||
| Keskiner et al. (2017) | RCT, parallel, double blind, 6 months | Chronic periodontitis | TG: SRP + ω‐3 fatty acids (6.25 mg EPA 19.19 mg DHA) 2 t.i.d. | The results demonstrated that dietary supplementation with low‐dose ω‐3 fatty acids improves salivary TNF‐alpha without any significant impact on clinical parameters in patients with chronic periodontitis, suggesting that the systemic benefits of dietary ω‐3 fatty acids may not translated into periodontal health |
| 60 (60) | 16♂/14♀ | CG: SRP + placebo 2 t.i.d. | ||
| Mean age: 41.7 (7.7) | ||||
|
Umrania et al. (2017) |
RCT, parallel, double blind, 3 months | Advanced chronic periodontitis | TG: SRP + ω‐3 fatty acids (700 mg) | Even though adjunct therapy with ω‐3 fatty acids can modulate cytokine levels and show pro‐resolution properties, its importance on clinical outcome may be controversial. Thus, this may be used as an adjunctive management of chronic periodontitis |
| 40 (40) | 25♂/15♀ | CG: SRP + placebo | ||
| Mean age: 43.75 (6.12) | ||||
| Elgendy and Kazem (2018) | RCT, parallel, double blind, 6 months | Generalized chronic periodontitis | TG: SRP + ω‐3 fatty acids (2000 mg) | Dietary supplementation with ω‐3 fatty acids may have potential benefits as a host modulatory agent in adjunctive management of chronic periodontitis in postmenopausal women, especially in patients with periodontal pockets |
| 50 (50) | 0♂/50♀ | CG: SRP + placebo (olive oil) | ||
| Mean age: 50.84 (3.2) | ||||
| Kujur et al. (2020) | RCT, parallel, single blind | Chronic moderate periodontitis | TG: SRP + ω‐3 fatty acids (1000 mg) | Adjunctive use of ω‐3 fatty acids proved to be beneficial over scaling and root planing alone in the treatment of chronic moderate periodontitis. The beneficial effects were in terms of significant improvements in clinical parameters, probing pocket depth, and clinical attachment level and gingival index. Hence, ω‐3 fatty acid may be used routinely in the management of chronic periodontitis |
| ?♂/?♀ | CG: SRP | |||
| Mean age: 45 years | ||||
| Age range: 30–60 | ||||
| Stańdo et al. (2020) | RCT, parallel, single blind | Generalized stage III and IV periodontitis | TG: SRP + ω‐3 fatty acids (2600 mg EPA 1800 mg DHA) | Dietary intervention with high dose of ω‐3 PUFA during non‐surgical therapy may have potential benefits in the management of periodontitis |
| 16♂/14♀ | CG: SRP | |||
|
Mean age: 49.0 (10.59) years |
||||
| Age range: ? |
Abbreviations: CG, control group; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; RCT, randomized controlled clinical trial; SRP, surgical periodontal therapy; TG, test group; ω‐3, omega‐3 fatty acids.
3.2.1. Side effects
The included papers did not report any adverse events or side effects.
3.2.2. Industry funding
None of the studies were sponsored by companies.
3.3. Methodological quality assessment
To estimate the potential risk of bias, the methodological quality of the included studies was based on a checklist as presented in Online appendix S2. All studies described random allocation. Blinding of the examiner as well as blinding of the subjects was described in all studies. There were two studies that exhibited unbalanced experimental groups (Salman et al., 2014; Elgendy & Kazem, 2018). Two of the studies did not report loss on follow‐up (Martinez et al., 2014; Salman et al., 2014), Stańdo et al. (2020) reported 25% loss to follow‐up in majority related to the COVID pandemic. All studies defined eligibility criteria for the participants. A summary of the proposed criteria indicates the estimated potential risk of bias to be “high” in one study (Salman et al., 2014), “moderate” in one study (Martinez et al., 2014), and “low” in eight studies.
3.4. Study outcome results
3.4.1. Descriptive analysis
Table 3 presents the results of the data extraction. Data regarding the PPD and CAL could be retrieved. The baseline and end data together with statistical significance within groups are presented. Additionally, modifications of some clinical indices are reported. Not all the included studies evaluated all the clinical parameters of interest. Table 3 descriptively summarizes statistically significant differences between using ω‐3 fatty acids (with or without ASA) besides SRP compared to SRP with placebo. Three comparisons indicated no significant difference (Martinez et al., 2014; Keskiner et al., 2017; Umrania et al., 2017) for both parameters of interest; Stańdo et al. (2020) only for the PPD did not reveal a significant effect. The other five studies showed a statistically significant improvement in favour of the ω‐3 fatty acids for both the PPD and CAL.
TABLE 3.
A descriptive summary of statistical significance levels
| Author, year | Risk of bias (Appendix 1) | Intervention | PPD | CAL | Control |
|---|---|---|---|---|---|
| El‐Sharkawy et al. (2010) | Low | SRP + ω‐3 + ASA | + | + | SRP + placebo |
| Elkhouli (2011) | Low | SRP + ω‐3 + ASA | + | + | SRP + placebo |
| Deore et al. (2014) | Low | SRP + ω‐3 | + | + | SRP + placebo |
| Salman et al. (2014) | High | SRP + ω‐3 | + | □ | SRP + placebo |
| Martinez et al. (2014) | Moderate | SRP + ω‐3 | ○ | ○ | SRP + placebo |
| Keskiner et al. (2017) | Low | SRP + ω‐3 | ○ | ○ | SRP + placebo |
| Umrania et al. (2017) | Low | SRP + ω‐3 | ○ | ○ | SRP + placebo |
| Elgendy and Kazem (2018) | Low | SRP + ω‐3 | + | + | SRP + placebo |
| Kujur et al. (2020) | Low | SRP + ω‐3 | + | + | SRP |
| Stańdo et al. (2020) | Low | SRP + ω‐3 | ○ | + | SRP |
Note: +: Significant difference in favour of SRP + ω‐3. ○: No significant difference between groups. □: not evaluated.
Abbreviations: ASA, aspirin; CAL, clinical attachment level; PPD, probing pocket depth; SRP, scaling and root planing; ω‐3, omega‐3 fatty acids.
3.4.2. Meta‐analysis
Table 4 summarizes the detailed outcomes of the MA performed on the primary data of interest. Two studies could not be used for MA because they only presented the median instead of the mean. A random MA was performed for an overall result, and besides that, the studies were separated based on only SRP + ω‐3 fatty acids or SRP + ω‐3 fatty acids combined with ASA.
TABLE 4.
Meta‐analysis for the baseline and end evaluating the adjunctive efficacy of ω‐3 fatty acids during non‐surgical periodontal therapy on PPD (a) and CAL (b)
| (a) PPD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurement moment | Analysis | # Included studies | Model | MD | Test overall | Test for heterogeneity | For details see Appendix | ||
| 95% CI | p value | I 2 value (%) | p‐value | ||||||
| Baseline | Overall | # 9 | Random | 0.01 | (−0.09; 0.11) | .88 | 0% | .57 | S5Ia |
| SRP + ω‐3 | # 7 | Random | 0.05 | (−0.07; 0.15) | .49 | 0% | .63 | S5Ia | |
| SRP + ω‐3 and ASA | # 2 | Fixed | −0.20 | (−0.48; 0.08) | .16 | 0% | 1.00 | S5Ib | |
| End | Overall | # 9 | Random | −0.51 | (−0.85; −0.17) | .003 | 92% | <.00001 | S5Ic |
| SRP + ω‐3 | # 7 | Random | −0.42 | (−0.81; −0.03) | .03 | 93% | <.00001 | S5Ic | |
| SRP + ω‐3 and ASA | # 2 | Fixed | −0.85 | (−1.12; −0.58) | <.00001 | 0% | .72 | S5Id | |
| Difference | Overall | # 5 | Random | −0.39 | (−0.58; −0.21) | .0001 | 78% | <.0003 | S5Ie |
| SRP + ω‐3 | # 4 | Random | −0.32 | (−0.62; −0.01) | .04 | 81% | <.0003 | S5Ie | |
| SRP + ω‐3 and ASA | # 1 | Fixed | NA | NA | NA | NA | NA | S5If | |
| (b) CAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurement moment | Analysis | # included studies | Model | MD | Test overall | Test for heterogeneity | For details see Appendix | ||
| 95% CI | p value | I 2 value (%) | p value | ||||||
| Baseline | Overall | # 8 | Random | 0.08 | (−0.04; 0.20) | .22 | 0% | .52 | S5IIa |
| SRP + ω‐3 | # 6 | Random | 0.12 | (−0.01; 0.25) | .07 | 0% | .73 | S5IIa | |
| SRP + ω‐3 and ASA | # 2 | Fixed | −0.20 | (−0.52; 0.12) | .22 | 0% | 1.00 | S5IIb | |
| End | Overall | # 8 | Random | −0.43 | (−0.74; −0.12) | .006 | 81% | <.00001 | S5IIc |
| SRP + ω‐3 | # 6 | Random | −0.42 | (−0.80; 0.03) | .03 | 83% | <.0001 | S5IIc | |
| SRP + ω‐3 and ASA | # 2 | Fixed | −0.34 | (−0.62; −0.05) | .02 | 84% | .01 | S5IId | |
| Difference | Overall | # 5 | Random | −0.41 | (−0.63; −0.19) | .0002 | 90% | <.00001 | S5IIe |
| SRP + ω‐3 | # 4 | Random | −0.34 | (−0.60; 0.09) | .009 | 91% | <.00001 | S5IIe | |
| SRP + ω‐3 and ASA | # 1 | Fixed | NA | NA | NA | NA | NA | S5IIf | |
Abbreviation: ASA, aspirin; CAL, clinical attachment level; NA, not applicable; MD, mean difference; PPD, probing pocket depth; SRP, scaling and root planing; ω‐3, omega‐3 fatty acids.
Regarding the overall PPD and CAL, there was no significant difference (p < .05) at the baseline point but there was a significant difference (p < .05) at the end. The analysis on the available end data from the intervention of SRP + ω‐3 fatty acids compared to SRP + placebo included nine studies for the PPD and eight for the CAL. For endpoint measurements, the analysis showed a significant MD in favour of SRP + ω‐3 fatty acids for both clinical parameters. PPD: −0.51 (p = .003, 95% CI: [−0.85; −0.17]) (Online appendix S5Ic) CAL: −0.43 (p = .006, 95% CI: [−0.74; −0.12]) (Online appendix S5IIc).
The analysis on the differences supports these findings PPD: −0.39 (p < .001, 95% CI: [−0.58; −0.21]) (Online appendix S5Ie) CAL: −0.41 (p = .002, 95% CI: [−0.63; −0.19]) (Online appendix S5IIe). A subgroup analysis was performed for SRP + ω‐3 fatty acids and ASA compared to SRP + placebo; for details, see Table 4 and online appendix S5.
Unexplained heterogeneity in the MAs was high for end and difference scores, I 2 = 78%–92%. Table 4 shows the data of the forest plots of the MA. Testing for publication bias could not be performed because fewer than 10 studies were included per MA, which would result in insufficient statistical power (Egger et al., 1997, Higgins & Green, 2009).
3.5. Evidence profile
Table 5 shows a summary of the various factors used to rate and assess the certainty for the quality of evidence and strength of recommendations according to GRADE (Guyatt et al., 2008). In magnitude of the effect of using ω‐3 fatty acids as an adjunct to SRP should be considered as “moderate”. The strength of the recommendation, based on the quality and body of evidence regarding the use of ω‐3 fatty acids as an adjunct to SRP, is that there is moderate certainty with a direction in favour of the use of ω‐3 fatty acids for a further reduction of the PPD and an improved CAL. For details concerning grading the body of evidence, see Table 5.
TABLE 5.
Summary of findings table
| Determinants of the quality | Overall | Sub analysis | |
|---|---|---|---|
| SRP + ω‐3 | SRP + ω‐3 and ASA | ||
| Study design | RCT | RCT | RCT |
| # Experiments (n = 10) | 10 | 8 | 2 |
| # Meta‐analysis (n = 10) | 10 | 8 | 2 |
| Risk of bias | Low to high | Low | Low to high |
| Consistency | Consistent | Consistent | Rather consistent |
| Directness | Generalizable | Generalizable | Generalizable |
| Precision | Rather precise | Rather precise | Rather precise |
| Reporting bias | Possible | Possible | Possible |
| Magnitude of the effect (Smiley et al., 2015) | Moderate | Moderate | Moderate |
| Strength of the recommendation based on the quality and body of evidence | Moderate | Moderate | Weak |
| Overall recommendation | If SRP is indicated, the use of ω‐3 fatty acids can be considered for a moderate extra added effect on PPD reduction and CAL gain | ||
| The strength of this recommendation is moderate | |||
Abbreviations: CAL, clinical attachment level; PPD, probing pocket depth; RCT, randomized controlled trial; SRP, surgical periodontal therapy; ω‐3, omega‐3 fatty acids.
4. DISCUSSION
The present systematic review is among the first ones reviewing randomized clinical trials comparing ω‐3 fatty acids on top of standard SRP. It focusses on systemically healthy patients with periodontal disease. It supports ω‐3 fatty acids do provide an additional effect to non‐SRP to be explained by their role to reduce inflammation. Two non‐included studies tested the use of ω‐3 fatty acids as a monotherapy (without SRP) for periodontitis in adults, in which no change was found in the PPD and CAL (Rosenstein et al., 2003; Parulkar et al., 2009). This underlines the critical role of SRP in the management of periodontal disease and suggests that ω‐3 fatty acids as a monotherapy does not significantly improve periodontal parameters.
In a systematic review, Donos et al. (2020) did not reveal a sufficient number of studies suitable for a MA on the additional effect of ω‐3 fatty acids to non‐SRP. Based on their review, the clinical practice guideline of the European Federation of Periodontology on the treatment of stage I–III periodontitis (Sanz et al., 2020) recommended “not to use omega 3 PUFAs as an adjunct to subgingival instrumentation”. The present results suggest a considerable effect of the adjunctive use of ω‐3 fatty acids to standard SRP. This update in the evidence tempts to a revision of the recommendation in the European federation of periodontology (EFP) Guideline.
4.1. Inclusion criteria
Recently, several studies were published on the effect of ω‐3 fatty acids as an adjunct to scaling and root planing in diabetes patients (Elwakeel & Hazaa, 2015, Rampally et al., 2019, Castro dos Santos et al., 2020). The present review did not include studies considering diabetes patients as study population. These studies were excluded to prevent the influence of the bidirectional relationship between diabetes and periodontal disease (Sanz et al., 2018). It is known that the diabetic status has an influence on the periodontal condition of a patient (Chapple & Genco, 2013). This was done to distinguish the direct effect of ω‐3 fatty acid supplementation on periodontal health from the indirect effect through the pathway of diabetic status of a patient. Three recent and previous published systematic reviews (Kruse et al., 2020; Corbella et al., 2021; Chatterjee et al., 2022) in non‐dental journals did not do this. Kruse et al. (2020) included one study on diabetes type 2 patients (Elwakeel & Hazaa, 2015) that showed one of the most outspoken effects on periodontal parameters among the other papers reviewed in that study. The design of the review by Kruse et al. (2020) did not allow for distilling the influence of ω‐3 fatty acids on the diabetic condition as a confounding factor. This supports the decision not to include studies concerning diabetic patients in the present review.
4.2. Heterogeneity
In this MA, considerable heterogeneity was observed for the end scores (I 2 = 81%–93%), while for the baseline scores, heterogeneity, as represented by I 2, was found to be 0% (see Table 4). At baseline, no heterogeneity was observed possibly because groups were well balanced at the start of the studies. The high I 2 observed at the end of the studies is a consequence of clinical or methodological diversity or a combination of both among studies and is to be expected (Higgins & Green, 2012). All of the studies had a parallel design, and none of them reported to have any conflicts of interest. Higgins and Green (2008) states that different studies performed by multiple teams in multiple places are a source of heterogeneity. Following the Cochrane handbook, a random‐effects MA allows for the study outcomes to vary in a normal distribution between studies (Higgins & Green, 2012). For a thorough investigation of heterogeneity, a large data set is needed and this was not the case in this review. Therefore, effect sizes and accompanying confidence intervals should be interpreted with caution.
4.3. Interpretation of the effect size
According to the “Evidence‐based clinical practice guideline on the non‐surgical treatment of periodontitis by means of scaling and root planing with or without adjuncts” (Smiley et al., 2015), the difference in weighted mean for gain in CAL with ω‐3 fatty acids of −0.41 mm (p = .0002) may be interpreted as a “moderate” effect. The weighted mean for the CAL gain with only ω‐3 fatty acids was −0.34 mm, which must be interpreted as a “small” effect. For ω‐3 fatty acids in combination with ASA, it could not be calculated as only one study provided data. However, the −0.41 mm CAL gain is an added effect next to the one of SRP. A systematic review on the clinical efficacy of subgingival debridement (Van der Weijden & Timmerman, 2003) reported a PPD reduction of 0.59–1.18 mm and 0.74 mm CAL gain. This means that the range for periodontal parameters to improve with additional treatments is limited. With this reference frame in mind, the additional effect (0.39 mm more pocket reduction and 0.41 mm more CAL gain than only SRP) of ω‐3 fatty acids are a considerable magnitude.
4.4. Dosage
From the studies that were reviewed, adjunctive use of ω‐3 fatty acids in patients with periodontal disease appears to be effective for periodontal parameters of inflammation. The dosage required to treat periodontitis is not specified. The reviewed studies that had a dosage of approximately 2000 mg/day of EPA and/or DHA suggested that dietary supplementation is sufficient to control inflammatory processes (El‐Sharkawy et al., 2010; Elkhouli, 2011; Salman et al., 2014; Elgendy & Kazem, 2018; Stańdo et al., 2020). The three studies that did not find improved clinical outcomes all used a daily dose lower than 2000 mg ω‐3 fatty acids. One of the studies that had failed (Keskiner et al., 2017) treated the patients with 6.25 mg of EPA and 19.19 mg of DHA per day, which is lower than the daily‐recommended dose of 250–500 mg of EPA and/or DHA for healthy adults (European Food Safety Authority, 2012). One thousand milligrams of ω‐3 fatty acids supply around 300 mg of EPA and/or DHA. In studies reporting that fish oil has therapeutic effects and preventive aspects on multiple chronic diseases, such as cardiovascular diseases, metabolic syndrome, and type II diabetes, a daily dose between 2 and 6 g of ω‐3 fatty acids a day was used (Kris‐Etherton et al., 2005; Calder, 2015; Gao et al., 2017; Mori, 2017). This suggests that the recommended daily dose for healthy patients may not be sufficient for a therapeutic effect. It can be inferred from the present MA that a dosage of 2000 mg of ω‐3 fatty acids a day may improve clinical outcomes in comparison with SRP alone, the dose which is in line with the therapeutic dose in chronic diseases.
4.5. Safety and tolerability of ω‐3 fatty acids
A limited storage capacity of polyunsaturated fatty acids in adipose tissue implies that a regular consumption of these fats is needed (Arterburn et al., 2006). The American Heart Association has suggested that a dose of 2–4 g/day of EPA plus DHA is generally regarded as safe (2018). Long‐term supplemental intakes of EPA and DHA that combined doses up to about 5 g/day appear to be safe (European Food Safety Authority, 2012). In the literature, potential side effects are mentioned: non‐serious events and mild laboratory abnormalities like “fishy taste”, bad breath, and gastrointestinal discomfort (Ägren et al., 1997; Rahman et al., 2009). The “Institute of Medicine” also reported that doses 2–15 g/day EPA and/or DHA might increase bleeding time, but it is noted that these doses have not been shown to cause bleeding problems (Bays, 2007). Current studies reported that high‐dose ω‐3 fatty acids have no effect on platelet aggregation or coagulation (Bagge et al., 2018). To improve or maintain the overall health, ω‐3 fatty acids are one of the most consumed dietary supplements (Bailey et al., 2013; Dickinson et al., 2014).
4.6. Collaboration of ω‐3 fatty acids with ASA
The anti‐inflammatory impact of ω‐3 fatty acids appear to lie in the production lipoxins (endogenous anti‐inflammatory agents) from arachidonic acid by lipoxygenase transformation circuits (Mittal et al., 2010). These endogenous resolution pathways are enhanced by the action of ASA on COX‐2. The metabolism of EPA and DHA into resolvins of the E and D variants, respectively, is enhanced by ASA transformation circuits (Dufresne et al., 2013). A reduced cellular inflammation is caused by resolvins, inhibiting the production and transportation of inflammatory cells and chemicals to the sites of inflammation (Dalli et al., 2013). A recent study (Naqvi et al., 2014) where the treatment group received ω‐3 fatty acids and the control group received placebo with ASA shows the efficacy of ω‐3 fatty acids in combination with ASA but not of ASA alone on PPD reduction and CAL gain.
In this present review, the two studies that used ω‐3 fatty acids combined with ASA (El‐Sharkawy et al., 2010; Elkhouli, 2011) tend to show more effect on the PPD reduction and the CAL gain than the studies using only ω‐3 fatty acids. However, these two particular studies also used a higher daily dose of ω‐3 fatty acids (3000 mg) than the studies not using adjunctive ASA. This may influence the results when comparing them to those with only SRP + ω‐3 fatty acids. Studies that used adjunctive ASA had a less‐balanced baseline, which was in favour of the treatment group. Therefore, the present study cannot be conclusive about the synergetic effect of ASA with ω‐3 fatty acids on periodontal health.
4.7. Oxidative stress
Nowadays, more attention is being paid on the role of free radicals and ROS to the progression of many inflammatory diseases like periodontitis (Wang et al., 2017). Free radicals and ROS are produced as superoxide ions by neutrophils at the site of infection. Free radicals are self‐reliant and contain one or more unpaired electrons (Halliwell & Gutteridge, 1995). They are highly reactive, and diverse species have the capacity of extracting electrons and oxidizing a variety of biomolecules that are essential to cell and tissue functions (Mittal et al., 2014). ROS can include oxygen‐free radicals, nitrogen, and chlorine species. In a healthy situation, ROS are neutralized by antioxidants. When inflammation occurs, ROS production is drastically increased (Mittal et al., 2014). This leads to oxidative stress and tissue damage because sequent high levels of ROS cannot be balanced by the antioxidant defence system (Sies, 1997). An increased level of free radicals, high levels of oxidative stress, and a decreased level of antioxidants are seen in patients with diabetes and post‐menopausal women (Maritim et al., 2003; Sejal & Agarwal, 2013). Several authors (Mori et al., 1999; Calder, 2005; Nälsén et al., 2006; Kiecolt‐Glaser et al., 2011) studied the effect of EPA and DHA on oxidative stress and found that EPA and DHA can reduce inflammation and decrease oxidative stress. In one of the included studies in this systematic review, the authors observed the effect of ω‐3 fatty acids on post‐menopausal women (Elgendy & Kazem, 2018) and found strongly statistically significant improved periodontal parameters. This finding suggests that more often, there may be a need for supplementation in patients with higher oxidative stress levels, like post‐menopausal women, but further research is needed to support this.
4.8. Comparison with other adjunctive treatments
The use of other adjuncts to mechanical periodontal treatment has also frequently been studied. For example, a systematic review with MA on the use of amoxicillin/metronidazole as an adjunct to SRP reported a significant CAL gain (MD = 0.21; 95% CI = 0.02–0.4) and PPD reduction (MD= 0.43; 95% CI = 0.24–0.63) (Sgolastra et al., 2012). Zandbergen et al. (2016) support these findings. The subgingival application of antimicrobials, as an adjunctive therapy to periodontal treatment, showed a significant improvement in PPD reduction and CAL gain, with a MD of 0.407 and 0.310 (Matesans‐Pérez et al., 2013). The effect of surgical debridement gives 0.6 mm more pocket reduction and 0.2 mm more CAL gain compared to non‐surgical debridement (Heitz‐Mayfield et al., 2002). Comparing these findings to the present MA, the amount of CAL gain and PPD reduction with the adjunct use of ω‐3 fatty acids was in the range with the adjunct use of antimicrobials or surgical debridement. This may imply that ω‐3 fatty acids are part of the next generation of adjuvant therapies to manage periodontitis, and it supports the used terminology in this review of “moderate effect” by Smiley et al. (2015).
4.9. Limitations of the study
In the MA of this systematic review, a high heterogeneity (>80%) was found for the studies that evaluated the effect of ω‐3 fatty acids. Higgins et al. (2003) and Sedgwick (2015) suggested that in a systematic review that includes studies that are clinically and methodologically diverse, heterogeneity in their results can be expected. Unfortunately, it was currently not possible to perform a MA with individual patient data; this would be possible if open data were available. Because of the unexplained high heterogeneity, the effect sizes and accompanying confidence intervals should be interpreted with caution. Nevertheless, given the clear direction of the observed positive effects of using ω‐3 fatty acids, one can follow the direction of results presented.
The present study documents an improvement of 0.39 mm of PPD reduction and 0.41 mm CAL gain after a dosage (≥2 g/day) of ω‐3 fatty acids as an adjunct to non‐surgical periodontal treatment. It is quite possible that a combination of ω‐3 fatty acids with low‐dose ASA might result in similar or better benefit. Further study will be required to specifically address the clinical relevance of ω‐3 fatty acids combined with ASA, compared to ω‐3 fatty acids alone. In combination with the theoretical background, the present study with encouraging clinical findings and safety profiles provides evidence for the application of adjunctive complementary supplements in the treatment of patients with periodontitis. Larger clinical trials with detailed ω‐3 fatty acid use are awaited with curiosity and great interest.
5. CONCLUSIONS
The collective evidence emerging from this systematic review and MA shows a positive effect of the use of ω‐3 fatty acids (potentially combined with ASA) as an adjunct to non‐SRP. In patients with periodontitis, dietary supplementation with ω‐3 fatty acids as an adjunct to SRP is more effective in reducing the PPD and improving the CAL than SRP alone. If SRP is indicated, the use of ω‐3 fatty acids can be considered for a moderate extra added effect on PPD reduction and CAL gain. The strength of this recommendation is moderate. The direction of the recommendation emerging from this review is that supplementation with ω‐3 fatty acids could be an effective adjunctive therapy for an additional effect in the management of periodontal treatment.
AUTHOR CONTRIBUTIONS
All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Myrlon M. Van Ravensteijn contributed to design, search and selection, and analysis and interpretation and drafted the manuscript. Mark F. Timmerman contributed to conception and design, and analysis and interpretation and critically revised the manuscript. Ester A. G. Brouwer contributed to analysis and interpretation and critically revised the manuscript. Dagmar E. Slot contributed to conception and design, search and selection, and analysis and interpretation and critically revised the manuscript.
FUNDING INFORMATION
The work for this study was funded by the regular academic appointments of Timmerman, Brouwer at the Radboud University, and Slot at the Academic Centre for Dentistry Amsterdam (ACTA). This paper was prepared as a part of the obligation of the first author Ravensteijn to fulfil the requirements of the Raboud University master's programme in Dentistry.
CONFLICT OF INTEREST AND SOURCE OF FUNDING STATEMENT
The authors declare that they have no conflicts of interest. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. The work for this paper was funded by the regular academic appointments of Timmerman, Brouwer at the Radboud University and Slot at the Academic Centre for Dentistry Amsterdam (ACTA). This paper was prepared as a part of the obligation of the first author Ravensteijn to fulfill the requirements of the Raboud University masters's program in Dentistry.
ETHICS STATEMENT
The protocol is registered by PROSPERO by number CRD42022136457.
Supporting information
Appendix S1 Methodological quality and potential risk of bias scores of the individual studies that were included for this review.
Appendix S2. Papers excluded after full‐text reading with details for rejection.
Appendix S3. Details with respect to heterogeneity of the studies that were included.
Appendix S4. Mean (SD) scores for the different intervention groups with various indices and their modifications. Within‐group analyses are presented. (A) Probing pocket depth (PPD). (B) Clinical attachment level (CAL).
Appendix S5. Forrest Plots of the meta‐analysis for the primary parameters of interest. Presented for the baseline, end, and difference scores. Presented for the use of a fixed model and random when appropriate. (A) Probing pocket depth (PPD). (B) Clinical attachment level (CAL).
ACKNOWLEDGEMENTS
The authors acknowledge the support of the following authors for their response, time, and effort to supply additional data: Dr Figueredo for the Martinez et al. (2014) study, Dr El‐Sharkawy, Dr Keskiner, Dr Umrania, Dr Elgendy, and Dr Kujur. This study was prepared as a part of the obligation of the first author to fulfil the requirements of the Radboud University Master in Dentistry.
Van Ravensteijn, M. M. , Timmerman, M. F. , Brouwer, E. A. G. , & Slot, D. E. (2022). The effect of omega‐3 fatty acids on active periodontal therapy: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 49(10), 1024–1037. 10.1111/jcpe.13680
Funding information Slot at the Academic Centre for Dentistry Amsterdam (ACTA); Radboud University
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- Ägren, J. J. , Väisänen, S. , Hänninen, O. , Muller, A. , & Hornstra, G. (1997). Hemostatic factors and platelet aggregation after a fish‐enriched diet or fish oil or docasahexaenoic acid supplementation. Prostaglandins Leukotriens Essential Fatty Acids, 57, 419–421. [DOI] [PubMed] [Google Scholar]
- Arterburn, L. M. , Hall, E. B. , & Oken, H. (2006). Distribution, interconversion, and dose response of n‐3 fatty acids in human. American Journal of Clinical Nutrition., 83, 1467–1476. [DOI] [PubMed] [Google Scholar]
- Bagge, A. , Schött, U. , & Kander, T. (2018). High‐dose omega‐3 fatty acids have no effect on platelet aggregation or coagulation measured with static and flow‐based aggregation instruments and Sonoclot; an observational study in healthy volunteers. Scandinavian Journal of Clinical and Laboratory Investigation., 78, 539–545. [DOI] [PubMed] [Google Scholar]
- Bailey, R. L. , Gahche, J. J. , Miller, P. E. , Thomas, P. R. , & Dwyer, J. T. (2013). Why US adults use dietary supplements. JAMA Internal Medicine, 173, 355–361. [DOI] [PubMed] [Google Scholar]
- Bannenberg, G. , & Serhan, C. N. (2010). Specialized pro‐resolving lipid mediators in the inflammatory response: An update. Biochimica et Biophysica Acta, 12, 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays, H. E. (2007). Safety considerations with omega‐3 fatty acid therapy. American Journal of Cardiology, 99, S35–S43. [DOI] [PubMed] [Google Scholar]
- Calder, P. C. (2005). Polyunsaturated fatty acids an inflammation. Biochemical Society Transactions, 33, 423–427. [DOI] [PubMed] [Google Scholar]
- Calder, P. C. (2006). N‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition, 83, 1505–1519. [DOI] [PubMed] [Google Scholar]
- Calder, P. C. (2009). Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie, 91, 791–795. [DOI] [PubMed] [Google Scholar]
- Calder, P. C. (2015). Functional roles of fatty acids and their effects on human health. Journal of Parenteral and Enteral Nutrition, 39(1 Suppl), 18S–32S. [DOI] [PubMed] [Google Scholar]
- Castro Dos Santos, N. C. , Furukawa, M. V. , Oliveira‐Cardoso, I. , Cortelli, J. R. , Feres, M. , Van Dyke, T. , & Rovai, E. S. (2022). Does the use of omega‐3 fatty acids as an adjunct to non‐surgical periodontal therapy provide additional benefits in the treatment of periodontitis? A systematic review and meta‐analysis. Journal of Periodontal Research, 57, 435–447. [DOI] [PubMed] [Google Scholar]
- Castro Dos Santos, N. C., Andere, N. M. R. B., Araujo, C. F., de Marco, A. C., Kantarci, A., Van Dyke, T. E., & Santamaria, M. P. (2020). Omega‐3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus: Randomized clinical trial. Journal of Periodontology, 91(10), 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, D. , Chatterjee, A. , Kalra, D. , Kapoor, A. , Vijay, S. , & Jain, S. (2022). Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta‐analysis. Journal of Oral Biology and Craniofacial Research, 12(1), 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple, I. L., & Genco, R; Working group 2 of joint EFP/AAP workshop (2013). Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Clinical Periodontology, 40, S106–S112. [DOI] [PubMed] [Google Scholar]
- Corbella, S. , Calciolari, E. , Alberti, A. , Donos, N. , & Francetti, L. (2021). Systematic review and meta‐analysis on the adjunctive use of host immune modulators in non‐surgical periodontal treatment in healthy and systemically compromised patients. Scientific Reports, 11(1), 12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli, J. , Winkler, J. W. , Colas, R. A. , Arnardottir, H. , Cheng, C. Y. , Chiang, N. , Petasis, N. A. , & Serhan, C. N. (2013). Resolvin D3 and aspirin‐triggered resolvin D3 are potent immunoresolvents. Chemistry & Biology, 20, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deore, G. D. , Gurav, A. N. , Patil, R. , Shete, A. R. , NaikTari, R. S. , & Inamdar, S. P. (2014). Omega 3 fatty acids as a host modulator in chronic periodontitis patients. A randomized, double‐blind palcebo‐controlled clinical trial. Journal of Periodontics and Implant Science., 44, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, A. B. , El‐Dash, N. , & Franco, J. C. (2014). Consumer usage and reasons for using dietary supplements: Report of a series of surveys. Journal of the American College Nutrition, 33, 176–182. [DOI] [PubMed] [Google Scholar]
- Donos, N. , Calciolari, E. , Brusselaers, N. , Goldoni, M. , Bostanci, N. , & Belibasakis, G. N. (2020). The adjunctive use of host modulators in non‐surgical periodontal therapy. A systematic review of randomized, placebo‐controlled clinical studies. Journal of Clinical Periodontology, 47(Suppl 22), 199–238. [DOI] [PubMed] [Google Scholar]
- Dufresne, M. , Dumas, G. , Asselin, E. , Carrier, C. , Pouliot, M. , & Reyes‐Moreno, C. (2013). Pro‐inflammatory type‐1 and anti‐inflammatory type‐2 macrophages differentially modulate cell survival and invasion of human bladder carcinoma T24 cells. Molecular Immunology., 48, 1556–1567. [DOI] [PubMed] [Google Scholar]
- Elwakeel, N. M., & Hazaa, H. H. (2015). Effect of omega 3 fatty acids plus low‐dose aspirin on both clinical and biochemical profiles of patients with chronic periodontitis and type 2 diabetes: a randomized double blind placebo‐controlled study. Journal of Periodontal Research, 50(6), 721–729. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products NaA . (2012). Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Journal, 10, 2815. [Google Scholar]
- Egger, M. , Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal, 13, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgendy, E. A. , & Kazem, H. H. (2018). Effect of omega‐3 fatty acids on chronic periodontitis patients in postmenopausal women: A randomized controlled clinical study. Oral Health and Preventive Dentistry, 4, 327–332. [DOI] [PubMed] [Google Scholar]
- Elkhouli, A. M. (2011). The efficacy of host response modulation therapy (omega‐3 plus low‐dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study). Journal of Periodontal Research, 46, 261–268. [DOI] [PubMed] [Google Scholar]
- El‐Sharkawy, H. , Aboelsaad, N. , Eliwa, M. , Darweesh, M. , Alshahat, M. , Kantarci, A. , Hasturk, H. , & Van Dyke, T. E. (2010). Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega‐3 fatty acids and low‐dose aspirin. Journal of Clinical Periodontology, 81, 1635–1643. [DOI] [PubMed] [Google Scholar]
- Gao, H. , Geng, T. , Huang, T. , & Zhao, Q. (2017). Fish oil supplementation and insulin sensitivity: A systematic review and meta‐analysis. Lipids Health Disorders, 16, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt, C. H. , Oxman, A. D. , Kunz, R. , Jaeschkle, R. , Helfand, M. , Liberati, A. , Schünemann, H. J. , & GRADE Working Group . (2008). Incorporating considerations of resources use into grading recommendations. British Medical Journal, 7654, 1170–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell, B. , & Gutteridge, J. M. (1995). The definition and measurement of antioxidants in biological systems. Free Radical Biology and Medicin, 1, 125–126. [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. , Trombelli, L. , Heitz, F. , Needleman, I. , & Moles, D. (2002). A systematic review of the effect of surgical debridement vs non‐surgical debridement for the treatment of chronic periodontitis. Journal of Clinical Periodontology., 29, 92–102. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Green, S. (2008). CCHB Cochrane handbook for systematic reviews of interventions. Cochrane Community (beta). http://community.cochrane.org.ru.idm.oclc.org/handbook.
- Higgins, J. P. T. , & Green, S. (2009). CCHB Cochrane handbook for systematic reviews of interventions. Cochrane Community (beta). http://community.cochrane.org.ru.idm.oclc.org/handbook.
- Higgins, J. P. T. , & Green, S. (2012). CCHB Cochrane handbook for systematic reviews of interventions. http://cochrane-handbook.org/.
- Keskiner, I. , Saygun, I. , Bal, V. , Serdar, M. , & Kantarci, A. (2017). Dietary supplementation with low‐dose omega‐3 fatty acids reduces salivary tumor necrosis factor‐a levels in patients with chronic periodontitis: A randomized controlled clinical study. Journal of Periodontal Research, 52, 695–703. [DOI] [PubMed] [Google Scholar]
- Kiecolt‐Glaser, J. K. , Epel, E. S. , Belury, M. A. , Andridge, R. , Lin, J. , Glaser, R. , Malarkey, W. B. , Hwang, B. S. , & Blackburn, E. (2011). Omega‐3 fatty acids, oxidative stress, and leukocyte telomere length: Randomized controlled trial. Brain, Behavior, and Immunity, 28, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman, K. S. (1999). Host modulation as a therapeutic strategy in the treatment of periodontal disease. Clinical Infectious Disease, 28, 520–526. [DOI] [PubMed] [Google Scholar]
- Kris‐Etherton, P. M. , Harris, W. S. , Appel, L. J. , & Committee, N. (2005). Fish consumption, fish oil, omega‐3 fatty acids, and cardiovascular disease. Arteriosclerosis Thrombosis Vascular Biology, 23, 20–30. [DOI] [PubMed] [Google Scholar]
- Kruse, A. B. , Kowalski, C. D. , Leuthold Vach, K. , Ratka‐Krüger, P. , & Woelber, J. P. (2020). What is the impact of the adjunctive use of omega‐3 fatty acids in the treatment of periodontitis? A systematic review and meta‐analysis. Lipids Health and Disease, 19, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujur, S. K. , Goswami, V. , Nikunj, A. M. , Singh, G. , Bandhe, S. , & Ghritlahre, H. (2020). Efficacy of omega 3 fatty acid as an adjunct in the management of chronic periodontitis: A randomized controlled trial. Indian Journal of Dental Research, 31, 229–235. [DOI] [PubMed] [Google Scholar]
- Maritim, A. C. , Sanders, R. A. , & Watkins, J. B. (2003). Diabetes, oxidative stress, antioxidants: A review. Journal of Biochemistry & Moleculair Toxicology, 17, 24–38. [DOI] [PubMed] [Google Scholar]
- Martinez, G. L. , Koury, J. C. , Brito, F. , Fischer, R. G. , Gustafsson, A. , & Figueredo, C. M. (2013). The impact of non‐surgical periodontal treatment on serum levels of long chain‐polyunsaturated fatty acids: A pilot randomized clinical trial. Journal of Periodontal Research, 49, 268–274. [DOI] [PubMed] [Google Scholar]
- Martinez, G. S. , Koury, J. C. , Martins, M. A. , Nogueira, F. , Fischer, R. G. , Gustafsson, A. , & Figueredo, C. M. (2014). Serum level changes of long chain‐polyunsaturated fatty acids in patients undergoing periodontal therapy combined with one year of omega‐3 supplementation: A pilot randomized clinical trial. Journal of Periodontal Implant Science, 44, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matesans‐Pérez, P. , Garcia‐Gargallo, M. , Figuero, E. , Bascones‐Martinez, A. , Sanz, M. , & Herrera, D. (2013). A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of periodontitis. Journal of Clinical Periodontology, 40, 227–241. [DOI] [PubMed] [Google Scholar]
- Mittal, A. , Ranganath, V. , & Nichania, A. (2010). Omega fatty acids and resolution of inflammation: A new twist in an old tale. Journal of Indian Society of Periodontology, 14, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, M. , Siddiqui, M. R. , Tran, K. , Reddy, S. P. , & Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling, 7, 1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & The PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, T. A. (2017). Marine omega‐3 fatty acids in the prevention of cardiovascular disease. Fitoterapia, 123, 51–58. [DOI] [PubMed] [Google Scholar]
- Mori, T. A. , Bao, D. Q. , Burke, V. , Puddey, I. B. , & Beilin, L. J. (1999). Docosahexaenoic acid but not eicosapentaenoic acid lowers blood pressure and heart rate in humans. Hypertension, 34, 253–260. [DOI] [PubMed] [Google Scholar]
- Nälsén, C. , Vessby, B. , Berglund, L. , Uusitupa, M. , Hermansen, K. , Riccardi, G. , Rivellese, A. , Storlien, L. , Erkkilä, A. , Ylä‐Herttuala, S. , Tapsell, L. , & Basu, S. (2006). Dietary (n‐3) fatty acids reduce plasma F2‐isoprostanes but not prostaglandin F2alpha in healthy humans. Journal of Nutrition, 136, 1222–1228. [DOI] [PubMed] [Google Scholar]
- Naqvi, A. Z. , Hasturk, H. , Mu, L. , Philips, R. S. , Davis, R. B. , Halem, S. , Campos, H. , Goodson, J. M. , Van Dyke, T. E. , & Mukamal, K. J. (2014). Docosahexaenoic acid and periodontitis in adults: A randomized controlled trial. Journal of Dental Research, 93, 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher, S. (1996). Periodontal diseases: Pathogenesis. Annals of Periodontology, 1, 821–878. [DOI] [PubMed] [Google Scholar]
- Parulkar, M. , Dawson, D. , Kryscio, R. , Novak, M. J. , Ebersole, J. L. , & Boissonneault, G. B. (2009). Lack of effect of omega‐3 fatty acids [PUFA] dietary supplement on clinical measures of periodontitis in humans. The FASEB Journal, 23, 454. [Google Scholar]
- Rahman, M. M. , Bhattacharya, A. , & Fernandes, G. (2009). Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. Journal of Cell Physiology, 214, 201–209. [DOI] [PubMed] [Google Scholar]
- Rampally, P., Koduganti, R. R., Ganapathi, S. N., Panthula, V. R., & Surya, P. J. (2019). Comparison of effectiveness of low‐dose aspirin versus omega‐3 fatty acids as adjuvants to nonsurgical periodontal therapy in Type II diabetic patients with chronic periodontitis. Journal of Indian Society of Periodontology, 23(3), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein, E. D. , Kushner, L. J. , Kramer, N. , & Kazandijan, G. (2003). Pilot study of dietary fatty acid supplementation in the treatment of adult periodontitis. Prostaglandins Leukotrienes and Essential Fatty Acids, 68, 213–218. [DOI] [PubMed] [Google Scholar]
- Ryan, R. (2016). Cochrane Consumers and Communication Review Group. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Review Group reviews: planning the analysis at protocol stage http://cccrg.cochrane.org.ru.idm.oclc.org.
- Salman, S. A. , Akram, H. M. , & Ali, O. H. (2014). Omega‐3 as an adjunctive to non surgical treatment of chronic periodontitis patients. ISOR journal of Dental and Medical Sciences, 13, 8–11. [Google Scholar]
- Salvi, G. E. , & Lang, N. P. (2005). Host response modulation in the management of periodontal diseases. Journal of Clinical Periodontology, 32, 108–129. [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Ceriello, A. , Buysschaert, M. , Chapple, I. , Demmer, R. T. , Graziani, F. , Herrera, D. , Jepsen, S. , Lione, L. , Madianos, P. , Mathur, M. , Montanya, E. , Shapira, L. , Tonetti, M. , & Vegh, D. (2018). Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the international diabetes federation and the European Federation of Periodontology. Journal of Clinical Periodontology, 45(2), 138–149. [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Herrera, D. , Kebschull, M. , Chapple, I. , Jepsen, S. , Beglundh, T. , Sculean, A. , Tonetti, M. S. , & EFP Workshop Participants and Methodological Consultants . (2020). Treatment of stage I‐III periodontitis‐the EFP S3 level clinical practice guideline. Journal of Clinical Periodontology, 47(Suppl 22), 4–60 (Erratum in: J Clin Periodontol. 2021 Jan;48(1):163). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick, P. (2015). Meta‐analyses: What is heterogeneity? British Medical Journal, 350, 350–435. [DOI] [PubMed] [Google Scholar]
- Sejal, B. D. , & Agarwal, A. (2013). The role of oxidative stress in menopause. Journal of Midlife Health, 4, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan, C. N. , Chiang, N. , & Van Dyke, T. E. (2008). Resolving inflammation: Dual anti‐inflammatory and pro‐resolution lipid mediators. Nature Review Immunology, 8, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgolastra, F. , Gatto, R. , Petrucci, A. , & Monaco, A. (2012). Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: A systematic review and meta‐analysis. Journal of Periodontology, 83, 1257–1269. [DOI] [PubMed] [Google Scholar]
- Sies, H. (1997). Oxidative stress: Oxidants and antioxidants. Experimental Physiology, 82, 219–295. [DOI] [PubMed] [Google Scholar]
- Simopoulos, A. P. (2008). The importance of the omega‐6/omega‐3 fatty acid ratio in cardiovascular disease and other chronic diseases. Experimental Biology and Medicine, 233, 674–688. [DOI] [PubMed] [Google Scholar]
- Smiley, C. J. , Tracy, S. L. , Abt, E. , Michalowicz, B. S. , John, M. T. , Gunsolley, J. , Cobb, C. M. , Rossman, J. , Harrel, S. K. , Forrest, J. L. , Hujoel, P. P. , Noraian, K. W. , Greenwell, H. , Frantsve‐Hawley, J. , Estrich, C. , & Hujoel, P. P. (2015). Evidence‐based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. The Journal of the American Dental Association, 146, 525–535. [DOI] [PubMed] [Google Scholar]
- Stańdo, M. , Piatek, P. , Namiecinska, M. , Lewkowicz, P. , & Lewkowicz, N. (2020). Omega‐3 polyunsaturated fatty acids EPA and DHA as an adjunct to non‐surgical treatment of periodontitis: A randomized clinical trial. Nutrients, 12(9), 2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaboration. (2020). RevMan 5 whenever its output is used: Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews
- Umrania, V. V. , Deepika, P. C. R. , & Kulkarni, M. (2017). Evaluation of dietary supplementation of omega‐3 polyunsaturated fatty acids as an adjunct to scaling and root planning on salivary interleukin‐1B levels in patients with chronic periodontitis: A clinico‐immunological study. Journal of Indian Society Periodontology, 21, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Weijden, F. , Dell'Acqua, F. , & Slot, D. E. (2009). Alveolar bone dimensional changes of post‐extraction sockets in humans: A systematic review. Journal of Clinical Periodontology, 36, 1048–1058. [DOI] [PubMed] [Google Scholar]
- Van der Weijden, G. A. , & Timmerman, M. F. (2003). A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. Journal of Clinical Periodontology, 29, 55–71. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Andrukhov, O. , & Rausch‐Fan, X. (2017). Oxidative stress and antioxidant system in periodontitis. Frontiers of Physiology, 8, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, R. C. (1990). Periodontal disease. The New England Journal of Medicine, 322, 373–382. [DOI] [PubMed] [Google Scholar]
- Zandbergen, D. , Slot, D. E. , Niederman, R. , & Van der Weijden, F. A. (2016). The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planing alone in treating periodontitis: A systematic review. BMC Oral Health, 29(16), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Methodological quality and potential risk of bias scores of the individual studies that were included for this review.
Appendix S2. Papers excluded after full‐text reading with details for rejection.
Appendix S3. Details with respect to heterogeneity of the studies that were included.
Appendix S4. Mean (SD) scores for the different intervention groups with various indices and their modifications. Within‐group analyses are presented. (A) Probing pocket depth (PPD). (B) Clinical attachment level (CAL).
Appendix S5. Forrest Plots of the meta‐analysis for the primary parameters of interest. Presented for the baseline, end, and difference scores. Presented for the use of a fixed model and random when appropriate. (A) Probing pocket depth (PPD). (B) Clinical attachment level (CAL).
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


