Abstract
Objective
Intermetatarsal bursae in the forefeet possess a synovial lining similar to joints and tendon sheaths. Inflammation of these bursae (intermetatarsal bursitis [IMB]) was recently identified as specific for early rheumatoid arthritis (RA). The present study was undertaken to determine if IMB is indeed an RA feature by assessing the following: 1) the association with other local inflammatory measures (synovitis, tenosynovitis, and osteitis), 2) the association with clinical signs, and 3) whether it responds to disease‐modifying antirheumatic drug (DMARD) therapy similarly to other local inflammatory measures.
Methods
One hundred fifty‐seven consecutive early RA patients underwent unilateral contrast‐enhanced 1.5T forefoot magnetic resonance imaging (MRI) at diagnosis. MRIs were evaluated for IMB presence and for synovitis, tenosynovitis, and osteitis in line with the RA MRI Scoring (RAMRIS) system (summed as RAMRIS inflammation). MRIs at 4, 12, and 24 months were evaluated for IMB presence and size in patients who had IMB at baseline and received early DMARD therapy. Logistic regression and generalized estimating equations were used. Anti–citrullinated protein antibody (ACPA) stratification was performed.
Results
Sixty‐nine percent of RA patients had ≥1 IMB. In multivariable analysis on bursa level, presence of IMB was independently associated with local presence of synovitis and tenosynovitis, with odds ratios (OR) of 1.69 (95% confidence interval [95% CI] 1.12, 2.57) and 2.83 (95% CI 1.80, 4.44), respectively, but not osteitis. On the patient level, IMB presence was most strongly associated with tenosynovitis (OR 2.92 [95% CI 1.62, 5.24]). IMB presence was associated with local joint swelling (OR 2.7 [95% CI 1.3, 5.3]) and tenderness (OR 1.7 [95% CI 1.04, 2.9]) independent of RAMRIS inflammation. During treatment, IMB size decreased between 0 and 12 months. This decrease associated with decrease in RAMRIS inflammation, which was driven by synovitis decrease. Within ACPA‐positive and ACPA‐negative RA, similar results were obtained.
Conclusion
IMB particularly accompanies inflammation of the synovial lining of joints and tendon sheaths, showed a similar treatment response after DMARD initiation, and associates with typical clinical signs. These findings suggest that IMB represents a frequently present novel RA feature of juxtaarticular synovial inflammation.
INTRODUCTION
Rheumatoid arthritis (RA)–related local inflammation in the hands and forefeet can be reliably and sensitively assessed using magnetic resonance imaging (MRI) (1, 2), which is recommended by the European Alliance of Associations for Rheumatology for early detection of RA (3). Three features of local inflammation are assessed according to the conventionally used RA MRI Scoring (RAMRIS) system: synovitis, tenosynovitis, and osteitis (4). Although RA is traditionally known for targeting the synovial lining of (small) joints (synovitis), MRI studies have shown that juxtaarticular synovial inflammation in the form of tenosynovitis is typical for the disease as well; tenosynovitis at the small joints represents inflammation of the synovial lining of tendon sheaths, is specific for early RA, and contributes to RA‐specific symptoms (5, 6, 7, 8).
SIGNIFICANCE & INNOVATIONS.
Inflammation of the synovium‐lined intermetatarsal bursae (intermetatarsal bursitis [IMB]) is frequently present at diagnosis of rheumatoid arthritis (RA) (69%), both in anti–citrullinated protein antibody (ACPA)–positive (75%) and ACPA‐negative (64%) patients, and associates with local joint tenderness and swelling.
IMB also associates with known RA‐related magnetic resonance imaging (MRI) inflammation (synovitis and tenosynovitis).
After initiation of disease‐modifying antirheumatic drugs, IMB decreases in a fashion similar to known RA‐related MRI inflammation and disease activity (according to the Disease Activity Score), suggesting a treatment response.
These findings imply that IMB is indeed a novel juxtaarticular inflammatory feature of RA.
Forefoot involvement is frequent in RA and an important cause of symptoms and disability (9). Specifically in the forefeet, in addition to synovial joints and tendon sheaths, another distinct tissue with a synovial lining but without connection to the metatarsophalangeal (MTP) joints is present and may become inflamed: the intermetatarsal bursae (10, 11, 12, 13). MRI‐detected intermetatarsal bursitis (IMB) was recently identified as highly specific for early RA and to be less frequent in healthy controls and non‐RA arthritides (14). Although IMB has been described in established RA and is associated with foot‐related disability (15, 16), its role in early disease has barely been explored. Two studies have thus far reported a prevalence of IMB in early RA of 63% and 69% (14, 17). One of these studies also showed that IMB associates with RA independently from clinical factors (age, sex, and body mass index) (14). Bursae have a function in reducing mechanical strain and friction. Mechanical strain (e.g., due to deformities or altered mechanical loading) is suggested to be involved in bursitis development, but reports on its role in IMB development in early RA are contradictory (18, 19, 20). In short, there is some evidence suggesting that IMB is a feature of early RA, but scientific data are scarce.
Because RA is the most common inflammatory disease in the field of rheumatology and foot symptoms are common in individuals with RA, we believe it is essential to understand the pathophysiology of forefoot symptoms. The forefeet undergo mechanical loading during walking. In recent‐onset RA, mechanical loading may possibly influence forefeet inflammation and/or aggravate forefeet symptoms. As such, IMB can be part of inflammation that relates to symptoms. We hypothesized that if IMB is indeed a feature of early RA, it should associate with known MRI inflammation measured by the RAMRIS (synovitis, tenosynovitis, and osteitis), as well as with typical signs related to RA (joint tenderness and swelling) at diagnosis. To determine this, we performed a large cross‐sectional MRI study. Finally, we hypothesized that IMB should also respond to treatment with disease‐modifying antirheumatic drugs (DMARDs), analogous to MRI inflammation measured by the RAMRIS (21, 22). Follow‐up MRIs were evaluated to study this.
PATIENTS AND METHODS
Patients
The Leiden Early Arthritis Clinic (EAC) is an inception cohort based in the Leiden University Medical Centre (LUMC) in The Netherlands and has been enrolling patients with clinically apparent arthritis of recent onset (symptom duration <2 years) since 1993. Its design has been described previously (23). At baseline, tender and swollen joint counts were conducted, Disease Activity Score (DAS) was assessed, and blood samples were taken to measure C‐reactive protein level, erythrocyte sedimentation rate, IgG anti–citrullinated protein antibodies (ACPAs), and IgM rheumatoid factor (24). Follow‐up visits were scheduled at 4 months, 12 months, and yearly thereafter. All patients provided written informed consent. This study was carried out in compliance with the Declaration of Helsinki, and all participating patients provided written informed consent. The Leiden EAC was approved by the Medical Ethics Committee of the LUMC (B19.008).
From June 2013 onwards, the EAC protocol included contrast‐enhanced MRI of the forefoot. For the current study, we included 157 consecutive DMARD‐naive, early RA patients from the EAC who were enrolled from June 2013 to March 2016. Fourteen patients with early RA were excluded because of missing baseline MRIs, and 5 others were excluded because of insufficient quality of MRIs. RA was defined as a clinical diagnosis plus fulfilment of the 2010 RA classification criteria within 1 year after inclusion (25).
Clinical signs typical for RA
Joint tenderness and swelling were assessed at physical examination by a trained research nurse. Joint swelling was also assessed independently by a rheumatologist and was considered positive if both assessors agreed on its presence in the same joint (26). Research nurses participate regularly in consensus exercises for joint examination led by a rheumatologist to maintain interobserver agreement.
MRI scanning and baseline evaluation
Shortly after the first visit (when clinical assessment was done) and before any DMARDs were started (the period between the first visit and MRI was 9 days on average), all patients underwent unilateral contrast‐enhanced 1.5T ONI MRI (GE) of the first through the fifth MTP joints on the most painful side. In the case of symmetrically severe symptoms, the dominant side was scanned. The MRI protocol is described in more detail in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640. All MRIs were scored blinded for clinical data.
The intermetatarsal bursae lie in the superior intermetatarsal spaces, which are bordered medially and laterally by the metatarsal heads, dorsally by the deep dorsal aponeurosis, and plantarly by the deep transverse metatarsal ligament (10, 12). IMB was therefore defined as contrast enhancement of the bursa in the superior intermetatarsal space, with or without rim enhancement, visible on ≥2 consecutive slices in both planes (axial and coronal). For each superior intermetatarsal space (4 per foot), presence of IMB was recorded by 2 independent readers (YJD and MR), who then determined the final scores by consensus; an IMB lesion was considered present if both agreed on this. This IMB scoring method was described previously; the specificity for RA of IMB presence assessed in this manner was 84% compared to healthy controls (14). Next to IMB presence, the size of the lesions in dorsoplantar direction (in mm) was recorded (14) to enable assessment of changes in size at follow‐up. Size measurements are described in more detail in Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640.
To assess the relation between IMB and other MRI measures of local inflammation, MRIs were also evaluated for synovitis, tenosynovitis, and osteitis in line with the RAMRIS system by 2 independent readers, as described previously (2, 27, 28). To obtain the total RAMRIS inflammation score for each patient, the scores for synovitis, tenosynovitis, and osteitis were summed; the average of the scores of both readers was used (29). At joint level, presence of RAMRIS inflammation was stringently defined based on consensus: synovitis, tenosynovitis, or osteitis were considered present per location if that feature was scored as ≥1 by both readers independently, concordant to the literature (26). Detailed information on RAMRIS inflammation scoring is presented in Supplementary Appendix A, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640.
Follow‐up MRIs
MRIs were repeated over time (scheduled at 4, 12, and 24 months from baseline) in patients included from June 2013 until February 2015; a flowchart illustrating patient selection for longitudinal analyses is presented in Supplementary Figure 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640. The course of IMB was evaluated longitudinally in patients who had ≥1 IMB lesion at baseline and received early DMARD therapy. “Early” therapy was defined as DMARD initiation (including glucocorticoids) within 100 days from first presentation at the outpatient clinic.
For IMB, both its presence and lesion size were evaluated. For the latter, a composite measure was used: the averaged IMB size (in mm), calculated by summing the dorsoplantar sizes of all IMB lesions in any intermetatarsal space and dividing the result by 4 (the maximum number of IMB lesions). The dorsoplantar size was used and not the transversal size because intermetatarsal bursae are confined transversally by the metatarsal heads and may, theoretically, distend dorsoplantarly more freely (30).
MRIs were scored in known time order. IMB presence and size were assessed without simultaneously performing RAMRIS scoring. In addition, the same set of MRIs was scored by another independent trained reader, who performed RAMRIS scoring. Interreader and intrareader intraclass correlation coefficients (ICCs) for IMB were 0.90 and 0.85, respectively (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640). For the RAMRIS system, interreader and intrareader ICCs were ≥0.90, as published previously (2).
Statistical analyses
First, the association between presence of IMB and RAMRIS inflammation at baseline was assessed at the patient and bursa levels. At the patient level, univariable logistic regression was used with continuous scores for RAMRIS inflammation (synovitis, tenosynovitis, osteitis, and total RAMRIS inflammation score) as independent variables. Bursa‐level analyses were performed using univariable generalized estimating equations (GEEs), with presence of RAMRIS inflammation (synovitis, tenosynovitis, osteitis, and presence of any of these 3) in the 2 joints neighboring the bursa as independent variables. Both at the patient and bursa levels, multivariable models with synovitis, tenosynovitis, and osteitis as separate independent variables were performed because these features often co‐occur.
Secondly, it was assessed at joint level whether presence of IMB at baseline contributes to 2 clinical signs typical for RA: joint tenderness and swelling. Univariable GEEs were used with tenderness or swelling of the MTP joint as outcome, and IMB presence in the adjacent intermetatarsal space as independent variable. Multivariable GEEs adjusted for concurrent presence of RAMRIS inflammation (synovitis, tenosynovitis, or osteitis). The first MTP joint was not included in these analyses because it is a predilection site for other diseases than RA (e.g., gout and osteoarthritis), which could introduce tenderness or swelling unrelated to RA (26).
Longitudinally, at patient level, the mean averaged IMB size and total RAMRIS inflammation were modeled over time using GEEs and visualized in 1 graph. The DAS score (calculated with a 4‐component formula based on 44 joints) (31) was plotted as well. Associations between the time courses of IMB and RAMRIS inflammation were assessed at the patient level using univariable GEEs, with changes in averaged IMB size as dependent variables, and changes in RAMRIS inflammation (synovitis, tenosynovitis, osteitis, and total scores) as independent variables. Again, a multivariable GEE with the 3 inflammation features as separate independent variables was performed. GEE models were limited to the 0–4 and 4–12 month intervals to optimize the fit because thereafter, patient numbers were lower and MRI‐detected inflammation was stable.
Sensitivity analyses were performed by repeating the patient‐level longitudinal analyses in the subgroup of patients who received methotrexate as initial DMARD therapy since methotrexate was most often used as a first‐line DMARD therapy, as recommended by international guidelines for RA management (32).
Analyses were repeated with stratification for ACPA status because ACPA‐positive and ACPA‐negative RA are considered different entities (22, 33, 34). Effects of ACPA status on the time courses of IMB and total RAMRIS inflammation at the patient level were assessed by adding ACPA status and the interaction between ACPA status and MRI time point as independent variables to the longitudinal models. SPSS, version 25, was used. Two‐sided P values less than 0.05 were considered statistically significant. Data are available from the corresponding author upon reasonable request.
RESULTS
Patient characteristics
Baseline characteristics are presented in Table 1. IMB was present in 109 patients (69%). IMB was more often present in patients with a higher swollen joint count and tended to be more often present in ACPA‐positive RA (75% versus 64% in ACPA negative; P = 0.13).
Table 1.
Baseline characteristics of all rheumatoid arthritis patients studied according to presence of IMB*
| Characteristic | IMB at baseline | ||
|---|---|---|---|
| All patients | Present | Absent | |
| (n = 157) | (n = 109) | (n = 48) | |
| Age, mean ± SD years | 59 ± 14 | 58 ± 14 | 61 ± 14 |
| Female, no. (%) | 109 (69) | 74 (68) | 35 (73) |
| BMI, mean ± SD | 26 ± 5 | 26 ± 4 | 26 ± 5 |
| Symptom duration, weeks | 11 (5–28) | 10 (5–27) | 12 (5–31) |
| SJC† | 7 (3–11) | 8 (4–11) | 4 (1–11) |
| TJC | 5 (3–7) | 5 (3–7) | 5 (3–8) |
| Swollen MTP joint(s), no. (%) | 57 (36) | 43 (39) | 14 (29) |
| ESR, mm/hour | 28 (14–45) | 28 (14–41) | 27 (10–49) |
| DAS, mean ± SD | 3.1 ± 0.8 | 3.1 ± 0.8 | 3.0 ± 0.8 |
| ACPA positive, no. (%) | 83 (53) | 62 (57) | 21 (44) |
| RF positive, no. (%)† | 101 (64) | 76 (70) | 25 (52) |
| No. of IMB lesions | 1 (0–3) | 2 (1–3) | – |
Values are the median (interquartile range) unless indicated otherwise. ACPA = anti–citrullinated protein antibody; BMI = body mass index; DAS = Disease Activity Score; ESR = erythrocyte sedimentation rate; IMB = intermetatarsal bursitis; MTP = metatarsophalangeal; RF = rheumatoid factor; SJC = swollen joint count; TJC = tender joint count.
Statistically significant at the 0.05 level.
IMB occurs together with tenosynovitis and synovitis
Patients with MRI‐detected IMB were more likely to have higher total RAMRIS inflammation scores (Table 2). Also, evaluation of synovitis, tenosynovitis, and osteitis separately showed that patients with IMB were more likely to have higher scores for all these inflammatory features. Multivariable analyses showed that IMB presence was associated with high tenosynovitis scores. Thus, patients with a higher severity of tenosynovitis had IMB more frequently.
Table 2.
The association between the presence of IMB and RAMRIS inflammation scores in the forefoot at the patient level at first presentation (n = 157)*
| Univariable | Multivariable† | |||||
|---|---|---|---|---|---|---|
| Range | Median (IQR) | OR (95% CI) | P | OR (95% CI) | P | |
| Total RAMRIS inflammation | 0–30 | 4 (1–9) | 1.51 (1.28, 1.78) | <0.001 | – | – |
| Synovitis | 0–10 | 1 (0–3) | 1.98 (1.45, 2.72) | <0.001 | 1.12 (0.77, 1.65) | 0.55 |
| Tenosynovitis | 0–12 | 1 (0–3) | 3.42 (1.97, 5.95) | <0.001 | 2.92 (1.62, 5.24) | <0.001 |
| Osteitis | 0–20 | 1 (0–3) | 1.70 (1.27, 2.27) | <0.001 | 1.38 (0.97, 1.97) | 0.074 |
95% CI = 95% confidence interval; IMB = intermetatarsal bursitis; IQR = interquartile range; OR = odds ratio; RAMRIS = Rheumatoid Arthritis Magnetic Resonance Imaging Scoring (system).
Including synovitis, tenosynovitis, and osteitis scores as independent variables.
Analyses were then done at the bursa level. IMB was more often present at locations with synovitis, tenosynovitis, or osteitis in the adjacent MTP joints (Table 3). In multivariable analyses, the presence of IMB was associated with local presence of synovitis and tenosynovitis, with odds ratios (ORs) of 1.69 (95% confidence interval [95% CI] 1.12, 2.57) and 2.83 (95% CI 1.80, 4.44), respectively. In contrast, it was not associated with presence of inflammation in the adjacent bones (osteitis) in multivariable analysis. Two example MRI images of IMB co‐occurring with tenosynovitis are presented in Figure 1. Additional example MRI images are presented in Supplementary Figure 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640.
Table 3.
The association between the presence of IMB and the presence of RAMRIS inflammation in neighboring joints at first presentation (n = 628 bursae)*
| Univariable | Multivariable† | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Any feature | 2.67 (1.91, 3.73) | <0.001 | – | – |
| Synovitis | 2.63 (1.84, 3.76) | <0.001 | 1.69 (1.12, 2.57) | 0.013 |
| Tenosynovitis | 3.69 (2.40, 5.67) | <0.001 | 2.83 (1.80, 4.44) | <0.001 |
| Osteitis | 1.99 (1.33, 2.98) | 0.001 | 1.30 (0.81, 2.08) | 0.28 |
95% CI = 95% confidence interval; IMB = intermetatarsal bursitis; OR = odds ratio; RAMRIS = Rheumatoid Arthritis Magnetic Resonance Imaging Scoring (system).
Including synovitis, tenosynovitis, and osteitis presence as independent variables.
Figure 1.
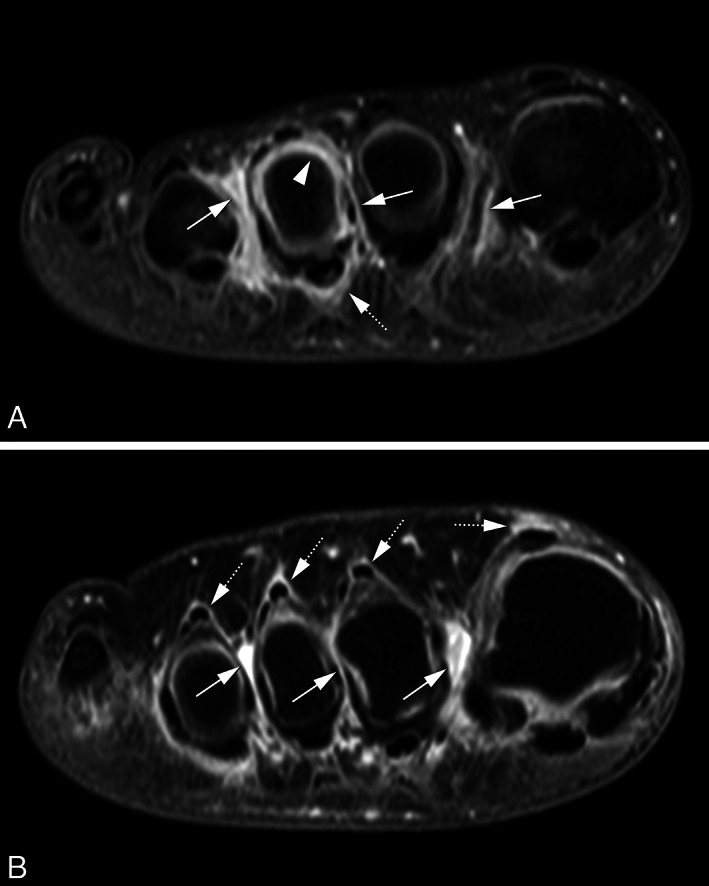
Magnetic resonance imaging (MRI) of 2 disease‐modifying antirheumatic drug–naive, early rheumatoid arthritis patients showing MRI‐detected intermetatarsal bursitis (IMB) co‐occurring with synovitis and flexor tenosynovitis (A) and with extensor tenosynovitis (B) using coronal T1‐weighted fat‐suppressed images after gadolinium administration of the forefoot at the level of the metatarsal heads. Both patients show enhancement of thickened synovium in the intermetatarsal spaces 1–3, consistent with IMB (arrows). Patient A (female, 33 years old) (A) shows peripheral enhancement in the third intermetatarsal space with a central area of lower signal intensity consistent with fluid. At the third metatarsophalangeal joint, there is enhancement surrounding the flexor tendon consistent with tenosynovitis (46) (dotted arrows) as well as synovitis (arrowhead). Patient B (female, 41 years old) (B) shows peripheral enhancement in the first intermetatarsal space with a central area of lower signal intensity consistent with fluid. In addition, there is enhancement surrounding extensor tendons consistent with tenosynovitis (dotted arrows) (46).
IMB contributes to joint tenderness and swelling independent of RAMRIS inflammation
One hundred fifty‐seven RA patients contributed 628 MTP joints, of which 200 (32%) were tender and 81 (13%) were swollen. Joints with adjacent IMB were more likely to be tender (OR 2.1 [95% CI 1.3, 3.4]) and swollen (OR 3.1 [95% CI 1.6, 6.2]). Multivariable analyses showed that IMB presence was associated with both clinical signs independent of synovitis, tenosynovitis, and osteitis (adjusted OR [ORadj] of 1.7 [95% CI 1.04, 2.9] for tenderness and ORadj of 2.7 [95% CI 1.3, 5.3] for swelling).
IMB decreases after DMARD initiation in a fashion similar to synovitis and tenosynovitis
Of the 109 patients who were IMB positive at baseline, 101 received early DMARD therapy, of whom 73 patients were included before February 2015 (the period wherein follow‐up MRIs were made; see Supplementary Figure 2, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640). Follow‐up MRI was available for 55 (75%) of these patients. Longitudinal MRIs (at 4, 12, and 24 months) of these 55 patients were evaluated to assess the time course of IMB after DMARD initiation.
The time courses of IMB and total RAMRIS inflammation are depicted in Figure 2A. Both measures decreased statistically significantly between baseline and 12 months: the mean averaged IMB size was 6.7 mm at baseline and decreased by 3.1 mm (95% CI 2.2, 4.1; P < 0.001), while the mean total RAMRIS inflammation was 7.7 points at baseline and decreased by 4.1 points (95% CI 2.4, 5.7; P < 0.001) between 0 and 12 months.
Figure 2.

Intermetatarsal bursitis (IMB) size (circles) and total Rheumatoid Arthritis Magnetic Resonance Imaging Scoring (RAMRIS) system inflammation (diamonds) in the forefoot over time (n = 55) in all patients (A) and in anti–citrullinated protein antibody (ACPA)–positive and ACPA‐negative patients separately (B). * = β signifies the association of change in averaged IMB size with change in total RAMRIS inflammation between 0 and 12 months, estimated using generalized estimating equations. IMB decrease was statistically significantly associated with total RAMRIS inflammation decrease at the 0.05 level in all patients (A) and in both ACPA subsets (B). 95% CI = 95% confidence interval.
Next, we assessed the relation between changes in IMB and simultaneous changes in RAMRIS inflammation over time. Between baseline and 12 months, greater decrease in averaged IMB size was statistically significantly associated with greater decrease in total RAMRIS inflammation (Figure 2A). The 3 RAMRIS inflammation features were also assessed separately for their relation to IMB decrease (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640). In univariable analyses, patients with greater decreases in synovitis and tenosynovitis on average underwent a greater simultaneous decrease in IMB. Multivariably, IMB decrease was associated with synovitis decrease in the same time interval. Notably, IMB decrease was not related to osteitis decrease, both in univariable and multivariable models. Last, DAS score over time in relation to IMB was plotted (see Supplementary Figure 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640).
Longitudinal MRI images of a patient showing decreasing IMB are presented in Figure 3. An additional series is presented in the supplementary file (see Supplementary Figure 5, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640).
Figure 3.

Longitudinal magnetic resonance imaging of decreasing intermetatarsal bursitis (IMB) in an early rheumatoid arthritis patient (female, 33 years old at baseline; corresponds to patient in Figure 1A) using coronal T1‐weighted fat‐suppressed images after gadolinium administration of the forefoot at the level of the metatarsal heads. The different time points are shown vertically: baseline (A), 4 months (B), 12 months (C), and 24 months (D). IMB (arrows) is visible in intermetatarsal spaces 1–3 with concomitant synovitis and flexor tenosynovitis at the third metatarsophalangeal joint. All inflammation decreased after initiation of disease‐modifying antirheumatic drugs; minimal IMB in the third space remained visible after 2 years.
Sensitivity analysis
Forty‐seven of 55 longitudinally studied patients (85%) received methotrexate as initial DMARD therapy. Longitudinal analyses were repeated in this subgroup. Results were similar to those of the main analyses (see Supplementary Tables 3–4 and Supplementary Figures 6–7, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640).
Analyses stratified for ACPA positivity
Analyses of the relation between IMB and RAMRIS inflammation at baseline and in the first year of follow‐up did not show meaningful differences between ACPA‐positive and ACPA‐negative RA (see Supplementary Tables 5–7, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640), except that in univariable analyses at baseline the association between IMB and osteitis was statistically significantly only in ACPA‐positive patients. IMB presence at baseline was associated with local joint tenderness independent of RAMRIS inflammation only in ACPA‐positive patients (ORadj 3.0 [95% CI 1.6, 5.6]; see Supplementary Table 8, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24640). The association with joint swelling seemed present in both groups but only reached statistical significance in ACPA‐positive patients (ORadj 3.3 [95% CI 1.2, 9.1]).
Longitudinally, decreases in both averaged IMB size and total RAMRIS inflammation between 0 and 12 months appeared to be more pronounced in ACPA‐negative RA (Figure 2B). This was statistically significant for IMB (2.4 mm [95% CI 0.4, 4.4] greater decrease in ACPA‐negative versus ACPA‐positive RA) but not for total RAMRIS inflammation (0.2 [95% CI –3.1, 3.6] points greater decrease in ACPA‐negative versus ACPA‐positive RA). Baseline values of averaged IMB size and total RAMRIS inflammation were not statistically significantly different.
DISCUSSION
RA is traditionally known as an autoimmune disease targeting the synovial lining of (small) joints. There is mounting evidence indicating that juxtaarticular synovial inflammation is an important trait of the disease as well. Recently, tenosynovitis was the first feature of such juxtaarticular inflammation to be identified as a trait of RA (6). Our study shows that IMB frequently occurs in RA at the time of diagnosis, especially when synovitis and tenosynovitis were also present, and that it contributes to joint tenderness and swelling independent of known MRI features. These data enhance our understanding of forefoot inflammation in RA and support the notion that IMB might be another feature of juxtaarticular synovial inflammation in RA.
The current study is the first to investigate IMB in early RA during follow‐up and in relation to known RA inflammation features (RAMRIS inflammation). We demonstrated that IMB decreased after DMARD initiation; a decrease that was most strongly related to a decrease in synovitis severity. This decrease of IMB was as one would expect from an RA treatment response. These findings may therefore further support the notion that IMB is truly a feature of RA.
Recognition of IMB is clinically relevant because it could add to the set of RA features and characteristics that physicians may consider when evaluating patients with (suspected) RA. Our findings suggest that IMB contributes to the clinical appearance of metatarsalgia and arthritis. More specifically, it could aid in the interpretation of forefoot symptoms and walking disabilities in the absence of synovitis on imaging.
While IMB in RA has been described in small case reports and larger studies in long‐standing disease (16, 35, 36, 37), the current study is the first large MRI study in early RA. The prevalence of MRI‐detected IMB in our baseline sample was published previously and amounted to 69% (14), which is in line with the 63% previously reported in a small MRI study in early RA (n = 30 patients) (17). The results of the present and previous imaging studies are concordant in their finding that IMB is prevalent in a majority of RA patients at diagnosis. In addition, data of our study suggest that IMB is especially present in patients presenting with extensive inflammation, measured by the swollen joint count or total RAMRIS inflammation.
The association between IMB and joint swelling was described previously in a study on early arthritis, which also included the RA patients studied here (26). However, the current finding that this association is present in RA patients specifically, independent of RAMRIS inflammation, is novel. Moreover, we now also show that IMB contributes to joint tenderness, which is a subject of utmost importance from the patient perspective (38). The association of IMB with joint swelling appeared somewhat stronger than its association with tenderness, generating the question whether the latter is partly caused by the former. When restricting analyses to nonswollen joints only (n = 540), the effect size changed only slightly (the ORadj for RAMRIS inflammation went from 1.7 [95% CI 1.04, 2.9] to 1.5 [95% CI 0.8, 2.8]). In our view, this suggests that IMB contributes not only to joint swelling but also to tenderness without clinical swelling.
IMB was frequently present in both ACPA‐positive and ACPA‐negative RA at diagnosis. The prevalence was higher in the ACPA‐positive group, but this finding was not statistically significant. The association of IMB presence with simultaneous presence of synovitis (at the joint level) and tenosynovitis (at the patient and joint level) was also positive in both groups. While ACPA‐positive patients were more likely to have IMB in the presence of osteitis in univariable analyses, this association was not statistically significant in ACPA‐negative patients. This difference between ACPA‐positive and ACPA‐negative RA is in line with previous findings showing that osteitis associates particularly with ACPA‐positive RA (39, 40). Despite similar associations of IMB with RAMRIS inflammation, associations with joint tenderness and swelling were more prominent in ACPA‐positive than ACPA‐negative RA. In both RA groups, however, IMB decreased significantly over time in a fashion similar to total RAMRIS inflammation. Moreover, patients in both groups showed greater IMB decrease when total RAMRIS inflammation decreased more strongly. Thus, although ACPA‐positive and ACPA‐negative RA have differences in risk factors, presumed etiology, and severity of disease course (33, 34, 41), IMB is prevalent and behaves similarly in relation to RAMRIS inflammation in both disease subsets.
Hypothetically, mechanical strain could promote development of bursitis. If so, one may assume that IMB decrease is secondary to decreasing mechanical pressure from reduction in neighboring synovitis (18, 19, 20). Exploratory analyses showed that IMB lesions with adjacent synovitis at baseline did not dissipate more often (44% after 12 months versus 57% for IMB lesions without adjacent synovitis; P = 0.17), arguing against a secondary treatment effect by decreasing mechanical pressure from neighboring synovitis.
We measured IMB size in dorsoplantar direction according to the literature and because this was expected to represent total lesion size more accurately than axial measurements. Intermetatarsal bursae are confined axially by the metatarsal heads and may therefore distend dorsoplantarly more freely (30). Influence of mechanical factors on dorsoplantar distension was most likely limited, as MRIs were made in supine, non–weight bearing position. Still, potentially relevant aspects of the time course of IMB might have gone unnoticed by focusing on the dorsoplantar dimension. Ideally, total IMB volume is used, but reliable measuring methods were not available and beyond the scope of the current investigation.
An important strength of the current study was the relatively large sample size at baseline compared to previous imaging studies of IMB in early RA (17, 35). Second, results were robust across patient‐ and joint‐level analyses. Last, owing to the design of the Leiden EAC, which is an inception cohort with extensive follow‐up including MRI scans at multiple time points, we were able to perform novel longitudinal analyses of MRI‐detected IMB in early RA.
There are also limitations. First, the method we used to score the presence and size of IMB lesions is novel and not yet systematically validated. On the other hand, it was developed in collaboration with a musculoskeletal radiologist (MR) with >20 years of experience, and interreader and intrareader reliability were good (ICCs ≥0.85). Second, MRIs were scored in chronological order to achieve better sensitivity to change, which is in line with the literature (2, 27, 42, 43, 44). Theoretically, this may have caused bias in the form of greater change scores than would have been the case with blinding for time order. However, impact on the main objective to assess associations between IMB and RAMRIS inflammation over time is assumed to be limited, as we have no reason to believe that the improvement in sensitivity to change is different between IMB and RAMRIS inflammation. Third, regression to the mean could have occurred in the longitudinal part of the study since only patients who were IMB positive at baseline were included. It might be interesting to assess in a subsequent study whether IMB‐negative patients may develop IMB over time despite receiving DMARDs or during flares. Furthermore, as RA patients were treated and we did not perform a randomized clinical trial with a placebo arm, we interpreted the decrease in DAS score, RAMRIS inflammation, and IMB as treatment response, but this was not formally proven. Although both IMB and RAMRIS inflammation decreased statistically and numerically significantly (by 46% and 53%, respectively), minimal reference values for determining a response in these measures are not available. We also had insufficient power to stratify analyses by individual DMARDs other than methotrexate. Finally, any association of IMB with deviations of forefoot bones (e.g., hallux valgus and hammer toes) that might hypothetically influence IMB could not be taken into account, as no weightbearing radiographs were made (18, 19, 20, 45).
Recognition of IMB as an RA feature paves the way for further study of its properties in the disease. A case report suggested that IMB can be recognized by the feature “opening toes” related to enlargement of the space between adjacent toes (37). Although such a clinical sign to detect IMB has the advantage of being less costly and time‐consuming than MRI, it has so far not been systematically studied. The contribution of IMB to walking disabilities, including the role therein of biomechanical factors such as pressure distribution, is another subject for further research. For RA patients with prominent foot symptoms and/or walking disabilities, it would be especially valuable to see if amelioration of IMB correlates with symptomatic and functional improvement and, if so, which individual DMARDs or additional therapeutic approaches influence IMB and forefoot symptoms. In addition, it could be studied whether IMB is of pathophysiologic relevance or just reflects extensive synovial inflammation pertaining to higher disease activity. For example, the causal relation between synovitis, tenosynovitis, and IMB could be investigated in a histopathologic study differentiating the types of synovitis. Last, although IMB has been reported to be detectable by ultrasound (15), which is more easily accessible in daily practice than MRI, its correlation with MRI‐detected IMB in early RA has not yet been studied.
In conclusion, IMB behaves in line with known RA characteristics; it particularly accompanies inflammation of the synovial lining of joints and tendon sheaths, shows a similar treatment response after DMARD initiation, and contributes to typical clinical signs. These findings support the notion that IMB is a novel inflammatory feature of early RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. van Dijk had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
van Dijk, van der Helm‐van Mil.
Acquisition of data
van Dijk, Dakkak, Matthijssen, Reijnierse.
Analysis and interpretation of data
van Dijk, Dakkak, Matthijssen, Niemantsverdriet, Reijnierse, van der Helm‐van Mil.
Supporting information
Supplementary Appendix A MRI scanning and scoring of RAMRIS‐inflammation
Supplementary Figure 1 Measurements of dorsoplantar IMB‐size on MRI
Supplementary Figure 2. Flowchart of patients studied at baseline and follow‐up
Supplementary Figure 3. (A‐D) Additional example MR‐images of IMB in axial and coronal planes
Supplementary Figure 4. Averaged IMB‐size and DAS44 over time (n = 55): (A) without stratification and (B) stratified by ACPA‐positivity
Supplementary Figure 5. Longitudinal MR‐images of decreasing IMB in an RA‐patient, without concurrent RAMRIS‐inflammation
Supplementary Figure 6. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy; averaged IMB‐size and RAMRIS‐inflammation in the forefoot over time (n = 47)
Supplementary Figure 7. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy; averaged IMB‐size and DAS44 over time (n = 47): (A) without stratification and (B) stratified by ACPA‐positivity
Supplementary Table 1 Intra‐ and interreader reliability of IMB scores
Supplementary Table 2. Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐size as outcome (n = 55)
Supplementary Table 3. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy: Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐size as outcome (n = 47)
Supplementary Table 4. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy: Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐ size as outcome, stratified for ACPA‐positivity
Supplementary Table 5. Logistic regression analyses at baseline of the association between presence of IMB and RAMRIS‐inflammation scores in the forefoot, stratified for ACPA‐positivity
Supplementary Table 6. Bursa‐level analyses using GEE of the association between presence of IMB and presence of RAMRIS‐inflammation in neighbouring joints, stratified for ACPA‐positivity
Supplementary Table 7. Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐size as outcome, stratified for ACPA‐positivity
Supplementary Table 8. Associations of IMB with local joint tenderness and swelling, assessed by GEEs stratified for ACPA‐status
ACKNOWLEDGMENTS
We thank G. Kracht for his assistance in preparing the MRIs. A. Boer, D. Boeters, W. Nieuwenhuis, and E. Newsum are acknowledged for RAMRIS scoring of baseline MRIs.
Supported by the European Union's Horizon 2020 Framework Programme for Research and Innovation (starting grant agreement 634886) and by ReumaNederland.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Østergaard M, Bird P, Gandjbakhch F, Eshed I, Haugen IK, Haavardsholm EA, et al. The OMERACT MRI in Arthritis Working Group: update on status and future research priorities. J Rheumatol 2015;42:2470–2. [DOI] [PubMed] [Google Scholar]
- 2. Dakkak YJ, Matthijssen XM, van der Heijde D, Reijnierse M, van der Helm‐van Mil AH. Reliability of magnetic resonance imaging (MRI) scoring of the metatarsophalangeal joints of the foot according to the rheumatoid arthritis MRI score. J Rheumatol 2020;47:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colebatch AN, Edwards CJ, Østergaard M, van der Heijde D, Balint PV, D'Agostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 2013;72:804–14. [DOI] [PubMed] [Google Scholar]
- 4. Østergaard M, Peterfy CG, Bird P, Gandjbakhch F, Glinatsi D, Eshed I, et al. The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) scoring system: updated recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol 2017;44:1706–12. [DOI] [PubMed] [Google Scholar]
- 5. Nieuwenhuis WP, van Steenbergen HW, Mangnus L, Newsum EC, Bloem JL, Huizinga TW, et al. Evaluation of the diagnostic accuracy of hand and foot MRI for early rheumatoid arthritis. Rheumatology (Oxford) 2017;56:1367–77. [DOI] [PubMed] [Google Scholar]
- 6. Rogier C, Hayer S, van der Helm‐van Mil A. Not only synovitis but also tenosynovitis needs to be considered: why it is time to update textbook images of rheumatoid arthritis. Ann Rheum Dis 2020;79:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mankia K, Agostino MA, Rowbotham E, Hensor EM, Hunt L, Möller I, et al. MRI inflammation of the hand interosseous tendons occurs in anti‐CCP‐positive at‐risk individuals and may precede the development of clinical synovitis. Ann Rheum Dis 2019;78:781. [DOI] [PubMed] [Google Scholar]
- 8. Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, et al. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology (Oxford) 2009;48:887–91. [DOI] [PubMed] [Google Scholar]
- 9. Brooks F, Hariharan K. The rheumatoid forefoot. Curr Rev Musculoskelet Med 2013;6:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theumann NH, Pfirrmann CW, Chung CB, Mohana‐Borges AV, Haghighi P, Trudell DJ, et al. Intermetatarsal spaces: analysis with MR bursography, anatomic correlation, and histopathology in cadavers. Radiology 2001;221:478–84. [DOI] [PubMed] [Google Scholar]
- 11. Awerbuch MS, Shephard E, Vernon‐Roberts B. Morton's metatarsalgia due to intermetatarsophalangeal bursitis as an early manifestation of rheumatoid arthritis. Clin Orthop Relat Res 1982:214–21. [PubMed] [Google Scholar]
- 12. Chauveaux D, Le Huec JC, Midy D. The supra‐transverse intermetatarsocapital bursa: a description and its relation to painful syndromes of the forefoot. Surg Radiol Anat 1987;9:13–8. [DOI] [PubMed] [Google Scholar]
- 13. Jovanovic MS, Royer J, Roy PE, Caron P, Houde G, Houde JP, et al. A comparative study of the spaces between the metacarpal and metatarsal heads. Surg Radiol Anat 1990;12:31–6. [DOI] [PubMed] [Google Scholar]
- 14. Dakkak YJ, Niemantsverdriet E, van der Helm‐van Mil AH, Reijnierse M. Increased frequency of intermetatarsal and submetatarsal bursitis in early rheumatoid arthritis: a large case‐controlled MRI study. Arthritis Res Ther 2020;22:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowen CJ, Culliford D, Dewbury K, Sampson M, Burridge J, Hooper L, et al. The clinical importance of ultrasound detectable forefoot bursae in rheumatoid arthritis. Rheumatology (Oxford) 2009;49:191–2. [DOI] [PubMed] [Google Scholar]
- 16. Hammer HB, Kvien TK, Terslev L. Intermetatarsal bursitis is frequent in patients with established rheumatoid arthritis and is associated with anti‐cyclic citrullinated peptide and rheumatoid factor. RMD Open 2019;5:e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boutry N, Lardé A, Lapègue F, Solau‐Gervais E, Flipo RM, Cotten A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol 2003;30:671. [PubMed] [Google Scholar]
- 18. Bowen CJ, Culliford D, Allen R, Beacroft J, Gay A, Hooper L, et al. Forefoot pathology in rheumatoid arthritis identified with ultrasound may not localise to areas of highest pressure: cohort observations at baseline and twelve months. J Foot Ankle Res 2011;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helliwell P, Siddle H, Redmond A, editors. The foot and ankle in rheumatology. Reports on the Rheumatic Diseases; 2011.
- 20. Nouh MR, Khalil AA. Forefoot: a basic integrated imaging perspective for radiologists. Clin Imaging 2014;38:397–409. [DOI] [PubMed] [Google Scholar]
- 21. Sundin U, Aga AB, Skare Ø, Nordberg LB, Uhlig T, Hammer HB, et al. Conventional versus ultrasound treat to target: no difference in magnetic resonance imaging inflammation or joint damage over 2 years in early rheumatoid arthritis. Rheumatology (Oxford) 2020;59:2550–5. [DOI] [PubMed] [Google Scholar]
- 22. Matthijssen XM, Niemantsverdriet E, Le Cessie S, van der Helm‐van Mil AH. Differing time‐orders of inflammation decrease between ACPA subsets in RA patients suggest differences in underlying inflammatory pathways. Rheumatology (Oxford) 2021;60:2969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm‐van Mil AH. Predicting arthritis outcomes: what can be learned from the Leiden Early Arthritis Clinic? Rheumatology (Oxford) 2011;50:93–100. [DOI] [PubMed] [Google Scholar]
- 24. Van der Linden MP, Batstra MR, Bakker‐Jonges LE, on behalf of the Foundation for Quality Medical Laboratory Diagnostics , Detert J, Bastian H, et al. Toward a data‐driven evaluation of the 2010 American College of Rheumatology/European League Against Rheumatism criteria for rheumatoid arthritis: is it sensible to look at levels of rheumatoid factor? Arthritis Rheum 2011;63:1190–9. [DOI] [PubMed] [Google Scholar]
- 25. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 26. Dakkak YJ, Boer AC, Boeters DM, Niemantsverdriet E, Reijnierse M, van der Helm‐van Mil AH. The relation between physical joint examination and MRI‐depicted inflammation of metatarsophalangeal joints in early arthritis. Arthritis Res Ther 2020;22:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haavardsholm EA, Østergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis 2007;66:1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA‐MRI scoring system. J Rheumatol 2003;30:1385–6. [PubMed] [Google Scholar]
- 29. Nieuwenhuis WP, Mangnus L, van Steenbergen HW, Newsum EC, Huizinga TW, Reijnierse M, et al. Older age is associated with more MRI‐detected inflammation in hand and foot joints. Rheumatology (Oxford) 2016;55:2212–9. [DOI] [PubMed] [Google Scholar]
- 30. Schwalbe G. The tendon sheaths and synovial bursae of the foot, 1896. Translated by Hartmann. Foot Ankle 1981;1:246–69. [PubMed] [Google Scholar]
- 31. Van der Heijde DM, van ‘t Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis 1990;49:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smolen JS, Landewé RB, Bijlsma JW, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685. [DOI] [PubMed] [Google Scholar]
- 33. Daha NA, Toes RE. Are ACPA‐positive and ACPA‐negative RA the same disease? Nat Rev Rheumatol 2011;7:202–3. [DOI] [PubMed] [Google Scholar]
- 34. Matthijssen XM, Niemantsverdriet E, Huizinga TW, van der Helm‐van Mil AH. Enhanced treatment strategies and distinct disease outcomes among autoantibody‐positive and ‐negative rheumatoid arthritis patients over 25 years: a longitudinal cohort study in the Netherlands. PLOS Med 2020;17:e1003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Albtoush OM, Xenitidis T, Horger M. Intermetatarsal bursitis as first disease manifestation in different rheumatological disorders and related MR‐imaging findings. Rheumatol Int 2019;39:2129–36. [DOI] [PubMed] [Google Scholar]
- 36. Bowen CJ, Hooper L, Culliford D, Dewbury K, Sampson M, Burridge J, et al. Assessment of the natural history of forefoot bursae using ultrasonography in patients with rheumatoid arthritis: a twelve‐month investigation. Arthritis Care Res (Hoboken) 2010;62:1756–62. [DOI] [PubMed] [Google Scholar]
- 37. Endo Y, Koga T, Eguchi M, Okamoto M, Tsuji S, Takatani A, et al. Utility of power Doppler ultrasonography for detecting forefoot bursae in early rheumatoid arthritis: a case report. Medicine (Baltimore) 2018;97:e13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heiberg T, Finset A, Uhlig T, Kvien TK. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stomp W, Krabben A, van der Heijde D, Huizinga TW, Bloem JL, van der Helm‐van Mil AH, et al. Are rheumatoid arthritis patients discernible from other early arthritis patients using 1.5T extremity magnetic resonance imaging? A large cross‐sectional study. J Rheumatol 2014;41:1630–7. [DOI] [PubMed] [Google Scholar]
- 40. Tamai M, Kawakami A, Uetani M, Takao S, Tanaka F, Nakamura H, et al. The presence of anti‐cyclic citrullinated peptide antibody is associated with magnetic resonance imaging detection of bone marrow oedema in early stage rheumatoid arthritis. Ann Rheum Dis 2006;65:133–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van der Woude D, van der Helm‐van Mil AH. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2018;32:174–87. [DOI] [PubMed] [Google Scholar]
- 42. Glinatsi D, Bird P, Gandjbakhch F, Haavardsholm EA, Peterfy CG, Vital EM, et al. Development and validation of the OMERACT Rheumatoid Arthritis Magnetic Resonance Tenosynovitis Scoring System in a multireader exercise. J Rheumatol 2017;44:1688–93. [DOI] [PubMed] [Google Scholar]
- 43. Glinatsi D, Lillegraven S, Haavardsholm EA, Eshed I, Conaghan PG, Peterfy C, et al. Validation of the OMERACT Magnetic Resonance Imaging Joint Space Narrowing Score for the wrist in a multireader longitudinal trial. J Rheumatol 2015;42:2480–5. [DOI] [PubMed] [Google Scholar]
- 44. Van Tuyl LH, van der Heijde D, Knol DL, Boers M. Chronological reading of radiographs in rheumatoid arthritis increases efficiency and does not lead to bias. Ann Rheum Dis 2014;73:391–5. [DOI] [PubMed] [Google Scholar]
- 45. Fuhrmann RA, Layher F, Wetzel WD. Radiographic changes in forefoot geometry with weightbearing. Foot Ankle Int 2003;24:326–31. [DOI] [PubMed] [Google Scholar]
- 46. Dakkak YJ, Jansen FP, DeRuiter MC, Reijnierse M, van der Helm‐van Mil AH. Rheumatoid arthritis and tenosynovitis at the metatarsophalangeal joints: an anatomic and MRI study of the forefoot tendon sheaths. Radiology 2020;295:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix A MRI scanning and scoring of RAMRIS‐inflammation
Supplementary Figure 1 Measurements of dorsoplantar IMB‐size on MRI
Supplementary Figure 2. Flowchart of patients studied at baseline and follow‐up
Supplementary Figure 3. (A‐D) Additional example MR‐images of IMB in axial and coronal planes
Supplementary Figure 4. Averaged IMB‐size and DAS44 over time (n = 55): (A) without stratification and (B) stratified by ACPA‐positivity
Supplementary Figure 5. Longitudinal MR‐images of decreasing IMB in an RA‐patient, without concurrent RAMRIS‐inflammation
Supplementary Figure 6. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy; averaged IMB‐size and RAMRIS‐inflammation in the forefoot over time (n = 47)
Supplementary Figure 7. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy; averaged IMB‐size and DAS44 over time (n = 47): (A) without stratification and (B) stratified by ACPA‐positivity
Supplementary Table 1 Intra‐ and interreader reliability of IMB scores
Supplementary Table 2. Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐size as outcome (n = 55)
Supplementary Table 3. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy: Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐size as outcome (n = 47)
Supplementary Table 4. Sensitivity analyses in patients who received methotrexate as initial DMARD‐therapy: Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐ size as outcome, stratified for ACPA‐positivity
Supplementary Table 5. Logistic regression analyses at baseline of the association between presence of IMB and RAMRIS‐inflammation scores in the forefoot, stratified for ACPA‐positivity
Supplementary Table 6. Bursa‐level analyses using GEE of the association between presence of IMB and presence of RAMRIS‐inflammation in neighbouring joints, stratified for ACPA‐positivity
Supplementary Table 7. Associations between decreases in RAMRIS‐inflammation and IMB from baseline to 12 months, assessed by GEEs with decrease in averaged IMB‐size as outcome, stratified for ACPA‐positivity
Supplementary Table 8. Associations of IMB with local joint tenderness and swelling, assessed by GEEs stratified for ACPA‐status


