Abstract
Intestinal nematode infections in rats or mice are accompanied by intestinal muscle hyper contractility that may contribute to parasite expulsion from the gut. Previous studies demonstrated that both the expulsion of nematode parasites and the associated muscle hyper contractility are dependent on CD4+ T helper cells. Nevertheless, the precise immunological mechanism underlying changes in intestinal muscle function remains to be determined. In this study, we investigated the role of interleukin 4 (IL-4) and signal transducer and activator of transcription factor 6 (STAT6) in the development of intestinal muscle hypercontractility and worm expulsion by infecting IL-4 and STAT6-deficient mice with Trichinella spiralis. Worm expulsion was almost normal in IL-4-deficient mice but substantially delayed in STAT6-deficient mice. Consistent with delayed worm expulsion, we also observed a marked attenuation of carbachol-induced muscle contraction in STAT6-deficient mice but only a moderate decrease in muscle hypercontractility in IL-4-deficient mice. In addition, we also observed severe impairment of T helper type 2 cytokine responses and intestinal mucosal mastocytosis in STAT6-deficient mice, although some degree of intestinal tissue eosinophilia was evident in these animals. These results are consistent with the hypothesis that STAT6-dependent changes in intestinal muscle function contribute to host protection in nematode infection.
CD4+ T helper (Th) cells are important in host protective immunity to many intestinal nematodes, including Trichinella spiralis (13, 18). Among the distinct CD4+ T-cell subsets (32), the Th2 type of immune response is predominantly associated with protective immunity in intestinal nematode infection (13, 19, 38, 39). Infection of mice with T. spiralis generates a strong Th2 response (13, 19), which regulates a variety of responses characteristic of this nematode infection, such as mucosal mastocytosis and intestinal eosinophilia. Th2 cells are derived from a naive, peripheral CD4+ T- cell population (Th0), and interleukin-4 (IL-4) is the primary determinant of differentiation of Th0 cells into Th2 cells. Although IL-4 is a key cytokine in the development of Th2 cell responses, recent studies demonstrate the involvement of a closely related cytokine, IL-13 (30). IL-4 and IL-13 share the alpha chain of the IL-4 receptor (IL-4Rα) and occupation of the IL-4 receptor results in the activation of at least two distinct signaling pathways (25, 34). One involves the activation of signal transducer and activator of transcription factor 6 (STAT6) through phosphorylation by Janus kinases 1 and 3. Once activated, STAT6 proteins form homodimers, translocate to the nucleus, and bind to promoter regions to regulate gene transcription. In addition to STAT6 activation, occupation of the IL-4 receptor has also been shown to activate insulin receptor substrate 2, which then associates with phosphatidylinositol 3-kinase and may be responsible for the proliferative response to IL-4. Although both signaling pathways can be activated through IL-4 receptor, recent studies with STAT6-deficient (STAT6−/−) mice clearly demonstrate that the STAT6 pathway is the principal signaling pathway involved in the differentiation of CD4+ T cells to the Th2 phenotype (24, 37).
Studies using animal models have demonstrated that as with other nematodes, infection with T. spiralis is associated with enhanced contractility of small intestinal muscle (11, 36, 41, 45). Studies of mice with different abilities to successfully expel T. spiralis demonstrate that the magnitude of infection-induced hypercontractility of intestinal muscle is greater in mouse strains that expel the parasite rapidly (e.g., NIH Swiss) than in those that expel the parasite slowly, such as B10.BR (41). Thus, during primary infection with T. spiralis, there exists a distinct relationship between muscle hypercontractility and the rapid expulsion of worms. We have hypothesized that during parasitic infections, the gut motor apparatus acts as an extension of the immune system, facilitating the eviction of worms through increased propulsive activity (11). In support of this hypothesis, we have shown that changes in intestinal muscle hypercontractility during primary T. spiralis infection are dependent on CD4+ Th cells and major histocompatibility complex class II molecules (42). Thus, while our studies invoke CD4 cell activation as a pre requisite for the development of muscle hypercontractility in this model, the T-cell-derived mediators remain to be identified. Clearly, Th2 cytokines are strong candidates, although we have recently shown that IL-5 is not a major contributor (44).
The aim of this study was to investigate the mechanisms by which the immune system induces muscle hypercontractility and regulates intestinal worm expulsion during T. spiralis infection. We studied infected IL-4-deficient (IL-4−/−) and STAT6−/− mice. Our results demonstrate that STAT6 signaling plays a critical role in the generation of muscle hypercontractility in response to primary infection with T. spiralis. Our results also confirm the importance of STAT6 in host defense (39) by demonstrating delayed worm expulsion in infected STAT6−/− mice. We also show that eosinophilia and to a lesser extent mastocytosis occur in the absence of STAT6 signaling.
MATERIALS AND METHODS
Animals.
STAT6−/− mice on a C57BL/6 background were originally produced by gene mutation as described by Takeda et al. (37). Breeding pairs of STAT6−/− mice and their wild-type (STAT6+/+) littermates were obtained from the John Curtin School of Medical Research, Australian National University, Canberra, Australia, and were kept and bred under specific-pathogen-free conditions at the animal facilities of McMaster University, Hamilton, Ontario, Canada. IL-4−/− mice on a C57BL/6 background were obtained from The Jackson Laboratory. All animals were kept in sterilized, filter-topped cages and fed autoclaved food; only male mice 8 to 10 weeks of age were used. The protocols employed were in direct accordance with guidelines drafted by the McMaster University Animal Care Committee and the Canadian Council on the Use of Laboratory Animals.
Parasitological techniques.
The T. spiralis parasites used in this study originated in the Department of Zoology at the University of Toronto, and the colony was maintained through serial infections alternating between male Sprague-Dawley rats and male CD1 mice. The larvae were obtained from infected rodents 60 to 90 days postinfection (p.i.), using a modification (45) of the technique described by Castro and Fairbairn (8). Mice were killed at various time points after infection. Adult worms were recovered from mice after the intestine had been opened longitudinally, rinsed, and placed in Hank's balanced salt solution for 3 h at 37°C. Worms were counted under a dissecting microscope.
Measurement of muscle contraction.
Preparation of the jejunal longitudinal muscle sections for muscle contractility experiments and analysis of the carbachol-induced contraction have been described previously (41). Briefly, the jejunum was removed and placed in oxygenated (95% O2, 5% CO2) Kreb's solution, and 1-cm sections of whole gut were cut from the jejunum, beginning at the ligament of Treitz and proceeding distally. The lumen of each segment was flushed with Krebs buffer prior to the insertion of short (2- β-mm) lengths of Silastic tubing (0.065-inch outside diameter; 0.030-inch inside diameter; Dow Corning, Midland, Mich.) into the open ends of the gut segments. Tubing was then tied in place with surgical silk. The insertion of the tubing was found to maintain patency of the gut segments over the course of experiments. Segments were then hung in the longitudinal axis and attached at one end to a Grass (Quincy, Mass.) FT03C force transducer, and responses were recorded on a Grass 7D polygraph. Tissues were equilibrated for 30 min at 37°C in Krebs buffer, oxygenated with 95% O2–5% CO2 before the start of the experiment. The previously identified optimal tension (400 mg) was then applied in carbachol dose-response experiments before the addition of the first dose of carbachol (41). Previous experiments indicated that this was optimal tension to determine the maximal responsiveness of both control and inflamed tissues. After the application of tension, gut segments were exposed to different concentrations of carbachol. After the maximal response to each dose was obtained, tissues were rinsed twice and equilibrated in fresh Krebs solution for 15 min before addition of the next dose. Contractile responses to carbachol were expressed as milligrams of tension per cross-sectional area as described previously (41). For each mouse, the mean tension was calculated from at least three segments.
Detection of cytokines in muscle by RT-PCR.
Expression of mRNAs of IL-4, IL-13 and gamma interferon (IFNγ) in the jejunal muscle was investigated by a method described previously (44). Briefly, following removal of the small intestine, the longitudinal muscle-myenteric plexus, including serosa, was stripped from the jejunum, beginning at the ligament of Trietz and proceeding 4 cm distally. Total cellular RNA was isolated based on a previously described guanidium isothiocyanate method (9). The concentration of RNA was determined by measuring absorbance at 260 nm, and its purity was confirmed using the ratio of absorbancy at 260 nm to that at 280 nm. RNA was stored at −70°C until used for reverse transcription-PCR (RT-PCR). mRNA was then reversed transcribed as described previously to yield cDNA, and the cDNA was amplified by PCR using gene-specific primers.
Fifty-nanogram aliquots of cDNA (0.1 μg) were then mixed with 20 pmol each of upstream (5′-GAA TGT ACC AGG AGC CAT ATC-3′) and downstream (5′-CTC AGT ACT ACG AGT AAT CCA-3′) primers for IL-4 (35). For detection of IL-13, the upstream primer 5′-TCT TGC TTG CCT TGG TGG TCT CGC-3′ and the downstream primer 5′-GAT GGC ATT GCA ATT GGA GAT GTT G-3′ were used (27). IFN-γ was investigated using the primers 5′-CAT GGC TGT TTC TGG CTG TTA C-3′ and 5′-TCG GAT GAG CTC ATT GAA TGC-3′ as upstream and downstream primers, respectively (17). The hypoxanthine phosphoribosyl transferase (HPRT) housekeeper gene was used as the positive control; to detect it, upstream (5′-GTT GGA TAC AGG CCA GAC TTT GTT G-3′) and downstream (5′-GAT TCA ACT TGC GCT CAT CTT AGG C-3′) primers were used (35). PCR was performed in 50-μl volumes containing deoxynucleoside triphosphate (200 μM), MgCl2 (1.5 mM), and 2.5 U of Taq polymerase (Gibco BRL) with corresponding buffer and distilled water. Messages for IL-4, IL-13, IFN-γ and HPRT were coamplified using the following parameters: denaturation 94°C for 30 s, annealing 55°C for 30 s, and extension at 72°C for 60 s. PCR products were loaded onto a 2.5% agarose gel and then visualized under UV light after ethidium bromide staining. The densities of the bands were determined for each sample (each lane representing one mouse), and the ratios of IL-4, IL-13, and IFN-γ gene expression compared to HPRT expression were calculated. The mean of the ratios was then calculated for uninfected and infected mice.
Evaluation of in vitro cytokine production from MLN and spleen cells.
Single-cell suspensions of spleen or mesenteric lymph node (MLN) were prepared in RPMI 1640 containing 10% fetal calf serum, 5 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 25 mM HEPES and 0.05 mM 2-mercaptoethanol (all from Gibco-BRL). Cells (107) were incubated in the presence of concanavalin A (ConA; 5 μg/ml). Culture supernatants were harvested after 24 h, and IL-4, IL-13, and IFN-γ concentrations in the supernatants were measured by enzyme immunoassay using commercially available kits purchased from R&D Systems (Minneapolis, Minn.).
Histology.
A segment of small intestine (1 cm in length) was taken at 10 cm from the pyloric sphincter, fixed in 10% neutral buffered formalin or in Carnoy's fluid, and processed using standard histological techniques. The sections from neutral buffered formation were stained with Congo red and lightly counterstained with hematoxylin for enumerating intestinal eosinophils; sections from Carnoy's fluid were stained with 0.5% toluidine blue (pH 0.3) for investigating numbers of intestinal mucosal mast cells. Numbers of eosinophils and mast cells were expressed per 10 villus crypt unit.
Statistical analysis.
Data were analyzed using Student's t test with P of <0.05 considered significant. All results are expressed as the mean ± standard error of the mean (SEM).
RESULTS
Worm expulsion is inhibited in STAT6−/− mice infected with T. spiralis.
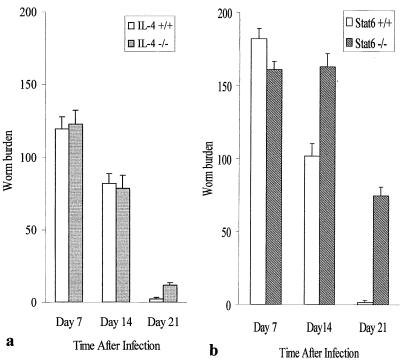
To investigate the role of IL-4 and STAT6 in T. spiralis expulsion, IL-4+/+, IL-4−/−, STAT6+/+, and STAT6−/− mice were infected with T. spiralis larvae and sacrificed on different days after infection. Worm expulsion was similar between IL-4−/− and IL-4+/+ mice and was almost complete by day 21 p.i. in both strains (Fig. 1a). In contrast, worm expulsion was significantly delayed in STAT6−/− mice; we recovered higher numbers of worms from STAT6−/− mice than from STAT6+/+ mice at all time points investigated. Almost all worms were expelled from the intestines of STAT6+/+ mice by day 21 p.i., whereas STAT6−/− mice had a substantial worm burden remaining on day 21 p.i. (Fig. 1b), indicating a prolonged infection.
FIG. 1.
Worm recovery from IL-4−/− (a) and Stat6−/− (b) mice after T. spiralis infection. Mice were infected with 375 T. spiralis larvae orally and killed on the days indicated to investigate the worm recovery from intestine. Each bar represents the mean ± SEM from five animals. ∗, significantly different between STAT6+/+ and STAT6−/− mice.
Infection-induced muscle hypercontractility is attenuated in STAT6−/−mice.
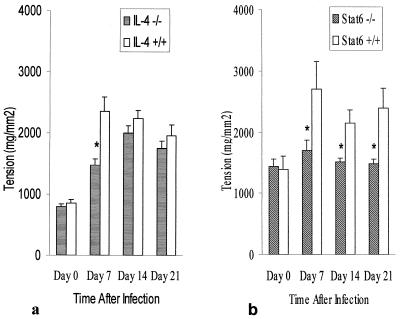
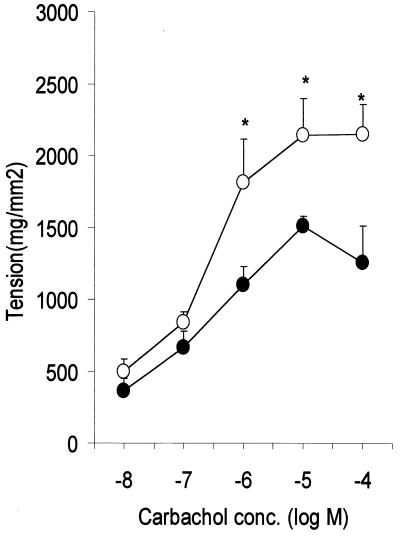
We investigated impact of IL-4 and STAT6 deficiency on intestinal muscle contraction during T. spiralis infection in IL-4−/− and STAT6−/− mice. As shown in Fig. 2, infection was accompanied by muscle hypercontractility evident in IL-4+/+ and STAT6+/+ mice at day 7 p.i. and persisting for up to 21 days p.i. This hypercontractility was significantly attenuated in IL-4−/− mice on day 7 of T. spiralis infection. Importantly, there was no significant difference between IL-4+/+ and IL-4−/− mice in muscle tension generated in response to carbachol on days 14 and 21 p.i. (Fig. 2a). In contrast, no smooth muscle hypercontractility was evident in infected STAT6−/− mice between days 7 and 21 p.i. (Fig. 2b). Indeed, muscle contractility in infected STAT6−/− mice was not significantly different from that seen in uninfected STAT6−/− mice. This was not a reflection of the carbachol dose used in these experiments as significant differences between STAT6−/− and STAT+/+ mice were observed over several doses (Fig. 3).
FIG. 2.
Maximum tension generated by intestinal muscle taken from IL-4−/− (a) and Stat6−/− (b) mice in response to 1 μM carbachol. IL-4+/+, IL-4−/−, STAT6+/+, and STAT6−/− mice were infected with 375 T. spiralis larvae orally and killed at the time points indicated. Day 0 represents data from control noninfected mice. Each value represents the mean ± SEM from four animals. ∗, significantly different between STAT6+/+ and STAT6−/− mice.
FIG. 3.
Dose-response relationships for carbachol-induced contraction of muscle from STAT6+/+ (○) and STAT6−/−(●) mice on day 14 p.i. Mice were infected with 375 T. spiralis larvae orally and killed at the time points indicated. Each value represents the mean ± SEM from four animals. ∗, significantly different between STAT6+/+ and STAT6−/− mice.
T. spiralis infection induces Th2 cytokine expression in muscularis externa.
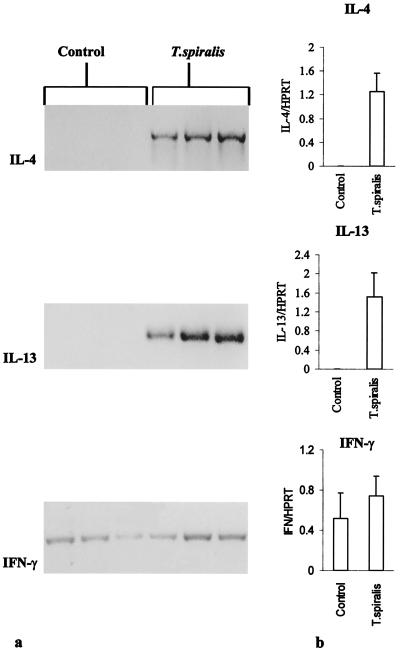
The PCR products for IL-4 and IL-13 were not detectable in the muscularis externa of uninfected control C57BL/6 mice but were intensely expressed in all three infected C57BL/6 mice on day 6 p.i. (Fig. 4a). IFN-γ was detected in both control and infected mice. As shown in Fig. 4ab, the expression of IL-4 and IL-13 mRNA was significantly higher in infected mice than in noninfected controls. However, there was no significant change in IFN-γ gene expression in the muscularis externa in after infection. In contrast, in T. spiralis-infected STAT6−/− mice, there was no expression of IL-4 or IL-13 mRNA after 40 cycles of RT-PCR (data not shown).
FIG. 4.
(a) Cytokines gene expression in muscularis externa of uninfected control (lanes 1 to 3) and T. spiralis-infected (lanes 4 to 6) mice. C57BL/6 mice were infected with T. spiralis orally and killed on day 6 p.i. to investigate expression of the IL-4, IL-13, and IFN-γ genes. (b) Mean ratios ± SEM of IL-4, IL-13, and IFN-γ band densities compared to HPRT band densities in infected and noninfected control mice.
Th2 cytokine response in spleen and MLN during T. spiralis infection is STAT6 dependent.
Measurement of in vitro cytokine production from MLN and spleen cells by stimulation with ConA revealed much less IL-4 and IL-13 production in STAT6−/− mice than in STAT6+/+ mice after T. spiralis infection (Table 1). IL-4 was not detected from MLN and spleen cells of STAT6−/− mice on day 14 p.i. Production of IL-13 was also impaired in STAT6−/− mice. IL-13 was not detected from MLN in STAT6−/− mice on day 14 p.i. Although IL-13 was detected from spleen cells in STAT6−/− mice, it was 81% less than the amount detected in STAT6+/+ mice. As expected, we observed high amounts of both IL-4 and IL-13 in STAT6+/+-infected mice. However, there was no significant difference in the levels of IFN-γ between STAT6+/+ and STAT6−/− mice. This observation further emphasized the importance of STAT6 in the development of a Th2-type immune response.
TABLE 1.
Cytokine production by in vitro ConA-stimulated MLN and spleen cells from STAT6+/+ and STAT6−/− mice infected with T. spiralisa
| Cytokine | Concn (pg/ml)
|
|||
|---|---|---|---|---|
| STAT6+/+
|
STAT6−/−
|
|||
| MLN | Spleen | MLN | Spleen | |
| IL-4 | 11.2 ± 6.6 | 212.4 ± 16.3 | 0 ± 0 | 0 ± 0 |
| IL-13 | 46.6 ± 6.4 | 288 ± 7.3 | 0 ± 0 | 54.5 ± 11 |
| IFN-γ | 9.6 ± 1.6 | 13.5 ± 1.7 | 7.5 ± 1.6 | 11.5 ± 2.7 |
STAT6+/+ and STAT6−/− mice were infected with T. spiralis orally and killed on day 14 p.i. MLN or spleen cells were stimulated with ConA for 24 h, and levels of IL-4, IL-13, and IFN-γ present in the supernatant were investigated by enzyme-linked immunosorbent assay. Each value represents the mean ± SEM from three mice.
Intestinal eosinophilia during T. spiralis infection is partially STAT6 dependent.
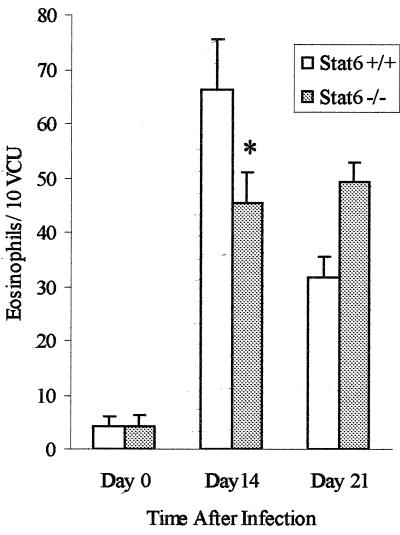
We next investigated intestinal tissue eosinophilia, which is considered to be a Th2-mediated characteristic of intestinal nematode infection. Significantly higher numbers of eosinophils were observed in the intestines of STAT6+/+ mice on days 14 and 21 after T. spiralis infection than in those of noninfected control mice. We also observed significantly more intestinal eosinophils in infected STAT6−/− mice than in non infected STAT6−/− mice on days 14 and 21 p.i. However, we observed significantly fewer eosinophils in STAT6−/− mice than in STAT6+/+ mice on day 14 p.i. (Fig. 5). These results indicate that intestinal eosinophilia in T. spiralis infection is only partially dependent on the STAT6 pathway.
FIG. 5.
Numbers of intestinal eosinophils in STAT6+/+ and STAT6−/− mice during T. spiralis infection. Mice were infected with T. spiralis orally and killed on the days indicated to determine intestinal eosinophil numbers. Day 0 represents data from control mice. Each value represents the mean ± SEM from four animals. ∗, significantly different between STAT6+/+ and STAT6−/− mice. VCU, villus crypt unit.
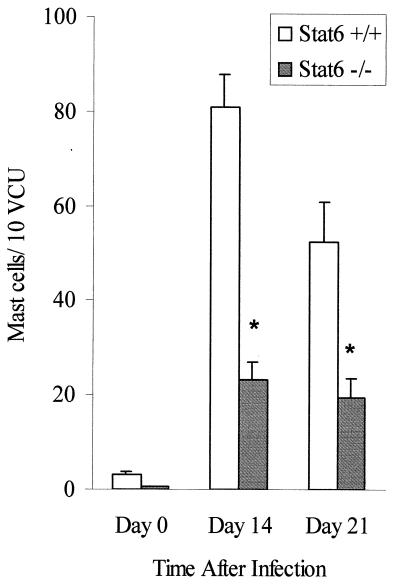
Intestinal mastocytosis during T. spiralis infection is STAT6 dependent.
We next investigated the development of intestinal mucosal mastocytosis as another Th2 parameter in STAT6+/+ and STAT6−/− mice. The number of mast cells in the small intestine increased after T. spiralis infection in STAT6+/+ mice. In contrast, we observed significantly fewer mast cells in STAT6−/− mice infected with T. spiralis (Fig. 6), which indicates a role for STAT6 in the development of mucosal mastocytosis following this nematode infection.
FIG. 6.
Intestinal mucosal mast cell responses in T. spiralis-infected STAT6+/+ and STAT6−/− mice. Mice were infected with 375 T. spiralis larvae and killed at the time points indicated to determine mucosal mast cell numbers in the small intestine. Day 0 represents data from control noninfected mice. Each value represents the mean ± SEM from four animals. ∗, significantly different between STAT6+/+ and STAT6 −/− mice. VCU, villus crypt unit.
DISCUSSION
The major finding of this is study is the demonstration of a critical role for STAT6 signaling in the generation of intestinal smooth muscle hypercontractility during primary infection with T. spiralis. Hypercontractility was evident postinfection in STAT6+/+ but not STAT6−/− animals. Since the expulsion of worms was significantly impaired in STAT6−/− mice, we postulate that the absence of STAT6 signaling prevents the development of infection-induced muscle hypercontractility, thus reducing propulsive forces within the gut, resulting in delayed eviction of the parasite from the gastrointestinal tract. These results provide the first evidence that STAT6 is essential for the development of intestinal muscle hypercontractility during primary nematode infection.
We consider muscle hypercontractility to be an important component of host defense during primary infection with nematode parasites, based on the following reasoning. First, altered gut motility is a robust finding during primary infection with several nematode parasites (14, 16, 41, 45), and smooth muscle, in addition to nerves and other cell types, is an important determinant of motility. Initial studies had demonstrated increased aboral intestinal transit in extrinisically denervated intestinal segments from nematode-infected animals, indicating that the factors responsible for the aboral force generation are located within the gut wall, rather than the autonomic or central nervous system (4). We had previously shown that inflammation-induced muscle hypercontractility is prominent in the proximal part of the intestine and that the distal regions such as the ileum and colon exhibit hypocontractility (21, 29). This distribution of changes would create an aboral gradient in muscle tension development during infection, promoting aboral propulsion of luminal contents. If such forces contribute to the expulsion of parasites, then one might expect to see a relationship between the magnitude of muscle hypercontractility and the ability of the host to evict worms from the gut. This is indeed the case, with strong responders to nematode infection such as NIH Swiss mice exhibiting the greatest degree of muscle hypercontractility and slow responders such B10. BR mice exhibiting only a mild degree of hypercontractility (41).
The case for a role for muscle hypercontractility in the process of worm expulsion is further strengthened by identifying a common underlying mechanism. Recent studies by Vallance et al. (42, 43) have demonstrated that the development of muscle hypercontractility is markedly attenuated in athymic, CD4- and major histocompatibility complex class II-deficient mice during T. spiralis infection. These results indicate that the integrity of the immune system is essential for the optimal development of muscle hypercontractility in this model. They also suggest that the processes underlying worm expulsion and muscle hypercontractility may share a common immunological basis.
IL-13, a pleiotropic immunoregulatory cytokine produced principally by activated T cells, shares a number of biological properties with IL-4 (30), including the activation of a common tyrosine kinase. Studies using Nippostrongylus brasiliensis and Trichuris muris infection of IL-4−/−, STAT6−/−, IL-4Rα−/−, and IL-13−/− animals indicated that IL-13 plays an essential role in Th2 cell-mediated expulsion of these parasites (5, 30, 38). Recently IL-13-dependent expulsion of worms in nematode-infected IL-4−/− mice has also been reported (6), indicating that IL-13 may compensate for the absence of IL-4.
Our finding of an increased expression of IL-4 and IL-13 mRNA in the muscularis externa of T. spiralis-infected STAT6+/+ mice provided a rational basis for considering these cytokines as mediators of the muscle hypercontractility. As there was no constitutive expression of IL-4 or IL-13 in the muscularis externa of STAT6+/+ mice, their expression postinfection seems to be due to influx of T cells into the muscle layers. Previous studies have demonstrated the infiltration of muscle layers by T lymphocytes during T. spiralis infection (12) and in patients with inflammatory bowel disease (15). It has been also reported that the infiltrating T cells in muscle layers in inflammatory bowel disease are both activated and divided forms, implying that they respond to antigen and antigen presentation within the muscle layer (15). Considering the interface between muscle changes and T cells (43) during T. spiralis infection, we postulate that hypercontractility is generated by the local production of IL-4 and IL-13 by T cells in the muscularis externa, as there was no expression of these cytokines in the tissue of STAT6−/− mice postinfection. We speculate that the generation of a smaller degree of muscle hypercontractility in infected IL-4−/− mice reflects the action of IL-13, a situation similar to that recently demonstrated in the context of worm expulsion in IL-4−/− mice (6).
Our interpretation of the local production of IL-4 and IL-13 inducing hypercontractility of muscle is supported by preliminary results from our laboratory (2). In that study, preincubation of dispersed murine intestinal muscle cells with either IL-4 or IL-13 resulted in an increased contractile response to subsequent stimulation by carbachol. This effect was abrogated when the STAT6 inhibitor leflunomide was added to the preincubation medium, indicating that these cytokines act directly on smooth muscle cells to induce hypercontractility via the STAT6 pathway.
Other components of the immune response in T. spiralis include intestinal mastocytosis and eosinophilia, and we examined the extent to which these responses are STAT6 dependent. We found a reduced mastocytotic response in infected STAT6−/− mice. Mast cells are generally considered to be important in the host response to infection with nematodes (1, 26), including T. spiralis (3, 20, 22), although some controversy exists (7, 28, 40). In a recent study of mast cell-deficient W/Wv mice, we also found that the expulsion of T. spiralis was delayed and intestinal motor function was altered (B. A. Vallance, P. A. Blennerhassett, J. D. Huizinga, and S. M. Collins, submitted for publication). The latter was due in part to the absence of c-kit, as the motor changes were not normalized after mast cell reconstitution by bone marrow graft, indicating a role for the c-kit-dependent interstitial cells of Cajal. These findings indicate that the motor response to nematode infection is complex and involves several effector cells including the interstitial cells of Cajal as well as other cells including smooth muscle cells.
The precise role of eosinophils in host protection against nematode infection is unclear. Despite a significant eosinophilia seen in primary T. spiralis infection, the precise role for eosinophils in host protective immunity remains to be determined. In STAT6−/− mice, with demonstrably defective Th2 development and delayed worm expulsion, we observed only a partial suppression of infection-induced eosinophilia. At least two mechanisms may produce eosinophilia. A STAT6-dependent mechanism involves IL-5 production (37), while a STAT6-independent mechanism(s) may involve activation of the eosinophil chemoattractant eotaxin, which recently has been shown to play a critical role in intestinal eosinophilia (31) and is effective in producing eosinophilia in IL-5-deficient mice (33). Eotaxin may also act cooperatively with IL-5 to promote the recruitment of eosinophils into tissues (10). Taken together, these findings explain why eosinophilia was observed in our infected STAT6−/− mice. Our results suggest that eosinophils are not critical for worm expulsion, consistent with a previous study in which treatment of mice with anti-IL-5 antibody ablated eosinophilia but failed to prevent the expulsion of worms in T. spiralis infection (23). The presence of eosinophilia in the absence of muscle hypercontractility in infected STAT6−/− mice in this study indicates that these cells do not play a major role in the development of muscle hypercontractility.
In conclusion, our study indicates that STAT6 is critical for the development of intestinal muscle hypercontractility during primary infection of mice with T. spiralis. Taken in conjunction with other work, our findings lead us to hypothesize that IL-4 and IL-13, acting via STAT6, mediate the hypercontractility of muscle and that this, in turn, contributes to the efficient eviction of adult worms from the gut following nematode infection.
ACKNOWLEDGMENT
This study was funded by a grant from Medical Research Council (MRC) of Canada.
REFERENCES
- 1.Abe T, Sugaya H, Yoshimura K, Nawa Y. Induction of the expulsion of Strongyloides ratti and retension of Nippostrongylus brasiliensis in athymic nude mice by repetitive administration of recombinant interleukin-3. Immunology. 1992;76:10–16. [PMC free article] [PubMed] [Google Scholar]
- 2.Akiho H, Blennerhassett P A, Collins S M. The roles of interleukins-4 and - IL-13, and Stat6 in inflammation-induced hypercontractility of murine isolated smooth muscle cells. Gastroenterology. 2000;118:4. doi: 10.1152/ajpgi.2002.282.2.G226. , A710. (Abstract.) [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh H, Murrell K D. The intestinal mast cell response to Trichinella spiralis infection in mast cell deficient W/Wv mice. J Parasitol. 1984;70:767–773. [PubMed] [Google Scholar]
- 4.Alizadeh H, Weems W A, Castro G A. Intrinsic jejunal propulsion in the guinea pig during parasitism with Trichinella spiralis. Gastroenterology. 1987;93:784–790. doi: 10.1016/0016-5085(87)90441-0. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft A J, McKenzie A N J, Grencis R K. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 6.Bancroft A J, Artis D, Donaldson D D, Sypek J P, Grencis R K. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur J Immunol. 2000;30:2083–2091. doi: 10.1002/1521-4141(200007)30:7<2083::AID-IMMU2083>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Betts C J, Else K J. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999;21:45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 8.Castro G A, Fairbairn D. Carbohydrates and lipids in Trichinella spiralis larvae and their utilization. J Parasitol. 1969;55:51–58. [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Collins P D, Marleau S, Griffiths-Johnson D A, Jose P J, Williams T J. Cooperation between interleukin-5 and the chemokine eotakine to induce eosinophil accommodation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins S M. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111:1683–1689. doi: 10.1016/s0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- 12.Dzwonkowski P, Stead R H, Blennerhasset M G, Collins S M. Induction of class II major histocompatilibity complex (MHC II) in enteric smooth muscle. Gastroenterology. 1991;100:A577. . (Abstract.) [Google Scholar]
- 13.Else K J, Finkelman F D. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145–1158. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 14.Farmer S G. Propulsive activity of the rat small intestine during infection with the nematode Nippostrongylus brasiliensis. Parasite Immunol. 1981;3:227–234. doi: 10.1111/j.1365-3024.1981.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 15.Fell J M E, Walker-Smith J A, Spencer J, McDonald T T. The distribution of dividing T cells throughout the intestinal wall in inflammatory bowel disease (IBD) Clin Exp Immunol. 1996;104:280–285. doi: 10.1046/j.1365-2249.1996.999701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhill J M, Finkelman F, Urban J, Morris S, Maliszewski C R, Shea-Donohue T. H. polygyrus and interleukin (IL)-4 enhance excitation of mouse small intestinal longitudinal muscle through leukotrine (LT) D4 modulation of cholinergic neurotransmission. Gastroenterology. 1995;108:A286. . (Abstract.) [Google Scholar]
- 17.Gray P W, Goeddel D V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci USA. 1983;80:5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grencis R K, Reidlinger J, Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985;56:213–218. [PMC free article] [PubMed] [Google Scholar]
- 19.Grencis R K, Hultner L, Else K J. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991;74:329–332. [PMC free article] [PubMed] [Google Scholar]
- 20.Grencis R K, Else K J, Huntley J F, Nishikawa S I. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–59. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 21.Grossi L, McHugh K, Collins S M. On the specificity of altered muscle function in experimental colitis in rats. Gastroenterology. 1993;104:1049–1056. doi: 10.1016/0016-5085(93)90273-f. [DOI] [PubMed] [Google Scholar]
- 22.Ha T Y, Reed N D, Croll P K. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herndon F J, Kayes S G. Depletion of eosinophils by anti IL-5 antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunological resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- 24.Kaplan M H, Schindler U, Smiley S T, Grusby M J. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 25.Keegan A D, Nelms K, Wang L, Pierce J H, Paul W E. Interleukin-4 receptor: signaling mechanisms. Immunol Today. 1994;15:423–431. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 26.Khan A I, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol. 1993;23:551–559. doi: 10.1016/0020-7519(93)90159-v. [DOI] [PubMed] [Google Scholar]
- 27.Krzesicki R F, Winterrowd G E, Brashler J R, Hatfield C A, Griffin R L, Filder S F, Kolbasa K P, Shull K L, Richard I M, Chin J E. Identification of cytokine and adhesion molecule mRNA in murine lung tissue and isolated T cells and eosinophils by semiquantitative reverse transcriptase polymerase chain reaction. Am J Respir Cell Mol Biol. 1997;16:693–701. doi: 10.1165/ajrcmb.16.6.9191471. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence C E, Paterson J C M, Higgins L M, Macdonald T T, Kennedy M W, Garside P. IL-4-regulated enteropathy in an intestinal nematode infection. Eur J Immunol. 1998;28:2672–2684. doi: 10.1002/(SICI)1521-4141(199809)28:09<2672::AID-IMMU2672>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Marzio L, Blennerhassett P, Chiverton S, Vermillion D L, Langer J, Collins S M. Altered smooth muscle function in worm-free regions in Trichinella infected rats. Am J Physiol. 1990;259:G306–G313. doi: 10.1152/ajpgi.1990.259.2.G306. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie G J, Emson C L, Bell S E, Anderson S, Fallon P G, Zurawski G, Murray R, McKenzie A N J. Impaired development of Th2 cells in IL-13 deficient mice. Immunity. 1998;9:423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 31.Mishra A, Hogan S P, Lee J J, Foster P S, Rothenberg M E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Investig. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 33.Mould A W, Matthaei K I, Yong I G, Foster P S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Investig. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelms K, Keegan A D, Zamorano J, Ryan J J, Paul W E. The IL-4 receptor: signaling mechanisms and biological functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 35.Svetic A, Finkelman F D, Jian Y C, Dieffenbach C W, Scott D E, McCarthy K F, Steinberg A D, Gause W C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- 36.Sukhdeo M V K, Croll N A. Gut propulsion in mice infected with Trichinella spiralis. J Parasitol. 1981;67:906–910. [PubMed] [Google Scholar]
- 37.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 38.Urban J, Noben-Trauth N, Donaldson D, Madden K, Morris S, Collins M, Finkelman F. IL-13, IL-4R and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 39.Urban J F, Jr, Schopf L, Morris S C, Orekhova T, Madden K B, Betts C J, Gamble H R, Byrd C, Donaldson D, Else K J, Finkelman F D. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell and T cell dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 40.Uber C L, Roth R L, Levy D A. Expulsion of Nippostrongylus brasiliensis by mice deficient in mast cells. Nature. 1980;287:226–228. doi: 10.1038/287226a0. [DOI] [PubMed] [Google Scholar]
- 41.Vallance B A, Blennerhassett P A, Collins S M. Increased intestinal muscle contractility and worm expulsion in nematode infected mice. Am J Physiol. 1997;35:G321–G327. doi: 10.1152/ajpgi.1997.272.2.G321. [DOI] [PubMed] [Google Scholar]
- 42.Vallance B A, Collins S M, Snider D P. CD4 T cells and major histocompatibility couple class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infect Immun. 1999;67:6090–6097. doi: 10.1128/iai.67.11.6090-6097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallance B A, Croitoru K, Collins S M. T lymphocytes dependent and independent intestinal smooth muscle dysfunction in the T. spiralis infected mouse. Am J Physiol. 1998;275:G1157–G1165. doi: 10.1152/ajpgi.1998.275.5.G1157. [DOI] [PubMed] [Google Scholar]
- 44.Vallance B A, Blennerhassett P A, Deng Y, Mathaei K I, Yong I G, Collins S M. IL-5 contributes to worm expulsion and muscle hypercontractility in primary T. spiralis infection. Am J Physiol. 1999;277:G400–G408. doi: 10.1152/ajpgi.1999.277.2.G400. [DOI] [PubMed] [Google Scholar]
- 45.Vermillion D L, Collins S M. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am J Physiol. 1988;254:G124–G129. doi: 10.1152/ajpgi.1988.254.1.G124. [DOI] [PubMed] [Google Scholar]