Abstract
Objectives
Normal features of the ST segment are poorly characterised in dogs. This study aimed to describe ST segment characteristics in a population of healthy dogs.
Materials and Methods
Medical records were reviewed to identify healthy dogs that underwent an electrocardiogram. Several ST segment qualitative parameters were evaluated: presence/absence of deviation, type of deviation (depression/elevation) and morphological patterns of depression (horizontal, downsloping, upsloping and sagging) and elevation (horizontal, concave and convex). Moreover, the amplitude of ST segment depression/elevation was measured. The potential effect of sex, bodyweight, age and somatotype on the presence/absence of ST segment deviation was evaluated through binary logistic regression.
Results
One hundred and eighty dogs were enrolled. The deviation was evident in 43 of 180 dogs (23.9%), among which 36 showed depression and seven showed elevation. The median depression amplitude was 0.1 (range 0.05 to 0.3) mV. The mean elevation amplitude was 0.136 ±0.055 mV. Concerning depression morphology, the horizontal pattern was overrepresented, followed by the downsloping and upsloping ones. Concerning elevation morphology, all dogs showed a concave pattern. No meaningful effect of sex, bodyweight, age and somatotype on the presence/absence of ST segment deviation was documented.
Clinical Significance
Normal features of canine ST segment were described and made available for clinical use.
INTRODUCTION
Although the history of electrocardiography dates back to the 19th century, the surface electrocardiogram still remains a valuable diagnostic test because it is relatively inexpensive, widely available and easy to perform (Tilley 1992a). Electrocardiography is a recording at the body surface of the average electrical potential generated in the heart graphed as voltage over time. The sequential movement of electrical currents during each cardiac cycle creates the main electrocardiographic components, namely the P wave, the PQ interval, the QRS complex, the ST segment, the T wave and the QT interval (Tilley 1992a, Santilli et al. 2018a). In human medicine, each waveform, segment and interval has been exhaustively studied in large populations of apparently healthy people to provide reliable reference intervals aimed at guiding cardiologists in performing a proper electrocardiographic analysis (Hiss et al. 1960, Dmitrienke et al. 2005, Rijnbeek et al. 2014). Conversely, veterinary studies analysing electrocardiograms from healthy dogs have focused predominantly on components of depolarisation (i.e., P wave and QRS complex); indeed, the assessment of ventricular repolarisation has been largely limited to the simple measurement of the QT interval duration and, to a lesser extent, to the analysis of a few T‐wave features (Hill 1968, Eckenfels & Trieb 1979, Hanton & Rabemampianina 2006, Mukherjee et al. 2020). Historically, poor attention has been paid by veterinary cardiologists to an important component of ventricular repolarisation, namely the ST segment, whose interpretation is currently performed almost empirically in small animal practice. This knowledge gap can have relevant clinical consequences, as it may not allow for the early identification of ventricular repolarisation abnormalities expressed in the form of ST segment changes that may arise from myocardial injury due to cardiac (Tilley 1992b, Davainis et al. 2004, Sleeper et al. 2015, Santilli et al. 2018b, Lekane et al. 2020) and extracardiac diseases (Tilley 1992b, Dvir et al. 2004, Surachetpong et al. 2016, Santilli et al. 2018b, Pugliese et al. 2020), the administration of drugs and medium contrast agents (Tilley 1992b, Khurana et al. 2014, Santilli et al. 2018b, Tamogi et al. 2020) and electrolyte abnormalities (Tilley 1992b, Santilli et al. 2018b).
The purpose of this study was to evaluate the electrocardiographic characteristics of the ST segment in a large population of healthy dogs, and to explore the possible influence of variables such as sex, age, bodyweight (BW) and somatotype on this electrocardiographic parameter.
MATERIALS AND METHODS
Study design and inclusion criteria
For the purpose of this multicentre retrospective analysis, the medical records of healthy dogs that underwent an electrocardiogram as part of their diagnostic evaluation between January 2014 and October 2021 were reviewed by a board‐certified cardiologist (G. R.). The reasons for electrocardiographic analysis in an apparently healthy subject could include preoperative evaluations before elective surgery (e.g., castration, spaying) or screening for specific cardiac disorders in dogs from highly predisposed breeds (e.g., dilated cardiomyopathy in Doberman Pinschers, arrhythmogenic right ventricular cardiomyopathy in boxers). To be included, dogs had to be at least 1 year of age and have a complete case record, including signalment, history, clinical findings, a minimum laboratory database (including serum electrolytes) performed less than 6 months before the examination and cardiac investigation. The latter had to include at least a transthoracic echocardiogram and 2‐minute six‐lead electrocardiogram performed according to standardised techniques (details below) (Tilley 1992a, Thomas et al. 1993). Dogs were considered healthy based on an unremarkable clinical history and physical examination as well as on normal laboratory, electrocardiographic and echocardiographic findings. Dogs were excluded if they had any cardiac or extra‐cardiac disease as well as if they needed sedation or were receiving any therapy at the time of cardiovascular examination.
Electrocardiographic analysis
In all dogs, an electrocardiogram was conducted with the dogs positioned and manually restrained in right lateral recumbency, with the front legs placed parallel to each other and perpendicular to the long axis of the body and the hind limbs in a neutral semiflexed position (Tilley 1992a). The animals were unsedated and were allowed time to acclimatise so that the electrocardiograms could be taken from relaxed dogs. All electrocardiograms were recorded using two commercially available machines (Cube ECG, Cardioline S.p.A., Caverano, Italy; ECG110S, Cardioline S.p.A.). The electrocardiographic leads were attached to the skin by flattened alligator clips at the level of the olecranon on the caudal aspect of the forelimb and over the patellar ligaments on the cranial aspect of the hind limbs (Tilley 1992a). Alcohol was applied to maintain electrical contact with the skin. Standard six‐lead electrocardiograms (leads I, II, III, aVR, aVL and aVF) were recorded for 1‐2minutes in all dogs at a paper speed of 50 mm/s and paper sensitivity of 10 mm/mV. For each animal, an effort was made to obtain an electrocardiographic tracing showing a clean isoelectric line with easily recognisable waveforms. The same investigator (G. R.) manually measured intervals and amplitudes using a calliper and ruler with 0.5‐mm graduations. Three representative consecutive beats were used to measure various electrocardiographic variables, and the results were averaged for each variable. Initially, the heart rhythm and conventional parameters (i.e., heart rate, amplitude and duration of the P wave, PQ‐interval duration, R‐wave amplitude, duration and mean electrical axis in the frontal plane of the QRS complex and QT‐interval duration) were assessed according to the standard technique to evaluate if there were electrocardiographic abnormalities. Such parameters were judged to be normal/abnormal according to previously reported reference intervals (Tilley 1992b, Santilli et al. 2018a; Romito et al. 2022). Subsequently, according to the aim of this study, particular attention was given to ST segment evaluation. For this purpose, pertinent measurements and morphological analysis were performed following indications from human medicine (Brady et al. 2001, Smith 2006, Rautaharju et al. 2009, Jeong et al. Jeong et al. 2010, Chugh & Chugh 2012). Specifically, the J point (i.e., the point of intersection between the descending branch of the R wave, or the ascending branch of the S wave when present and the baseline) was used as the start point of the ST segment; the endpoint of the ST segment was the start point of the T wave. The ST segment features were all assessed in lead II (Fig 1) and included:
absence/presence ST segment deviation: defined as “absent” or “present” when the segment was isoelectric (i.e., flat, neither positive nor negative) or displaced compared with the baseline, respectively. In the case of inconstant baseline due to a discrepancy between the PQ and TP intervals, the former interval was used for comparison;
type of ST segment deviation: defined as “depressed” or “elevated” when the ST segment deviation was below or above the baseline, respectively;
type of ST segment depression: possible patterns that were looked for included “horizontal” (i.e., the J point is below the baseline and the ST segment remains straight thereafter), “upsloping” (i.e., the ST segment is depressed below the baseline at its origin and then slopes upward, returning to baseline), “downsloping” (i.e., the ST segment is depressed below the baseline at its origin and then it slopes further downward) and “sagging” depression (i.e., the ST segment sags downward and becomes cup‐shaped);
type of ST segment elevation: possible patterns that were looked for included “horizontal” (i.e., the J point is above the baseline and the ST segment remains straight thereafter), “concave” (i.e., the J point is above the baseline and the ST segment, instead of remaining straight, tends to be arched upward) and “convex” elevation (i.e., the J point is above the baseline and the ST segment, instead of remaining straight, tends to be arched downward);
the maximal amplitude of the ST segment deviation (mV): measured from the baseline to the peak of the ST segment.
FIG 1.
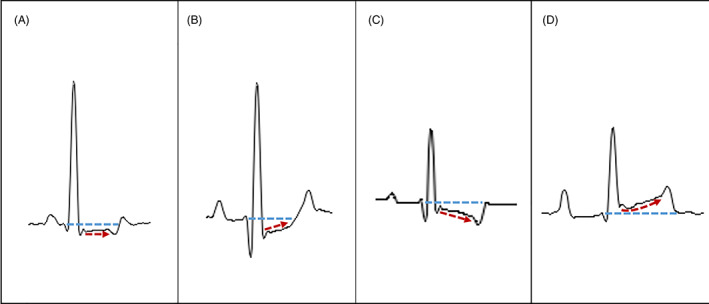
Selected close‐ups from electrocardiographic tracings obtained from four healthy dogs. In all cases, a single complex recorded in lead II was selected to illustrate in detail the distinct ST segment morphological patterns. (A) Horizontal depression. (B) Upsloping depression. (C) Downsloping depression. (D) Concave elevation. Fine undulations of the black lines which represent the contour of the electrocardiographic waves, intervals and segments are inevitably due to overmagnification of the tracings. In each panel, the blue and red dotted lines help to identify the reference baseline and ST segment morphologies, respectively
Statistical analysis
All electrocardiographic data were collected into electronic spreadsheets (Microsoft Excel version 2016, Microsoft Corporation, Redmond, WA) and then imported into a statistical software package (MedCalc Statistical Software version 19.5.1, Ostend, Belgium) for further analysis. All continuous variables were tested for their distribution with a Shapiro‐Wilk normality test. Initial descriptive statistics included mean ±standard deviation for normally distributed data and median and range (minimum to maximum) for data that were not normally distributed. For statistical analysis, when needed, dogs were divided according to their BW, age and somatotype adapting criteria from previous literature (Groppetti et al. 2017, Harvey 2021, Wallis et al. 2021). Specifically, dogs were purposefully classified as small (<15 kg) and medium‐to‐large dogs (≥15 kg), as adult (1 to 6 years) and senior‐to‐geriatric dogs (≥7 years) and as brachymorphic and non‐brachymorphic dogs (i.e., mesomorphic and dolichomorphic dogs were grouped together). The potential effect of sex, BW, age and somatotype (as predictors) on presence/absence of ST segment deviation (as the outcome) was evaluated through a binary logistic regression assessing odds ratios with 95% confidence intervals. A P‐value of less than 0.05 was considered significant.
RESULTS
One hundred and eighty dogs fulfilled the inclusion criteria. Seventy‐six were male (62 entire and 14 neutered) and 104 were female (82 entire and 22 spayed) with a median age of 4 years (1 to 17 years). One hundred and twenty‐nine and 51 were classified as adult and senior‐to‐geriatric dogs, respectively. Median BW was 12.9 kg (1.7 to 78 kg). One hundred and eight and 72 were classified as small and medium‐to‐large dogs, respectively. Sixty‐eight dogs were French Bulldogs; 18 were mixed‐breed dogs; 16 dogs were Doberman Pinschers; nine dogs were Labrador retrievers; and six dogs were German shepherds. Other breeds (i.e., those including less than six dogs) are reported in Supporting Information Data S1. One hundred and ten and 70 were classified as non‐brachymorphic and brachymorphic dogs, respectively. One hundred and thirty‐two dogs had a regular sinus rhythm, while the remaining 48 dogs showed sinus arrhythmia. In all dogs, standard electrographic parameters were within the reference intervals.
Regarding the ST segment, deviation was evident in 43 of 180 dogs (23.9%) while the remaining 137 of 180 dogs (76.1%) had an isoelectric ST segment. Thirty‐six dogs showed ST segment depression. This corresponded to 20% when considering the entire study population (i.e., 36 of 180) and to 83.7% when considering exclusively dogs with ST‐deviation (i.e., 36 of 43). Among these dogs, there were 15 of 36 male and 21 of 36 female dogs (42% and 58%, respectively), 25 of 36 small and 11 of 36 medium‐to‐large dogs (69% and 31%, respectively), 30 of 36 adult and six of 36 senior‐to‐geriatric dogs (83% and 17%, respectively) and 14 of 36 brachymorphic and 22 of 36 non‐brachymorphic dogs (39% and 61%, respectively). Seven dogs showed ST segment elevation. This corresponded to 3.9% when considering the entire study population (i.e., seven of 180) and to 16.3% when considering exclusively dogs with ST deviation (i.e., seven of 43). Among these dogs, there were five of seven male and two of seven female dogs (71% and 29%, respectively), three of seven small and four of seven medium‐to‐large dogs (43% and 57%, respectively), five of seven adult and two of seven senior‐to‐geriatric dogs (71% and 29%, respectively) and two of seven brachymorphic and five of seven non‐brachymorphic dogs (29% and 71%, respectively). The median value of depression was 0.1 (0.05 to 0.3) mV. In 34 of 36 (94.4%) dogs with ST segment depression, the segment amplitude was less than 0.2 mV, while only two of 36 (5.6%) dogs showed higher values (i.e., 0.25 mV and 0.3 mV). The mean value of elevation was 0.136 ±0.055 mV. In 5 of 7 (71.4%) dogs with ST segment elevation, the segment amplitude was less than 0.15 mV, while only two of seven (28.6%) dogs showed higher values (i.e., both 0.2 mV). In the overall population, depression morphological patterns were distributed as follows: horizontal in 22 of 36 (61.1%) dogs, downsloping in eight of 36 (22.2%) dogs and upsloping in six of 36 (16.7%) dogs; whereas no dog showed a sagging pattern. Concerning elevation morphological patterns, seven of seven (100%) dogs showed a concave pattern, while horizontal and convex elevations were not identified. The investigated predictors (i.e., sex, BW, age and somatotype) did not affect significantly the probability to develop the ST segment deviation (Table 1).
Table 1.
. Binary logistic regression assessing the probability to develop the ST segment deviation among the considered predictors (sex, BW, age and somatotype) in 180 healthy dogs
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | Baseline | Baseline | |
| Female | 0.79 | 0.39 to 1.58 | 0.51 |
| BW category | |||
| Small | Baseline | Baseline | |
| Medium‐to‐large | 0.75 | 0.37 to 1.53 | 0.43 |
| Age category | |||
| Adult | Baseline | Baseline | |
| Senior‐to‐geriatric | 0.50 | 0.21 to 1.17 | 0.11 |
| Somatotype category | |||
| Non‐brachymorphic | Baseline | Baseline | |
| Brachymorphic | 0.91 | 0.45 to 1.85 | 0.79 |
BW bodyweight, OR odds ratio, 95% CI 95% confidence intervals, P P value
DISCUSSION
In this study, we systematically analysed several ST segment features in a large population of healthy subjects. The main findings of the present study revealed that in healthy dogs: (1) mild ST segment deviation is not an uncommon finding; (2) ST segment depression is more common than elevation; (3) ST segment depression and elevation have a similar amplitude that frequently, but not always, is below 0.2 mV; and (4) the horizontal and the concave morphological patterns are overrepresented compared with other morphologies in dogs with ST segment depression and elevation, respectively.
Knowledge on physiology of ST segment genesis represents an essential prerequisite to understand our results. Moreover, in light of the extensive human literature on ST segment analysis as well as the scant veterinary data on this electrocardiographic parameter, knowledge of the characteristics of the human ST segment is extremely useful for comparison with findings from our study population. On the electrocardiogram, the ST segment encompasses the time between the end of ventricular depolarisation (i.e., the end of the QRS complex, namely the J point) and the beginning of ventricular repolarisation (i.e., beginning of the T wave). Hemodynamically, the ST segment corresponds to ventricular systole, whereas electrophysiologically, it correlates with phase 2 (plateau phase) of the transmembrane action potential (Tilley 1992b, Rautaharju et al. 2009, Chugh & Chugh 2012). This phase occurs after phase 0 and phase 1, during which the majority of myocardial cells have gone through rapid depolarisation and early repolarisation, respectively. Physiologically, during phase 2, there are slow and similar transmembrane voltage changes in the ventricular myocardial cells. In other words, in this phase, all ventricular myocytes attain about the same membrane potential. Therefore, no significant difference in potential is recorded by the surface electrocardiogram, which consequently often displays an almost horizontal segment on the isoelectric line (Tilley 1992b, Rautaharju et al. 2009, Chugh & Chugh 2012). Thus, any significant change in voltage gradients during the plateau phase of the action potential can result in variations in the ST segment (Rautaharju et al. 2009, Chugh & Chugh 2012, Klabunde 2017). A perfect example of this is represented by acute myocardial ischemia, as it induces a loss of membrane integrity and leakage of potassium from the cell to the extracellular region, moving down its concentration gradient. As a result, the resting membrane potential becomes less negative (or more positive). However, the remaining portion of the non‐ischemic myocardium has resting membrane potential physiologically set at −90 mV. This creates a voltage gradient between ischemic and non‐ischemic cardiomyocytes, which, in turn, leads to an abnormal electrical current (also known as “current of injury” or “injury current”). During diastole, this current flows intracellularly from the ischemic region toward normal myocytes and tends to depolarise the latter, leading to the emergence of the ST segment deviation on the surface electrocardiogram (Rautaharju et al. 2009, Chugh & Chugh 2012, Klabunde 2017).
However, it is important to acknowledge that the human ST segment may vary not only as a consequence of disease states, but also under physiological conditions. Indeed, factors such as age, sex, ethnicity and physical training may influence the ST segment amplitude in healthy people. For example, although no sex difference is apparent before puberty, both the J point and ST segment undergo a marked elevation after puberty in males, but not in females (Surawicz & Parikh 2002, Ezaki et al. 2010). In males, the peak of the J point and ST segment amplitudes is observed between 20 and 29 years of age; then, with advancing age, a gradual and progressive reduction is observed after the third decade of life (Ezaki et al. 2010). Regarding ethnicity and physical training, several studies have demonstrated a higher rate of ST segment elevation in black athletes, both male and female, compared with sex‐matched white athletes (Papadakis et al. 2011). This explains why different limits of normality have been reported in the scientific statement on the assessment of ST segment amplitude from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, the American College of Cardiology Foundation and the Heart Rhythm Society (e.g., 0.35 mV in black men, 0.3 mV in white men, 0.25 mV in black women and 0.2 mV in white women) (Rautaharju et al. 2009). Interestingly, the aforesaid demographic variables can also affect the ST segment morphology. For example, a steeper ST segment slope appears more common at puberty in men in comparison to women (Bidoggia et al. 2000, Surawicz & Parikh 2002), while the convex ST segment pattern is identified more frequently in black athletes compared with black sedentary individuals and white athletes (Papadakis et al. 2011). For this reason, physicians are encouraged to interpret the ST segment features in the context of the individual patient characteristics, with the aim of properly discerning normal variants that may apparently mimic myocardial ischemia from true abnormal patterns that require urgent therapeutic decisions.
In light of the complexity of interpretation of human ST segment and its variability in healthy individuals, we felt it necessary not only to evaluate ST segment features in the entire study population, but also in selected categories. Interestingly, contrary to human literature, we were unable to demonstrate any apparent potential effect of sex, BW, age and somatotype on the presence/absence of ST segment deviation. Despite the high number of dogs that we enrolled, the number of subjects showing ST segment deviation was relatively low. Therefore, it is possible that such a discrepancy may arise from a limited sample size. It is also possible that some differences may become clearer by investigating in future further possible influencing variables, such as the endocrine status (e.g., sex hormone levels), body condition score and athletic level (e.g., working/sporting dogs versus companion dogs) of healthy dogs. Lastly, it can be even speculated that the disagreement between results from the present study and findings reported in human literature is an expression of intrinsic species‐related differences concerning ventricular action potential (Detweiler 2010). In any case, from a purely clinical point of view, findings from the present report may be advantageous in daily small animal practice. Indeed, the probable absence of a significant variability between healthy dogs of different sex, age, BW and somatotype would make it easier to identify normality limits that can be applied to the entire canine population.
Comparison of present results on the amplitude of ST segment deviation with previous veterinary literature is significantly limited by the fact that, although veterinary textbooks cite cutoffs of ST segment depression (i.e., <0.2 mV) and elevation (i.e., <0.15 mV), the reported limits appear not to be based on a solid scientific data derived from original studies (Tilley 1992b, Santilli et al. 2018a,b). Nevertheless, it is interesting to note that the majority of dogs enrolled herein had ST segment depression and elevation within the cutoffs mentioned by textbooks (i.e., 94.4% had a depression <0.2 mV and 71.4% had an elevation <0.15 mV). Even more intriguingly, it should be noted that some dogs had higher values, as the maximum values that with measured were 0.3 mV and 0.2 mV for depression and elevation, respectively. Accordingly, we believe that ST segment depression less than 0.2 mV and elevation less than 0.15 mV may represent appropriate cutoffs for many but not all dogs. We also believe that depression between 0.2 and 0.3 mV as well as elevation between 0.15 and 0.2 mV should not be systematically interpreted as a certain pathological sign, especially when these values are observed in healthy dogs with an otherwise normal electrocardiographic tracing. On the other hand, based on results from the previous study, ST segment depression more than 0.3 mV and ST‐segment elevation more than 0.2 mV could raise suspicion and prompt further investigation. However, in dogs, further studies are needed to accurately correlate magnitude of ST segment changes on the surface electrocardiogram with echocardiographic evidence of myocardial damage and other tests established in the human field such as serum cardiac troponin I measurements.
Even more difficult, if not impossible, is the comparison between this study and the previous veterinary literature concerning the prevalence of ST segment deviation and that of each morphological pattern. As mentioned above, this is due to the lack of studies aimed at comprehensively analysing this electrocardiographic parameter in healthy dogs. As humans represent the only mammalian species in which normal ST segment features have been thoroughly evaluated (Detweiler 2010) many results of this study could be only interpreted in the light of data from human medicine. Concerning the frequency of ST segment deviation, we identified this electrocardiographic sign in 23.9% of the study population. Interestingly, this finding closely resembles the results of a large‐scale electrocardiographic survey conducted in 6014 people, where the prevalence of ST segment deviation in the frontal plane was 24.3% (Hiss et al. 1960). Also, the prevalence of ST segment elevation documented in the current study (i.e., 3.9%) is within the range reported in human medicine (i.e., 1 to 14% in healthy individuals). In contrast, the prevalence of ST segment depression observed in dogs in this report (i.e., 20%) is higher than that found in healthy humans (i.e., 1 to 10%) (Srikantia et al. 1964, de Bacquer et al. 1995, Mansi & Nash 2001). Concerning the morphological pattern of ST segment deviation, the horizontal one was overrepresented among dogs with depression and all dogs with elevation showed a concave pattern. Intriguingly, no dogs showed a sagging depression, a horizontal elevation or a convex elevation. The lack of sagging depression among healthy dogs is in line with the human literature, as this electrocardiographic sign is typical of pathologic states in people (e.g., digitalis toxicity; Pollehn et al. 2002). In contrast, present findings on horizontal and convex elevation disagree with human cardiology, as these morphologies have been documented in some healthy individuals (Hiss et al. 1960, Thomas et al. 1960, Rautaharju et al. 2009, Chugh & Chugh 2012). As previously stated for ST segment amplitude, it remains to be conclusively established whether the aforesaid discrepancies may be due to intrinsic species‐related differences or to the relatively limited number of dogs showing ST segment deviation. However, it is important to underline that, based on our current experience, a sagging depression as well as horizontal or convex elevation may be advised as suspicious in dogs, although future studies enrolling a larger number of subjects with ST segment deviation are needed to expand further our preliminary findings.
A further consideration should be made concerning the measurement of ST segment amplitude. Indeed, it is important to highlight that, in the absence of definitive guidelines on measurement of the ST segment relative to the J point in dogs, we standardised our analysis by simply measuring the point of maximal ST segment amplitude irrespective of distance from the J point. Should J waves be clearly visible in an individual dog (of note, this may occur under physiological conditions as well as disease states such as myocardial ischemia, hypothermia or hypercalcaemia; Agudelo & Schanilec 2015), then care should be taken not to include these in ST segment measurements. However, it should be noticed that no patient in our study showed evidence of visible J waves affecting the J point or ST segment analysis. Another possible confounding factor that may affect the ST segment assessment is represented by the Ta wave (i.e., the deflection of atrial repolarization). In patients with normal sinus rhythm, Ta wave has a very minimal impact on the surface electrocardiogram since the QRS complex normally obscures its evidence on tracings (as, physiologically, they occur almost simultaneously and the Ta wave has a very low amplitude; mean value in dogs, −0.09 mV) (Perego et al. 2014,Srour & Hyder 2014, Manne 2018). However, the Ta wave may become evident in patients with some disturbances of cardiac rhythm, including short and long PR intervals, second‐degree and third‐degree atrioventricular blocks and ectopic atrial rhythms (Perego et al. 2014, Srour & Hyder 2014, Manne 2018). In these cases, the Ta wave may superimpose on the ST segment and alter its morphology and/or amplitude (Srour & Hyder 2014, Manne 2018). However, such a risk may be reasonably excluded in the current study population, as all dogs were healthy subjects showing sinus rhythm and normal standard electrographic parameters.
This study has some limitations that should be highlighted. First, the retrospective design prevented standardising the time between electrocardiographic examinations and laboratory analysis, which made it impossible to completely exclude the possibility that some hematologic abnormalities could have occurred at the time of the electrocardiogram. However, in our opinion, it is not likely that relevant laboratory changes able to remarkably affect the ST segment (e.g., severe potassium disturbances) would have arisen in the time between the blood tests and the electrocardiogram, since all enrolled dogs appeared clinically healthy at enrolment. Another possible limitation concerning blood tests is represented by the lack of measurement of the serum conconcentration of cardiac troponin I. Indeed, such a test could have been useful to exclude the possibility of an ongoing, occult myocardial damage potentially capable of causing a change in the ST segment. Nevertheless, it should be noted that previous veterinary studies aimed at describing the electrocardiographic features of apparently healthy dogs did not include any laboratory investigations as part of the inclusion criteria (Hill 1968, Eckenfels & Trieb 1979, Hanton & Rabemampianina 2006, Mukherjee et al. 2020). Accordingly, although the time lapse of this study may appear relatively wide (i.e., laboratory database performed ≤6 months from the cardiologic examination) and cardiac troponin I was not measured, the methodology of our investigation remains one of the most rigorous in the pertinent canine literature. The retrospective nature of the study also precluded us from obtaining complete data concerning the systemic arterial blood pressure. Nevertheless, dogs with moderate/severe systemic hypertension were very unlikely to be inadvertently enrolled given the lack of typical physical (e.g., blindness, centrally localising neurological signs) and echocardiographic (e.g., left ventricular wall thickening) signs at the time of the electrocardiogram (Acierno et al. 2018). Concerning mild systemic hypertension, although this condition could be sometimes clinically/echocardiographically occult, it is unlikely able to cause relevant electrocardiographic changes (Cortadellas et al. 2006). Secondly, our analysis was conducted exclusively on six‐lead electrocardiograms and all the ST segment features were assessed only in lead II. This technical choice was adopted as, in daily small animal practice, standard leads are the most commonly employed, and lead II represents the one conventionally used by the majority of veterinarians for electrocardiographic measurements as it is expected to give the biggest deflections (since it most accurately aligns with the physiological mean electrical vector of depolarization) (Tilley 1992b, Santilli et al. 2018a,b). Thirdly, although the overall number of dogs enrolled was high, the number of subjects showing ST segment deviation was relatively low. This was inevitably due to the fact that such electrocardiographic sign does not manifest in all subjects. Fourthly, the sample size of groups composing each category was not always equally balanced (e.g., female and adult dogs were overrepresented compared with male and senior‐to‐geriatric dogs, respectively). Finally, the high prevalence of French Bulldogs, due to the current great diffusion of this breed in our country (Romito et al. 2022), may have represented a bias.
In conclusion, a detailed description of several features of the normal canine ST segment is now available for clinical use as well as future research on canine ventricular repolarisation. Because our findings were obtained using a standardised electrocardiographic analysis in a large population of healthy dogs, they likely represent a reliable and broadly applicable guide for the interpretation of the ST segment in this species. For the same reason, our maximum amplitude values of ST segment depression and elevation could be incorporated into future textbooks on canine electrocardiography to replace the currently cited cutoffs.
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Author Contributions
Giovanni Romito: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (lead); writing – original draft (lead). Prisca Castagna: Conceptualization (supporting); project administration (supporting); resources (supporting); supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting). Nazzareno Giuseppe Pelle: Resources (supporting); supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting). Fabio Testa: Resources (supporting); supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting). Maria Chiara Sabetti: Data curation (supporting); project administration (supporting); software (supporting); supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting). Mario Cipone: Methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting).
Supporting information
Appendix S1: Supporting Information.
Acknowledgements
Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement.
References
- Acierno, M. J. , Brown, S. , Coleman, A. E. , et al. (2018) ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. Journal of Veterinary Internal Medicine 32, 1803‐1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo, C. F. & Schanilec, P. (2015) The canine J wave. Veterinární Medicína 60, 208‐212 [Google Scholar]
- Bidoggia, H. , Maciel, J. P. , Capalozza, N. , et al. (2000) Sex‐dependent electrocardiographic pattern of cardiac repolarization. American Heart Journal 140, 430‐436 [DOI] [PubMed] [Google Scholar]
- Brady, W. J. , Syverud, S. A. , Beagle, C. , et al. (2001) Electrocardiographic ST‐segment elevation: the diagnosis of acute myocardial infarction by morphologic analysis of the ST segment. Academic Emergency Medicine 8, 961‐967 [DOI] [PubMed] [Google Scholar]
- Chugh, S. N. & Chugh, K. (2012) Myocardial infarction. In: Textbook of Clinical Electrocardiography. 3rd edn. Eds Chugh S. N. and Chugh K.. Jaypee Brothers Medical Publisher Ltd., New Delhi, India. pp 193‐210 [Google Scholar]
- Cortadellas, O. , del Palacio, M. J. , Bayón, A. , et al. (2006) Systemic hypertension in dogs with leishmaniasis: prevalence and clinical consequences. Journal of Veterinary Internal Medicine 20, 941‐947 [DOI] [PubMed] [Google Scholar]
- Davainis, G. M. , Meurs, K. M. & Wright, N. A. (2004) The relationship of resting S‐T segment depression to the severity of subvalvular aortic stenosis and the presence of ventricular premature complexes in the dog. Journal of the American Animal Hospital Association 40, 20‐23 [DOI] [PubMed] [Google Scholar]
- de Bacquer, D. , Martins Pereira, L. S. , de Backer, G. , et al. (1995) Prevalence and correlates of ECG abnormalities in the adult Belgian. Journal of Electrocardiology 28, 1‐11 [DOI] [PubMed] [Google Scholar]
- Detweiler, D. K. (2010) The mammalian electrocardiogram: comparative features. In: Comprehensive Electrocardiology. 2nd edn. Eds Macfarlane P. W., van Oosterom A., Pahlm O., Kligfield P., Janse M. and Camm J.. Springer London Ltd, London, UK. pp 1911‐1941 [Google Scholar]
- Dmitrienke, A. A. , Sides, G. D. , Winters, K. J. , et al. (2005) Electrocardiogram reference ranges derived from a standardized clinical trial population. Therapeutic Innovation & Regulatory Science 39, 395‐405 [Google Scholar]
- Dvir, E. , Lobetti, R. G. , Jacobson, L. S. , et al. (2004) Electrocardiographic changes and cardiac pathology in canine babesiosis. Journal of Veterinary Cardiology 6, 15‐23 [DOI] [PubMed] [Google Scholar]
- Eckenfels, A. & Trieb, G. (1979) The normal electrocardiogram of the conscious Beagle dog. Toxicology and Applied Pharmacology 47, 567‐584 [DOI] [PubMed] [Google Scholar]
- Ezaki, K. , Nakagawa, M. , Taniguchi, Y. , et al. (2010) Gender differences in the ST segment. Effect of androgen‐deprivation therapy and possible role of testosterone. Circulation Journal 74, 2448‐2454 [DOI] [PubMed] [Google Scholar]
- Groppetti, D. , Pecile, A. , Palestrini, C. , et al. (2017) A national census of birth weight in purebred dogs in Italy. Animals 7, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton, G. & Rabemampianina, Y. (2006) The electrocardiogram of the Beagle dog: reference values and effect of sex, genetic strain, body position and heart rate. Laboratory Animals 40, 123‐136 [DOI] [PubMed] [Google Scholar]
- Harvey, N. D. (2021) How old is my dog? Identification of rational age groupings in pet dogs based upon normative age‐linked processes. Frontiers in Veterinary Science 8, 643085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, J. D. (1968) The electrocardiogram in dogs with standardized body and limb positions. Journal of Electrocardiology 1, 175‐182 [DOI] [PubMed] [Google Scholar]
- Hiss, R. G. , Lamb, L. E. & Allen, M. F. (1960) Electrocardiographic findings in 67,375 asymptomatic subjects. X. Normal values. American Journal of Cardiology 6, 178‐189 [DOI] [PubMed] [Google Scholar]
- Jeong, G. Y. , Yu, K. H. , Yoon, M. J. , et al. (2010) ST shape classification in ECG by constructing reference ST set. Medical Engineering & Physics 32, 1025‐1031 [DOI] [PubMed] [Google Scholar]
- Khurana, A. , Kumar, A. , Sharma, S. K. , et al. (2014) Electrocardiographic and hemato‐biochemical effects of two balanced anesthetic protocols in dogs. Veterinary World 7, 835‐841 [Google Scholar]
- Klabunde, R. E. (2017) Cardiac electrophysiology: normal and ischemic ionic currents and the ECG. Advances in Physiology Education 41, 29‐37 [DOI] [PubMed] [Google Scholar]
- Lekane, M. , Connolly, D. , Smets, P. , et al. (2020) Clinical, ECG and echocardiographic findings in a canine case series of presumptive myocardial infarction. Journal of Veterinary Internal Medicine 34, 403‐404 [Google Scholar]
- Manne, J. R. R. (2018) Atrial repolarization waves (Ta) mimicking inferior wall ST segment elevation myocardial infarction in a patient with ectopic atrial rhythm. Case Reports in Medicine 2018, 1015730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi, I. A. & Nash, I. S. (2001) Ethnic differences in the ST segment of the electrocardiogram: a comparative study among six ethnic groups. American Journal of Emergency Medicine 19, 541‐544 [DOI] [PubMed] [Google Scholar]
- Mukherjee, J. , Mohapatra, S. S. , Jana, S. , et al. (2020) A study on the electrocardiography in dogs: reference values and their comparison among breeds, sex, and age groups. Veterinary World 13, 2216‐2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis, M. , Carre, F. , Kervio, G. , et al. (2011) The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro‐Caribbean origin. European Heart Journal 32, 2304‐2313 [DOI] [PubMed] [Google Scholar]
- Perego, M. , Skert, S. & Santilli, R. A. (2014) Analysis of the atrial repolarization wave in dogs with third‐degree atrioventricular block. American Journal of Veterinary Research 75, 54‐58 [DOI] [PubMed] [Google Scholar]
- Pollehn, T. , Brady, W. J. , Perron, A. D. , et al. (2002) The electrocardiographic differential diagnosis of ST segment depression. Emergency Medical Journal 19, 129‐135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese, M. , La Maestra, R. , Passantino, A. , et al. (2020) Electrocardiographic findings in bitches affected by closed cervix pyometra. Veterinary Sciences 7, 183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaharju, P. M. , Surawiczm, B. , Gettes, L. S. , et al. (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 119, 241‐250 [DOI] [PubMed] [Google Scholar]
- Rijnbeek, P. R. , van Herpen, G. , Bots, M. L. , et al. (2014) Normal values of the electrocardiogram for ages 16‐90 years. Journal of Electrocardiology 47, 914‐921 [DOI] [PubMed] [Google Scholar]
- Romito, G. , Castagna, P. , Sabetti, M. C. , et al. (2022) Physiological shift of the vetricular mean electrical axis in healthy French Bulldogs: a retrospective electrocardiographic analysis of 80 healthy dogs. Journal of Veterinary Cardiology 42, 34‐42 [DOI] [PubMed] [Google Scholar]
- Santilli, R. A. , Moïse, N. S. , Pariaut, R. , et al. (2018a) Formation and interpretation of the electrocardiographic waves. In: Electrocardiography of the Dog and Cat ‐ Diagnosis of Arrhythmia. 2nd edn. Eds Santilli R. A., Moïse N. S., Pariaut R. and Perego M.. Edra, Milan, Italy. pp 35‐70 [Google Scholar]
- Santilli, R. A. , Moïse, N. S. , Pariaut, R. , et al. (2018b) Electrocardiographic changes secondary to systemic disorders and drugs. In: Electrocardiography of the Dog and Cat ‐ Diagnosis of Arrhythmia. 2nd edn. Eds Santilli R. A., Moïse N. S., Pariaut R. and Perego M.. Edra, Milan, Italy. pp 293‐312 [Google Scholar]
- Sleeper, M. M. , Maczuzak, M. E. & Bender, S. J. (2015) Myocardial infarct associated with a partial thickness left atrial tear in a dog with mitral insufficiency. Journal of Veterinary Cardiology 17, 229‐236 [DOI] [PubMed] [Google Scholar]
- Smith, S. W. (2006) ST segment elevation differs depending on the method of measurement. Academic Emergency Medicine 13, 406‐412 [DOI] [PubMed] [Google Scholar]
- Srikantia, S. G. , Padmavati, S. & Gopalan, C. (1964) The electrocardiogram in some Indian population groups. Circulation 29, 118‐123 [DOI] [PubMed] [Google Scholar]
- Srour, J. F. & Hyder, O. (2014) Catch the Ta wave: a source of ST‐segment elevation. American Journal of Medicine 127, 288‐290 [DOI] [PubMed] [Google Scholar]
- Surachetpong, S. D. , Vichit, P. & Hunprasit, V. (2016) Measurements of cardiac troponin I and creatine kinase myocardium isoform in dogs with diabetic ketoacidosis. Comparative Clinical Pathology 25, 1185‐1191 [Google Scholar]
- Surawicz, B. & Parikh, S. R. (2002) Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. Journal of the American College of Cardiology 40, 1870‐1876 [DOI] [PubMed] [Google Scholar]
- Tamogi, H. , Itami, T. , Hori, A. , et al. (2020) ST segment depression and ventricular fibrillation in a dog after contrast agent administration. Journal of Veterinary Medical Science 82, 1714‐1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, W. P. , Gaber, C. E. , Jacobs, G. J. , et al. (1993) Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. Journal of Veterinary Internal Medicine 7, 247‐252 [DOI] [PubMed] [Google Scholar]
- Thomas, J. , Harris, E. & Lassiter, G. (1960) Observations on the T wave and S‐T segment changes in the precordial electrocardiogram of 320 young Negro adults. American Journal of Cardiology 5, 468‐472 [DOI] [PubMed] [Google Scholar]
- Tilley, L. P. (1992a) Principles of electrocardiographic recording. In: Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment. 3rd edn. Ed Tilley L. P.. Lippincott Williams & Wilkins, Philadelphia, PA, USA. pp 21‐39 [Google Scholar]
- Tilley, L. P. (1992b) Analysis of canine P‐QRS‐T deflections. In: Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment. 3rd edn. Ed Tilley L. P.. Lippincott Williams & Wilkins, Philadelphia, PA, USA. pp 59‐99 [Google Scholar]
- Wallis, C. , Saito, E. K. , Salt, C. , et al. (2021) Association of periodontal disease with breed size, breed, weight, and age in pure‐bred client‐owned dogs in the United States. Veterinary Journal 275, 105717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.


