Abstract
Background and objectives
Cystic fibrosis (CF)‐related diabetes (CFRD) affects 50% of CF adults. Gut microbial imbalance (dysbiosis) aggravates their inflammatory response and contributes to insulin resistance (IR). We hypothesized that probiotics may improve glucose tolerance by correcting dysbiosis.
Methods
A single‐center prospective pilot study assessing the effect of Vivomixx® probiotic (450 billion/sachet) on clinical status, spirometry, lung clearance index (LCI), and quality of life (QOL) questionnaires; inflammatory parameters (urine and stool metabolomics, blood cytokines); and glucose metabolism (oral glucose tolerance test [OGTT]), continuous glucose monitoring [CGM], and homeostasis model assessment of IR (HOMA‐IR) in CF patients.
Results
Twenty‐three CF patients (six CFRD), mean age 17.7 ± 8.2 years. After 4 months of probiotic administration, urinary cysteine (p = 0.018), lactulose (p = 0.028), arabinose (p = 0.036), mannitol (p = 0.041), and indole 3‐lactate (p = 0.046) significantly increased, while 3‐methylhistidine (p = 0.046) and N‐acetyl glutamine (p = 0.047) decreased. Stool 2‐Hydroxyisobutyrate (p = 0.022) and 3‐methyl‐2‐oxovalerate (p = 0.034) decreased. Principal component analysis, based on urine metabolites, found significant partitions between subjects at the end of treatment compared to baseline (p = 0.004). After 2 months of probiotics, the digestive symptoms domain of Cystic Fibrosis Questionnaire‐Revised improved (p = 0.007). In the nondiabetic patients, a slight decrease in HOMA‐IR, from 2.28 to 1.86, was observed. There was no significant change in spirometry results, LCI, blood cytokines and CGM.
Conclusions
Changes in urine and stool metabolic profiles, following the administration of probiotics, may suggest a positive effect on glucose metabolism in CF. Larger long‐term studies are needed to confirm our findings. Understanding the interplay between dysbiosis, inflammation, and glucose metabolism may help preventing CFRD.
Keywords: CFRD, cystic fibrosis, glucose metabolism, metabolomics, probiotics
1. INTRODUCTION
The most common endocrine complication in cystic fibrosis (CF) is CF‐related diabetes (CFRD). By the fourth decade of life, 50% of patients are affected, and another 35% have impaired glucose tolerance (IGT). 1 CFRD is a specific form of diabetes, with some characteristics resembling type 1 (e.g., occurrence at a young age) and type 2 diabetes (e.g., preceded by a long period of glucose intolerance). 1 , 2 The central cause is pancreatic endocrine dysfunction with reduced insulin secretion due to beta‐cell destruction, but insulin resistance (IR) also contributes to the pathogenesis.
The Cystic Fibrosis Foundation (CFF) and the American Diabetes Association (ADA) recommend to perform annual diabetes screening, starting at the age 10 years, with an oral glucose tolerance test (OGTT). 2 Continuous glucose monitoring (CGM) measures subcutaneous interstitial glucose levels every 5–10 min, and reflects physiologic real‐life glucose fluctuations. 1
Disease‐associated microbiome alterations are often referred to as a “dysbiosis,” the imbalance due to deficiency of beneficial function, or the presence of a detrimental microbial activity. CF is associated with profound dysbiosis, both pulmonary and intestinal, from a very young age. Prolonged antibiotic treatment may further disturb the microbial balance. 3 , 4 Dysbiosis aggravates the pro‐inflammatory response in CF, and may contribute to pulmonary deterioration, as well as to IR.
The World Health Organization defines probiotics as “live micro‐organisms which, when administered in adequate amounts, confer a health benefit on the host.” 5 In CF, studies have suggested that probiotics may help in restoring intestinal microbial balance. 6 Probiotics may influence the “gut–lung axis,” a microbial link between the respiratory and gastrointestinal tracts; it involves the transfer of metabolites and immunomodulatory signals between the gut and lungs. 7
A few studies have assessed probiotics administration for the prevention and treatment of type 2 diabetes, all in non‐CF patients. Kassaian et al. demonstrated beneficial effect on the glycemic control of prediabetic adults. 8 Promising results on glycemic control were also demonstrated by Naito et al. in obese, prediabetic adults. 9 The mechanism by which probiotics may affect glycemic control is not well understood; suggested mechanisms include reduction of intestinal permeability, reduction of pro‐inflammatory cytokines and oxidative stress, regulation of nuclear factor κ B and insulin signaling pathway, and increased insulin sensitivity. 10 We are unaware of studies assessing the effect of probiotics on glycemic control in CF.
Vivomixx® is a high potency probiotic food supplement containing eight strains of live bacteria in concentrations of 450 billion per sachet. 11 In a small pilot study of prediabetic adolescents, Vivomixx® supplementation resulted in lower fasting blood glucose concentrations after the first month compared to the control group. After 4 months, the glycemic control was similar in the two groups. This was attributed to suboptimal compliance with probiotics and the small sample size. However, intestinal bacteriome analysis after 4 months revealed a statistically significant decrease in bacterial lipopolysaccharides levels, which may lead to increased insulin sensitivity. 12
The metabolome is the ensemble of the low weight metabolites of urine and feces; it has been found to be greatly informative about diseases involving inflammatory processes. Moreover, the concentration of specific molecules may be influenced by alterations of gut microbiota related to inflammation. 13
Taken together, dysbiosis in CF may increase inflammation, which may induce IR and affect clinical status. We aimed to assess the effect of Vivomixx® administration on clinical outcomes, metabolomics, inflammatory parameters, and glucose metabolism in CF patients.
2. METHODS
This was a prospective, single‐center pilot study evaluating the effect of a probiotic formulation (Vivomixx®) administration on glucose metabolism and clinical outcome in CF patients. The study was reviewed and approved by the institutional board, and we obtained informed consent from subjects or their legal guardians before recruitment. The study population included patients with CF aged >8 years, who are treated at our tertiary center. Patients who had symptoms consistent with a pulmonary exacerbation during the visit or the preceding week were excluded.
2.1. Study intervention and evaluations
For 4 months, the patients received daily the probiotic product 450 billion/sachet (Vivomixx® in the European Union, Visbiome in the US; De Simone Formulation in Korea). The preparation contains eight live freeze‐dried bacterial species (four strains of lactobacilli [Lactobacillus paracasei DSM24733®/NCIMB 30439, Lactobacillus plantarum DSM24730®/NCIMB 30437, Lactobacillus acidophilus DSM24735®/NCIMB 30442, and Lactobacillus helveticus DSM24734®/NCIMB 30440]); three strains of bifidobacteria (Bifidobacterium animals subsp. lactis DSM24736®/NCIMB 30435, B. animalis subsp.lactis DSM24737®/NCIMB 30436, and Bifidobacterium breve DSM24732®/NCIMB 30441) and the Streptococcus salivarius subsp. thermophilus DSM24731®/NCIMB 30438). The patient's age defined the dosage: <12 years to one sachet/day: 12–18 years to two sachets/day; and >18 years to two sachets twice daily.
The patients underwent evaluations at baseline and after 4 months of probiotic administration:
Clinical and demographics variables were extracted from patients' files, including age, gender, pancreatic status, and CFRD.
Spirometry was performed in accordance with the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force, using a KoKo spirometer (n‐Spire Healthcare, Inc.). 14 Results are expressed as absolute values and percent predicted (mean ± SD) derived from Polgar and Quanjer. 15
Lung clearance index (LCI) : Multiple breath washout (MBW) measurements were performed using the Easy‐One Pro, MBW Module (NDD Medical Technologies), as first described by Fuchs et al. in 2008. 16 LCI is the number of functional residual capacity (FRC) turnovers required to washout the nitrogen, and was calculated as the total expired volume during the washout phase divided by the FRC. 17 An increased LCI (>7) indicates more FRC turnovers required for the washout, reflecting inhomogeneous ventilation. 18 , 19
Quality of life (QOL) questionnaire : The Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) is a validated disease‐specific instrument designed to measure the impact on overall health, daily life, perceived well‐being, and symptoms. The questionnaire includes nine QoL domains (physical, role/school, vitality, emotion, social, body image, eating, treatment burden, health perceptions) and three symptom scales (weight, respiratory, and digestion). Each item is summed to generate a domain score and standardized. Scores range from 0 to 100, with higher scores indicating better health. 20
Spirometry, LCI, and QOL were also evaluated after 2 months of probiotic administration.
Metabolomic analysis : Before and after treatment, urine and stool samples were collected for metabolomics analysis. Among the platforms employed for investigations on the fecal and urinary metabolome, proton nuclear magnetic resonance (1H‐NMR) has been found to grant high reproducibility and minimal sample preparation. 21 Analysis was performed starting from 350 µl of urine added to 350 µl of water and to 200 μl of a D2O solution of 3‐(trimethylsilyl)‐propionic‐2,2,3,3‐d4 acid sodium salt (TSP) 10 mM set to pH 7.0 using a 1 M phosphate buffer. The signals were assigned by comparing their multiplicity and chemical shift with Chenomx software data bank (ver 8.3, Chenomx Inc.). Water‐soluble molecules were extracted from 80 mg of feces by vortex mixing with 1 ml of water followed by centrifugation. The supernatant (700 µl) was subjected to metabolomics analysis as described above for urine.
Inflammatory cytokines : Venous blood was collected, processed, and stored at −80°C until use. IL‐6, TNF‐α, and α1AT were measured by commercial kits as follows: LEGEND MAX™ human IL‐6 enzyme‐linked immunosorbent assay (ELISA) kit (BLG‐430507), LEGEND MAX™ human TNF‐a ELISA Kit (BLG‐430207), and human alpha 1‐antitrypsin ELISA kit (E‐80A1T), respectively.
Evaluation of glucose metabolism : OGTT, with measurement of insulin and C‐peptide levels, was performed before the initiation of probiotics and at the end of treatment; for the nondiabetic patients, HOMA‐IR index was calculated from OGTT values (fasting insulin (pmol/L)/6 × fasting glucose(mg/dl)/405); CGM was performed using FreeStyle Libre sensors (Abbott Ltd.) for 2 weeks—before the initiation of probiotic supplementation and during the last 2 weeks of treatment.
2.2. Statistical methods
Statistical analysis was performed using SPSS version 25. Descriptive statistics were used for the demographic and clinical variables. Results are expressed as absolute values, mean ± SD or median and interquartile range (IQR), as appropriate.
The primary outcome was metabolomics before and after probiotics. Secondary outcomes were parameters of glucose metabolism (CGM, OGTT, and HOMA‐IR), pulmonary function tests (spirometry and LCI), inflammatory cytokines, and QoL (CFO‐R).
The Kolmogorov–Smirnov test was used to assess the normality of the quantitative parameters; parametric and nonparametric tests were used as appropriate. For metabolomics, both univariate and multivariate statistical approaches were used. The univariate Wilcoxon signed‐rank test and the paired t‐test were performed to compare differences before and after probiotics, for the not‐normally and normally distributed continuous variables, respectively. The multivariate principal component analysis (PCA) was performed as ordination method to confirm the potential of metabolites to discriminate between the groups. The presence of statistically significant partitions between groups was evaluated by applying the analysis of similarities (ANOSIM) with 1000 permutations on the matrix of Euclidean distances between samples. Where necessary, calculated p value was adjusted by using the Benjamini–Hochberg procedure to take account of multiple comparisons.
In each case, a p ≤ 0.05 was considered statistically significant.
3. RESULTS
Twenty‐three CF patients (11 males, 48%), mean age 17.65 ± 8.2 years, participated in the study. Baseline patient characteristics are presented in Table 1. As can be seen, forced expiratory volume in 1 s was mildly reduced (mean 69.7 ± 18.5% predicted). Six patients (26%) had CFRD, treated with insulin. Patients with CFRD had lower FEF 25%–75% (30 ± 14.99 vs. 58.94 ± 29.91, p = 0.035) and higher LCI (16.14 ± 4.06 vs. 11.56 ± 3.1, p = 0.013). As expected, patients with CFRD also had higher hemoglobin A1C levels. For the nondiabetic patients (n = 17), the median HOMA‐IR at baseline was 2.28 (IQR range 1.29–2.74). Cytokine levels before and after probiotic were available for the whole group. The levels of α‐1‐antitrypsin (α1AT), interleukin‐6 (IL‐6), and tumor necrosis factor‐alpha (TNF‐α) did not change following the administration of probiotics (621.33 ± 143.45 vs. 664.56 ± 197.17, p = 0.57; 29.94 ± 25.95 vs. 26.94 ± 24.21, p = 0.33; and 3.36 ± 0.46 vs. 3.88 ± 5.41, p = 1.00, respectively).
Table 1.
Baseline patient characteristics
| Total (n = 23) | CFRD (n = 6) | Nondiabetic (n = 17) | p value | |
|---|---|---|---|---|
| Age (years) | 17.65 ± 8.2 | 23.1 ± 10.99 | 16.2 ± 5.8 | 0.16 |
| Male (%) | 11 (48%) | 3 (50%) | 8 (47%) | 1.00 |
| PI | 15 (65%) | 4 (66.7%) | 11 (64/7%) | 0.37 |
| BMI (kg/m2) | 19.6 ± 3.4 | 19.3 ± 3.98 | 19.7 ± 3.3 | 0.83 |
| FVC (%) | 85.3 ± 14.02 | 81.33 ± 12.2 | 85.3 ± 14.02 | 0.43 |
| FEV1 (%) | 69.7 ± 18.5 | 58.3 ± 17.6 | 73.7 ± 17.6 | 0.08 |
| FEF25–75 (%) | 51.4 ± 29.5 | 30 ± 14.99 | 58.9 ± 29.9 | 0.035 |
| LCI | 12.6 ± 3.8 | 16.1 ± 4.1 | 11.6 ± 3.1 | 0.013 |
| HbA1C | 5.61 ± 0.59 | 6.2 ± 0.6 | 5.4 ± 0.5 | 0.003 |
| HOMA‐IRa | 2.28 (1.29–2.74) |
Note: Values are presented as mean ± SD.
Abbreviations: BMI, body mass index; CFRD, cystic fibrosis‐related diabetes: FEF25–75, forced expiratory flow between 25% and 75% of FVC; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HbA1C, hemoglobin A1C; HOMA‐IR, Homeostatic Model Assessment for Insulin Resistance; IQR, interquartile range; LCI, lung clearance index; PI, pancreatic insufficient.
Median (IQR).
No significant side effects, such as GI intolerance, were found. Some patients complained about the taste and were reinstructed about the correct administration of the probiotics (mixture of the powder with cold food, yogurt, or ice cream). For 16 patients, urine samples were available at both the beginning and the end of probiotic administration. Figure 1 presents the significant results of urine metabolites. After 4 months of probiotic administration, there was a significant increase in urinary levels of cysteine (p = 0.018), lactulose (p = 0.028), arabinose (p = 0.036), mannitol (p = 0.041), and indole 3‐lactate (p = 0.046), while a significant decrease was observed in 3‐methylhistidine (p = 0.046) and N‐acetyl glutamine (p = 0.047). Furthermore, significant decreases in stool levels of 2‐hydroxyisobutyrate (p = 0.022) and 3‐methyl‐2‐oxovalerate (p = 0.034) were found at the end of the 4 months of probiotic administration. The multivariate PCA analysis was based on the metabolic compounds that significantly changed at the end of the probiotic therapy. The urinary profile of specific metabolites was effective in determining a significant separation between groups of subjects, before and after the administration of Vivomixx® (ANOSIM R = 0.126, p = 0.004) (Figure 2).
Figure 1.

Box and Whisker plots showing levels of urinary metabolites before and after probiotic therapy (only compounds with significant changes are presented, with p values). [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.

Principal component analysis plot—multivariate analysis of urinary metabolomics. For each group, the 95% confidence interval was drawn. Numbers in parenthesis represent the percentage of the total variance explained by the principal components. [Color figure can be viewed at wileyonlinelibrary.com]
When stratifying the population of samples according to the presence of CFRD, no statistically significant differences in metabolic compounds between the groups were found at both time points. However, in the multivariate PCAs, based on metabolic compounds significantly modified in the whole population, significant partitions could still be found (patients with CFRD before vs. after, ANOSIM R: 0.187 p = 0.033; nondiabetic before vs after, ANOSIM R: 0.101 p = 0.030).
Seven (30.4%) of our patients were treated with CFTR modulators at the time of probiotics administration. Of them, urine metabolites were available for four. When stratifying the population of samples according to the administration of CFTR modulators, no statistically significant differences nor partition were observed at both time points. In the multivariate PCA analysis, significant partitions before and after probiotic administration were found only in subjects not taking CFTR modulators (ANOSIM R: 0.1 68 p = 0.003).
Paired stool samples were available for seven patients. Figure 3 presents the significant results. The metabolomics analysis of stool samples found a significant decrease in the concentration of 2‐hydroxyisobutyrate (p = 0.022) and 3‐methyl‐2‐oxovalerate (p = 0.034) compared to baseline.
Figure 3.
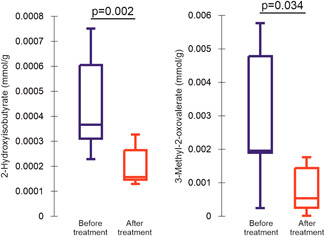
Box and Whisker plots showing levels of stool metabolites before and after probiotic therapy (only compounds with significant changes are presented, with p values). [Color figure can be viewed at wileyonlinelibrary.com]
After 2 months of probiotic therapy, the digestive symptoms domain of CFQ‐R improved (78.74 ± 21.95 at baseline vs. 85.51 ± 18.78 after 2 months, p = 0.007); while at the end of the 4 months of probiotic administration, the score returned to baseline values (78.26 ± 22.09). Additionally, there was a slight decrease in HOMA‐IR in the nondiabetic patients at the end of the study, from a median of 2.42 to 1.86 (p = 0.86). Table 2 presents the OGTT results before and after probiotics. As can be seen, no significant differences were found in glucose, insulin, and c‐peptide levels at the different time points. In the CGM measurements, there was no significant change in mean glucose levels (93.58 ± 16.91 vs. 98.16 ± 24.67, p = 0.48) or number of events with glucose >200 mg/dl (6.29 ± 3.15 vs. 4.71 ± 4.309, p = 0.15). Similarly, there was no significant change in spirometry results, LCI, and other domains of QOL.
Table 2.
OGTT results before and after probiotic administration.
| Glucose (mg/dl) | p value | Insulin (pmol/L) | p value | C‐peptide (pmol/L) | p value | |
|---|---|---|---|---|---|---|
| 0′ before | 87.76 ± 13.4 | 0.14 | 58.9634.53 | 0.084 | 571.89 ± 330.32 | 0.55 |
| 0′ after | 92.48 ± 10.72 | 45.5 ± 30.66 | 498.04 ± 208.31 | |||
| 30′ before | 156.52 ± 32.63 | 0.11 | 323.81 ± 349.15 | 1.00 | 1751.05 ± 1200.72 | 0.58 |
| 30′ after | 168.86 ± 46.73 | 368.2 ± 597.58 | 1515.98 ± 782.77 | |||
| 60′ before | 152.29 ± 47.49 | 0.29 | 395.03 ± 393.77 | 0.22 | 2121.17 ± 1165.25 | 0.88 |
| 60′ after | 248.43 ± 378.05 | 257.99 ± 191.75 | 2004.38 ± 1006.1 | |||
| 90′ before | 142.9 ± 61.86 | 0.61 | 282.40 ± 261.29 | 0.15 | 2147.71 ± 1085.06 | 0.6 |
| 90′ after | 142.95 64.29 | 230.73 ± 174.19 | 1993.66 ± 973.84 | |||
| 120′ before | 119.00 ± 53.76 | 0.35 | 232.56 ± 167.11 | 0.22 | 1897.07 ± 925.64 | 0.58 |
| 120′ after | 126.33 ± 69.83 | 191.25 ± 190.99 | 1761.22 ± 927.09 |
Abbreviations: OGTT, oral glucose tolerance test; ′, minutes.
4. DISCUSSION
In this single‐center prospective pilot study, probiotic administration in CF patients resulted in significant changes in stool and urine metabolomics. We also evaluated the effect of probiotics on clinical, inflammatory parameters and glucose metabolism, and found a slight, nonstatistically significant decrease in the HOMA‐IR index.
As mentioned previously, CFRD is one of the most common complications in CF and shares characteristics of type 1 and type 2 diabetes. The central cause is pancreatic endocrine dysfunction with reduced insulin secretion due to beta‐cell destruction, but IR also contributes to the pathogenesis. A phase of glucose intolerance with periods of repeated postprandial hyperglycemia, accompanied by worsening pulmonary status and nutritional decline, commonly precedes CFRD. 1 , 2
Microbial dysbiosis in CF enhances the inflammatory state, which in turn contributes to IR. Treatment with periodic acute courses of antimicrobials, as well as chronic therapy with antibiotics to treat airway infections, facilitate dysbiosis. Even when administered by inhalation, significant amounts of antibiotics are ingested and thus also affect the GI tract. 22
A systematic review found a reduction in pulmonary exacerbations in CF patients receiving probiotics. There was an improvement in subjective GI symptoms, without significant differences in fat absorption. 6 Probiotic supplementation did not change changes fecal calprotectin, clinical status, or microbiome profile, in a pilot study from the ESPGHAN Working Group. However, they found normalization of gut permeability in 13% of patients. 23 Notably, our study showed a transient improvement in the digestive symptom domain in CFQ‐R after 2 months of probiotic therapy, with return to baseline values at the end of the treatment period. This may be attributed to better compliance at the beginning of the study, but further conclusions cannot be made. Interestingly, the digestive symptom domain improved by 6.77 points. In a study examining the effect of pulmozyme on QOL, a year of treatment resulted in a significant improvement in several CFQ‐R domains, with the improvement of 5.5 points in caregivers' CFQ‐R digestive symptoms. 24 It should be noted that four patients required antibiotic therapy for pulmonary exacerbations during the study period; as mentioned earlier, antibiotics have been shown to have a detrimental effect on the gut microbiota. 25
Several studies examined the ability of Vivomixx® to restore the normal microbiome. In dogs with hemorrhagic diarrhea, Vivomixx® accelerated the normalization of the intestinal microbiome. Bacteria belonging to the Blautia and Faecalibacterium genera increased, and Clostridium decreased. 26 The ESPEN guidelines recommended using Vivomixx® in patients with mild to moderate ulcerative colitis (UC), confirming its efficacy in protecting the gut barrier and normalizing gut flora. 27 Vivomixx® was found to decrease the gut microbial Firmicutes to Bacteroidetes (F/B) ratio (representing dysbiosis markers) in obese adolescents 28 as well as in mice. 29 In CF patients, decreased levels of Bacteroidetes and Firmicutes were found compared to healthy controls, while reduced microbial richness correlated with intestinal inflammation. Postprobiotics, there was a significant increase in the proportion of Bacteroidetes (3.6%–16.9%) and Firmicutes (18.0%–38.2%). 6 We are not aware of studies assessing the effect of probiotics on glycemic control in CF.
In the metabolomic analysis, our study found a significant increase in urinary cysteine, lactulose, arabinose, mannitol, and indole 3‐lactate after 4 months of probiotic administration, as opposed to decreased levels of 3‐methylhistidine and N‐acetyl glutamine. In stool, 2‐hydroxyisobutyrate and 3‐methyl‐2‐oxovalerate decreased postprobiotics. The significant differences remained also when stratifying the group according to diabetic status. Several studies conducted in animals and humans considered metabolomics to identify and highlight modifications of molecules involved in inflammatory processes and blood glucose control. S‐ethyl cysteine (SEC) and s‐methyl cysteine (SMC) provided antioxidative, anti‐inflammatory, and protective effects on the kidneys of diabetic mice. 30 SCP‐80‐I (composed of arabinose, mannose, glucose, and galactose) exerted a potential hypoglycemic and hypolipidemic effect in streptozotocin‐induced diabetic rats. 31 The excretion of 3‐methylhistidine was significantly higher in patients with poor diabetic control 32 ; 13 metabolites (including N‐acetyl glutamine) exhibited a strong association with low GFR in nonproteinuric type 2 diabetes. 33 In a large cohort (859 subjects), a metabolic triplet containing 2‐hydroxybutyric acid was sensitive for the detection of prediabetes and diabetes 34 ; 2‐hydroxybutyrate and 3‐ methyl‐2‐oxovalerate were higher in serum and plasma of patients with inflammatory bowel disease. 35 Moreover, 2‐hydroxybutyrate and 2‐hydroxyisobutyrate increased in the plasma of undernourished mice. 36 Taken together, our results imply an anti‐inflammatory and a possible favorable effect on glucose metabolism after probiotic administration.
CFTR modulators may affect the intestinal microbiome. Ooi et al. found a favorable effect of Ivacaftor on gut flora and intestinal inflammation. 37 When performing subanalysis of our results according to treatment with CFTR modulators, we were not able to determine the significant effect of probiotic administration for subject taking CFTR modulators. This lack of significance could be due to the effect of CFTR modulators on the intestinal microbiome or by the reduced sample size determined by the adopted stratification.
In our study, there was a slight decrease in HOMA‐IR after probiotic administration, from 2.28 to 1.86. Although IR is defined as a value greater than the 75th percentile for nondiabetic subjects, the reported cut‐off values vary widely in the literature. 38 In a large cohort of nondiabetic individuals (2459 Spanish adults) the best HOMA‐IR cut‐off for IR ranged from 1.85 in men to 2.07 in women. 39 In a cross‐sectional study with 79 CF patients, HOMA‐IR was positively associated with fat‐free mass index and fat mass index. 40 Our small sample size may have resulted in a type II error; a larger group of patients may have resulted in more significant results.
Our study has several limitations. The main limitation is the small number of patients and the lack of a control group. In addition, as with other CF therapies, the adherence to probiotic administration may have been suboptimal. Some patients complained about the taste of the powder and admitted that they were not fully compliant (e.g., took two sachets instead four a day for several days), but we did not have objective measures of compliance. The lack of effect of probiotics on CGM, OGTT, inflammatory cytokines, and pulmonary functions may reflect the small sample size and suboptimal compliance. We did assess the effect of probiotics on the intestinal microbiome. As it was a real‐life study, four patients experienced a respiratory exacerbation while on probiotics, necessitating antibiotic treatment which may have affected the results. As metabolomics were available only for two of them, subanalysis could not be performed. Given the small sample size, other confounding variables such as proton pump inhibitors, enteral nutrition, transplant status, and steroid administration were not controlled for or analyzed. Urine and stool samples were not available for all patients. Patients were instructed to bring urine and stool samples for the study visits. Most of the patients agreed to give urine samples during the study visits, even if forgotten at home. Eventually, paired urine samples were available for 16/23 (69.6%) of patients. However, patients were more reluctant with the stool samples, and only 7/23 (30.4%) paired samples were available. We assessed one type of probiotics and the results of this pilot study should not be generalized to other probiotic formulations with different numbers/strains of bacteria.
However, to the best of our knowledge, this is the first study to examine the effect of probiotics on glucose metabolism and metabolomics in CF patients. The change in metabolites profile suggests a positive effect of Vivomixx® on glucose metabolism in these patients. Larger, randomized double‐blind placebo‐controlled studies using different probiotics products, will help in extending our knowledge. Understanding the interplay between microbial imbalance, inflammation, and glucose metabolism in CF may be beneficial in preventing CFRD.
AUTHOR CONTRIBUTIONS
Michal Gur: Conceptualization; investigation; funding acquisition; writing—original draft; data curation; methodology. Nehama Zuckerman‐Levin: Methodology; validation; data curation; writing—review and editing. Kamal Masarweh: Writing—review and editing; data curation; methodology. Moneera Hanna: Data curation; investigation; visualization. Luca Laghi: Formal analysis; methodology; validation. Massimiliano Marazzato: Formal analysis; methodology; investigation. Shir Levanon: Data curation; visualization. Fahed Hakim: Writing—review and editing; formal analysis; supervision. Ronen Bar–Yoseph: Validation; visualization; data curation. Michael Wilschanski: Data curation; writing—review and editing; methodology. Lea Bentur: Conceptualization; methodology; supervision; writing—original draft; funding acquisition; investigation; project administration.
CONFLICT OF INTEREST
The authors declare no conflict of interest. Perrigo had no role in the design of the study, in the collection or analysis of data, in the manuscript preparation or the decision to publish the results.
ACKNOWLEDGMENT
The authors acknowledge the statistical help of Mrs. R. Leiba from the Medical Statistics Unit, Rambam Health Care Campus. Perrigo Israel provided the probiotics sachets. The Israeli Lung Association partially funded the study.
Gur M, Zuckerman‐Levin N, Masarweh K, et al. The effect of probiotic administration on metabolomics and glucose metabolism in CF patients. Pediatric Pulmonology. 2022;57:2335‐2343. 10.1002/ppul.26037
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Boudreau V, Reynaud Q, Dubois CL, et al. Screening for cystic fibrosis‐related diabetes: matching pathophysiology and addressing current challenges. Can J Diabetes. 2016;40(5):466‐470. [DOI] [PubMed] [Google Scholar]
- 2. Kim RJ. Cystic fibrosis–related diabetes in children: an update. Pediatr Ann. 2016;45(9):e321‐e326. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld M, Gibson RL, McNamara S, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32(5):356‐366. [DOI] [PubMed] [Google Scholar]
- 4. Huang YJ, LiPuma JJ. The microbiome in cystic fibrosis. Clin Chest Med. 2016;37(1):59‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 6. Anderson JL, Miles C, Tierney AC. Effect of probiotics on respiratory, gastrointestinal and nutritional outcomes in patients with cystic fibrosis: a systematic review. J Cyst Fibros. 2017;16(2):186‐197. [DOI] [PubMed] [Google Scholar]
- 7. Marsland BJ, Trompette A, Gollwitzer ES. The gut‐lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150‐S156. [DOI] [PubMed] [Google Scholar]
- 8. Kassaian N, Feizi A, Aminorroaya A, Jafari P, Ebrahimi MT, Amini M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double‐blind randomized clinical trial. Acta Diabetol. 2018;55(10):1019‐1028. [DOI] [PubMed] [Google Scholar]
- 9. Naito E, Yoshida Y, Kunihiro S, et al. Effect of Lactobacillus casei strain Shirota‐fermented milk on metabolic abnormalities in obese prediabetic Japanese men: a randomised, double‐blind, placebo‐controlled trial. Biosci Microbiota Food Health. 2018;37(1):9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Zheng S, Cui J, Guo T, Zhang J. Lactiplantibacillus plantarum Y15 alleviate type 2 diabetes in mice via modulating gut microbiota and regulating NF‐κB and insulin signaling pathway. Braz J Microbiol. 2022;53(2):935‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedorak RN, Feagan BG, Hotte N, et al. The probiotic VSL#3 has anti‐inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clin Gastroenterol Hepatol. 2015;13(5):928‐935.e2. [DOI] [PubMed] [Google Scholar]
- 12. Stefanaki C, Michos A, Mastorakos G, et al. Probiotics in adolescent prediabetes: a pilot RCT on glycemic control and intestinal bacteriome. J Clin Med. 2019;8(10):1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel KP, Luo FJ‐G, Plummer NS, Hostetter TH, Meyer TW. The production of p‐cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol. 2012;7(6):982‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 15. Quanjer PH, Borsboom GJJM, Brunekreef B, et al. Spirometric reference values for White European children and adolescents: Polgar revisited. Pediatr Pulmonol. 1995;19(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 16. Fuchs SI, Sturz J, Junge S, Ballmann M, Gappa M. A novel sidestream ultrasonic flow sensor for multiple breath washout in children. Pediatr Pulmonol. 2008;43(8):731‐738. [DOI] [PubMed] [Google Scholar]
- 17. Kent L, Reix P, Innes JA, et al. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros. 2014;13(2):123‐138. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs SI, Eder J, Ellemunter H, Gappa M. Lung clearance index: normal values, repeatability, and reproducibility in healthy children and adolescents. Pediatr Pulmonol. 2009;44(12):1180‐1185. [DOI] [PubMed] [Google Scholar]
- 19. Robinson PD, Latzin P, Verbanck S, et al. Consensus statement for inert gas washout measurement using multiple‐ and single‐breath tests. Eur Respir J. 2013;41(3):507‐522. [DOI] [PubMed] [Google Scholar]
- 20.Cystic Fibrosis Questionnaire (CFQ); Cystic Fibrosis Questionnaire Revised (CFQ‐R). Accessed January 15, 2022. https://qol.thoracic.org/sections/instruments/ae/pages/cfq-cfq-r.html
- 21. Laghi L, Picone G, Capozzi F. Nuclear magnetic resonance for foodomics beyond food analysis. Trends Analyt Chem. 2014;59:93‐102. [Google Scholar]
- 22. De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med. 2013;3(9):a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Biervliet S, Hauser B, Verhulst S, et al. Probiotics in cystic fibrosis patients: a double blind crossover placebo controlled study: pilot study from the ESPGHAN working group on pancreas/CF. Clin Nutr ESPEN. 2018;27:59‐65. [DOI] [PubMed] [Google Scholar]
- 24. Rozov T, de Oliveira VZ, Santana MA, et al. Dornase alfa improves the health‐related quality of life among Brazilian patients with cystic fibrosis‐a one‐year prospective study. Pediatr Pulmonol. 2010;45:874‐882. [DOI] [PubMed] [Google Scholar]
- 25. Zhou P, Zhang X, Xu Z, et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017;17(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ziese AL, Suchodolski JS, Hartmann K, et al. Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS One. 2018;13(9):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321‐347. [DOI] [PubMed] [Google Scholar]
- 28. Verma A, Nelson MT, Depaolo WR, Hampe C, Roth CL. A randomized double‐blind placebo controlled pilot study of probiotics in adolescents with severe obesity. J Diabetes Metab Disord. 2021;20(2):1289‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mestre L, Carrillo‐Salinas FJ, Mecha M, et al. Manipulation of gut microbiota influences immune responses, axon preservation, and motor disability in a model of progressive multiple sclerosis. Front Immunol. 2019;10(JUN):1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin MC, Hsu CC, Chiang PF, Wu WJ. Antiinflammatory and antifibrogenic effects of s‐ethyl cysteine and s‐methyl cysteine in the kidney of diabetic mice. Mol Nutr Food Res. 2007;51(5):572‐579. [DOI] [PubMed] [Google Scholar]
- 31. Ma YQ, Wang X, Gao S. Hypoglycemic activity of polysaccharides from sweet corncob on streptozotocin‐induced diabetic rats. J Food Sci. 2017;82(1):208‐213. [DOI] [PubMed] [Google Scholar]
- 32. Marchesini G, Forlani G, Zolp M, Vannin P, Pisi E. Muscle protein breakdown in uncontrolled diabetes as assessed by urinary 3‐methylhistidine excretion. Diabetologia. 1982;23:456‐458. [DOI] [PubMed] [Google Scholar]
- 33. Ng DPK, Salim A, Liu Y, et al. A metabolomic study of low estimated GFR in non‐proteinuric type 2 diabetes mellitus. Diabetologia. 2012;55(2):499‐508. [DOI] [PubMed] [Google Scholar]
- 34. Wang L, Zhang Y, Liu X, et al. Metabolite triplet in serum improves the diagnostic accuracy of prediabetes and diabetes screening. J Proteome Res. 2021;20(1):1005‐1014. [DOI] [PubMed] [Google Scholar]
- 35. Schicho R, Shaykhutdinov R, Ngo J, et al. Quantitative metabolomic profiling of serum, plasma, and urine by 1H NMR spectroscopy discriminates between patients with inflammatory bowel disease and healthy individuals. J Proteome Res. 2012;11(6):3344‐3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Preidis GA, Keaton MA, Campeau PM, Bessard BC, Conner ME, Hotez PJ. The undernourished neonatal mouse metabolome reveals evidence of liver and biliary dysfunction, inflammation, and oxidative stress. J Nutr. 2014;144(3):273‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ooi CY, Syed SA, Rossi L, et al. Impact of CFTR modulation with ivacaftor on gut microbiota and intestinal inflammation. Sci Rep. 2018;8(1):17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang Q, Li X, Song P, Xu L. Optimal cut‐off values for the homeostasis model assessment of insulin resistance (HOMA‐IR) and pre‐diabetes screening: developments in research and prospects for the future. Drug Discov Ther. 2015;9(6):380‐385. [DOI] [PubMed] [Google Scholar]
- 39. Gayoso‐Diz P, Otero‐González A, Rodriguez‐Alvarez MX, et al. Insulin resistance (HOMA‐IR) cut‐off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross‐sectional study. BMC Endocr Disord. 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nielsen BU, Faurholt‐Jepsen D, Oturai PS, et al. Associations between glucose tolerance, insulin secretion, muscle and fat mass in cystic fibrosis. Clin Med Insights Endocrinol Diabetes. 2021;14:11795514211038259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


