Abstract
BACKGROUND
Biocompatible Pickering emulsions (PE) stabilized by tailor‐made antioxidant‐loaded particles have been known for some time now, but antioxidant‐rich natural plant particle‐based emulsions are much less well known. This study aimed to investigate the physico‐chemical properties of commercial Zingiber officinale powders obtained from biological and conventional agricultural practice and ginger powder‐based PE.
RESULTS
The physico‐chemical and biological properties of Zingiber officinale powders (GDPs) obtained from conventional (GDPC1 and GDPC2) and biological agricultural (GDPBIO) practices, and the properties of derived PE (PE_GDPs) were examined. All GDPs showed weak aggregation in aqueous media and a sufficiently hydrophobic surface to stabilize oil‐in‐water (O/W) PE against coalescence for at least 1 month. Zingiber officinale powders (2% w/w) derived from biological agricultural practices (GDPBIO) demonstrated the best emulsifying properties. The Zingiber officinale powders and PE_GDPs were also characterized by their phytochemical profiles. All the investigated samples exhibited ferric reducing ability power greater than the positive control, butylated hydroxytoluene (BHT), with values ranging from 91.21 to 102.63 μmol L−1 Fe (II) g−1 for GDPC2 and 05PE_GDPC1 (O/W=1:1), respectively. In β‐carotene bleaching test the following trend GDPC1 > GDPBIO > GDPC2 was observed. A 05PE_GDPBIO sample with the oil volume fraction equal to 50% was stable to oxidation and exhibited a promising α‐amylase inhibitory activity.
CONCLUSION
The results suggest that ginger powder should be used as a starting point to design biocompatible PEs for different applications in the functional food, nutraceutical, and pharmaceutical industries. In fact, powder and based PE are characterized by a promising antioxidant activity, carbohydrate hydrolyzing enzyme and lipase inhibitory properties. Further in vivo studies are necessary to confirm these findings. © 2022 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Pickering emulsions, physical stability, antioxidant, dual functional stabilizers, natural emulsifier, ginger powder
INTRODUCTION
Zingiber officinale Rosc. (Zingiberaceae) is used extensively worldwide to flavor dishes and beverages. It is also used as functional food and nutraceutical product with an annual sales increase of 6.5%. 1 Several works confirmed the healthy properties of ginger with particular reference to its antioxidant, anti‐diabetic, and anti‐obesity effects. 2 , 3 At the same time, researchers have shown that the bioactive compounds that it contains and that reach the bloodstream following oral intake are relatively low due to losses during gastro‐enteric absorption. For this reason, technological approaches are investigated to enhance the bioavailability of those compounds. 1 , 3 , 4 Recently, Pickering emulsions (PEs) encapsulating bioactive molecules have gained increasing interest. 5 Pickering emulsions are an important class of emulsion, in which stabilization is not the consequence of the use of surfactants but arises from solid particles. 6 To the best of our knowledge, few studies have proposed the use of natural particles as Pickering stabilizers without mechanical, biological, or chemical pretreatment. 7 Recently, some research groups have addressed the possibility of Pickering stabilization using plant‐based particles to improve their physico‐chemical stability and increase their bio‐accessibility under simulated gastrointestinal conditions. 8 , 9
The present study is aimed to design emulsions by employing Pickering particles that act both as physical emulsion stabilizers and as interfacial reservoirs of bioactive compounds. For this purpose, ginger powders (GDPs) obtained by biological and conventional agricultural practices were used. The essential difference between biological and conventional agricultural practices is that conventional practice relies on chemical intervention to fight pests and weeds and provide plant nutrition. These are not used in biological agricultural practice. Total phenol content (TPC), total flavonoid content (TFC), and total carotenoid content (TCC) were quantified in both GDPs and derived Pickering emulsions (PE_GDPs). Moreover, high‐performance liquid chromatography (HPLC) was used to identify the chemical profile of the GDP samples. The physico‐chemical properties of GDPs were first investigated based on morphology, size, surface charge, and wettability of particles. Subsequently the physical stability of PE_GDPs was evaluated over time. The physical characteristics of the aqueous dispersions of raw GDPs were also reported, after which their emulsifying capacity was discussed. Moreover, GDPs and 05PE_GDPs were analyzed for their in vitro antioxidant activity. The inhibition of lipase and carbohydrate hydrolyzing enzymes (α‐glucosidase and α‐amylase) was also assessed.
MATERIALS AND METHODS
Chemicals and reagents
The oil phase of the emulsions consisted of a commercially available oil known as Miglyol® 812 N, a mixture of medium chain triglycerides (95% capric and caprylic acids, with hydrosolubility less than 0.01 g L−1 at 20 °C) (Eigenmann & Veronelli S.p.A., RHO (MILAN) Italy). Acarbose from Actinoplanes sp. was obtained from Serva (Heidelberg, Germany). 6‐Gingerol, 8‐gingerol, 6‐shogaol, 6‐paradol, and 10‐gingerol were purchased from Chengdu Biopurify Phytochemicals Ltd (Shangai, China). All solvents, standards, enzymes, and reagents used in the present work were purchased from Sigma‐Aldrich S.p.a. (Milan, Italy).
Plant sample
GDPs obtained from biological and conventional agricultural practices were purchased at a local market. All powders were used as they are, without any delipidation, sieving, grinding, or milling pre‐treatment.
Sample preparation for phytochemical determination
For phytochemical analysis and the evaluation of biological activity, GDP was subjected to extraction by applying ultrasound‐assisted maceration using ethanol (EtOH) (3 cycles × 1 h, 40 kHz at a temperature of 30 °C). After each extraction cycle, the solution was filtered (Whatman filter Paper 4), and the solvent was removed.
Preparation of Pickering emulsions and ginger particle dispersions
In all prepared PEs, the oil phase (from 10 to 90% v/v) refers to the entire volume of the emulsion, while the GDP concentration C (mass%) (from 1.1 to about 29.0% w/w) is relative to the mass of the continuous phase, including the water mass (mw) and that of GDP material (mp), C = mp/(mp + mw). The volume of each emulsion was fixed at 5 mL. The PEs were prepared using an ultrasonic method at a controlled temperature of 25 °C with an ice bath. The ultrapure water, the oil, and the powder were introduced consecutively in a vial and were gently vortexed for 1 min. A 3 mm ultrasonic probe (Ultrasonic UP400S, Hielscher, Teltow, Germany) was immersed in the blend. Ultrasound was applied for 5 min, at 400 W and 100% of duty cycle. After the addition of each component, as well as after the sonication, a step under a N2 gas constant flow was provided. All emulsions were stored at 4 °C until further use. The composition of all the samples of ginger powder‐based PE discussed in this work is reported in the supplementary material (Table S1). Aqueous dispersions of each GDP (1% w/w) were prepared with a different procedure using mixing by vortex or hand for 1 min or following the same PE preparation scheme, including the steps under the N2 gas constant flow. For details of the characterization of the GDPs and derived PE, refer to the supplementary material (section SI.1).
Total phenol, flavonoid and carotenoid contents
Several research papers have evaluated the spectrophotometric content of phenol, flavonoid, and carotenoid in different medicinal and edible plants. 10 , 11 , 12 , 13
The total phenol content (TPC) was evaluated using the Folin–Ciocalteu method. 14 The absorbance was read at 765 nm. Results were expressed as mg gallic acid equivalent (GAE)g−1 dried weight (DW). The total flavonoid content (TFC) was determined using the method based on the formation of a flavonoid–aluminium complex. 15 Results were expressed as mg quercetin equivalent (QE)g−1 DW. The total carotenoid content (TCC) was quantified spectrophotometrically at 460 nm. 13 The TCC was expressed as μg β‐carotene (βC)g−1 DW.
Ginger powder chemical profile
The GDP extract was analyzed using an ultra‐high‐performance liquid chromatography (UHPLC) system that consisted of an UHPLC PLATIN blue (Knauer, Berlin, Germany) equipped with a binary pump system using a Knauer Blue Orchid column C18A (1.8 μm, 100 mm × 2 mm) coupled with a photo diode array retector (PLATINblue, Knauer, Berlin, Germany). Compound quantification was carried out following the method reported by You et al. 16 6‐,8‐,10‐Gingerol, 6‐shogaol, and 6‐paradol were selected as external standards and the results, expressed as mg g−1 of extract, were elaborated with Clarity 6.2 software. Calibration curves, detection limits (LOD), and quantification limits (LOQ) of analytical methods were reported in the supplementary material (Table S2).
Ginger powder and ginger‐based PE antioxidant activity
The in vitro antioxidant activity of GDP extract at different concentrations, and 05PE_GDP (1:1 ratio in water), were tested following the procedure previously described by Zheng et al. 17 Ferric reducing ability power (FRAP) was assessed following a previously published method. 13 The FRAP value was expressed as μM Fe(II)g−1. Butylated hydroxytoluene (BHT) was used as a positive control. For the β‐carotene bleaching test, a solution of β‐carotene, Tween 20, and linoleic acid, was prepared. 15 Samples were tested and the absorbance was read at 470 nm after 30 and 60 min of incubation. Propyl gallate was used as a positive control. For 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid) (ABTS) assay the methodology followed the protocol reported by Leporini et al. 14 Samples were tested and, after 6 min, the absorbance was read at 734 nm. To evaluate 1,1‐diphenyl‐2‐picrylhydrazyl (DPPH) radical scavenging activity, the methodology followed the protocol reported by Loizzo et al. 18 The bleaching of DPPH was determined at 517 nm. Ascorbic acid was used as a positive control in both tests.
Pancreatic lipase and carbohydrate hydrolyzing enzyme inhibition assays
The inhibition of pancreatic lipase was assessed as previously reported. 19 A mixture was prepared of 4‐nitrophenyl octanoate (NPC), 5 mmol L−1 in a dimethyl sulfoxide solution, aqueous solution of porcine pancreatic lipase (1 mg mL−1), and Tris–HCl buffer (pH 8.5). Samples at different concentrations (2.5–40 mg mL−1) were added to a well with 6 μL of the enzyme, 6 μL of NPC, and 279 μL of buffer. After incubation at 37 °C for 30 min, the absorbance was measured at 405 nm. Experiments were performed in triplicate. Orlistat was used as a positive control.
For α‐glucosidase and α‐amylase inhibitory activity, previously published procedures were applied. 20 In the α‐glucosidase inhibitory activity test, a maltose (12 g of maltose in 300 mL of 50 mmol L−1 sodium acetate buffer), α‐glucosidase (EC 3.2.1.20) (1 mg of enzyme in 10 mL of ice‐cold distilled water) and O‐dianisidine (DIAN, 1 tablet in 25 mL of distilled water) solutions were prepared. The peroxidase/glucose oxidase (PGO) system‐color reagent solution was obtained by dissolving 1 capsule in 100 mL of ice‐cold distilled water. A mixture of 5 μL of sample (at concentrations ranging from 1000 to 25 μg mL−1), 250 μL maltose solution, and 5 μL enzyme was left to incubate at 37 °C for 30 min. Then, 50 μL of perchloric acid was added, and the mixture was centrifuged. The supernatant was collected and mixed with 5 μL of DIAN and 300 μL of PGO and left to incubate at 37 °C for 30 min. The absorbance was read at 500 nm. In the α‐amylase inhibitory assay, the enzyme solution was prepared by adding 0.0253 g of enzyme to 100 mL of cold water; the starch solution was prepared by stirring (at 65 °C for 15 min) 0.125 g of potato starch in 25 mL of sodium phosphate buffer (20 mmol L−1) and sodium chloride (6.7 mmol L−1). 13 Samples were dissolved in ethanol at concentrations ranging from 1000 to 25 μg mL−1, added to starch solution, and left to react with the enzyme at room temperature for 5 min. The absorbance was read at 540 nm. In both tests, acarbose was used as a positive control.
Statistical analysis
Data are expressed as means ± standard deviations (SD) (n = 3). Prism Graph Pad Prism version 4.0 for Windows (Graph Pad Software, San Diego, CA, USA) was used to calculate the concentration‐response curve. A one‐way ANOVA was used to evaluate the differences within and between groups, followed by a Tukey test to determine any significant difference in physico‐chemical parameters (**P < 0.01, ***P < 0.001, ****P < 0.0001) among investigated samples whereas Dunnett's test (α = 0.05) was used to compare each group with the positive control. Microsoft Excel 2010 software was used to calculate linear regression, assessment of repeatability, calculation of average, relative standard deviation, and Pearson's correlation coefficient (r).
RESULTS AND DISCUSSION
Physico‐chemical characterization of ginger powders
The morphology of GDPs tested here as Pickering particles is shown by scanning electron microscopy (SEM) images in Fig. 1. GDPC1, GDPC2 and GDPBIO appeared to have a rather similar surface, somewhat smooth and homogeneous. All powders contained particles of different sizes and various shapes, some with a more amorphous or compact superface structure. This morphological anisotropy may facilitate jamming effects through lateral particle‐particle interactions. A different aspect ratio of Pickering particles is known to have a significant impact on interfacial properties and plays a fundamental role in the self‐assembly of particles at the water–oil interface. No particular difference in peak position and absorption of characteristic bands was observed in the GDPs’ Fourier‐transform infrared (FTIR) spectra (supporting information, Figure S1, Table S3).
Figure 1.
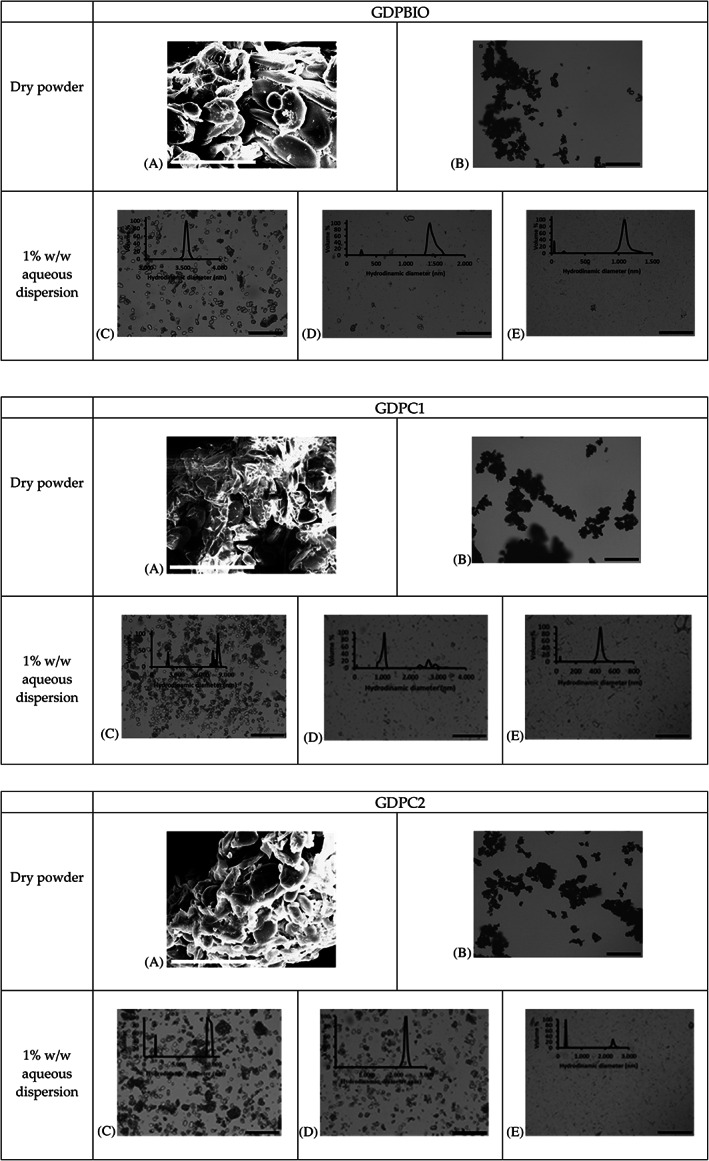
Characterization of raw ginger powders GDPBIO, GDPC1, and GDPC2. (A) Scanning electron microscopy images (scale bar is 50 μm). (B) Optical microscopy images (scale bar is 200 μm) of dry powders; optical microscopy images (scale bar is 200 μm) with particle size distributions (PSD) of GDP aqueous dispersions (1% w/w) after (C) hand shake, (D) vortex, and (E) emulsifying procedure used for our PE_GDP emulsions.
Independent of provenance, all raw GDPs appeared to be well dispersed in water at pH 6.5 after handshaking, but if they were not processed, powders contained large particles, some of undefined shape, as confirmed by optical microscopy images (Fig. 1). Since it is preferable that the Pickering particles size is at least one order of magnitude smaller than the targeted emulsion droplet size, 6 , 21 , 22 we investigated the impact of the emulsification process used for this study. Aqueous dispersions of 1% of different GDPs were submitted to the same treatment applied for PE_GDPs formation. Characteristic particle size distributions and their respective microscope images are given in Fig. 1 which also reports those of the unprocessed samples as reference. Interestingly, the complete emulsification process has a significant impact on the particles’ morphology as well as on their size distribution, effectively changing their original structure. The mixing intensity induced by our emulsifying procedure was more than enough to produce a good disentanglement of large primary particles and fine particle fragmentation. Some residues of more aggregated or cohesive particles were observed only for GDPC2. This is confirmed by the particle‐size distribution showing the absence of objects larger than 2 μm for the other two GDPs types. In the case of GPDBIO, the particle size decreased considerably, leading to a fraction of particles between 40 and 200 nm and a slightly larger polydispersity index (PDI). 23
The zeta potential (ζ) of the Pickering particles is an important property as it not only provides information about their surface charge but also accounts for particle dispersion/aggregation. Aveyard et al. 24 reported that interparticle interactions can potentially influence their adsorption at the oil–water interface, which in turn affects PE stability. Generally, the particles are unstable against aggregation when the ζ potential does not exceed the absolute value of 20 mV. 25 Table S4 in the supplementary material shows the ζ potentials of the GDPs’ aqueous dispersions as a function of pH. All ζ potentials increased with increasing pH value. GPDBIO, possessing the highest ζ potential of −20.00 mV at pH 6.0, is likely to be less surface‐active and less hydrophobic at a neutral pH.
However, the slightly aggregating behavior and dispersibility of the different GDPs in water at pH 6.0 was indicative of moderate hydrophobicity and, under specific emulsification conditions, of a potential ability as effective Pickering stabilizers of oil‐in‐water (O/W) emulsions. To verify that, we measured the wettability of different raw GDPs. Food‐grade particle arrangement at the oil–water interface is difficult to assess, but is undoubtedly very important for understanding potential emulsion droplet formation. The wettability of GDPs, as reflected by the three‐phase contact angle measurements with water (WCA) and also with oil (OCA), can be used as a landmark to characterize the type of emulsion that different GDPs would prefer to stabilize. Accordingly, where WCA significantly exceeds the OCA, Pickering particles can be classified as relatively hydrophobic, whereas the reverse is true for hydrophilic particle. 6 Ginger powder derived from biological agricultural practices and GDPC2 has a WCA greater than the OCA (Fig. 2) and consequently a real but moderate hydrophobic character can be attributed to it.
Figure 2.
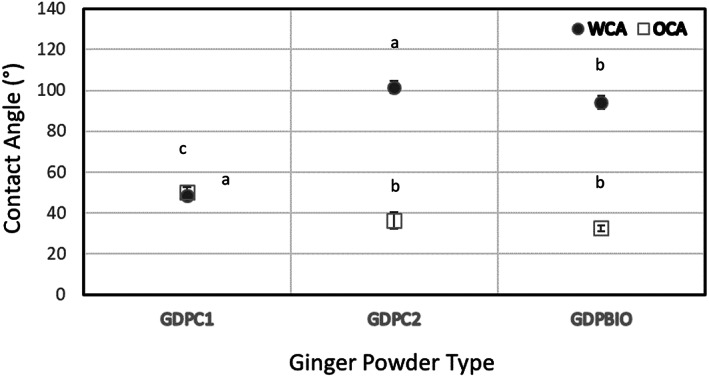
Contact angles with oil (OCA) (open square symbols) and with water WCA (filled circle symbols) (measured in triplicate and error bars represent 2 SDs and where not visible are smaller than symbols) for GDPC1, GDPC2, and GDPBIO powders. Sign: significant. Differences were evaluated by one‐way ANOVA followed by a multicomparison Tukey test **P < 0.01.
For particle WCA values between 85° and 95°, it is known that both O/W and water‐in‐oil (W/O) PEs can be obtained, but of significantly reduced stability (it depends on the oil/water volume ratio and on the phase in which the particles are dispersed initially). 26 On the other hand, stable W/O PEs are expected for values of 95° ≤ WCA ≤ 115°. 22 As reported in Fig. 2, GPDC2 and GPDBIO would preferentially stabilize W/O PE. In the case of GDPC1, the difference between WCA and OCA is much smaller, indicating that there is no significant preference for either phase. The contact angles are also quite low for both phases to indicate an affinity for both phases; accordingly, there can be no prediction of the preferential formation of the emulsion type based only on the wettability character of GPDC1 particles.
Berton‐Carabin and Schroën 27 argued that the wettability of the food‐grade particles is related, in part, to the chemical structure of the materials but also to the surface roughness resulting from the fractal structure of the particles (in turn modifiable by the table compression conditions used by the sessile drop method). Furthermore, the wettability of plant particulate material can be changed by soluble components eventually originating from the emulsification process because they may also physically absorb at their surface. Generally, in case of plant particles, the presence of other interfacially active ingredients able to compete with same Pickering particle for the physical stabilization/destabilization of emulsions may also dependent on proportions of two liquids (oil and water), oil phase composition, pH and temperature, and then control the type of emulsion by adopted emulsification process. This seems to suggest that food‐grade particles may have a distribution of effective and real wettability values depending, inter alia, on being initially wetted by a particular phase or both liquids. 28
Physical stability of ginger particle based Pickering emulsions
In this study, naturally antioxidant‐rich GDPs were investigated as potential stabilizers for biocompatible PE for value‐added applications because of their clean‐label feature. Thus, in the first experiments, a set of emulsions was prepared at variable oil/water volume ratios (φw), from 0.1 to 0.9 while maintaining the total mass of powder constant (Table S1 in the supplementary material). Migloyl® 812 N, used here for our PE_GDPs, is a mixture of medium‐chain triglycerides (MCT), clear and slightly viscous, able to stabilize O/W Pickering emulsions in the presence of more hydrophobic silica particles and W/O with the more hydrophilic ones. 26 According to Binks et al., 28 O/W PE are more stable when prepared with moderately hydrophobic particles. Miglyol® 812 N, mainly composed of the fractionated vegetable caprylic (C8) and capric (C10) fatty acids, has a higher solvent capacity and is less susceptible to oxidation 29 than similar MCT edible oils present in many foods and dietary supplements for weight management. Le Bars et al. 30 confirmed the oral safety of Miglyol® 812 (GRAS Notice 000449), identifying it as a potential vehicle to improve the solubility and eventually the bioavailability of orally administered drugs. As far as we know, this is the first report addressing the Miglyol® 812 N based PE stabilized by GDP. In this work, we showed that every GDP used here was a good emulsifier for potential novel edible PE. Regardless of the GDP type, we observed that the PE_GDPs’ stability to both sedimentation and coalescence increased progressively with particles’ concentration and for increasing values of oil/water volume ratio, φw starting from 0.5. Here we presented also the first bioactivity results on PEs stabilized by 2% (w/w) of different GDPs. The emulsification procedure was performed as indicated above and every PE_GDP was stored at 4 °C after preparation. Figure S2 in the supplementary material shows the visual appearance of the PE_GDP formulation series containing 100 mg of GDP (2%w/w) during a storage period of 4 weeks. All formulations have a color between beige and yellow and, just immediately after preparation, they are not all equally homogeneous. A rapid indication of PEs’ stability was given using the bottle test method by monitoring the extent of eventual phase separation/sedimentation/creaming phenomena over time. Sedimentation of GDPs was observed in PE_GDPC2 and PE_GDPBIO at very low water fractions. On the other hand, at every O/W volume ratio of our PE_GDP formulation series, almost no clear oil phase separation was noticed during the entire storage period.
Within the first 24 h after preparation (Supporting Information, Fig. S2), for φw values lower than 0.4 and higher than 0.6, the formation of multiphase emulsions is clearly distinguishable, although stable to coalesce (no clear oil released), in all PE_GDPs series. By contrast, creaming is minimal at φw = 0.5 of PE_GDPC1, at which point we observed that the resolved aqueous phase was clear, implying that GDPC1 particles could be anchored at the droplets’ oil–water interface and associated with the creamed emulsion. A marked maximum in stability occurs for 05PE_GDPC2 and 05PE_GDPBIO, for which no clear separated water layer was observed after up to 1 month of storage at 4 °C. To select the most suitable PE_GDPs for further biological study, we carried out a complete in‐depth study on emulsion type and physical stability over time and respect to temperature changes of some PE_GDPs. The experimental results (data not shown) confirmed that their maximum emulsification capacity and greatest shelf life (stability with time at 25 °C) occurred in the samples at an O/W ratio of 50/50, hereafter referred to as 05PE_GDPs. The drop test indicated a good and rapid dispersibility in water suggesting that all 05PE_GDPs may be oil‐in‐water emulsions, in spite of expectations based only on contact angle measurements (Fig. 2). Light microscopy images revealed the appearance of water‐in‐oil‐in‐water multiple drops (W/O/W) in all 05PE_GDP samples immediately after preparation (Fig. 3).
Figure 3.
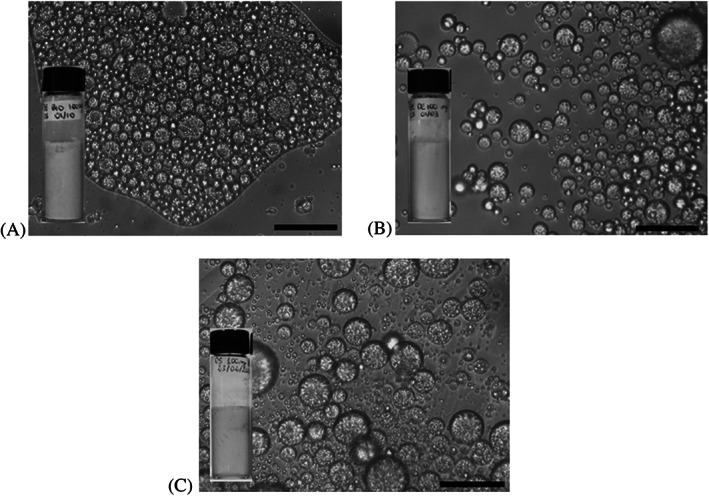
Optical microscopy images of fresh samples (scale bar is 25 μm) and corresponding visual appearance of (A) 05PE_GDPBIO, (B) 05PE_GDPC1, and (C) 05PE_GDPC2.
GDPC2 or GDPBIO powder‐stabilized droplets showed network formation in the continuous phase, most likely due to particle bridging. In contrast with the other two samples, in the 05PE_GDPC1 emulsion there were less strongly aggregated or bridged droplets, most of which turned out to be of the W/O/W type. When a drop of the 05PE_GDP was dispersed in water for a better microscopic visualization, we confirmed the coexistence of discrete multiple drops (W/O/W), both large and small, and smaller single drops O/W; however these were spherical in shape. The existence of multiple droplets in preferred emulsions (φw = 0.5) can be associated with the coalescence of drops with the inclusion of the continuous phase as a result of insufficient coverage of freshly formed interfaces during the particular mixing process adopted here. As mentioned, the shear imposed during the mechanical emulsifying treatments (vortex plus ultrasonication) can influence the GDPs morphology significantly and, thus, it can be expected to give rise to various soluble surface‐active components in variable quantities. Our emulsifying scheme allows us to recognize the effective initial particle location (by gravity all GDP particles settle on the bottom in vials) but without knowing the exact particle partition between the oil and aqueous phases as they pass through them. This implies that some particles (the amount is not known) transfer from oil to water rapidly, modifying the original wettability of the GDP particles and reducing, in particular, the already moderate hydrophobicity of GDPC2 and GDPBIO. 26
Long‐term stability is a basic prerequisite, regardless of the field of application of biodegradable and biocompatible PE. None of 05PE_GDPs formulations exhibited (Fig. S2) evidence of oiling‐off before 1 month storage. However, the very thin serum layer at the bottom of the vial observed in all samples completely disappeared after gentle shaking. At the same time, the mean droplet size was measured to characterize quantitatively the stability of 05PE_GDPs in storage for 4 weeks at 4 °C, with the result that some formulations remained mostly nanometer sized.
The droplet size distribution was monomodal immediately after 1 h from preparation of 05PE_GDPC2 and it stayed like this after storage for 4 weeks. On the other hand, 05PE_GDPC1 showed an initial multimodal droplet‐size distribution, which reduced to a monomodal distribution during storage. Instead, the particle‐size distribution of 05PE_GDPBIO was monomodal initially but bimodal at the end of storage (Fig. S3 in the supplementary material). As shown in Fig. 4, the PDI value of the 05PE‐GDPC1 sample stabilized at 0.4 indicating that its droplets remained relatively inhomogeneous in size over time. The PDI of 05PE_GDPBIO and 05PE_GDPC2 ranged between 0.067 and 0.1, confirming that their droplet size distributions were narrow and homogeneous after 4 weeks. After storing for 7 days, the mean droplet diameter of 05PE_GDPC2 (832 nm) and 05PE_GDPBIO (923 nm) was similar to that observed after 1 day, the former remaining unchanged while the latter increased slightly after almost 1 month. Nevertheless, it was observed that both 05PE_GDPs exhibited a uniform and stable state after up to 90 days storage (Fig. S2 in the supplementary information). As shown in Fig. 4, different long‐term stability can be attributed at 05PE_GDPC1, probably due to incomplete coverage of some oil droplets during sonication or some molecular‐based stabilizing interference from small molecules (surface‐active component or protein) coming from the GDPC1 powder itself. So, after 1 h, the mean droplet diameter of 05PE_GDPC1 was larger than 1 μm, indicating that some slight droplet coalescence occurred immediately after sonication. The very thin phase separation observed visually (Fig. S2 in the supplementary material) at the bottom of the 05PE_GDPC1 vial was consistent with halving of the droplet size. The influence of the mixing procedure on the final 05PE_GDP emulsions probably differs for the different GDPs because they are not a priori dispersed in either of the two phases. Additional experiments are planned to understand whether different emulsifying behavior can result from a initial dispersion of GDPs in water or oil.
Figure 4.
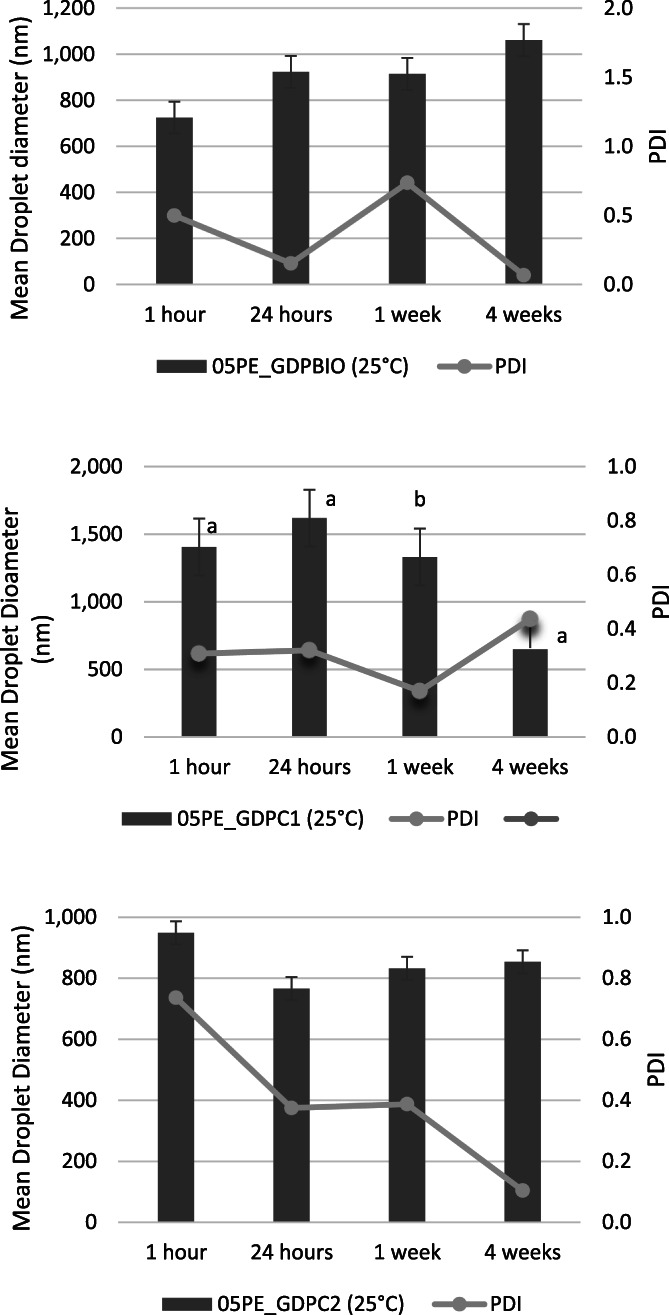
Mean droplet diameter and polydispersity index (PDI) at 25 °C of 05PE‐GDP samples acquired during storage time after preparation. Sign: significant. Differences were evaluated by a one‐way ANOVA followed by a multicomparison Tukey test **P < 0.01 for 05PE_GDPC1.
The ζ‐potential value of all 05PE_GDPs emulsions was higher than that of the GPD present in the aqueous dispersion (Fig. 5). This increase in the negative surface charge might be attributed to the concentration of GDP at the droplet surface as compared to being simply dispersed in the aqueous phase. Generally, a high interfacial charge (ζ‐potential| ≥ 30 mV) 31 implies good stability of the emulsion whereas coalescence, flocculation, or aggregation of oil droplets can occur with lower values. Measured values of ζ‐potential ≥ ± 30 mV are indicative of strong electrostatic stabilization of oil droplets in addition to the mechanical stabilization provided by the GDP particles. We therefore believe that the good long‐term stability of our PEs stabilized by different GDPs can be attributed not only to the irreversible adsorption of the GDP particles at the oil droplet surfaces but also to strong electrostatic repulsion due to the high surface potential of dispersed oil droplets. In fact, even after almost 30 days of storage, the ζ potential values of all formulations was found to be negative and within the range of ≈ −43.0 mV and ≈ −51.2 mV (Fig. S4 in the supplementary material). Particle behavior at the oil–water interface is highly important in understanding the origin of the emulsifying and stabilizing ability of different GDPs. Despite this, particle arrangement at the interface is undoubtedly difficult to assess. Further CSM characterization and investigations of the rheological properties of 05PE_GDPs are planned to provide us with some information on the role played by its different components in structuring the aqueous phase surrounding the oil droplets. All 05PE_GDPs showed a gel‐like appearance, probably due to high viscosity conferred by the relatively high oil content, suggesting the improved long‐term stability of the GDP particles accumulated at the oil–water interface.
Figure 5.
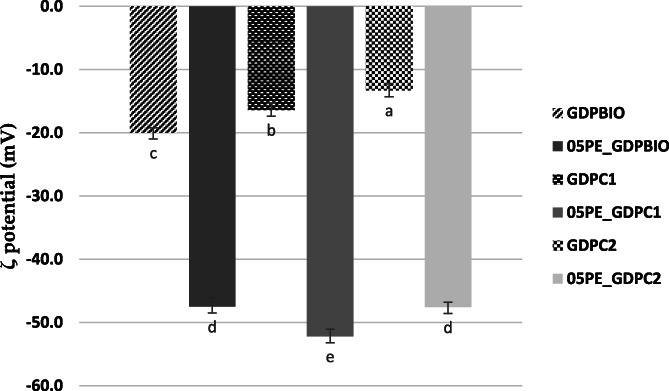
ζ potential values of GDP dispersions 1% and emulsions 05PE_GDP at 25 °C. Sign: significant. Differences were evaluated by one‐way ANOVA followed by a multicomparison Tukey test **P < 0.01.
Ginger‐powder and ginger‐derived PEs, TPC, TFC and TCC
Samples derived from biological agricultural practice showed the highest total phytochemical content in both GDPBIO and 05PE_GDPBIO, with values of 39.27 mg GAEg−1 DW, 15.38 mg QEg−1 DW, and 14.67 μg βCg−1 DW, and 36.52 mg GAEg−1 DW, 15.11 mg QEg−1 DW, and 13.51 μg βCg−1 DW for TPC, TFC and TCC, respectively (Table 1). A high degree of variability was observed in phytochemical content in the samples that were investigated. This is probably due to the different geographical origins of the ginger. Previously, Mošovská et al. 32 investigated the phytochemical content of Z. officinale, finding TPC and TFC levels of 181.41 mg GAEg−1 extract and 14.15 QEg−1 extract, respectively.
Table 1.
TPC, TFC and TCC in ginger powder extract derived Pickering emulsions
| Samples | TPC mg GAE/g DW | TFC mg QE/g DW | TCC μg βC/g DW | Reference |
|---|---|---|---|---|
| Powder | ||||
| GDPC1 | 35.66 ± 2.3b | 13.84 ± 1.01b | 6.38 ± 0.74b | 38 |
| GDPC2 | 22.64 ± 1.93c | 8.36 ± 0.83c | 5.37 ± 0.71c | 38 |
| GDPBIO | 39.27 ± 2.4a | 15.38 ± 1.08a | 14.67 ± 1.56a | 38 |
| Sign. | ** | ** | ** | |
| Pickering emulsion | ||||
| 05PE_GDPC1 | 29.97 ± 1.96b | 12.93 ± 1.05b | 5.96 ± 0.72b | |
| 05PE_GDPC2 | 19.83 ± 1.43c | 7.58 ± 0.81c | 4.76 ± 0.69c | |
| 05PE_GDPBIO | 36.52 ± 2.15a | 15.11 ± 1.06a | 13.51 ± 1.39a | |
| Sign. | ** | ** | ** |
Data are expressed as means ± SDs (n = 3). TPC, total phenol content. TFC, total flavonoid content. TCC, total carotenoid content. GAE, gallic acid equivalent. QE, quercetin equivalent. βC, β‐carotene, Sign, significant. Differences between samples were evaluated by a one‐way ANOVA followed by a multicomparison Tukey test. ** P < 0.01. Means in the same column with different small letters differ significantly.
Ginger powder chemical profile
Five phytochemicals were selected as markers and were quantified in GDP extracts (Table 2). 6‐Gingerol was the main abundant compound in all of the samples that were investigated, with values ranging from 37.97 to 63.54 mgg−1 for GDPC1 and GDPBIO, respectively, followed by 6‐shogaol (values ranging from 20.27 to 31.83 mg g−1 for GDPC2 and GDPC1, respectively). A high degree of variability was observed in the 10‐gingerol content; indeed, GDPC1 shows content between seven and eight times higher than the other ginger samples. Previously, Shao et al. 33 quantified the major ginger constituents (6‐, 8‐, and 10‐gingerol, 6‐, 8‐, and 10‐shogaol, 6‐paradol, and 1‐dehydrogingerdione) in 11 ginger commercial products including ground ginger powders (dried ginger rhizome). 6‐Gingerol was the main abundant compound, with values ranging from 554.78 to 772.33 mg 100 g−1 of product followed by 10‐gingerol, 6‐shoagol, and 8‐gingerol. In particular, 8‐gingerol specifying ginger from India, whereas 10‐gingerol can be individually employed as a marker for Chinese ginger. 34
Table 2.
Quantification of non‐volatile ginger extracts compounds (mg g−1)
| Sample | [6]‐gingerol | [8]‐gingerol | [6]‐shogaol | [6]‐paradol | [10]‐gingerol |
|---|---|---|---|---|---|
| GDPC1 | 37.97 ± 3.31c | 2.55 ± 1.12b | 31.83 ± 2.87a | 6.06 ± 0.65a | 33.23 ± 3.64a |
| GDPC2 | 44.10 ± 3.98b | 2.19 ± 1.32c | 20.27 ± 2.12c | 2.85 ± 0.07c | 4.63 ± 0.11c |
| GDPBIO | 63.54 ± 4.52a | 3.28 ± 2.00a | 21.15 ± 1.25b | 3.45 ± 0.23b | 5.56 ± 0.91b |
| Sign. | ** | ** | ** | ** | ** |
Data are expressed as means ± SD (n = 3). Sign: significant. Differences between powders were evaluated by a one‐way ANOVA followed by a multicomparison Tukey test. ** P < 0.01. Means in the same column with different small letters differ significantly.
Antioxidant potential of ginger powder extracts and ginger powder based Pickering emulsions
The antioxidant power of samples was investigated using a multi‐target approach, given the complexity of the oxidative process (Tables 3 and 4). In this study, the half‐maximal inhibitory concentration (IC50) of the positive control, ascorbic acid, used in the antioxidant assays was comparable to a previous finding. 35 , 36 , 37 All samples could reduce iron with a potency greater than the BHT positive control with values ranging from 91.21 to 102.50 μmol L−1 Fe (II) g−1 for GDPC2 and GDPC1, respectively, whereas values from 101.87 to 102.63 μmol L−1 Fe (II) g−1 were recorded for 05PE_GDPBIO and 05PE_GDPC1, respectively. Relative to ginger powders, analyzing the protection from lipid peroxidation, the following trend was observed at both incubation times: GDPC1 > GDPBIO > GDPC2. 38 The conversion of powder in the emulsion did not affect protection from lipid peroxidation as it was observed for all tested PE. The 05PE_GDPBIO sample showed 94.78% inhibition of lipid peroxidation after 60 min of incubation. In this study, two different radical systems (DPPH and ABTS tests) were applied to investigate the radical scavenging activity of both GDP extracts and derived PE_GDPs. All powder samples exhibited strong ABTS+·radical scavenging potential in comparison with the positive control ascorbic acid with reference to GDPBIO and GDPC2 (IC50 values of 0.79 and 0.81 μg mL−1, respectively). A similar observation should be made in the DPPH test, where the same samples have IC50 values comparable with the positive control, ascorbic acid (IC50 values of 7.91 and 9.80 μg mL−1 for GDPBIO and GDPC2, respectively). Lower DPPH and ABTS radical scavenging activity (IC50 values of 4.25 and 0.40 mg mL−1) was found for biological ginger extract by Mošovská et al. 32 despite higher TPC content and comparable TFC value.
Table 3.
Antioxidant potential of ginger powder extracts
| Sample | FRAP | β‐carotene bleaching test | DPPH | ABTS | Reference | |
|---|---|---|---|---|---|---|
| t = 30 min | t = 60 min | |||||
| Powder | ||||||
| μM Fe (II)/g | IC50 (μg mL−1) | IC50 (μg mL−1) | IC50 (μg mL−1) | IC50 (μg mL−1) | ||
| GDPC1 | 102.50 ± 3.91 | 6.63 ± 0.94*** | 6.81 ± 2.93*** | 10.79 ± 1.01 | 1.76 ± 0.70 | 38 |
| GDPC2 | 91.21 ± 3.54 | 16.57 ± 1.47**** | 13.95 ± 2.96**** | 9.80 ± 0.98 | 0.81 ± 0.05 | 38 |
| GDPBIO | 100.95 ± 3.99 | 8.13 ± 1.02**** | 7.80 ± 2.91**** | 7.91 ± 0.74 | 0.79 ± 0.06 | 38 |
| Control positive | 63.2 ± 2.3 | 0.09 ± 0.04 | 0.08 ± 0.03 | 5.09 ± 0.09 | 1.70 ± 0.06 | |
Abbreviations: ABTS: 2,2′‐azino‐bis‐3‐thylbenzothiazoline‐6‐sulfonic acid; DPPH: 2,2‐diphenyl‐1‐picrylhydrazyl; FRAP: reducing antioxidant power.
Differences within and between groups were evaluated by one‐way ANOVA followed by a multicomparison Dunnett's test: **** P < 0.0001. *** P < 0.001, compared with positive controls: BHT in FRAP test; Propyl gallate in β‐carotene bleaching test; Ascorbic acid in DPPH and ABTS tests.
Table 4.
Antioxidant potential of ginger‐based Pickering emulsions
| Sample | FRAP | β‐carotene bleaching test | DPPH | ABTS | |
|---|---|---|---|---|---|
| t = 30 min | t = 60 min | ||||
| Pickering emulsion | |||||
| μM Fe (II)/g † | % Inhibition † | % Inhibition † | % Inhibition † | % Inhibition † | |
| 05PE_GDPC1 | 102.63 ± 4.81a | 80.45 ± 3.75b | 97.89 ± 3.32b | 86.65 ± 3.78b | 80.47 ± 4.02a |
| 05PE_GDPC2 | 101.94 ± 4.93b | 76.36 ± 2.24a | 97.72 ± 2.61b | 88.34 ± 3.82c | 82.43 ± 3.75b |
| 05PE_GDPBIO | 101.87 ± 3.87b | 88.64 ± 3.76c | 94.78 ± 4.03a | 82.59 ± 2.97a | 83.38 ± 4.08c |
| Sign. | ** | ** | ** | ** | ** |
PE_GDPs were tested as they are. FRAP, reducing antioxidant power; DPPH, 2,2‐diphenyl‐1‐picrylhydrazyl; ABTS, 2,2′‐azino‐bis‐3‐thylbenzothiazoline‐6‐sulfonic acid. Sign, significant. Differences between samples were evaluated by one‐way ANOVA followed by a multicomparison Tukey test. ** P < 0.01. Means in the same column with different small letters differ significantly.
No statistically significant differences were observed in PE radical scavenging potential. Pearson's correlation coefficient revealed positive correlations between TPC and TFC, and the FRAP test, with r values of 0.94 and 0.94 whereas for PE a positive correlation was only found between TPC, TFC and TCC and β‐carotene bleaching test (0.95, 0.91 and 0.98 at 30 min of incubation, respectively). Previously, Shah et al. 39 demonstrated that curcumin‐encapsulated PE showed a higher DPPH radical scavenging activity than free curcumin dissolved in oil phase and they confirmed that this is a possible strategy to enhance the antioxidant potential of naturally derived products.
Anti‐lipase, ‐α‐amylase, and ‐α‐glucosidase activity
The inhibitory activity of key enzymes involved in the metabolism of fats and sugars was assessed. Generally, no statistically significant differences were recorded among GDP extracts in lipase inhibition (Table 5). The IC50 values of 32.37 and 39.57 μg mL−1 were recorded for GDPC1 and 2, respectively. These values are comparable to those reported for the positive control Orlistat (IC50 value of 37.42 μg mL−1). 38 A promising lipase inhibitory assay was also observed when PE were tested. In this case, the following order of potency should be observed: 05PE_GDPC2 > 05PE_GDPBIO > 05PE_GDPC1. A high degree of variability was found against α‐glucosidase and α‐amylase enzymes; sample GDPC2 was the most active with IC50 values of 43.85 and 55.49 μg mL−1, respectively. Pearson's correlation coefficient showed that both TPC and TFC are positive related to α‐amylase inhibitory activity with an r values of 0.85 in both cases whereas values of 0.92, 0.92 and 0.94 were found for TPC, TFC and TCC and α‐glucosidase inhibitory activity. Analysis of PE bioactivity showed that 05PE_GDPBIO exerted the highest anti‐α‐amylase inhibitory activity with 100% inhibition, followed by 05PE_GDPC2 whereas sample 05PE_GDPC1 showed the highest activity against α‐glucosidase (99.08%) (Fig. 6).
Table 5.
Anti‐lipase, ‐α‐amylase, and ‐α‐glucosidase ginger powder extracts
| Samples | Anti‐lipase | Anti‐α‐glucosidase | Anti‐α‐amylase | Reference |
|---|---|---|---|---|
| Powder | ||||
| IC50 (μg mL−1) | IC50 (μg mL−1) | IC50 (μg mL−1) | ||
| GDPC1 | 32.37 ± 1.41ns | 61.31 ± 1.43**** | 74.88 ± 1.80**** | 38 |
| GDPC2 | 39.57 ± 1.87ns | 43.85 ± 1.31*** | 55.49 ± 1.61*** | 38 |
| GDPBIO | 34.48 ± 1.96ns | 78.76 ± 1.97**** | 68.09 ± 1.57**** | 38 |
| Positive controls | 37.42 ± 1.05 | 35.51 ± 0.94 | 50.12 ± 1.37 |
Data are expressed as means ± SD (n = 3). Acarbose was used as a positive control in the α‐amylase and α‐glucosidase tests. Orlistat was used as a positive control in the lipase test. Differences within and between groups were evaluated by one‐way ANOVA followed by a multicomparison Dunnett's test: ****P < 0.0001, ***P < 0.001, compared with acarbose in α‐glucosidase and α‐amylase, and Orlistat in a lipase inhibition assay (IC50 = μg mL−1).
Figure 6.
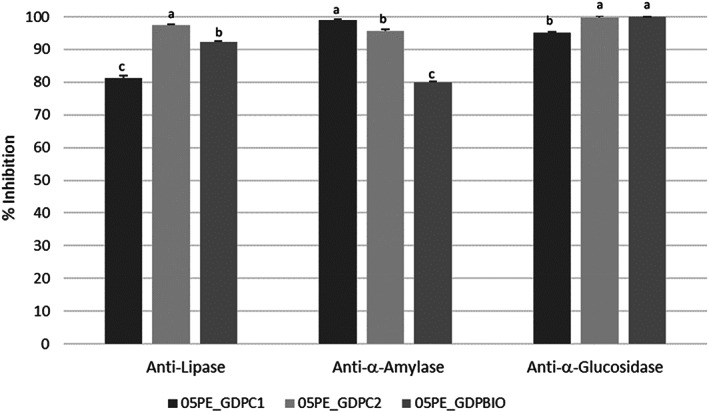
Anti‐lipase, ‐α‐amylase, and ‐α‐glucosidase ginger powder‐based PE. Pickering emulsions were tested as they were. Differences between samples were evaluated by one‐way ANOVA followed by a multicomparison Tukey test. **P < 0.01. Means in the same column with different small letters differ significantly.
Several research articles investigated anti α‐amylase, anti‐α‐glucosidase and anti‐lipase activity for possible use as a strategy for prevention of hyperglycemia and obesity with aim of using these bioactive ingredients for the development of functional foods or nutraceutical products. 11 , 12 , 13
No statistically significant differences were recorded between samples derived from biological and conventional practices. Previously, the investigation on the effect of red (Z. officinale var. Rubra) and Z. officinale Roscoe on both enzymes revealed that Z. officinale Roscoe had a significantly stronger inhibitory effect against both enzymes than red ginger. 40 Rani et al. 41 investigated the anti‐α‐amylase and ‐α‐glucosidase activity of different GDP extracts. Ethyl acetate extract showed the highest activity, with an IC50 value of145.04 μg mL−1 against α‐amylase. Ginger lipase inhibitory activity was previously reported by Bae et al., 42 who found IC50 values of 68.4 and 84.8 μg mL−1 for butanol and chloroform, respectively.
CONCLUSION
In conclusion, the present study has designed, for the first‐time, Ginger dried powders stabilized Pichering emulsions (PE_GDPs). The physico‐chemical properties (morphology, particle size distribution, ζ potential, and contact angle) of different GDPs obtained by biological and conventional agricultural practices were investigated. We have reported here the physical characteristics of ginger aqueous dispersions, after which the emulsifying capacity of raw GDPs was discussed. The physical stability of 05PE_GDPs was assessed for up to 1 month. The GDPs and PE_GDPs were also characterized for total phenol, flavonoid, and carotenoid content as well as for quantification of selected markers (6‐gingerol, 8‐gingerol, 6‐shogaol, 6‐paradol and 10‐gingerol). The GDPs and 05PE_GDPs were investigated for their in vitro antioxidant activity, carbohydrate hydrolyzing enzyme and lipase inhibitory properties. GDPBIO showed greater ability than other powders to form O/W emulsions that were highly stable to coalescence. Further investigations to better understand the physico‐chemical properties at the origin of the best emulsifying ability of biological ginger powders are planned. Among the investigated PEs, it was found that 05PE_GDBIO was characterized by a high stability to oxidation and a promising antioxidant and α‐amylase inhibitory activity. In vitro release studies as a function of the different content of stabilizer in more stable 05PE_GDPs are already under way. The aim is to confirm the optimum 05PE_GDP with the highest bioavailability of bioactive ingredients, of which GDPs naturally have a rich content. Preliminary results indicate their possible application for targeted delivery of nutraceuticals or checking lipid digestion. However, further studies are necessary to confirm the PE_GDP physical stability in different matrix and to investigate the in vivo biological effect.
AUTHOR CONTRIBUTIONS
Conceptualization: Patrizia Formoso and Monica Rosa Loizzo. Formal analysis: Maria Carmela Pellegrino, Giuseppina Anna Corrente, Mariarosaria Leporini, Rosa Romeo, and Luigia Gervasi. Data curation: Amerigo Beneduci, Rosa Tundis, Vincenzo Sicari. Writing – original draft preparation: Patrizia Formoso, Vincenzo Sicari. Writing – review and editing: Patrizia Formoso and Rosa Tundis. Supervision: Patrizia Formoso and Monica Rosa Loizzo. All authors have read and agreed to the published version of the manuscript.
FUNDING
Italian Ministry for University and research (MIUR) – EX 60%.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
The authors wish to thank to Eigenmann & Veronelli S.p.A., Italy for providing Miglyol® 812 N, Dr Sabrina Morelli, Institute on Membrane Technology of the National Research Council of Italy, and Dr Domenico Sturino, Native English Speaker and lecturer at the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria for manuscript proofreading.
This work is dedicated to the memory of Professor Francesco Menichini, Head of Pharmaceutical Biology and Food Science Technology Research Group at University of Calabria from 1978 to 2016. Professor Menichini played a key role in the development of bioactive phytochemicals, not only within Italy but also in the international arena. Open Access Funding provided by Universita della Calabria within the CRUI‐CARE Agreement.
Contributor Information
Patrizia Formoso, Email: patrizia.formoso@unical.it.
Rosa Tundis, Email: rosa.tundis@unical.it.
REFERENCES
- 1. Santos Braga S, Ginger: Panacea or consumer's hype? Appl Sci 9:1570 (2019). [Google Scholar]
- 2. Seo SH, Fang F and Kang I, Ginger (Zingiber officinale) attenuates obesity and adipose tissue remodeling in high‐fat diet‐fed C57BL/6 mice. Int J Environ Res Public Health 18:631–638 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganji S and Sayyed‐Alangi SZ, Encapsulation of ginger ethanolic extract in nanoliposome and evaluation of its antioxidant activity on sunflower oil. Chem Pap 71:1781–1789 (2017). [Google Scholar]
- 4. Kalarikkal SP, Prasad D, Kasiappan R, Chaudhari SR and Sundaram GM, A cost‐effective polyethylene glycol‐based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci Rep 10:4456 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei Y, Zhou D, Mackie A, Yang S, Dai L, Zhang L et al., Stability, interfacial structure, and gastrointestinal digestion of β‐carotene‐loaded Pickering emulsions co‐stabilized by particles, a biopolymer, and a surfactant. J Agric Food Chem 69:1619–1636 (2021). [DOI] [PubMed] [Google Scholar]
- 6. Binks BP, Particles as surfactants‐similarities and differences. Curr Opin Colloid Interface Sci 7:21–41 (2002). [Google Scholar]
- 7. Khan A, Wen Y, Huq T and Ni Y, Cellulosic nanomaterials in food and nutraceutical applications: a review. J Agric Food Chem 66:8–19 (2018). [DOI] [PubMed] [Google Scholar]
- 8. Tan C and McClements DJ, Application of advanced emulsion technology in the food industry: a review and critical evaluation. Foods 10:812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bai L, Huan S, Rojas OJ and McClements DJ, Recent innovations in emulsion science and technology for food applications. J Agric Food Chem 69:8944–8963 (2021). [DOI] [PubMed] [Google Scholar]
- 10. Ng ZX, Samsuri SN and Yong PH, The antioxidant index and chemometric analysis of tannin, flavonoid, and total phenolic extracted from medicinal plant foods with the solvents of different polarities. J Food Process Preserv 44:e14680 (2020). [Google Scholar]
- 11. Ng ZX and See AN, Effect of in vitro digestion on the total polyphenol and flavonoid, antioxidant activity and carbohydrate hydrolyzing enzymes inhibitory potential of selected functional plant‐based foods. J Food Process Preserv 43:e139032019 (2019). [Google Scholar]
- 12. Ng ZX and Rosman NF, In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate‐digestive enzymes inhibitory potential of selected edible mushrooms. J Food Sci Technol 56:865–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leporini M, Loizzo MR, Sicari V, Pellicanò TM, Reitano A, Dugay A et al., Citrus × Clementina Hort. juice enriched with its by‐products (peels and leaves): chemical composition, in vitro bioactivity, and impact of processing. Antioxidants 9:298 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leporini M, Tundis R, Sicari V, Pellicanò TM, Dugay A, Deguin B et al., Impact of extraction processes on phytochemicals content and biological activity of Citrus × Clementina Hort. Ex Tan leaves: new opportunity for under‐utilized food by‐products. Food Res Int 127:108742 (2020). [DOI] [PubMed] [Google Scholar]
- 15. Sicari V, Loizzo MR, Sanches Silva A, Romeo R, Spampinato G, Tundis R et al., The effect of blanching on phytochemical content and bioactivity of Hypochaeris and Hyoseris species (Asteraceae), vegetables traditionally used in southern Italy. Foods 10:32 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. You H, Ireland B, Moeszinger M, Zhang H, Snow L, Krepich S et al., Determination of bioactive nonvolatile ginger constituents in dietary supplements by a rapid and economic HPLC method: analytical method development and single‐laboratory validation. Talanta 194:795–802 (2019). [DOI] [PubMed] [Google Scholar]
- 17. Zheng H, Mao L, Yang J, Zhang C, Miao S and Gao Y, Effect of oil content and emulsifier type on the properties and antioxidant activity of sea buckthorn oil‐in‐water emulsions. J Food Qual 2020:1540925 (2020). [Google Scholar]
- 18. Loizzo MR, Tundis R, Sut S, Dell'acqua S, Ilardi V, Leporini M et al., High‐performance liquid chromatography/electrospray ionization tandem mass spectrometry (HPLC‐ESI‐MSn) analysis and bioactivity useful for prevention of “diabesity” of Allium commutatum Guss. Plant Foods Hum Nutr 75:124–130 (2020). [DOI] [PubMed] [Google Scholar]
- 19. Tundis R, Conidi C, Loizzo MR, Sicari V, Romeo R and Cassano A, Concentration of bioactive phenolic compounds in olive mill wastewater by direct contact membrane distillation. Molecules 26:1808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loizzo MR, Napolitano A, Bruno M, Geraci A, Schicchi R, Leporini M et al., LC‐ESI/HRMS analysis of glucosinolates, oxylipins and phenols in Italian rocket salad (Diplotaxis erucoides subsp. erucoides (L.) DC.) and evaluation of its healthy potential. J Sci Food Agric 101:5872–5879 (2021). [DOI] [PubMed] [Google Scholar]
- 21. Dickinson E, Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci Technol 1:4–12 (2012). [Google Scholar]
- 22. Schulman HJ and Leja J, Control of contact angles at the oil‐water‐solid interfaces. Emulsions stabilized by solid particles (BaSO4). Trans Faraday Soc 50:598–605 (1954). [Google Scholar]
- 23. Hao H, Tarun B, Songbai L, Zhenhua D and Zisheng L, Novel multi‐phase nano‐emulsion preparation for co‐loading hydrophilic arbutin and hydrophobic coumaric acid using hydrocolloids. Food Hydrocolloids 93:92–101 (2019). [Google Scholar]
- 24. Aveyard R, Binks BP and Clint JH, Emulsions stabilised solely by colloidal particles. Adv Colloid Interface Sci 100:503–546 (2003). [Google Scholar]
- 25. Zembyla M, Lazidis A, Murray BS and Sarkar A, Water‐in‐oil Pickering emulsions stabilized by synergistic particle‐particle interactions. Langmuir 35:13078–13089 (2019). [DOI] [PubMed] [Google Scholar]
- 26. Binks BP and Rodrigues JA, Types of phase inversion of silica particle stabilized emulsions containing triglyceride oil. Langmuir 19:4905–4912 (2003). [Google Scholar]
- 27. Berton‐Carabin C and Schroën K, Towards new food emulsions: designing the interface and beyond. Curr Opin Food Sci 27:74–81 (2019). [Google Scholar]
- 28. Binks BP, Colloidal particles at liquid interfaces. Phys Chem Phys 9:6298–6299 (2007). [DOI] [PubMed] [Google Scholar]
- 29. Kaukonen AM, Boyd BJ, Porter CJ and Carman WN, Drug solubilization behavior during in vitro digestion of simple triglycerides lipid solution formulations. Pharm Res 21:245–253 (2004). [DOI] [PubMed] [Google Scholar]
- 30. Le Bars G, Dion S, Gauthier B, Mhedhbi S, Pohlmeyer‐Esch G, Comby P et al., Oral toxicity of Miglyol 812® in the Göttingen® minipig. Regul Toxicol Pharmacol 73:930–937 (2015). [DOI] [PubMed] [Google Scholar]
- 31. Albright LF, Measuring physical properties, in Albright's Chemical Engineering Handbook, 1nd edn, ed. by Albright L. CRC Press, NY, pp. 1531–1537 (2008). [Google Scholar]
- 32. Mošovská S, Nováková D and Kaliňák M, Antioxidant activity of ginger extract and identification of its active components. Acta Chim Slovaca 8:115–119 (2015). [Google Scholar]
- 33. Shao X, Lv L, Parks T, Wu H, Ho CT and Sang S, Quantitative analysis of ginger components in commercial products using liquid chromatography with electrochemical array detection. J Agric Food Chem 58:12608–12614 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yudthavorasit S, Wongravee K and Leepipatpiboon N, Characteristic fingerprint based on gingerol derivative analysis for discrimination of ginger (Zingiber officinale) according to geographical origin using HPLC‐DAD combined with chemometrics. Food Chem 158:101–111 (2014). [DOI] [PubMed] [Google Scholar]
- 35. Ng ZX and Umah R, Effects of different heat treatments on the antioxidant activity and ascorbic acid content of bitter melon, Momordica charantia. J Food Technol 22:1–9 (2019). [Google Scholar]
- 36. Ng ZX and Phaik MI, Peperomia pellucida (L.) Kunth herbal tea: effect of fermentation and drying methods on the consumer acceptance, antioxidant and anti‐inflammatory activities. Food Chem 344:128738 (2021). [DOI] [PubMed] [Google Scholar]
- 37. Ng ZX, Koick YTT and Yong PH, Comparative analyses on radical scavenging and cytotoxic activity of phenolic and flavonoid content from selected medicinal plants. Nat Prod Res 35:5271–5276 (2021). [DOI] [PubMed] [Google Scholar]
- 38. Loizzo MR, Formoso P, Leporini M, Sicari V, Falco T and Tundis R, Influence of organic and conventional agricultural practices on chemical profile, in vitro antioxidant and anti‐obesity properties of Zingiber officinale Roscoe. In: The 1st International E‐Conference on Antioxidants in Health and Disease Session. The Biology of Natural Products in Disease Pathophysiology: Mechanisms of Action. 2020.
- 39. Shah BR, Zhang C, Li Y and Li B, Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan‐tripolyphosphate nanoparticles. Food Res Int 89:399–407 (2016). [DOI] [PubMed] [Google Scholar]
- 40. Oboh G, Akinyemi A, Ademiluyi A and Adefegha A, Inhibitory effects of aqueous extracts of two varieties of ginger on some key enzymes linked to type‐2 diabetes in vitro . J Food Nutr Res 49:14–20 (2010). [Google Scholar]
- 41. Rani M, Padmakumari K, Sankarikutty B, Cherian L, Nisha VM and Raghu KG, Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int J Food Sci Nutr 62:106–110 (2011). [DOI] [PubMed] [Google Scholar]
- 42. Bae J‐S and Kim T‐H, Pancreatic lipase inhibitory and antioxidant activities of Zingiber officinale extracts. Korean J Food Preserv 18:390–396 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


