Abstract
In pneumococcal meningitis it is assumed that bacteria cross the blood-brain barrier (BBB), which consists mainly of cerebral endothelial cells. The effect of Streptococcus pneumoniae on the BBB was investigated with an in vitro BBB model using a human brain microvascular endothelial cell line (HBMEC) and primary cultures of bovine brain microvascular endothelial cells (BBMEC). Within a few hours of incubation with pneumococci, rounding and detachment of the HBMEC were observed, and the transendothelial electrical resistance of the BBMEC monolayer decreased markedly. An S. pneumoniae mutant deficient in pneumolysin did not affect the integrity of the endothelial cell monolayer. Neither cell wall fragments nor isolated pneumococcal cell walls induced changes of endothelial cell morphology. However, purified pneumolysin caused endothelial cell damage comparable to that caused by the viable pneumococci. The cell detachment was dependent on de novo protein synthesis and required the activities of caspase and tyrosine kinases. The results show that pneumolysin is an important component for damaging the BBB and may contribute to the entry of pneumococci into the cerebral compartment and to the development of brain edema in pneumococcal meningitis.
Pneumococcal infection is the main cause of severe bacterial meningitis in children and adults (31). As a correlate of neuronal cell damage, apoptosis of neurons has been observed in humans suffering from bacterial meningitis as well as with experimental pneumococcal meningitis (7, 20, 38). Two major mechanisms are thought to be responsible for brain damage resulting from pneumococcal meningitis: neurotoxicity of host mediators and bacterial products, and brain edema caused by loss of the integrity of the blood-brain barrier (BBB) (7, 18, 19, 25, 35, 39). The brain edema—the most serious complication during bacterial meningitis—is caused by microvascular changes and damage to the BBB (23, 24).
The BBB consists of endothelial cells which are sealed by tight junctions (6). Tight junction formation is characteristic of cerebral endothelial cells and is enhanced by astrocytes (17, 21). The interaction of pneumococci with cerebral endothelial cells is poorly understood. It has been reported recently that pneumococci are able to invade cerebral endothelial cells and that the interaction with the platelet-activating factor receptor contributes to pneumococcal invasion (26). Pneumococci exert a cytotoxic effect on human umbilical vein endothelial cells, apparently mediated by the pneumococcal cell wall (11). Since pneumococci are the main cause of bacterial pneumonia, their effect has been investigated in other experimental systems. It has been shown that pneumococci invade the lung epithelium and pulmonary endothelial cells through the action of pneumolysin. This protein, a major virulence factor of Streptococcus pneumoniae, slows ciliary beating of the epithelial cells, disrupts tight junctions, and contributes to pneumococcal adherence to the disrupted bronchial epithelium (for a review, see reference 30). Pneumolysin injures alveolus epithelial cells (28) and pulmonary artery endothelial cells (29), resulting in disruption of the cells that form the alveolus-capillary barrier. In addition to these direct effects of pneumolysin on endothelial and epithelial cells, the protein interacts with the human inflammatory and immune responses. Whereas pneumolysin inhibits neutrophil and monocyte function, it induces the production of proinflammatory mediators, such as nitric oxide, COX-2, tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 production in macrophages (7, 16, 30). The cytotoxicity of pneumolysin is probably due to its ability to bind cholesterol and to the formation of transmembrane pore complexes (12, 27). The mechanism by which pneumolysin induces the proinflammatory mediators remains speculative.
Studies in experimental pneumococcal meningitis revealed that pneumolysin is the causative agent of hearing loss and cochlear damage, one of the major sequelae of pneumococcal meningitis (37). This effect appears to be related to the pneumolysin-induced tissue damage rather than to an inflammatory host response. In addition, pneumolysin when injected into the subarachnoid space caused a brisk inflammatory response and contributed to BBB damage. However, pneumolysin-deficient mutants and wild-type cells induced subarachnoid inflammation to a similar degree (10). These in vivo experiments strongly suggest that pneumolysin is an important component in the pathogenicity of meningitis.
We investigated the role of pneumolysin and pneumococcal cell walls in an in vitro system of the BBB using cerebral endothelial cells. Pneumococci induced severe damage in brain microvascular endothelial cells. Production of pneumolysin was important for disintegration of the BBB, but pneumococcal cell walls had no effect. The cytopathology of human brain microvascular endothelial cells (HBMEC) in response to pneumolysin was dependent on de novo protein synthesis, tyrosine phosphorylation, and induction of caspase activity.
MATERIALS AND METHODS
Bacterial strains.
Streptococcus pneumoniae D39 (serotype 2) (1) was used in cell culture experiments. Bacteria were grown in Todd-Hewitt broth containing 0.5% yeast extract. Cells were harvested at mid-exponential phase by centrifugation, washed once with phosphate-buffered saline, and resuspended in cell culture medium to a final concentration of 107 CFU/ml. In some experiments, 10-fold dilutions of the pneumococcal suspension were used. A pneumolysin-negative mutant of D39 (D39ply::pJDC9) was constructed by insertion-duplication mutagenesis. A 418-bp internal gene fragment, amplified from chromosomal DNA using the primers ply-1 (TTG TTG GAT CCG AAA GAA AGA AGC) and ply-2 (GAG TTA GGA TCC ATA TCA AGA GAA), was ligated with the insertion vector pJDC9 (8) by standard DNA techniques. After transformation of the plasmid into S. pneumoniae D39, erythromycin-resistant transformants were selected with 1 μg of erythromycin/ml of Luria-Bertani agar containing 5% sheep blood. Insertion of the plasmid into the pneumolysin gene ply was confirmed by PCR analysis and DNA sequencing. For preparation of purified pneumococcal cell walls, the unencapsulated pneumococcal strain R6x (36) was used.
Sixteen clinical pneumococcal strains isolated from the blood or cerebrospinal fluid of patients suffering from pneumococcal pneumonia and/or meningitis were additionally used. The strains were isolated in our laboratory.
Cell culture materials and chemical reagents.
M199, RPMI 1640, newborn calf serum (NCS), fetal calf serum (FCS), glutamine, nonessential amino acids, minimal essential medium (MEM)-vitamins, Na pyruvate, bovine plasma fibronectin, penicillin-streptomycin (P/S), and amphotericin were obtained from Gibco BRL (Eggenstein, Germany). Collagenase-dispase, dispase II, and trypsin-EDTA solution were obtained from Roche Molecular Biochemicals (Mannheim, Germany). Dulbecco's modified Eagle's medium (DMEM), HEPES, bovine serum albumin, gelatin, heparin, and anti-glial fibrillary acidic protein antibodies were from Sigma (Deisenhofen, Germany), dextran was from IGN (Eschwege, Germany), Percoll was from Pharmacia Biotech (Freiburg, Germany), and NuSerum IV was from Becton Dickinson (Heidelberg, Germany). Multiwell culture dishes and Transwell Clear were obtained from Corning Costar (Bodenheim, Germany). All chemical reagents were of analytical grade.
HBMEC cultures.
HBMEC were isolated from a brain biopsy of an adult female with epilepsy. The cells retained morphological and functional characteristics of cerebral endothelial cells as previously described (34). HBMEC were plated at a density of 105 cm−2 in 25-cm2 cell culture flasks or 24-well plates, respectively. Cells were cultured in RPMI 1640 supplemented with heat-inactivated 10% FCS, 10% NuSerum IV, 1% nonessential amino acids, 1% MEM- vitamins, 1 mM Na pyruvate, 2 mM glutamine, and 1% P/S. Cultures were incubated at 37°C in a humid atmosphere enriched with 5% CO2. After 2 days of culture, the medium was changed to culture medium free of antibiotics. After an additional day, the culture medium was replaced with medium containing pneumococci.
Cell detachment assay.
Cells (105) were seeded on 24-well plates and cultured for 72 h with medium changes as described above. Following treatment, the supernatant and two washes from each well were pooled, and the cells were counted. The remaining cells were detached by 0.5-mg/ml trypsin–0.2-mg/ml EDTA, and the cells were counted. Percent detachment was expressed as (total cells in the supernatant and wash)/(total cells in the supernatant, wash, and detached from the culture plate) × 100.
BBMEC cultures.
Bovine brain microvascular endothelial cells (BBMEC) were prepared from the cerebral cortices of freshly slaughtered cows. The cells were isolated by a modified method described by Bowman et al. (5). Briefly, the meninges and the white matter were removed from the brain. The cortex was cut into 2-mm3 pieces, and tissue fragments were resuspended in M199 containing 1% dispase II and 2% P/S to a final volume of 160 ml. The cell suspension was incubated for 2 to 3 h at 37°C. The tissue suspension was passed through a 180-μm-pore-size sieve, and the filtrate, containing microvessels, was diluted 2:5 with dextran dissolved in M199 to a final dextran concentration of 9%. The preparation was vortexed for 30 s and spun down at 3,000 × g. The cell pellets were resuspended in 50 ml of M199 containing 0.1% collagenase, 0.08% dispase, and 2% P/S. The suspension was incubated for 12 h at 37°C with mild agitation. The cell suspension was centrifuged at 200 × g for 10 min at 37°C. The cell pellet was resuspended in 12 ml of M199, and aliquots (2 ml each) were placed on top of a Percoll gradient (derived by centrifugation of 45% Percoll in M199 at 37,500 × g for 40 min) and centrifuged at 800 × g for 20 min at 37°C. The middle band containing the capillary endothelial cells was removed and washed in 50 ml of M199. After centrifugation at 200 × g for 10 min, cells were resuspended in 5 ml of trypsin-EDTA solution and incubated at 37°C. After 5 min the reaction was stopped by addition of 10 ml of M199 containing 20% newborn calf serum (NCS), 1% P/S, and 0.1% glutamine (M199–20% NCS), washed once, and finally resuspended in 10 ml of M199–20% NCS. Cells (270,000) were seeded on polyester filter membranes (Transwell Clear; 12 mm; pore size, 0.4 μm) coated with 2% bovine plasma fibronectin for 1 h. In total, 20 h were used for preparation of the cells before plating. The BBMEC were grown over 7 days before stimulation experiments were performed. At that time, more than 97% of the cells were viable.
Rat astrocyte culture.
Primary cultures of astrocytes were prepared from newborn rat cerebral cortex. After the meninges and the white matter were removed, the brain tissue was placed in DMEM containing 10% FCS–1% P/S–1% glutamine. The tissue was forced gently through a 180-μm- and a 70-μm-pore-size sieve consecutively. The cells were washed once with culture medium. Cells (105 per well) were seeded on 12-well dishes precoated with 0.2% gelatin. Medium was changed daily during the first 3 days. Starting from day 4, medium was changed every second day. By this method astrocyte cultures were obtained with a purity of more than 95%, estimated by immunohistochemistry for anti-glial fibrillary acidic protein. The purity of astrocyte cultures was controlled daily by analyzing the cell morphology.
BBB in vitro model.
After reaching confluence, astrocyte cultures were used for coculture of BBMEC seeded on polyester filters precoated with bovine plasma fibronectin (see above). The endothelial cells and astrocytes were cocultured for 7 days. Cell culture medium was exchanged every second day. Under these conditions, the endothelial cells reached confluence within 3 to 4 days, and the transendothelial electrical resistance (TEER) increased continuously until day 7 to 8. The effect of bacteria was investigated after the TEER reached a value of about 500 to 600 Ω × cm2, typically at day 7 of in vitro culture. The TEER was monitored with a Millicell ERS device (Millipore, Eschborn, Germany). The resistance measurement chamber was obtained from WPI (Berlin, Germany).
Prior to stimulation with bacteria, antibiotics were removed by two washes with culture medium free of P/S. At each time point the TEER was measured in triplicate. One single filter could be used only once for measuring the TEER to avoid an artificial decrease of the TEER. The TEER of the stimulated filters is given as a percentage of the mean calculated from quadruplicates of results for nonstimulated controls.
Preparation of purified pneumococcal cell walls.
For cell wall preparation, a procedure described previously by Heumann et al. (14) was used with minor modifications. R6x cells were obtained from 1,000 ml of exponentially growing culture. Cells were washed in 50 mM Tris buffer (pH 7.0), added to preheated 5% sodium dodecyl sulfate (SDS) solution, and boiled for 15 min. Bacterial cell walls were centrifuged at 17,000 × g for 10 min. The pellet was washed twice in 1 M NaCl and three times in distilled water to remove SDS and resuspended with the help of a glass bead stirrer. Remaining cells were removed by centrifugation at 700 × g, and cell walls were obtained from the supernatant by high-speed centrifugation (38,000 × g; 15 min). The pellet was resuspended in 100 mM Tris buffer (pH 7.5) containing 0.05% NaN3, 20 mM MgSO4, 10 μg of DNase/ml, and 50 μg of RNase/ml and incubated at 37°C for 2 h. Trypsin was added to a final concentration of 50 μg/ml, and CaCl2 was added to a concentration of 10 mM. The solution was digested overnight at 37°C. SDS was added to a final concentration of 1%, and the mixture was incubated at 60°C for 15 min. The pneumococcal cell walls were pelleted at 38,000 × g and washed four times with distilled water until the SDS was completely removed. The cell wall pellet was lyophilized and resuspended in distilled water to give a final concentration of 1 mg/ml.
Preparation of pneumolysin.
Pneumolysin was purified as previously described (22). Briefly, recombinant toxin was overexpressed in Escherichia coli strain JM109. The bacteria were lysed by sonication, and the pneumolysin was purified by hydrophobic and ion-exchange chromatography. Toxin purity was assessed by SDS-polyacrylamide gel electrophoresis followed by Coomassie blue staining, which showed a single 52-kDa band accounting for 95% of the protein.
Detection and neutralization of pneumolysin.
Pneumolysin production was tested using the hemolysis assay as described previously (4). A pneumolysin-neutralizing polyclonal rabbit serum was used (T.J. Mitchell, University of Glasgow, Glasgow, United Kingdom). The serum was used at dilutions of 1:10 to 1:1,000. For a control, sera of nonimmunized rabbits were used at the appropriate concentrations.
Measurement of the oxidative burst of neutrophil granulocytes.
Neutrophil granulocytes were isolated from buffy coats derived from healthy human donors using plasma Percoll gradients (Pharmacia Biotech) and were cultured in DMEM with 10% FCS in a concentration of 106 ml−1 at 37°C. Pneumococcal cell walls were added to the in vitro cultures immediately after isolation of the cells. The release of oxidative radicals was measured by chemiluminescence using 5-amino-2,3-dihydro-1,4-phthalazinedione (Sigma). Chemiluminescence was measured over 240 min using the AutoLumat LB 953 (E&G Berthold, Bad Wildbad, Germany). Three distinct preparations were used for the experiments.
Inhibition studies.
Cycloheximide (Sigma) was used at a concentration of 5 and 50 μg/ml, herbimycin A (Sigma) was used at 0.25 and 2.5 μg/ml, and N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl-ketone (z-VAD-fmk; Calbio-chem) was used at 10 and 100 μM. HBMEC were preincubated with herbimycin A for 4 h and with z-VAD-fmk for 2 h prior to the experiment.
Statistical analysis.
To test for differences between the groups, analysis of variance followed by the Student-Newman-Keuls multiple comparison test was used. A P value of <0.05 was considered statistically significant.
RESULTS
In vitro model of BBB.
In the present study an HBMEC line and primary BBMEC were used. HBMEC were originally isolated from a brain biopsy of an adult female with epilepsy and immortalized by simian virus 40 transformation and retained characteristics of cerebral endothelial cells (33). The cells were positive for γ-glutamyl transpeptidase, low-density lipoprotein uptake, and factor VIII-Rag (data not shown). However, the cells attained a TEER of about 60 Ω × cm2 which could not be enhanced in coculturing with astrocytes. Therefore, we used primary cultures of BBMEC. BBMEC when cocultured with astrocytes were able to reach a TEER value of approximately 500 to 600 Ω × cm2 within 7 days of culture. The TEER was monitored daily, and stimulation experiments with pneumococci were performed after a plateau of the TEER was reached. Since the TEER depended slightly on the individual preparation, the values are given in relation to the mean of those the unstimulated control cultures cultivated in parallel.
Morphological changes of HBMEC induced by S. pneumoniae.
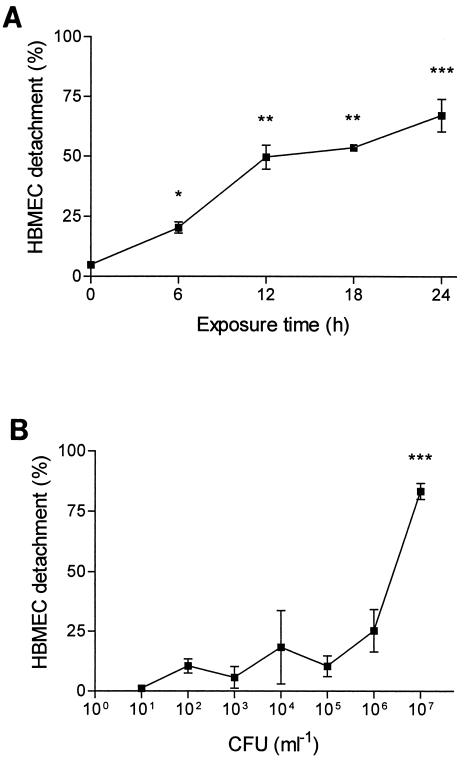
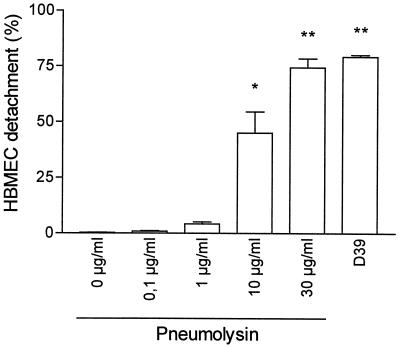
HBMEC changed their morphology after exposure to pneumococci. After 6 h of bacterial stimulation, the morphological changes were obvious and progressed over the 24-h observation period. The changes in HBMEC morphology started with rounding and detaching from the culture plate. For quantification of the damage of the endothelial cell monolayer, the percentage of detached cells was determined. The effect was dependent on the number of bacteria and the time of exposure. Pneumococci at a concentration of 107 CFU/ml caused detachment of up to 90% of the HBMEC. At 106 CFU/ml, the effect on HBMEC was less strong (25%), and no significant changes of cell morphology and cell detachment were observed at 105 CFU/ml and lower (Fig. 1). In culture supernatant, the lactate dehydrogenase concentration was elevated, demonstrating cell damage (data not shown). The detached cells showed signs of necrosis as revealed by vital staining with acridine orange-ethidium bromide. All further experiments were performed with 107 CFU/ml.
FIG. 1.
Detachment of HBMEC after addition of pneumococci. (A) Significant HBMEC detachment was measured 6 h after addition of 107 CFU of D39/ml; the detachment progressed over 24 h. (B) After 24 h of incubation time, significant cell detachment was observed after addition of pneumococci at concentrations of 106 to 107 CFU/ml. Values are means ± standard deviations of duplicate results. ∗, ∗∗, and ∗∗∗, P values of <0.05, 0.01, and 0.001 with respect to the controls.
Influence of S. pneumoniae on the BBMEC monolayer.
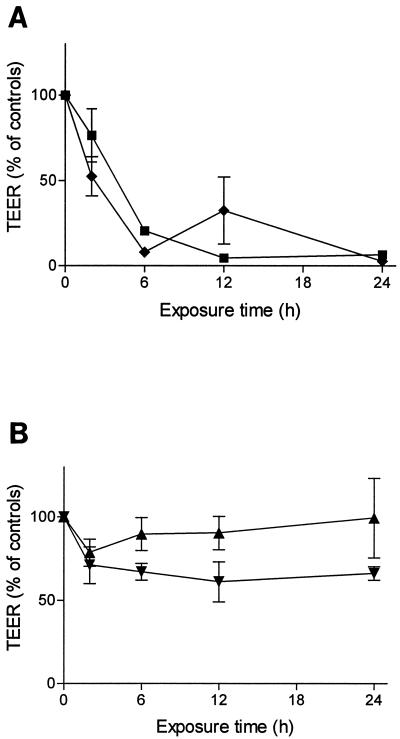
The primary culture of BBMEC retained the typical appearance of endothelial cells. After a tight monolayer was achieved, the endothelial cell compartment of the cultures was supplemented with pneumococci at a final concentration of 107 CFU/ml. After 6 to 8 h, the TEER decreased rapidly (Fig. 2A). The morphological changes differed from those observed for HBMEC cultures: the cytoplasm of the BBMEC shrank distinctly. However, the cells did not detach during the stimulation period of 24 h. Most of the BBMEC stained positive for apoptosis using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick labeling method. The lactate dehydrogase concentrations in the culture medium ranged within the background of the serum-supplemented culture medium.
FIG. 2.
TEER of BBMEC cultures after addition of D39 (107 CFU/ml). (A) The TEER decreased rapidly after the wild-type D39 was added, both without (■) and with (⧫) antibiotic supplementation. (B) Heat-killed wild-type D39 (▾) or viable pneumolysin-deficient pneumococci (D39ply::pJDC9) (▴) caused only minor changes to the tightness of the endothelial cell monolayer. The experiments were conducted in parallel using the same preparation of BBMEC. Values are means ± standard deviations of triplicate results in relation to the mean of results for four unstimulated controls.
Influence of pneumococcal inactivation by heat and treatment with antibiotics on cytotoxicity for cerebral endothelial cells.
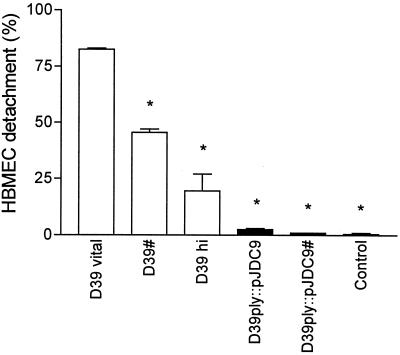
The use of heat-killed pneumococci (60°C; 20 min; 107 CFU/ml) caused minor morphological changes in BBMEC and HBMEC monolayers. The TEER of the BBMEC monolayer decreased slightly within 24 h (Fig. 2B). Only 25% of the HBMEC detached after a 24-h exposure to heat-killed pneumococci (Fig. 3). These experiments suggest that the causative factor is either heat labile, actively secreted, or released during autolysis of the pneumococci.
FIG. 3.
Effect of antibiotical treatment, heat inactivation, and pneumolysin activity on HBMEC detachment. Endothelial cell detachment was quantitated 24 h after addition of D39 and the pneumolysin-deficient mutant (D39ply::pJDC9; 107 CFU/ml). Viable pneumococci were added without and with (#) antibiotic supplementation. Antibiotical inactivation of the wild-type D39 caused significant cell cytotoxicity, whereas antibiotical treatment of the pneumolysin-deficient mutant did not. Heat inactivation of the wild-type D39 (D39 hi) attenuated the cytotoxic potential distinctly. Values are means ± standard deviations of duplicate results. ∗, P value of <0.001 with respect to results for viable D39.
To investigate the influence of pneumococcal components which were released into the medium during antibacterial treatment, live pneumococci (107 CFU/ml) were added in the presence of penicillin-streptomycin. Within 1 h, no viable pneumococci could be recovered from the endothelial cell culture. In contrast to the results obtained with heat-killed bacteria, a rapid decrease of the TEER of the BBMEC monolayer was observed (Fig. 2A). Approximately 50% of the HBMEC detached after 24 h of incubation time with antibiotically treated bacteria, in contrast to a detachment of less than 25% in experiments with heat-killed bacteria (Fig. 3).
Role of pneumococcal pneumolysin on cytotoxicity to the cerebral endothelium.
Since pneumolysin has been identified as the major pneumococcal cytotoxin which is released during autolysis of the bacterial cells (30), the effect of the cytotoxin on the cerebral endothelial cells was investigated. A pneumolysin-deficient mutant of S. pneumoniae D39 was constructed by insertion-duplication mutagenesis in the structural gene ply (D39ply::pJDC9). No pneumolysin activity could be detected in mutant cell lysates and in the culture supernatant.
D39ply::pJDC9 had no effect on the tightness of the cerebral endothelial cell monolayer. After addition of up to 107 CFU of the pneumolysin-deficient mutant/ml, the TEER of the BBMEC remained nearly unchanged over 24 h (Fig. 2B). Furthermore, the human endothelial cell line was also unaffected by the pneumolysin-deficient strain D39ply::pJDC9, and no significant cell detachment was observed (Fig. 3). The pneumolysin mutant was also investigated during antibiotic-induced inactivation. No morphological changes and no cell detachment were observed with these preparations, documenting that it was pneumolysin itself, rather than cell walls or cell wall fragments, that contributed to pneumococcus-induced cytotoxicity to cerebral endothelial cells (Fig. 3).
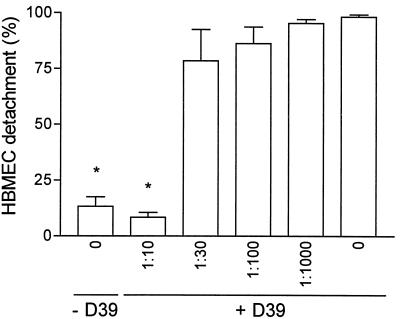
We complemented these results by coincubating the pneumolysin-producing pneumococcal strain D39 with a pneumolysin-neutralizing polyclonal rabbit antiserum in HBMEC cultures for 24 h. The results of that experiment were similar to those obtained with the pneumolysin-deficient mutant. No effect on cell shape or cell detachment was apparent during the 24-h period in the presence of the neutralizing antiserum at a dilution of 1:10 (Fig. 4). Only a slight inhibition of the pneumococcal cytotoxicity to the HBMEC was observed if sera of nonimmunized rabbits were used at the same dilution.
FIG. 4.
Effect of pneumolysin-neutralizing antiserum on HBMEC detachment 24 h after addition of wild-type D39 (107 CFU/ml). The antiserum abolished the pneumococcal cytotoxicity completely at a final dilution of 1:10; at dilutions of more than 1:30, no effect was evident over 24 h. Values are means ± standard deviations of duplicates. ∗, P value of <0.001 with respect to results for viable D39 in the absence of antiserum.
Recombinant pneumolysin was used as the stimulus for HBMEC cultures demonstrating comparable morphological changes. Cytotoxicity of recombinant pneumolysin (30 μg/ml) compared well with 107 CFU of viable pneumococci (D39)/ml 24 h after addition of the stimulus. The effect of pneumolysin was dose dependent. Culture medium was checked for pneumolysin activity at the end of the experiment. Hemolytic activity of the culture medium supplemented with 30 μg (final concentration) of recombinant pneumolysin/ml compared well with results for culture supplemented with viable pneumococci (Fig. 5).
FIG. 5.
Effect of purified pneumolysin on HBMEC detachment 24 h after addition of the protein in comparison to results for pneumococci (D39; 107 CFU/ml). Values are means of duplicate results; error bars are standard deviations. ∗ and ∗∗, P values of <0.01 and <0.001 with respect to controls. The hemolytic titers of the culture medium after supplementation with 30 μg of pneumolysin/ml and 107 CFU of pneumococci/ml were comparable.
Effect of clinical pneumococcal isolates on the cerebral endothelium.
Sixteen pneumococcal strains isolated from the cerebrospinal fluid or the blood of patients suffering from pneumococcal meningitis and/or pneumococcal bacteremic pneumonia, respectively, were used at a concentration between 1 × 107 and 6 × 107 CFU/ml. Cell detachment was quantitated 24 h after the bacteria were added to the culture medium. Although all S. pneumoniae isolates are believed to contain the ply gene, the amount of the protein released into the growth medium varies. It had been shown that the extracellular titer of pneumolysin can be low while the cytoplasmic titer is high (4). Therefore, pneumolysin activity released into the culture medium was determined for all S. pneumoniae isolates during the early phase of stationary growth. Strains releasing large amounts of pneumolysin induced cell damage similar to that caused by the D39 strain, whereas those producing small amounts of pneumolysin had a tendency to cause less endothelial cell damage. In fact, the strains 18.B (serotype 7F) and 20.B (serotype 1), which showed no detectable pneumolysin in the culture supernatant, did not affect the cell monolayer integrity at all. Lysed bacteria of strains 18.B and 20.B caused only weak pneumolysin activity in the hemolysis assay, regardless of whether exponentially growing cells or stationary-phase cells were used, in agreement with little but detectable cytoplasmic pneumolysin activity. These results are in agreement with an important role of pneumolysin release in the disruption of the endothelial cell monolayer. No obvious difference between pneumococcal strains from patients displaying the syndrome of meningitis versus pneumonia was evident (Table 1).
TABLE 1.
HBMEC detachment after 24 h of incubation time with clinical pneumococcal isolates
| Straina | Capsular serotype | Clinical syndromeb | CFU (107/ml)c | Pneumolysin activityd | Endothelial cell detachment ± SD (%) |
|---|---|---|---|---|---|
| D39 | 2 | 1 | 5 | 78.9 ± 0.7 | |
| 1.L | 9A | M | 6 | <5 | 86.6 ± 4.2 |
| 2.L | 11A | M | 3 | <5 | 21.2 ± 0.3 |
| 13.L | 19F | M | 2 | 15 | 96.8 ± 3.2 |
| 14.B | 23F | M | 5 | 45 | 67.0 ± 4.2 |
| 16.L | 5 | M | 2 | 15 | 65.4 ± 7.6 |
| 51.L | 23A | M | 1 | 15 | 94.5 ± 0.5 |
| 53.L | 14 | M | 5 | 45 | 44.9 ± 8.7 |
| 3.B | 1 | M/P | 1 | 5 | 18.5 ± 4.1 |
| 12.L | 6B | M/P | 2 | 5 | 50.3 ± 2.8 |
| 18.B | 7F | P | 1 | X | 1.9 ± 0.4 |
| 20.B | 1 | P | 5 | X | 12.9 ± 2.2 |
| 29.B | 18F | P | 3 | 45 | 52.3 ± 8.8 |
| 30.B | 6A | P | 4 | 45 | 11.1 ± 2.2 |
| 31.B | 14 | P | 4 | 45 | 81.6 ± 4.1 |
| 38.B | 1 | P | 6 | 45 | 94.7 ± 0.5 |
| 40.B | 18B | P | 2 | 15 | 79.5 ± 3.1 |
The pneumococcal strains were isolated from either the cerebrospinal fluid (L) or the blood (B).
The patients suffered from pneumococcal meningitis (M) and/or pneumococcal pneumonia (P).
Final concentration of pneumococci in the culture at the beginning of the experiment.
Pneumolysin activity of the pneumococcal culture supernatant was reported as the reciprocal of the greatest dilution which caused complete lysis (4) (X, no reactivity; <5, incomplete lysis of the lowest dilution).
Effect of purified pneumococcal cell wall on the cerebral endothelium.
To investigate whether cell walls released during the antibiotic treatment described above are responsible for the detrimental effects on the HBMEC, purified cell walls were added to the HBMEC culture at concentrations up to 200 μg/ml. Whereas cell walls have been shown to strongly induce the oxidative burst of neutrophils, they did not induce any morphological changes or cell detachment of the HBMEC over a 24-h period.
Role of HBMEC protein synthesis, tyrosine phosphorylation, and caspase activation.
Since pneumolysin acts on eukaryotic cells by forming pores in the cell membrane but also might have properties similar to those of lipopolysaccharide (7), three of the mechanisms which had been identified for lipopolysaccharide in inducing cell cytotoxicity were investigated for S. pneumoniae.
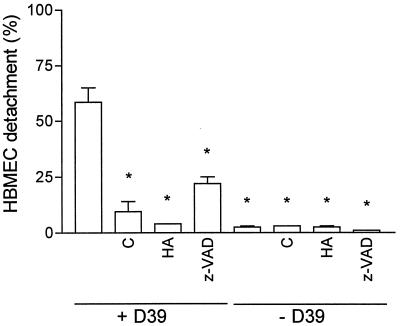
The stimulation of the HBMEC culture with pneumococci was performed in the presence of cycloheximide (5 and 50 μg/ml), the protein kinase inhibitor herbimycin A (2.5 and 0.25 μg/ml), and the broad-spectrum caspase inhibitor z-VAD-fmk (10 and 100 μM) for inhibition of de novo protein synthesis, tyrosine phosphorylation, and caspase activity. Results of these experiments were obtained 8 h after the addition of pneumococcal cells. Cycloheximide, herbimycin A, and z-VAD-fmk alone had no effect on the HBMEC during this period (Fig. 6). At a concentration of 107 CFU/ml, pneumococci induced detachment of about 50% of the HBMEC. Cycloheximide abolished the cytopathic effect of the endothelial cells completely at 5 μg/ml, indicating that it was dependent on de novo protein synthesis. Herbimycin A prevented the effect induced by pneumococci on the HBMEC at a concentration of 2.5 μg/ml completely, and at 0.25 μg/ml, 17% of the cells detached from the culture plate. The caspase inhibitor z-VAD-fmk (10 μM) reduced the pneumococcal cell damage. However, z-VAD-fmk did not inhibit the effect completely (Fig. 6). Increasing the z-VAD-fmk concentration to 100 μM did not amplify the effect.
FIG. 6.
Inhibition of HBMEC protein synthesis, tyrosine phosphorylation, and caspase activity. Cycloheximide (C), herbimycin A (HA), and z-VAD-fmk (z-VAD) were used at 5 μg/ml, 2.5 μg/ml, and 10 μM, respectively. HBMEC were preincubated with herbimycin A for 4 h and with z-VAD-fmk for 2 h prior to addition of 107 CFU of D39/ml. Detachment of HBMEC was quantified 8 h thereafter. Values are means ± standard deviations of duplicates. ∗, P value of <0.001 with respect to viable D39 in the absence of inhibitors.
DISCUSSION
The primary purpose of this study was to investigate the effect of pneumococci on the integrity of cerebral endothelial cell monolayers. Cerebral endothelial cells differ functionally and morphologically from endothelial cells of peripheral origin, especially in the capability to form a monolayer sealed by tight junctions. These cells form the basis of the BBB, which is penetrated by the pneumococcus in the case of pneumococcal meningitis.
In the present study we used two different endothelial cell types of cerebral origin. The advantage of primary prepared BBMEC was that these cells form tight monolayers and thus represent an excellent in vitro model reflecting the BBB. The disadvantage of these cells is the difficult and time-consuming preparation; also, cattle do not serve as hosts to the pneumococcus. Therefore, an HBMEC line was used as an additional model system. Unlike the BBMEC, tight junction formation could not be assumed, since the monolayers did not demonstrate a high TEER comparable to that for the BBMEC. The advantage of these cells lies in their origin, availability, and ease of culture. The results obtained with the two cell types complemented each other.
It has been shown with human umbilical vein endothelial cells that pneumococci caused the disruption of endothelial cell monolayers. In these experiments, pneumococcal cell walls were identified as a main cause of the cytopathic effects induced by the pathogen (11). These results were obtained from in vitro experiments with human umbilical vein endothelial cells which do not express characteristic properties of cerebral endothelial cells. Therefore, these experiments are not a suitable model of pneumococcal disturbance of the BBB. It has also been shown recently that pneumococci bind to HBMEC, and invasion and transmigration through the cells was observed (26). The platelet-activating factor receptor seemed to play a role in the transmigration process, and the effect was enhanced by pretreatment of the cells with TNF-α. Over the first 5 h after pneumococcal stimulation, no cytotoxic effect of HBMEC was reported. The authors stated that for at least 14 h, the TEER remained unchanged in their experiments, suggesting that pneumococci did not compromise tight junctions (26). In contrast, the data we obtained from the BBMEC cultures clearly demonstrated that the TEER decreased and the cytoplasm shrank within 4 to 6 h after pneumococci had been added to the endothelial cell culture. After 24 h, apoptotic endothelial cells were detected. In parallel with the decrease of the TEER of BBMEC cultures, the morphology of HBMEC changed, i.e., the cells showed signs of necrosis and detached. Destruction of the cell monolayer depended on a functional pneumolysin secreted by the pneumococci. In contradiction to results of previous experiments using human umbilical vein endothelial cells (11), pneumococcal cell walls did not affect the integrity of the cerebral endothelial cell culture. Neither cellular lysis of a pneumolysin-deficient mutant nor a preparation of purified cell walls resulted in cell destruction or caused any morphological changes of the HBMEC. The discrepancy between previous results and those reported here might be due to the different cell types used in the two studies. In support of our results, experiments performed on pulmonary endothelial cells in vitro also demonstrated that pneumolysin activity was a major cause of damage to pulmonary endothelial cells (29).
Our observations strongly suggest that the pneumococcus uses the same virulence mechanisms for damaging the BBB in cerebral infection as for damaging lung tissue. This observation underlines the importance of pneumolysin in BBB disruption and as a major virulence factor in general. We could not detect any obvious differences between endothelial cell damage caused by pneumococci isolated from the cerebrospinal fluid of patients with pneumococcal meningitis and that caused by blood isolates of patients suffering from bacteremic pneumonia. In fact, the pneumolysin gene has been detected in all clinical isolates of S. pneumoniae. It is therefore likely that pneumococcal meningitis develops as a consequence of an impairment of the host defense system rather than being related to special properties of individual pneumococcal strains. One may assume that the pneumolysin released by pneumococci adhering to the cerebral endothelial cells or pneumolysin in the blood during pneumococcal bacteremia has a concentration sufficient to induce damage to the tight junctions of the cerebral endothelial cells, enabling penetration of the pneumococci into the cerebral compartment through an impaired BBB.
Apart from pneumolysin, the production of hydrogen peroxide has been described as contributing to pneumococcal toxicity. However, additional cytotoxicity was observed in experiments using pneumolysin-negative pneumococci at higher concentrations (108 CFU/ml) than we used in this study (up to 107 CFU/ml) (9, 15). The effect of pneumolysin is more severe than the effect of hydrogen peroxide.
Pneumolysin belongs to a group of bacterial thiol-activated cytolysins which is involved in several aspects of the pathophysiology of pneumococcal infection. The protein forms transmembrane pores, resulting in cytolysis of eukaryotic cells; it also interferes with functional components of the immune system (for a review, see reference 30). Recently, pneumolysin was reported to induce the production of TNF-α, IL-1β (16), and nitric oxide (7) in monocytes. In the present study we demonstrated that pneumococci induced cytopathology of cerebral endothelial cells. The effect depended on de novo protein synthesis, tyrosine phosphorylation and, at least in part, caspase activation. The results indicate that pneumolysin acts on eukaryotic cells in a manner similar to that of LPS of gram-negative bacteria. LPS increases paracellular permeability and endothelial cell detachment of bovine pulmonary artery endothelial cells in vitro, changes caused by caspase-mediated cleavage of adherence junction proteins (2) and mediated through tyrosine phosphorylation (3). However, LPS-induced changes in endothelial barrier function increased upon inhibition of protein synthesis (13), while the effect of pneumolysin decreased after inhibition of protein synthesis.
In conclusion, pneumolysin appears to be the main inducer of cerebral endothelial cell barrier dysfunction caused by S. pneumoniae. Pneumolysin-induced endothelial cell dysfunction involves a protein synthesis-dependent pathway, tyrosine phosphorylation, and caspase activation. The pneumococcal cytotoxin might enable the entry of pneumococci into the cerebrum by an impairment of the BBB.
ACKNOWLEDGMENTS
This work was supported by the Stiftung für Altersforschung, Universität Düsseldorf.
We thank Gabriele Zysk, Bernd Nietzgen, and Doris Schmidt for excellent technical assistance and Colin MacKenzie for critically reading the manuscript.
REFERENCES
- 1.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannerman D D, Sathyamoorthy M, Goldblum S E. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- 3.Bannerman D D, Goldblum S E. Endotoxin induces endothelial barrier dysfunction through protein tyrosine phosphorylation. Am J Physiol. 1997;273:L217–L226. doi: 10.1152/ajplung.1997.273.1.L217. [DOI] [PubMed] [Google Scholar]
- 4.Benton K A, Paton J C, Briles D E. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect Immun. 1997;65:1237–1244. doi: 10.1128/iai.65.4.1237-1244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman P D, Ennis S R, Rarey K E, Betz A L, Goldstein G W. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann Neurol. 1983;14:396–402. doi: 10.1002/ana.410140403. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury M W B. The structure and function of the blood-brain barrier. Fed Proc. 1984;43:186–190. [PubMed] [Google Scholar]
- 7.Braun J S, Novak R, Herzog K H, Bodner S M, Cleveland J L, Tuomanen E I. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 8.Chen J D, Morrison D A. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene. 1988;64:155–164. doi: 10.1016/0378-1119(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 9.Duane P G, Rubins J B, Weisel H R, Janoff E N. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun. 1993;61:4392–4397. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedland I R, Paris M M, Hickey S, Shelton S, Olsen K, Paton J C, McCracken G H., Jr The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J Infect Dis. 1995;172:805–809. doi: 10.1093/infdis/172.3.805. [DOI] [PubMed] [Google Scholar]
- 11.Geelen S, Bhattacharyya C, Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993;61:1538–1543. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert R J C, Rossjohn J, Parker M W, Tweten R K, Morgan P J, Mitchell T J, Errington N, Rowe A J, Andrew P W, Byron O. Self-interaction of pneumolysin, the pore-forming protein toxin of Streptococcus pneumoniae. J Mol Biol. 1999;284:1223–1237. doi: 10.1006/jmbi.1998.2258. [DOI] [PubMed] [Google Scholar]
- 13.Goldblum S E, Ding X, Brann T W, Campbell-Washington J. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells: concurrent F-actin depolymerization and new actin synthesis. J Cell Physiol. 1993;157:13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- 14.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst R A, Sikand K S, Rutman A, Mitchell T J, Andrew P W, O'Callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun. 2000;68:1557–1562. doi: 10.1128/iai.68.3.1557-1562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janzer R C, Raff M C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 18.Leib S L, Kim Y S, Chow L L, Sheldon R A, Täuber M G. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Investig. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leib S L, Kim Y S, Ferriero D M, Täuber M G. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J Infect Dis. 1996;173:166–171. doi: 10.1093/infdis/173.1.166. [DOI] [PubMed] [Google Scholar]
- 20.Nau R, Soto A, Brück W. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol. 1999;58:265–274. doi: 10.1097/00005072-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Neuhaus J, Risau W, Wolburg H. Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann N Y Acad Sci. 1991;633:578–580. doi: 10.1111/j.1749-6632.1991.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 22.Paton J C, Andrew P W, Boulnois G C, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 23.Pfister H W, Scheld W M. Brain injury in bacterial meningitis: therapeutic implications. Curr Opin Neurol. 1997;10:254–259. doi: 10.1097/00019052-199706000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Pfister H W, Feiden W, Einhäupl K M. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol. 1993;50:575–581. doi: 10.1001/archneur.1993.00540060015010. [DOI] [PubMed] [Google Scholar]
- 25.Pfister H W, Fontana A, Täuber M G, Tomasz A, Scheld W M. Mechanism of brain injury in bacterial meningitis: workshop summary. Clin Infect Dis. 1994;19:463–479. doi: 10.1093/clinids/19.3.463. [DOI] [PubMed] [Google Scholar]
- 26.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossjohn J, Gilbert R J C, Crane D, Morgan P J, Mitchell T J, Rowe A J, Andrew P W, Paton J C, Tweten R K, Parker M W. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J Mol Biol. 1998;284:449–461. doi: 10.1006/jmbi.1998.2167. [DOI] [PubMed] [Google Scholar]
- 28.Rubins J B, Duane P G, Clawson D, Charboneau D, Young J, Niewoehner D E. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993;61:1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubins J B, Duane P G, Charboneau D, Janoff E N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992;60:1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubins J B, Janoff E N. Pneumolysin: a multifunctional pneumococcal virulence factor. J Lab Clin Med. 1998;131:21–27. doi: 10.1016/s0022-2143(98)90073-7. [DOI] [PubMed] [Google Scholar]
- 31.Schuchat A, Robinson K, Wenger J D, Harrison H H, Farley M, Reingold A L, Lefkowity L, Perkins B A. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 32.Steinfort C, Wilson R, Mitchell T, Feldman C, Rutman A, Todd H, Sykes D, Walker J, Saunders K, Andrew P, Boulnois G J, Cole P J. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun. 1989;57:2006–2013. doi: 10.1128/iai.57.7.2006-2013.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 34.Stins M F, Gilles F, Kim K S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–89. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 35.Täuber M G, Sachdeva M, Kennedy S L, Loetscher H, Lesslauer W. Toxicity in neuronal cells caused by cerebrospinal fluid from pneumococcal and gram-negative meningitis. J Infect Dis. 1992;166:1045–1050. doi: 10.1093/infdis/166.5.1045. [DOI] [PubMed] [Google Scholar]
- 36.Tiraby J G, Fox M S. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci USA. 1973;70:3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter A J, Comis S D, Osborne M P, Tarlow M J, Stephen J, Andrew P W, Hill J, Mitchell T J. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental meningitis in guinea pigs. Infect Immun. 1997;65:4411–4418. doi: 10.1128/iai.65.11.4411-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zysk G, Brück W, Gerber J, Brück Y, Prange H W, Nau R. Anti-inflammatory treatment influences apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996;55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Zysk G, Brück W, Huitinga I, Fischer F R, Flachsbarth F, van Rooijen N, Nau R. Elimination of blood-derived macrophages inhibits the release of interleukin-1 and the entry of leukocytes into the cerebrospinal fluid in pneumococcal meningitis. J Neuroimmunol. 1997;73:77–80. doi: 10.1016/s0165-5728(96)00173-7. [DOI] [PubMed] [Google Scholar]