Abstract
Objective
To compare the proportion of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) in cervical biopsies with that in large loop excision of the transformation zone (LLETZ) specimens in women aged ≥45 years with transformation zone type 3 (TZ3).
Design
Multicentre cross‐sectional study.
Setting
Three colposcopy clinics in the Central Denmark Region.
Population
Women aged ≥45 years referred to colposcopy as a result of a positive human papillomavirus (HPV) test and/or abnormal cytology and with TZ3 at colposcopy.
Methods
Women had multiple biopsies taken and an LLETZ was performed.
Main outcome measures
Histologically confirmed CIN2+ in biopsies compared with that in LLETZ specimens.
Results
Of 166 eligible women at colposcopy, 102 women with paired data from biopsies and LLETZ specimens were included for final analysis. The median age was 67.7 years (IQR 62.6–70.4 years), and most were postmenopausal (94.1%) and had undergone HPV‐based screening (81.3%). The CIN2+ detection rate was significantly higher in LLETZ specimens than in biopsies (32.4% vs 14.7%, difference 17.7%, 95% CI 6.3–29.0%), resulting in more than half of CIN2+ cases being missed in biopsies (54.5%, 95% CI 36.4–71.9%). The overall agreement between biopsies and LLETZ was 82.4% (95% CI 73.6–89.2%).
Conclusions
CIN2+ detection is underestimated in women aged ≥45 years with TZ3 if detection relies on the results of biopsies alone. To reduce the risk of underdiagnosis and overtreatment, future studies should explore the use of new biomarkers for risk stratification to improve discrimination between women at increased risk of CIN2+ who need to undergo LLETZ and women who may undergo follow‐up.
Keywords: biopsies, cervical intraepithelial neoplasia, colposcopy, human papillomavirus, LLETZ, postmenopausal, transformation zone type 3
Short abstract
Linked article: This article is commented on by Sarah Feldman, pp. 2141 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471‐0528.17260
1. INTRODUCTION
Cervical cancer is the fourth most common cancer in women worldwide, with approximately 570 000 cases yearly and 311 000 deaths in 2018. 1 In Denmark, cervical cancer is diagnosed in nearly 400 women each year, with approximately 100 deaths annually. 2 Recent studies have reported higher incidence and mortality rates in older women than in younger women. 3 , 4 Moreover, older women are more commonly diagnosed with advanced‐stage disease and, hence, have a poorer prognosis. 5 This may be because of insufficient screening, screening failure, diagnostic challenges and insufficient follow‐up of older women.
In postmenopausal women, the transformation zone (TZ) is often retracted into the cervical canal as a result of age‐dependent changes. Combined with epithelial atrophy, these factors challenge the complete visualization of the squamous–columnar junction (i.e. TZ type 3), including a potential precancerous lesion. These issues hamper the collection of targeted biopsies and increase the risk of missing disease located in the cervical canal. Histopathological examination of colposcopy‐directed biopsies is used for diagnosis, and several studies indicate that collecting multiple biopsies improves the chances for detection of cervical intraepithelial neoplasia grade 2 or higher (CIN2+). 6 , 7 , 8 However, in women with TZ3, the collection of biopsies is likely to be compromised, resulting in an increased risk of underdiagnosis. Consequently, several guidelines suggest the use of large loop excision of the transformation zone (LLETZ) in women referred with abnormal cytology and TZ3 at colposcopy, to ensure correct diagnosis. 9 , 10 , 11 , 12 In women aged ≥45 years with TZ3, a diagnostic LLETZ may be offered to women either immediately or after insufficient sampling of histological material. However, performing an LLETZ on all women referred to colposcopy is likely to increase the risk of overtreatment and complications such as bleeding and stenosis, and stenosis could hamper subsequent follow‐up. 13 , 14 Our understanding of the magnitude of potential underdiagnosis when only taking biopsies and overtreatment following diagnostic LLETZ is lacking.
Therefore, we aimed to compare the CIN2+ detection rate in cervical biopsies with that in LLETZ specimens in women aged ≥45 years with TZ3.
2. METHODS
2.1. Study design and setting
This multicentre, cross‐sectional study was conducted from March 2019 through June 2021 at the Departments of Obstetrics and Gynaecology in the Central Denmark Region, Denmark (i.e. the Regional Hospital of Randers, Viborg and Horsens).
In Denmark, cervical cancer screening is offered to women aged 23–64 years. All procedures related to screening, diagnostic, follow‐up and treatment are free of charge for all citizens. Women aged 23–59 years are invited for cytology‐based screening every third year (age 23–49 years) or fifth year (age 50–59 years), whereas women aged 60–64 years are offered high‐risk human papilloma virus (HPV)‐based screening. Since January 2021, HPV‐based screening has been offered to women aged 30–59 years born on odd numbered dates, whereas women born on even numbered dates have continued with cytology‐based screening. 15 Additionally, as a part of an interventional study in the Central Denmark Region that started in April 2019, women aged 65–69 years have been invited for one additional HPV screening test, with women testing positive for HPV undergoing cytology triage. 16 Women participating in the present study were referred as summarised in Table S1.
2.2. Participants
Women were prospectively assessed for eligibility if they were aged ≥45 years, had an abnormal cervical cancer screening result (Table S1) and were referred to colposcopy. At colposcopy, women were considered eligible for inclusion if they had TZ type 3, according to the 2011 International Federation of Cervical Pathology and Colposcopy nomenclature. 17 Women were excluded if they had previous excisional treatment or hysterectomy, received anticoagulant medical treatment, intended to get pregnant or underwent follow‐up for a previously diagnosed CIN. Moreover, women were excluded if LLETZ was not technically possible because of pain, a narrow vagina or severe atrophy of the vagina or cervix, making it difficult to distinguish the cervix from the vaginal wall.
All women received written and verbal information on the study, and upon inclusion the participating women signed an informed consent form.
2.3. Clinical management
Colposcopic examination of the cervix was performed after the application of acetic acid (3%) and biopsies were obtained with 3‐mm forceps from abnormal areas identified by colposcopy. If no abnormalities appeared, four blind biopsies were taken according to Danish national guidelines and the study protocol. 18 At colposcopy, but prior to taking biopsies and performing the LLETZ, a liquid‐based cytology sample (SurePath™; BD, Franklin Lakes, NJ, USA) was collected using Cervex‐Brush® and/or EndoCervex‐Brush® (Rovers Medical Devices, Oss, the Netherlands). Finally, an LLETZ was performed immediately after colposcopy. The procedure was performed using local anaesthesia (Citanest Dental Octapressin®; Dentsply Sirona, York, PA, USA).
2.4. Data sources
From the medical records, the following data were obtained: colposcopy description, parity, medical history and daily medication. After colposcopy, women completed a questionnaire with questions on basic characteristics and behavioural risk factors. Information on previous cervical cancer screening results and results of study‐related cervical cytology, biopsies and LLETZ specimens were obtained from the nationwide Danish Pathology Databank, which stores information on all cyto‐ and histopathological examinations performed in Denmark since 1998 at an individual level. 19
2.5. Cytology and histopathological examination
The Department of Pathology, Randers Regional Hospital, Denmark, is responsible for analysing all cervical cytology samples in the Central Denmark Region (approx. 90 000 samples annually). Cytology slides were interpreted by experienced cyto‐technicians using computer‐assisted microscopy (BD FocalPoint™ GS Imaging System). Results were categorised according to the Bethesda 2014 grading system. 20 HPV DNA testing was performed using the cobas® 4800 system (Roche Diagnostic, Basel, Switzerland), which enables the individual detection of HPV16 and HPV18, and the pooled detection of 12 other high‐risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). 21 Biopsies and LLETZ specimens were routinely examined at local pathology departments in Randers and Viborg. Histopathological outcomes were graded according to the cervical intraepithelial neoplasia (CIN) classification: 22 normal, CIN1, CIN2, CIN3, unclassifiable CIN (i.e. the full height of the epithelium is not discernible), adenocarcinoma in situ (AIS) or cancer. Histopathological outcomes for biopsies and LLETZ were divided into two categories: <CIN2, defined as normal (including no dysplasia and inflammation) and CIN1; and CIN2+, defined as unclassifiable CIN, CIN2, CIN3 and cancer. The TZ was considered to be represented in biopsies if the specimen contained both squamous and glandular epithelium (on the surface or in a crypt). The LLETZ specimen was considered to be representative if the squamous–columnar junction (SCJ) was present on the surface of the tissue specimen.
2.6. Statistics
We used CIN2+ as the primary outcome, as this is the threshold for excisional treatment in women aged ≥45 years. CIN2+ detection in biopsies and LLETZ was reported using proportions and 95% confidence intervals (95% CIs), and McNemar’s χ2 (i.e. paired samples) was used for comparison of proportions across groups. The overall percentage agreement in histological diagnoses between paired biopsies and LLETZ was calculated and presented with 95% CIs. To test the robustness of the agreement calculation, we performed a sensitivity analysis excluding cases with unclassifiable CIN. Continuous variables were reported as medians and interquartile ranges (IQRs).
Data were entered and stored at Research Electronic Data Capture (REDCap, https://www.project‐redcap.org/). 23 , 24 STATA 16 (StataCorp LLC, College Station, TX, USA) was used for statistical analyses. A p‐value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Study population
Of 166 women eligible at colposcopy, 35 (21.1%) declined to participate. For 24 women (14.5%), LLETZ was not technically possible (Figure 1), and another five women (3.0%) had no biopsies taken, leaving 102 women (61.4%) with paired samples for the final analyses (Figure 1). Although anticoagulant treatment was an exclusion criterion, three women receiving anticoagulant medical treatment were included by accident. The three women showed no excessive bleeding after LLETZ, and we decided to include these for the final analysis as they did not differ from other study participants with respect to basic characteristics.
FIGURE 1.
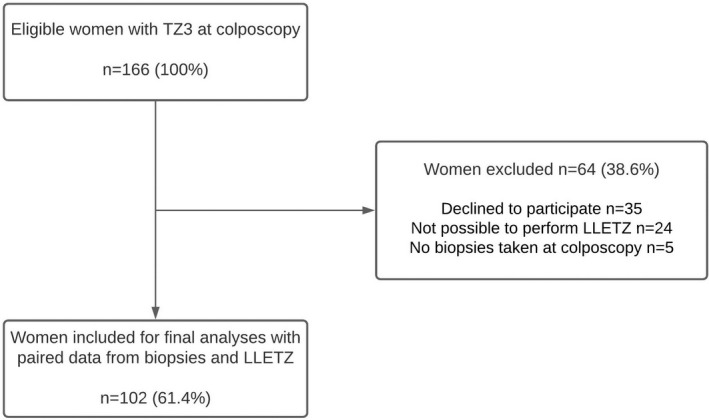
Flow chart of the study population
The median age of the included women was 67.6 years (IQR 62.6–70.4 years), with the majority being postmenopausal (n = 96; 94.1%) (Table 1). Most women were non‐smokers (n = 72, 70.6%) and did not use vaginal hormone (n = 79, 77.5%). The majority had undergone primary HPV screening (n = 83, 81.3%), and 69 women (67.7%) had no previous record of atypical squamous cells of undetermined significance or worse (ASC‐US+). With regards to sexual behaviour, 58 women (56.9%) had had more than five lifetime sexual partners, and most women (82.4%) reported no new sexual partner within the past 2 years (Table 1).
TABLE 1.
Basic characteristics of the women aged ≥45 years referred to colposcopy
| Women n, (%)(N = 102) | |
|---|---|
| Median age (IQR) | 67.6 (62.6–70.4) |
| Age groups (years) | |
| 45–59 | 14 (13.7) |
| ≥60 | 88 (86.3) |
| Body mass index (BMI) a | |
| Median BMI (IQR) | 25.4 (21.8–28.2) |
| Current smoking a | |
| No | 72 (70.6) |
| Yes | 23 (22.6) |
| Parity a | |
| Nulliparous | 8 (7.8) |
| Parous | 90 (88.2) |
| Menopause status a | |
| Postmenopausal | 96 (94.1) |
| Premenopausal | 2 (2.0) |
| Current use of vaginal hormone a | |
| No | 79 (77.5) |
| Yes | 15 (14.7) |
| Use of hormone therapy during menopause a | |
| No | 88 (86.3) |
| Yes | 6 (5.9) |
| Referral status b | |
| Primary HPV screening | 83 (81.3) |
| Primary cytology screening | 19 (18.7) |
| Previous history of abnormal cytology (ASC‐US+) b | |
| No | 69 (67.7) |
| Yes | 33 (32.4) |
| Lifetime sexual partners a | |
| <5 | 31 (30.4) |
| 5–10 | 36 (35.3) |
| >10 | 22 (21.6) |
| New sexual partners within the past 2 years a | |
| Yes | 8 (7.8) |
| No | 84 (82.4) |
| HPV vaccination a | |
| Yes | 7 (6.9) |
| No | 88 (86.3) |
Self‐reported data.
Data on referred status and previous history of abnormal cytology was obtained from the Danish Pathology Databank. ASC‐US+ is defined as atypical squamous cells of undetermined significance or worse. The numbers and proportions vary because of missing data.
3.2. Colposcopy and histopathological outcomes
Fifteen women (14.0%) had abnormalities detected at colposcopy, with acetowhitening being the most common finding (73.3%). Five women (33.3%) had atypical vessels (data not shown). Most women (63.7%) had four biopsies taken, as recommended in the Danish guidelines; however, as a result of technical difficulties, 37 women (36.3%) had fewer than four biopsies taken (Table 2). The median depth of the LLETZ specimen was 10 mm (IQR 8–13 mm).
TABLE 2.
Histopathological results detected in biopsies and LLETZ specimens
| Variable | Biopsies | LLETZ |
|---|---|---|
| Histopathological result overall and grouped into <CIN2 a and CIN2+ b | ||
| n %, (95% CI) | n %, (95% CI) | |
| Normal |
83 81.4, (72.4–88.4) |
62 60.8, (50.6–70.3) |
| CIN1 |
4 3.9, (1.1–9.7) |
7 6.9, (2.8–13.6) |
| CIN2 |
3 2.9, (0.6–8.4) |
5 4.9, (1.6–11.1) |
| CIN3 |
4 3.9, (1.1–9.7) |
17 16.7, (10.0–25.3) |
| Unclassifiable CIN |
8 7.8, (3.4–14.9) |
11 10.8, (5.5–18.5) |
| <CIN2 |
87 85.3, (76.9–91.5) |
69 67.7, (57.7–76.6) |
| CIN2+ |
15 14.7, (8.5–23.1) |
33 32.4, (23.4–42.3) |
| Total | 102 (100.0) | 102 (100.0) |
| Representation of squamous and endocervical epithelium in the specimen | ||
|---|---|---|
| n %, (95% CI) | n %, (95% CI) | |
| <CIN2 | ||
| Yes |
48 55.2, (44.1–65.9) |
64 92.8, (83.9–97.6) |
| No |
39 44.8, (34.1–55.9) |
5 7.2, (2.4–16.1) |
| CIN2+ | ||
| Yes |
12 80.0, (51.9–95.7) |
33 100, (89.4–100.0) |
| No |
3 20.0, (4.3–48.1) |
0 |
| CIN2+ result stratified by referral status c | ||
|---|---|---|
| n %, (95% CI) | n %, (95% CI) | |
| HPV+ and normal cytology | ||
| <CIN2 |
43 42.2, (32.4–52.3) |
42 41.2, (31.5–51.4) |
| CIN2+ | <3 | <3 |
| HPV+ and ASC‐US+ | ||
| <CIN2 |
28 27.5, (19.1–37.2) |
13 12.7, (7.0–20.8) |
| CIN2+ |
8 7.8, (3.4–14.9) |
23 22.5, (14.9–31.9) |
| Cytology‐based screening | ||
| <CIN2 |
13 12.7, (7.0–20.8) |
11 10.8, (5.5–18.5) |
| CIN2+ |
6 5.9, (2.2–12.4) |
8 7.8, (3.4–14.9) |
| Histopathological result statisfied by the number of biopsies taken at colposcopy | ||||
|---|---|---|---|---|
| n %, (95% CI) | n %, (95% CI) | |||
| Biopsies <4 | Biopsies (≥4 biopsies) | Biopsies <4 | Biopsies (≥4 biopsies) | |
| <CIN2 |
33 32.4, (23.4–42.3) |
54 52.9, (42.8–62.9) |
23 22.5, (14.9–31.9) |
46 45.1, (35.2–55.3) |
| CIN2+ |
4 3.9, (1.1–9.7) |
11 10.8, (5.5–18.5) |
14 13.7, (7.7–22.0) |
19 18.6, (11.6–27.6) |
| Total |
37 36.3, (27.0–46.4) |
65 63.7, (53.6–73.0) |
37 36.3, (27.0–46.4) |
65 63.7, (53.6–73.0) |
Abbreviation: CIN, cervical intraepithelial lesions.
<CIN2 is defined as: normal or CIN1.
CIN2+ is defined as: CIN unclassifiable, CIN grades 2 and 3, and cancer. LLETZ: large loop excision of the transformation zone.
Women who underwent HPV‐based screening without reflex cytology are excluded (n = 3). HPV+: Any high‐risk HPV type.
Histopathological examination of biopsies showed that 87 women (85.3%, 95% CI 76.9–91.5%) had <CIN2, and 15 women (14.7%, 95% CI 8.5–23.1%) had CIN2+ detected (Table 2). Histopathological examination of the LLETZ specimens revealed that 69 women (67.7%, 95% CI 57.7–76.6) had <CIN2, which was statistically lower compared to the biopsies (85.3% vs 67.7%, p < 0.01). Similarly, the CIN2+ detection was significantly higher in the LLETZ specimens than in the biopsies (32.4% vs 14.7%, p < 0.01) (Table 2), corresponding to 54.5% of CIN2+ cases being missed in biopsies (95% CI 36.4–71.9) (Table 3). The overall percentage agreement between biopsies and LLETZ specimens was 82.4% (95% CI 73.6–89.2%) (Table 3). Excluding unclassifiable CIN from the analysis did not change the percentage agreement (82.4% vs 86.2%, p = 0.47).
TABLE 3.
Agreement in histopathological results between biopsies and LLETZ
| Cervical biopsy result | LLETZ result | |||
|---|---|---|---|---|
| <CIN2 a | CIN2+ b | Total | ||
| <CIN2 a | 69 (67.6%) | 18 (17.6%) | 87 (85.3%) | |
| CIN2+ b | 0 | 15 (14.7%) | 15 (14.7%) | |
| Total | 69 (67.6%) | 33 (32.4%) | 102 (100%) | |
| Agreement | 84/102 = 82.4% (95% CI 73.6–89.2%) | |||
| Biopsy underestimates disease in all women | 18/102 = 17.6% (95% CI 10.8–26.4%) | |||
| Biopsy underestimates disease in women with CIN2+ detected | 18/33 = 54.5% (95% CI 36.4–71.9%) | |||
| Biopsy overestimates or removes disease | 0/102 (0%) | |||
Abbreviation: CIN, cervical intraepithelial lesion.
<CIN2 is defined as: normal or CIN1.
CIN2+ is defined as: CIN unclassifiable, CIN grades 2 and 3, and cancer.
Of the 18 women with no evidence of CIN2+ in biopsies but with CIN2+ detected in the LLETZ specimen, 12 women (66.7%) had the TZ represented in the biopsies. All 18 women with CIN2+ detected by LLETZ had the SCJ included in the LLETZ specimen (Table 2). Of these 18 women, 16 (88.9%) were referred with a positive HPV test (81.3% non‐16/18‐HPV) and ASC‐US+ in their reflex cytology (data not shown).
With respect to the referral status of the women and risk of CIN2+, we found that women testing positive for HPV with ASC‐US+ on reflex cytology had an increased risk of being diagnosed with CIN2+, as compared with women testing positive for HPV with normal reflex cytology (Table 2). The positive predictive value of ASC‐US+ on reflex cytology in women testing positive for HPV (n = 80) was 63.9% (95% CI 46.2–79.2%).
A total of 33 women (32.4%) had a previous abnormal screening history, of which 23 (22.5%) and 10 (9.8%) women were diagnosed with <CIN2 and CIN2+ detected in the LLETZ specimen, respectively. In women with a normal screening history, 46 women (45.1%) had <CIN2 and 23 women (22.5%) had CIN2+. In women with previous abnormal cytology (n = 33, 32.4%), 24 women (72.7%) had previously noted low‐grade changes and nine women (27.3%) had previously noted high‐grade changes (data not tabulated). Moreover, in women with colposcopic abnormalities (n = 15), six (40.0%) had <CIN2 and nine (60.0%) had CIN2+ in their LLETZ specimens (data not tabulated).
The results of women with the representation of the TZ in biopsies and the SCJ in the LLETZ specimen are summarised in Table 2. Of note, in women with representation of the TZ in at least one of the biopsies, the biopsies missed CIN2+ lesions in 21 out of 33 (63.6%, 95% CI 45.1–79.6%) cases detected in the LLETZ specimens (Table 2).
4. DISCUSSION
4.1. Main findings
In this multicentre, cross‐sectional study on women aged ≥45 years with TZ3, we found that biopsies underestimated over half of the CIN2+ cases detected in the LLETZ specimens. Relying on the results of biopsies in women with TZ3 carries a risk of delaying diagnosis and treatment, as the TZ was significantly less represented in biopsies compared with LLETZ specimens. However, as 67.7% of women in the present study had <CIN2 detected in the LEETZ specimen, our study also suggests a profound risk of overtreatment. Thus, weighing the risk of underdiagnosis against overtreatment is important in the clinical management of women with TZ3 at colposcopy.
4.2. Strengths and limitations
A major strength of this multicentre study was the use of paired samples (i.e. multiple biopsies and LLETZ specimens), which minimised the risk of confounding. The majority (93.9%) of biopsies and LLETZ specimens were analysed at the same pathology department in Randers, limiting inter‐laboratory variation. The present study contributes results from a unique population of women aged ≥45 years attending colposcopy. Our results may be generalisable to other populations of women with TZ3 and with a comparable cervical cancer incidence who have undergone a similar screening and triage algorithm.
A number of limitations should also be addressed. The sample size was rather small, which makes the results less robust. We did not use an external reference reviewer for histopathological examination, which could increase the risk of inter‐ and intra‐observer variation. The pathologists were aware of the histopathological result of the biopsies before the LLETZ specimens were assessed. However, this reflects real‐life practice and despite this knowledge half of the biopsies were still evaluated as normal. We cannot rule out that some women may have been misclassified as having a TZ3 instead of TZ2. However, as the outcome (CIN2+) is not affected by the type of TZ, the risk of bias is considered minimal, but potential misclassification might explain the high number of biopsies with the TZ being represented. We cannot rule out that selection bias might have occurred when women were booked for colposcopy; however, we consider selection bias to be minimal as women were enrolled consecutively. We acknowledge that results based on multiple biopsies may not be generalisable to other countries where fewer biopsies are taken. As a result, implementation of diagnostic LLETZ in such settings may be even more efficient than in our setting. In our study, some women (aged 65–69 years) participated in a continuing trial offering them an additional HPV test. 16 These women could have been HPV‐positive for several years, which could have contributed to the high CIN2+ detection rate found in our study.
4.3. Interpretation
The CIN2+ detection rate in this multicentre study is somewhat higher than detection rates reported in other studies investigating women aged ≥55 years (32% vs 15–25%). 25 , 26 , 27 , 28 , 29 A recent study including women (aged ≥70 years) undergoing HPV‐based screening reported that 18% of women who had histological sampling performed (514 of 2782) had CIN2+ detected. 25 Taking the age into consideration, we would expect these women to have a TZ3 at colposcopy. Our findings suggest that the CIN2+ detection rate in the previous study may have been underestimated, as not all women had biopsies collected and the histopathological outcome was based on the combined results of biopsy and LLETZ. Aarnio et al. explored risk of CIN2+ among women (n = 40, mean age 58 years) with persistent HPV infection and normal cytology by collecting biopsies and performing an LLETZ at the same visit. 28 They reported CIN2+ in 15% (6/40) of women with LLETZ, whereas biopsies failed to detect any of these CIN2+ cases. Another study on women who were HPV‐positive/cytology‐normal (mean age 59.5 years) reported CIN2+ in 25% (6/24) in a subgroup of women who were offered diagnostic conisation as a result of TZ3. 27 The higher CIN2+ detection rate in our study compared with previous studies may be explained by differences in clinical management and the characteristics of study participants across studies, such as the inclusion of women testing positive for HPV with abnormal cytology in the present study, unlike previous studies.
The current Danish National Guidelines state that four biopsies should be taken at colposcopy regardless of a visible lesion and referral status. 18 These recommendations are based on results from studies showing that collecting multiple biopsies increases the possibility of detecting CIN2+. 6 , 7 , 8 In women with a visible TZ, biopsies may be used as the reference standard instead of LLETZ. Thus, a recent study reported a 95% concordance in detecting CIN2+ between representative biopsies and LLETZ in women with a visible TZ. 8 However, despite taking multiple biopsies in our study, more than 50% of CIN2+ lesions were missed. In women who had biopsies with the TZ being represented, 63.6% of CIN2+ cases diagnosed in the LLETZ specimen were missed. Caution is therefore advised when interpreting non‐representative biopsies from women with TZ3. To improve the visualization of the TZ and a potential lesion, a few trials have suggested pre‐colposcopic treatment with estrogen or misoprostol. 30 , 31 , 32 Unfortunately, these studies suffer from a small sample size, and future well‐conducted clinical trials are therefore needed to clarify the potential impact of this treatment, including the appropriate dose and duration of treatment. Endocervical curettage (ECC) is another possibility for obtaining histopathological material in women with TZ3. Studies have indicated that the use of ECC may increase the diagnostic yield of CIN2+, particularly in older women. 33 , 34 However, the use of ECC remains controversial as ECC has a higher rate of unsatisfactory histopathological results than biopsies and may be more painful for the women. For these reasons, ECC is not routinely recommended in Denmark. 18
Cervical cancer screening and the diagnostic work‐up of women screening positive is a delicate balance between benefits, in terms of preventing cancer cases, and harms, in terms of complications related to preventive treatment. The choice of management strategy should preferably be based on the result of the referral test, screening history, the women’s preferences and other risk factors. Offering LLETZ to all women testing positive for HPV, regardless of triage and risk profile, would lead to overtreatment and possible complications. This statement is supported by our study data showing that more than half of the women (60.8%) had a normal histopathological result in their LLETZ specimen. This estimate of overtreatment corresponded to the 50–75% with normal histopathological results reported in the above‐mentioned studies. 26 , 28 , 29 Although women aged ≥60 years have been shown to prefer the adverse effects of overtreatment over the risk of underdiagnosis, 35 additional studies are needed to better identify women at increased risk of CIN2+, while allowing women at lower risk to undergo follow‐up. p16/Ki67 dual stain could be a potential risk marker as studies have demonstrated that it has a higher sensitivity and negative predictive value for detecting CIN2+ than cytology in a screening population of younger women. 36 , 37 However, not much is known about the clinical utility of the p16/Ki67 dual stain in a referral population of women aged ≥45 years. Likewise, studies on methylation markers as a triage strategy for women testing positive for HPV have shown promising results. 38 Exploring whether these or other biomarkers could enable a reliable risk stratification of women with TZ3 is of utmost clinical importance to reduce the risk of overtreatment while simultaneously securing adequate diagnosis. Until these biomarkers are properly validated for use as risk markers in a similar population, our results suggest that women testing positive for HPV with normal cytology could undergo follow‐up, whereas women testing positive for HPV with ASC‐US+ in their reflex cytology could undergo LLETZ.
5. CONCLUSION
Our findings indicate that CIN2+ detection in women aged ≥45 years with TZ3 is underestimated when detection relies solely on the results of biopsies, compared with LLETZ specimens. CIN2+ lesions are even missed in women with both endocervical and squamous cells represented in the biopsies. However, performing LLETZ for all women with TZ3 at colposcopy is likely to raise the risk of overtreatment significantly. To individualise diagnostic work‐up and treatment, and to minimise the risk of underdiagnosis and overtreatment, future studies should explore the use of new biomarkers for individual risk stratification.
CONFLICT OF INTERESTS
LWG and LKP have received speaker’s fees from Astra Zeneca outside of the submitted work. LWG and BA are participating in other studies with HPV test kits and CINtec® PLUS kits sponsored by Roche. AH reports receiving reagents from Roche Denmark at a reduced cost, outside of the submitted work. MHB, CBK, HM and PB report no conflicts of interest. Completed disclosure of interests form available to view online as supporting information.
AUTHOR CONTRIBUTIONS
Conceptualisation and study design: LWG, AH, BA, PB and LKP. Recruitment, data collection, data management and analysis of samples: CBK, MHB, HM, PB and LWG. Data analyses: PB, AH, LWG, BA and LKP. LWG prepared the first draft with guidance/supervision from BA, LKP, PB and AH. Critical revision of the article for important intellectual content was provided by CBK, HM, MHB, LWG, BA, PB, AH and LKP. All authors approved the final version for publication.
DETAILS OF ETHICS APPROVAL
This study was listed at the record of processing activities for research projects in the Central Denmark Region (J.no: 1–16–02‐528‐18). The Central Denmark Region Committees on Health Research Ethics decided that according to the Consolidation Act on Research Ethics Review of Health Research Projects, Consolidation Act number 1083 of 15 September 2017, section 2 (1), this study was not notifiable to the committee (J.no.: 1–10–72‐4‐17).
Supporting information
Table S1
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
ACKNOWLEDGEMENTS
The authors wish to thank the clinical staff at the Department of Obstetrics and Gynaecology, Viborg, Horsens and Randers Regional Hospital, who recruited women to this study.
Gustafson LW, Hammer A, Bennetsen MH, Kristensen CB, Majeed H, Petersen LK, et al. Cervical intraepithelial neoplasia in women with transformation zone type 3: cervical biopsy versus large loop excision. BJOG. 2022;129(13):2132–2140. 10.1111/1471-0528.17200
ClinicalTrials.gov identifier: NCT04298957.
Funding information
Health Research Foundation of Central Denmark Region (A3234), Dagmar Marshalls Foundation, Else and Mogens Wedell‐Wedellsborgs Foundation, Fabricant Einar Willumsens Mindelegat and AP Moller Foundation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Data can be made available on request from researchers who meet the criteria for access to patient's confidential data and upon approval from the Danish Data Protection Agency.
REFERENCES
- 1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danckert B, Ferlay J, Engholm G, Hansen HL, Johannesen TB, Khan S, Køtlum JE, Ólafsdóttir E, LKH Schmidt, Virtanen A and Storm HH. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2 (26.03.2019). Association of the Nordic Cancer Registries. Danish Cancer Society. 2021 13/09/2021. Available from: http://www.ancr.nu.
- 3. Hammer A, Kahlert J, Rositch A, Pedersen L, Gravitt P, Blaakaer J, et al. The temporal and age‐dependent patterns of hysterectomy‐corrected cervical cancer incidence rates in Denmark: a population‐based cohort study. Acta Obstet Gynecol Scand. 2017;96(2):150–7. [DOI] [PubMed] [Google Scholar]
- 4. Hammer A, Kahlert J, Gravitt PE, Rositch AF. Hysterectomy‐corrected cervical cancer mortality rates in Denmark during 2002‐2015: a registry‐based cohort study. Acta Obstet Gynecol Scand. 2019;98(8):1063–9. [DOI] [PubMed] [Google Scholar]
- 5. Darlin L, Borgfeldt C, Widen E, Kannisto P. Elderly women above screening age diagnosed with cervical cancer have a worse prognosis. Anticancer Res. 2014;34(9):5147–51. [PubMed] [Google Scholar]
- 6. Wentzensen N, Walker JL, Gold MA, Smith KM, Zuna RE, Mathews C, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33(1):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pretorius RG, Belinson JL, Burchette RJ, Hu S, Zhang X, Qiao Y‐L. Regardless of skill, performing more biopsies increases the sensitivity of colposcopy. J Low Genit Tract Dis. 2011;15(3):180–8. [DOI] [PubMed] [Google Scholar]
- 8. Booth BB, Petersen LK, Blaakaer J, Johansen T, Mertz H, Dahl K, et al. Accuracy of colposcopy‐directed biopsy vs dynamic spectral imaging directed biopsy in correctly identifying the grade of cervical dysplasia in women undergoing conization: a methodological study. Acta Obstet Gynecol Scand. 2020;99(8):1064–70. [DOI] [PubMed] [Google Scholar]
- 9. Petersen LK, Schroll J, Dolleris BB, Bundgaard A, Kirschner B, Booth BB, et al. National clinical guideline for cervical dysplasia. Examination, treatment, and management of women aged 60 and older [Only in Danish]: DSOG 2019. Available from: https://www.sst.dk/‐/media/Opgaver/Patientforl%C3%B8b‐og‐kvalitet/NKR/Puljefinansierede‐NKR/pdf‐version‐af‐ublished_guideline_2633.ashx?la=da&hash=0809DD9A773B341B3D08AE73C9361E2AB84029E8
- 10. Gynecology SSoOa . SFOG‐riktlinje av NVP för cervixcancerprevention 2019 [updated Uppdaterad 190330.; SFOG‐riktlinje av Nationellt vårdprogram livmoderhalscancerprevention (NVP) för cervixcancerprevention.[Only in Swedish]]. Available from: https://www.sfog.se/media/336482/sfog‐riktlinje‐190330_revhs‐190413.pdf.
- 11. Frazer I. Guidelines for the management of screen‐detected abnormalities, screening in specific populations and investigation of abnormal vaginal bleeding.: cancer council Australia cervical cancer screening guidelines working party. 2020. National Cervical Screening Program Sydney: Cancer Council Australia; [cited 2020 October 30]. Available from: https://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Screening.
- 12. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk‐based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El‐Nashar SA, Shazly SA, Hopkins MR, Bakkum‐Gamez JN, Famuyide AO. Loop electrosurgical excision procedure instead of cold‐knife conization for cervical intraepithelial neoplasia in women with unsatisfactory Colposcopic examinations: a systematic review and meta‐analysis. J Low Genit Tract Dis. 2017;21(2):129–36. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka Y, Ueda Y, Kakuda M, Kubota S, Matsuzaki S, Iwamiya T, et al. Predictors for recurrent/persistent high‐grade intraepithelial lesions and cervical stenosis after therapeutic conization: a retrospective analysis of 522 cases. Int J Clin Oncol. 2017;22(5):921–6. [DOI] [PubMed] [Google Scholar]
- 15. NSLS . The National Steering Committee for Cervical Cancer Screening 2021. Available from: https://www.regioner.dk/nsls/viden
- 16. Tranberg M, Petersen LK, Elfström KM, Hammer A, Blaakær J, Bennetsen MH, et al. Expanding the upper age limit for cervical cancer screening: a protocol for a nationwide non‐randomised intervention study. BMJ Open. 2020;10(11):e039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colposcopy TIFoCPa . IFCPC Nomenclature. Available from: https://ifcpc.org/medical‐professionals/ifcpc‐nomenclature/
- 18. Petersen L. Cervix dysplasia ‐ Guideline: Danish Society of Obstetrics and Gynecology; 2012. Available from: https://www.dsog.dk/gynkologi
- 19. Erichsen R, Lash TL, Hamilton‐Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and data Bank. Clin Epidemiol. 2010;2:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nayar RWD. The Bethesda system for reporting cervical cytology. Definitions, criteria, and explanatory notes. 3rd ed. New York: Springer Nature Switzerland AG; 2015. [Google Scholar]
- 21. Heideman DA, Hesselink AT, Berkhof J, van Kemenade F, Melchers WJ, Daalmeijer NF, et al. Clinical validation of the cobas 4800 HPV test for cervical screening purposes. J Clin Microbiol. 2011;49(11):3983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richart RM. Natural history of cervical intraepithelial neoplasia. Clin Obstet Gynecol. 1967;10(4):748–84.4172733 [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. St‐Martin G, Viborg PH, Andersen ABT, Andersen B, Christensen J, Ejersbo D, et al. Histological outcomes in HPV‐screened elderly women in Denmark. PLoS One. 2021;16(2):e0246902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergengren L, Lillsunde‐Larsson G, Helenius G, Karlsson MG. HPV‐based screening for cervical cancer among women 55‐59 years of age. PloS One. 2019;14(6):e0217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahlgren H, Elfström KM, Lamin H, Carlsten‐Thor A, Eklund C, Dillner J, et al. Colposcopic and histopathologic evaluation of women with HPV persistence exiting an organized screening program. Am J Obstet Gynecol. 2020;222(3):253.e1–e8. [DOI] [PubMed] [Google Scholar]
- 28. Aarnio R, Wikström I, Gustavsson I, Gyllensten U, Olovsson M. Diagnostic excision of the cervix in women over 40 years with human papilloma virus persistency and normal cytology. Eur J Obstet Gynecol Reprod Biol. 2019;3:100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergengren L, Karlsson MG, Helenius G. Prevalence of HPV and pathological changes among women 70 years of age, 10 years after exclusion from the Swedish cervical cancer screening program. Cancer Causes Control. 2020;31(4):377–81. [DOI] [PubMed] [Google Scholar]
- 30. Beniwal S, Makkar B, Batra S, Gandhi G, Goswami D, Zutshi V. Comparison of vaginal versus Oral estradiol Administration in Improving the visualization of transformation zone (TZ) during colposcopy. J Clin Diagn Res. 2016;10(7):QC18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Makkar B, Batra S, Gandhi G, Zutshi V, Goswami D. Vaginal misoprostol versus vaginal estradiol in overcoming unsatisfactory colposcopy. Gynecol Obstet Invest. 2014;77(3):176–9. [DOI] [PubMed] [Google Scholar]
- 32. Richards A, Dalrymple C. Abnormal cervicovaginal cytology, unsatisfactory colposcopy and the use of vaginal estrogen cream: an observational study of clinical outcomes for women in low estrogen states. J Obstet Gynaecol Res. 2015;41(3):440–4. [DOI] [PubMed] [Google Scholar]
- 33. Gage JC, Duggan MA, Nation JG, Gao S, Castle PE. Detection of cervical cancer and its precursors by endocervical curettage in 13,115 colposcopically guided biopsy exams. Am J Obstet Gynecol. 2010;203(5):481.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomon D, Stoler M, Jeronimo J, Khan M, Castle P, Schiffman M. Diagnostic utility of endocervical curettage in women undergoing colposcopy for equivocal or low‐grade cytologic abnormalities. Obstet Gynecol (New York 1953). 2007;110(2), Part 1:288–95. [DOI] [PubMed] [Google Scholar]
- 35. Kirkegaard P, Gustafson LW, Petersen LK, Andersen B. ‘I want the whole package’. Elderly Patients' preferences for follow‐up after abnormal cervical test results: a qualitative study. Patient Prefer Adherence. 2020;14:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wentzensen N, Fetterman B, Castle PE, Schiffman M, Wood SN, Stiemerling E, et al. p16/Ki‐67 dual stain cytology for detection of cervical precancer in HPV‐positive women. J Natl Cancer Inst. 2015;107(12):djv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wentzensen N, Clarke MA, Bremer R, Poitras N, Tokugawa D, Goldhoff PE, et al. Clinical evaluation of human papillomavirus screening with p16/Ki‐67 dual stain triage in a large organized cervical cancer screening program. JAMA Intern Med. 2019;179(7):881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonde J, Floore A, Ejegod D, Vink FJ, Hesselink A, van de Ven PM, et al. Methylation markers FAM19A4 and miR124‐2 as triage strategy for primary human papillomavirus screen positive women: a large European multicenter study. Int J Cancer. 2021;148(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Data can be made available on request from researchers who meet the criteria for access to patient's confidential data and upon approval from the Danish Data Protection Agency.


