Abstract
Background
Allergy to dogs affects around 10% of the population in developed countries. Immune therapy of allergic patients with dog allergen extracts has shown limited therapeutic benefit.
Methods
We established a mouse model of dog allergy by repeatedly administering dog dander and epithelium extracts via the intranasal route. We also assessed the efficacy of a recombinant multimeric protein containing Can f 1, f 2, f 4 and f 6 in preventing inflammatory responses to dog extracts.
Results
Repeated inhalation of dog extracts induced infiltration of the airways by TH2 cells, eosinophils and goblet cells, reminiscent of the house dust mite (HDM) model of asthma. Dog extracts also induced robust airway hyperresponsiveness and promoted TH17 cell responses, which was associated with a high neutrophilic infiltration of the airways. scRNA‐Seq analysis of T helper cells in the airways pinpointed a unique gene signature for TH17 cells. Analysis of T‐cell receptors depicted a high frequency of clones that were shared between TH17, TH2 and suppressive Treg cells, indicative of a common differentiation trajectory for these subsets. Importantly, sublingual administration of multimeric Can f 1‐2‐4‐6 protein prior to sensitization reduced airway hyperresponsiveness and type 2‐mediated inflammation in this model.
Conclusion
Dog allergen extracts induce robust TH2 and TH17 cell‐mediated responses in mice. Recombinant Can f 1‐2‐4‐6 can induce tolerance to complex dog allergen extracts.
Keywords: allergen immune therapy, dog allergy, single cell RNA‐Seq, TH2, TH17
Intranasal administration of dog allergen extracts induces airway hyperresponsiveness and robust TH17 cell‐driven neutrophilia. Single cell RNA‐Sequencing pinpoints distinct gene expression signatures between canonical TH cell subsets and shows considerable clonal overlap between TH2, TH17, Treg and TFH cells. A recombinant multimer of lipocalin allergens (Can f 1‐2‐4‐6) reduces TH2 cell responses and airway hyperresponsiveness when administered prophylactically.Abbreviations: AHR, airway hyperresponsiveness; CCR6, C‐C motif chemokine receptor 6; CXCR5, C‐X‐C chemokine receptor 5; EOS, eosinophil; IL, interleukin; NEU, neutrophil; Tfh, follicular T helper cell; Treg, regulatory T helper cell

Abbreviations
- AHR
airway hyperresponsiveness
- CCR6
C‐C motif chemokine receptor 6
- CXCR5
C‐X‐C chemokine receptor 5
- EOS
eosinophil
- IL
interleukin
- NEU
neutrophil
- Tfh
follicular T helper cell
- Treg
regulatory T helper cell
1. INTRODUCTION
Asthma is a chronic inflammatory disorder of the airways affecting over 300 million people worldwide, leading to substantial mortality and loss of quality of life. 1 Furthermore, the incidence of allergic airway disease is rising globally. 2
A central player in the asthmatic and allergic response is the CD4+ T cell. CD4+ T cells respond to peptides presented in the context of MHC‐II molecules. When activated by pathogens or allergens, CD4+ T cells differentiate into several functionally‐distinct T helper (TH) cell subsets; TH1 cells secrete the cytokine interferon‐gamma (IFN‐γ) and promote immunity to viruses, TH2 cells secrete interleukin‐ (IL‐) 4, IL‐5 and IL‐13 and mediate helminth clearance, TH17 cells secrete IL‐17 and promote immunity to fungi and Treg cells suppress inflammation through various mechanisms. 3 TH2 cells are known to drive several features of childhood‐onset asthma including airway eosinophilia, goblet cell hyperplasia and IgE secretion. 4 However, roles for other TH cell subsets have also been proposed to impact on the development of asthma. 4 , 5 Adult‐onset asthma, which is increasing in prevalence world‐wide, is associated with higher levels of TH17 cells and neutrophils and is more resistant to corticosteroids. 6
The only curative treatment available for allergic diseases is allergen‐specific immunotherapy (AIT). Patients are administered allergen in low doses over a long period with the aim of developing immune tolerance. 7 Tolerance is thought to be achieved by the induction of Treg cells, by decreasing the TH2 cell response and by increasing serum concentrations of allergen‐specific IgG1, IgG4 and IgA. 8 While AIT is generally proposed as a strategy to confer tolerance to already sensitized individuals, prophylactic AIT has also been proposed as a strategy to combat the alarming rise in allergic disorders across the globe. 9 , 10 , 11
Over the last few decades, antigens including ovalbumin and extracts from house dust mite, cat, peanut and fungi have been used to study allergic immune responses. 12 , 13 , 14 , 15 , 16 It is clear that different allergen extracts elicit distinct immune responses, with some inducing strong innate allergic responses and others inducing primarily adaptive immune responses. Furthermore, the quality of the T helper cell response induced by allergens has been found to vary. For instance, house dust mite induces a dominant TH2 cell response in mice, while Aspergillus versicolor induces strong TH17 responses side‐by‐side with TH2 cell responses. 17 , 18
Dog allergens are found in dander, hair, saliva and urine and can easily become airborne. 19 , 20 Eight dog allergens have been identified of which Can f 1, Can f 2, Can f 4 and Can f 6 are part of the lipocalin family, which share structural similarities with serum albumin proteins. Between 50 and 70% of dog‐allergic people are sensitized to Can f 1, approximately 25% are sensitive to Can f 2, 35%–81% to Can f 4 and 38% to Can f 6. 21 , 22 , 23 , 24 , 25 , 26 Members of the lipocalin superfamily can be found in all common mammalian allergen sources and have been proposed to promote TH2 cell responses and allergy. 27 Recently, we developed a recombinant multimeric molecule comprising all four known dog lipocalins and demonstrated its diagnostic efficacy and immunogenicity in animal studies. 28 In this study, we characterize airway inflammation in mice administered dog allergen extracts and test the ability of a recombinant dog lipocalin multimer to induce immune tolerance.
2. METHODS
2.1. Experimental animals
Wildtype C57BL/6J and Rag1 −/− mice were bred and maintained at the Comparative Medicine animal facility located at Karolinska Institutet. Mice were 8–10 weeks old at the start of experiments and both female and male mice were used in experiments but only one gender was used per experiment. All mice were housed in individually ventilated cages with food and water ad lib and under specific pathogen‐free conditions. Experiments were approved by Stockholms jordbruskverket (8971/2017 and 3649/2019).
2.2. Dog and house dust mite model
Mice were intranasally sensitized with 1 μg of house dust mite (HDM) (Greer, XBP70D3A lot 329779) extract in 40 μl PBS, 1 μg each of dog dander (Greer, XPE64D3A lot 338515 was used in all experiments, except in Figure 4A, where lot 398826 was used) and dog epithelial extract (Greer, XPE7D3A lot 343971 was used in all experiments), referred to as dog allergen extracts, in 40 μl PBS or with PBS as control. One week after sensitization, mice were challenged with 5 daily administrations of 10 μg of HDM in 40 μl PBS or 5 μg each of dog dander and dog epithelial extract in 40 μl PBS or with PBS as control. Mice were sacrificed and organs harvested on Day 15. Bronchoalveolar lavage was performed by cannulating the trachea and flushing out the airways with 2 × 1 ml PBS. 29 In experiments aimed at quantifying airway neutrophilia, mice were challenged one additional time 3 h before sacrifice. All instillations were done under isoflurane anaesthesia. Recombinant anti‐IL‐17 (clone 17F3) or control Ig (clone MOPC21) was injected i.p. at a dose of 500 μg each one day before final dog allergen extract challenge.
FIGURE 4.
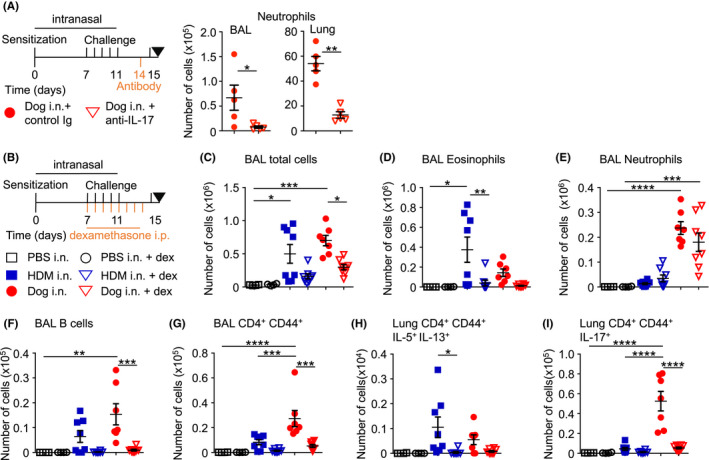
Dexamethasone reduces airway inflammation after administration of dog allergen extracts. (A) Regimen of intranasal allergen challenge with intraperitoneal anti‐IL‐17 or control Ig (500 μg each) administration. Levels of airway and lung tissue neutrophils are shown, n = 5 mice in each group. (B) Regimen of intranasal allergen challenge with intraperitoneal dexamethasone/vehicle control administration: PBS i.n. vehicle i.p. n = 4 (open square), PBS i.n. dexamethasone i.p. n = 4 (open circle), HDM i.n. vehicle i.p. n = 8 (blue square), HDM i.n. dexamethasone i.p. n = 8 (open blue triangle), dog i.n. vehicle i.p. n = 8 (red circle), or dog i.n. dexamethasone i.p. n = 8 (open red triangle). (C) Total number of cells in the BAL. (D) Number of eosinophils in the BAL. (E) Number of neutrophils in the BAL. (F) Number of B cells in the BAL. (G) Number of effector T helper cells in the BAL. (H) Number of IL‐5+ IL‐13+ T helper cells in the lung. (I) Number of IL‐17+ T helper cells. One‐way ANOVA with Bonferroni's post‐test was performed to adjust for multiple comparisons. *p < .05, p < .01, ***p < .001, ****p < .0001
2.3. Flow cytometry
Flow cytometry was performed on a BD LSRII using combinations of the following antibodies: from BD: B220 (RA3‐6B2), CD3 (145‐2C11), CD4 (RM4‐5 and GK1.5), CD8 (53–6.7), CD44 (IM7), GR‐1 (RB6‐8C5), IFN‐γ (XMG1.2), IL‐4 (11B11), IL‐17 (TC11‐18H10), Siglec‐F (E50‐2440) and CD16/32 (2.4G2); from Invitrogen: CD11c (N418), FOXP3 (FJK‐16 s), IL‐13 (ebio13A); from Biolegend: IL‐5 (TRFK5), TCRγδ (GL3), TCRβ (H57‐597), Vγ1 (2.11), Vγ4 (UC3‐10A6), Vδ6.3 (C504.17C). For intracellular staining, cells were fixed and permeabilized using the eBioscience FOXP3/Transcription factor staining buffer set from Invitrogen. PBS57‐CD1d tetramers were obtained from the NIH Tetramer Core Facility.
2.4. Restimulation with phorbol 12‐myristate 13‐acetate and ionomycin
For detection of cytokine‐producing cells from the airways and lung tissue, cells were stimulated with phorbol 12‐myristate 13‐acetate (PMA) and ionomycin in the presence of Brefeldin A and/or Golgistop (containing Monensin, BD) for 3 h at 37°C and analysed by flow cytometry.
2.5. Restimulation of lymph node cells and quantification of cytokine production by cytometric bead array
2.5 × 105 cells from the mediastinal lymph node were cultured for 2 days in IMDM medium and restimulated by either HDM (20 μg/ml), dog allergen extracts (20 μg/ml), Can f 1, Can f 2, Can f 3, Can f 4 or Can f 6 (10 μg/ml). The supernatant was collected and analysed with the BD™ cytometric bead array (CBA) Mouse Enhanced Sensitivity kit (BD). CBA samples were run on a CyAn™ ADP Analyzer (Beckman Coulter).
2.6. Quantification of endotoxin levels in allergen extracts
Pierce™ LAL Chromogenic Endotoxin Quantitation Kit (Thermofisher) was used to measure endotoxin content of HDM, dog dander and dog epithelium extracts.
2.7. ELISA
ELISA was performed by coating ELISA plates (nunc) either with unconjugated anti‐IgE (R35‐92, BD) allergen extracts (5 μg/ml) or recombinant Can f 1 (5 μg/ml). Plates were incubated at 4°C for 12 h, washed with PBS and blocked with 2% milk in PBS. Serum was added in three‐fold or five‐fold serial dilution and incubated for 2 h at room temperature. Plates were washed and then incubated for one hour with secondary antibody, either HRP coupled anti‐IgG1 (SouthernBiotech) or biotin coupled anti‐IgE (R35‐72, BD) followed by streptavidin—HRP (Mabtech). TMB substrate (KPL) followed by H2SO4 were used to develop and stop the assay. The Asys Expert 96 ELISA reader (Biochrom) was used to read OD at 450 nm.
2.8. Histopathology
Lungs were fixed with 10% formalin for a minimum of 24 h before being embedded in paraffin. Periodic acid‐Schiff‐diastase (PAS‐D) and Haematoxylin and eosin (H&E) stains were performed. Complete airways in PAS‐D stained lung sections were scored in a blinded fashion on a 0–4 point scale with points awarded based on the percentage of the airway covered by positively stained cells; 0 points for 0% of the airway affected, 1 point for 1%–25%, 2 points for 26%–50%, 3 points for 51%–75% and 4 points for more than 75% PAS positive. 22–72 full airways were counted per mouse.
2.9. Measurement of airway function
Mice were sensitized and challenged following the standard regimen with one additional challenge on Day 14. Animals were anaesthetized with 10 ml/kg i.p. mixture of hypnorm (Fentanyl 0.315 mg/ml, fluanisone 10 mg/ml, VetaPharma), midazolam (5 mg/ml, hameln) and saline (Apoteket) in 1:1:2. After being tracheostomized and cannulated, mice were connected to the FlexiVent apparatus equipped with module 1 (SCIREQ) where animals were ventilated at respiratory rate of 150 breaths/min, tidal volume of 10 ml/kg and positive end expiratory pressure (PEEP) of 3 cmH2O. Following stabilization, lung resistance was measured using forced oscillation technique (FOT) at baseline and under increasing concentrations of nebulized methacholine (Sigma). Respiratory mechanics parameters were calculated by flexiWare version 8 (SCIREQ) based on a single compartment model and constant phase model. These included total respiratory system resistance (Rrs), elastance (Ers) calculated from single compartment model and Newtonian resistance (Rn), tissue damping (G) and tissue elastance (H) from constant phase model.
2.10. RNA‐seq of single T helper cells from the BAL
T helper cells were purified from the bronchoalveolar lavage of mice sensitized and challenged with dog allergen extracts. The BAL was kept cold and processed rapidly. Cells were stained for CD4, CD3, Siglec‐F and B220. CD4+ CD3+ SiglecF− B220− cells were sorted into pure FCS using a BD FACSAria Fusion. Cells were washed and resuspended in cold PBS. Single cells were isolated with the droplet‐based microfluidic system Chromium (10x Genomics). Libraries were prepared by the Eukaryotic Single Cell Genomics national facility at SciLife Laboratory, Stockholm. Gene expression matrices were pre‐processed and filtered using Seurat v3. 30 , 31 TCR frequencies and expression patterns were analysed and graphed with Loupe V(D)J Browser (10x Genomics) and by combining the barcodes and clusterfiles generated with Seurat v3. For each cluster, the clonotypes occurring in that cluster were counted. To visualize the distribution of counts in each cluster, ball‐packing plots (Figure 6C) were created using the Julia package APackOfTheClones (https://github.com/MurrellGroup/APackOfTheClones). To further represent these distributions, the clonotype counts in each cluster were ordered from highest to lowest and Lorenz curves plotted (Figure 6D). The co‐occurrence of clonotypes between clusters is summarized by a plot of pairwise correlations of square‐root‐transformed counts of all clonotypes (Figure 6E).
FIGURE 6.
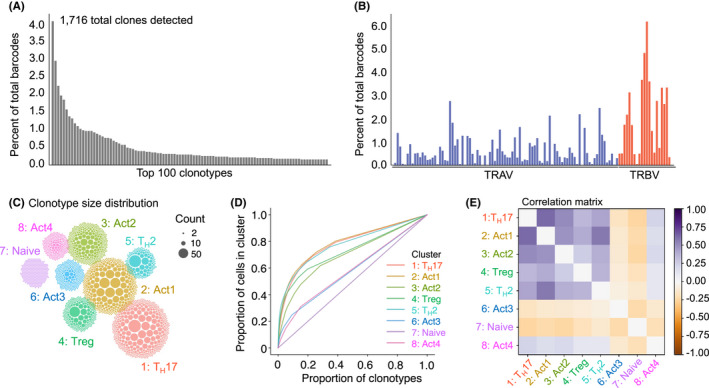
scRNA‐Seq of airway TH cells pinpoints large clonal outgrowths and a shared differentiation trajectory for TH17, TH2, Treg and other activated TH cell clusters. Side‐by‐side with a 5′ gene transcription library (Figure 5), a VDJ library was amplified and sequenced on TH cells isolated from the airways of mice. (A) The frequency of the top 100 expressed TCRs is shown. (B) The frequency of V gene usage across the TRAV and TRBV gene loci. (C) Clonotype count distributions. Each ball corresponds to a clonotype in the given cluster, with the size of the ball proportional to the number of cells of that clone within the cluster. The clusters are placed in similar positions to the UMAP plot (Figure 5A). (D) Lorenz curves, plotting the proportion of clonotypes against the proportion of cells in a cluster. (E) Correlation matrix of the number of cells expressing each TCR between each pair of clusters, after a variance stabilizing square root transformation
2.11. Sublingual immunotherapy
Mice were anaesthetized with isoflurane and administered either 10 μg of Can f 1‐2‐4‐6 protein 28 in 20 μl PBS or PBS as control sublingually three times per week for four weeks before the standard intranasal allergen instillations.
2.12. Dexamethasone treatment
To test for corticosteroid resistance mice were injected either with 1 mg/kg dexamethasone intraperitoneal daily from Day 7 to 14 after sensitization or with PBS as control.
2.13. Quantification and statistical analysis
Nonparametric Mann–Whitney U test was used to compare two groups. For multiple comparisons, one‐way analysis of variance (ANOVA) and Bonferroni's test were used. In Figure 1, to compare AHR of mice administered HDM or dog allergen extracts to PBS control mice, ANOVA and Dunnett's multiple comparisons test were used. *p < .05, **p < .01, ***p < .001, ****p < .0001.
FIGURE 1.
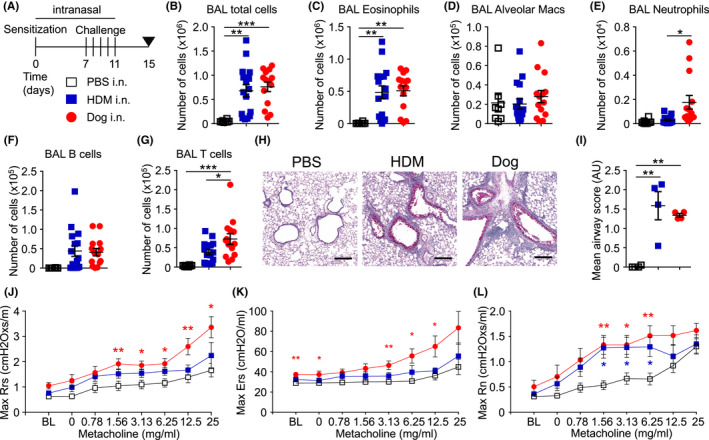
Intranasal administration of dog allergen extracts leads to airway inflammation and airway hyperresponsiveness. (A) Regimen of intranasal administration of PBS (open square), HDM (blue square) or dog allergen extracts (red circle). (B–G) PBS n = 8, HDM n = 16, dog allergen extracts n = 14. (B) Total number of cells in the BAL. (C) Number of eosinophils in the BAL. (D) Number of alveolar macrophages in the BAL. (E) Number of neutrophils in the BAL. (F) Number of B cells in the BAL. (G) Number of T cells in the BAL. (H) Periodic acid‐Schiff‐diastase staining of lung sections (line = 200 μm). (I) Mean airway score of lung sections (n = 4 per group). (J–L) Airway resistance to increasing doses of methacholine as measured by FlexiVent (PBS n = 6, HDM n = 6, dog allergen extracts n = 7). (J) Overall resistance Rrs. (K) Elastance Ers. (L) Newtonian resistance Rn. Red stars indicate a comparison between dog and PBS, while blue stars indicate a comparison between HDM and PBS. In (B–I), One‐way ANOVA with Bonferroni's post‐test was performed. In (J–L), One‐way ANOVA and Dunnett's test was used to compare dog‐ or HDM‐challenged mice to PBS
3. RESULTS
3.1. Intranasal administration of dog allergen extracts leads to immune cell infiltration of the airways, goblet cell hyperplasia and airway hyperresponsiveness
In line with protocols for HDM instillation, 32 1 μg each of dog dander and epithelium extract was administered i.n. to sensitize mice followed by five daily administrations of 5 μg of each extract (10 μg total) on Days 7–11. On Day 15, mice were sacrificed and analysed for signs of airway and lung tissue inflammation (Figure 1A). Instillations with HDM and dog allergen extracts led to a comparable influx of cells into the airways (Figure 1B), hallmarked by a high number of eosinophils (Figures 1C and S1A for gating). The number of alveolar macrophages in the lavage was not significantly different between mice after HDM, dog allergen extracts or PBS instillation (Figure 1D). Significantly greater neutrophil infiltration of the airways was observed in mice administered dog allergens compared with those administered HDM (Figure 1E). B cell numbers in the lavage were comparable between mice administered dog allergen or HDM extracts (Figure 1F), whereas more T cells were present in the airways of mice administered dog allergen extracts (Figure 1G). Haematoxylin and eosin (H&E) staining showed inflammation in the lung and a thickening of the basement membrane and smooth muscle cell layer surrounding the airways (Figure S1B). Lungs of HDM and dog allergen extract‐administered mice showed signs of goblet cell hyperplasia and mucus production (Figure 1H,I). Furthermore, dog allergen extract‐sensitized and ‐challenged mice had higher levels of total airway resistance (Rrs) and elastance (Ers) when compared with mice administered PBS, reacting more strongly at most doses of methacholine (Figure 1J,K). Newtonian resistance (Rn) was increased in both HDM or dog allergen extract‐administered mice compared with control mice (Figure 1L). Thus, intranasal administration of dog allergen extracts induced signs of allergic airway inflammation reminiscent of that seen after instillation of HDM.
3.2. Dog allergen extract induces differentiation of TH2 and TH17 cells and promotes a dual eosinophilic and neutrophilic infiltration of the airways
To investigate the role of adaptive immunity for the inflammatory response to dog allergens, we administered dog allergen extracts to Rag1 −/− mice. This failed to induce appreciable airway eosinophilia or neutrophilia, such as that seen in WT mice (Figure 2A). Administration of either HDM or dog allergens to WT mice led to increased numbers of effector (CD44+) CD4+ and CD8+ T cells in the airways and increased proportions of Treg cells (% Foxp3+ of CD4+ cells) in the lungs (Figures 2B,C and S2A). Cytokine production was measured after stimulating airway or lung tissue cells with PMA and ionomycin for 3 hours. In the airways, T helper cells expressing IL‐5, IL‐13 and IFN‐γ were prominent in mice administered dog allergen extracts, comparable with the frequency in mice administered HDM (Figure 2D). In mice administered dog allergen extracts, a significantly higher proportion of cells produced IL‐17 compared with HDM‐sensitized and HDM‐challenged mice (Figure 2D). CD4+ T cells from the lung showed a similar pattern of cytokine expression, with the highest frequencies of IL‐17‐producing cells observed in dog‐sensitized and dog‐challenged mice (Figure 2E). In comparison, CD8+ T cells in the airways produced IFN‐γ but not IL‐5, IL‐13 or IL‐17 (Figure S2B). To determine whether NKT and γδ T cells may also contribute to the response to dog allergen extracts, these cells were enumerated (Figure S3). NKT cell numbers in the lung parenchyma were not altered in mice administered either HDM or dog allergens (Figure S3A). However, the frequency of γδ T cells in the lung was increased after dog allergen instillation and moreover, a specific increase in the frequency of Vγ4+ γδ T cells was observed (Figure S3B). In line with the elevated frequency of Vγ4+ γδ T cells in the lung, γδ T cells in the airways were capable of producing IL‐17 (Figure S3C).
FIGURE 2.
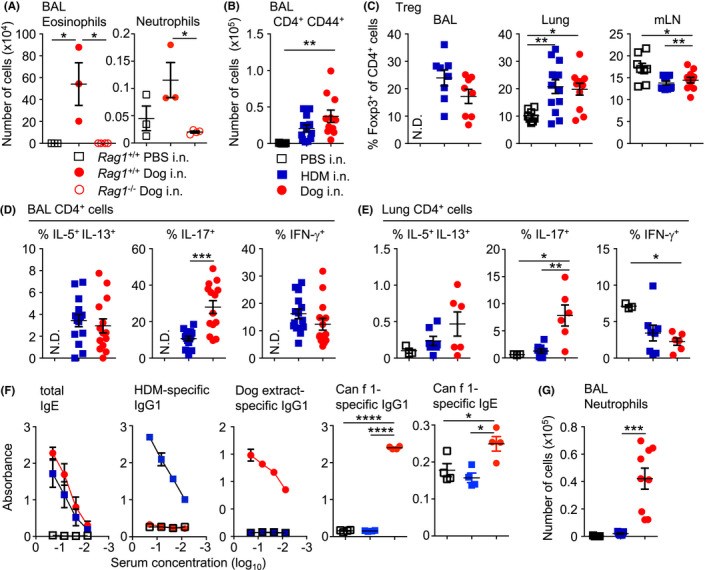
Intranasal administration of dog allergen extracts induces differentiation of both TH2 and TH17 cells. (A) PBS (open square), HDM (blue square) or dog allergen extracts (red circle) were administered to C57Bl6/J mice and the number of eosinophils and neutrophils was quantified (C57Bl6/J PBS n = 3, C57Bl6/J dog n = 3, Rag1 −/− dog n = 4). (B) Number of CD4+ CD44+ cells in the BAL (PBS n = 5, HDM = 12, dog allergen extracts n = 11). (C) Graphs of frequency of Foxp3+ cells among total CD4+ cells from the BAL, lung and mLN (BAL: HDM = 8, dog allergen extracts n = 8; lung and mLN PBS n = 8, HDM = 13, dog allergen extracts n = 11). (D) Graphs of the frequency of IL‐5+ IL‐13+, IL‐17+ and IFN‐γ+ cells among total CD4+ cells from the BAL (HDM = 14, dog allergen extracts n = 14). (E) Graphs of the frequency of IL‐5+ IL‐13+, IL‐17+ and IFN‐γ+ cells among total CD4+ cells from the lung (PBS n = 3, HDM = 8, dog allergen extracts n = 6). (F) ELISAs were performed on the serum of mice. Dog allergen‐specific and HDM‐specific IgG1 and total IgE graphs are the representative of 3 experiments (PBS n = 3, HDM = 5, dog allergen extracts n = 3). Can f 1‐specific IgG1 and IgE was also tested at a 1:3 dilution (PBS n = 4, HDM = 4, dog allergen extracts n = 4). (G) Regimen of intranasal administration with one additional challenge on day fifteen, three hours before harvesting organs, number of neutrophils from the BAL (PBS n = 5, HDM = 9, dog allergen extracts n = 9). N.D. Denotes not determined due to low cell numbers. One‐way ANOVA with Bonferroni's post‐test was performed except to compare frequencies of cytokine+ cells in the airways, where Mann–Whitney U test was used *p < .05, p < .01, ***p < .001, ****p < .0001
High endotoxin levels have been reported to promote a strong TH17 response in murine asthma models. 33 , 34 , 35 While both HDM and dog allergen extracts contained endotoxin, the levels in the dog dander extracts were around two orders of magnitude higher than in the HDM extract and about 17‐fold higher in dog epithelium extract compared with HDM extract (Figure S4A). Administration of dog epithelium or dander allergen extracts separately, induced inflammation in the airways that was characterized by airway eosinophilia and Th2, Th1 and Th17 cell cytokine production in the airways and lung tissue. Although extracts of dander appeared to induce more inflammation, epithelial extracts also promoted considerable inflammation and Th17 cell differentiation (Figure S4B–D). The abundance of Can f 1, Can f 2, Can f 3 and Can f 6 in each extract was also quantified, with dander containing the highest levels of Can f 1, Can f 2 and Can f 6, while Can f 3 was highly abundant in both extracts (Figure S4E).
We also analysed serum antibody levels by ELISA. Mice administered HDM or dog allergens showed high levels of allergen‐specific IgG1 antibodies (Figure 2F) and showed comparable levels of total IgE in serum. Whole dog allergen extract‐specific IgE was not detected in the serum of mice (not shown), potentially due to competition from IgG1, as has been previously described. 36 However, IgG1 and IgE specific for Can f 1 was detected from the serum of mice administered dog allergen extracts (Figure 2F).
TH17 cell responses have been linked to the recruitment of neutrophils. Since neutrophils are typically recruited rapidly to sites of inflammation, we challenged mice an additional time on Day 15, three hours before sacrifice. Challenge on Day 15 with dog allergens markedly increased the infiltration of neutrophils into the airways, in a manner not observed with HDM (Figure 2G). In all, dog allergen extracts induce strong type 2‐ and type 17‐mediated airway inflammation marked by airway eosinophilia and neutrophilia, goblet cell hyperplasia, airway hyperresponsiveness and IgG1/IgE production.
3.3. Restimulation of lymph node cells with dog allergen extracts leads to the production of IL‐13, IL‐5, IL‐10, IL‐17 and IFN‐γ
To investigate the specificity of cytokine production in response to dog allergens, we restimulated mediastinal lymph node cells from mice administered PBS, HDM or dog allergen extracts for 48 h in the presence of allergen extracts or recombinant Can f 1 or Can f 2 (Figure 3A–E). HDM and dog allergen extracts induced comparable IL‐13, IL‐5 and IL‐10 production in lymph node cell cultures from mice that had been sensitized and challenged with the respective allergen (Figure 3A–C). Only cells from mice administered dog allergen extracts produced high levels of IL‐17 and IFN‐γ (Figure 3D,E), whereas lymphocytes from mice administered HDM produced very little of these cytokines. Recombinant Can f 1 appeared to induce some production of IL‐5, IL‐13 and IL‐10 but this was significantly less than that induced by whole dog allergen extracts (Figure 3A–C).
FIGURE 3.
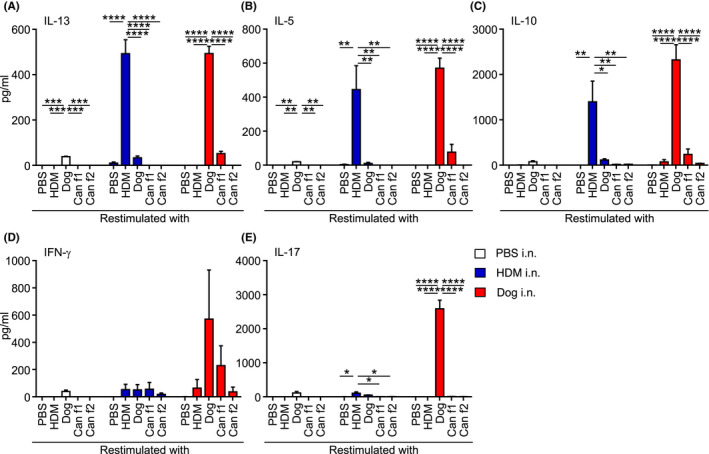
Mixed TH2/TH17 cytokine response is induced by dog allergen extracts. (A–E) Concentration of the indicated cytokine in supernatant from mediastinal lymph node cell cultures after 48 h. Cells from mice administered PBS n = 2 (white), HDM n = 3 (blue), dog allergen extracts n = 3 (red) were restimulated with PBS, HDM, dog allergen extracts, Can f 1 or Can f 2. (A) IL‐13. (B) IL‐5. (C) IL‐10. (D) IFN‐γ. (E) IL‐17. One representative of two independent experiments is shown. One‐way ANOVA with Bonferroni's post‐test was performed to compare the responses between PBS, HDM, Dog, Can f 1 and Can f 2, *p < .05, p < .01, ***p < .001, ****p < .0001
3.4. Airway neutrophilia in response to dog allergen extract administration is dependent on IL‐17
To test whether neutrophil recruitment into the lung and airways was dependent on the cytokine IL‐17, we administered 500 μg anti‐IL‐17 (clone 17F3) or isotype control antibody (clone MOPC21) one day before the final challenge. Blockade of IL‐17 significantly reduced the number of airway and lung tissue neutrophils (Figure 4A), suggesting that dog allergen extracts induced neutrophil recruitment in an IL‐17‐dependent manner.
3.4.1. Dexamethasone reduces airway eosinophilia and cytokine production but not airway neutrophilia
To test the effect of corticosteroids on dog allergen‐induced airway inflammation, we administered dexamethasone daily from the first day of allergen challenge up to one day before sacrifice (Day 7 to Day 14, Figure 4B). Mice were administered an additional dose of allergen three hours before sacrifice. Dexamethasone reduced the overall number of airway‐infiltrating cells including B and effector T helper cells but did not significantly reduce the number of airway neutrophils following exposure to dog allergen extracts (Figure 4C–G). Dexamethasone administration appeared to reduce TH2 and TH17 cytokine‐producing cells in the lungs of mice administered HDM or dog allergen extracts respectively (Figure 4H,I). γδ T cells were also reduced in the airways of mice administered dexamethasone (Figure S3C). Thus, corticosteroid treatment was effective in reducing several parameters of airway inflammation in response to dog allergen extracts but failed to reduce airway neutrophilia.
3.5. Single cell RNA‐Sequencing reveals several distinct T helper cell clusters in the BAL
We next analysed CD3+CD4+ cells from the airways of mice administered dog allergen extracts by Single cell RNA‐Sequencing (scRNA‐Seq). Unsupervised hierarchical clustering and visualization of 5489 cells identified 8 distinct clusters (Figure 5A,B). The majority of cells expressed transcripts for Cd3e, Cd3g and Cd3d (Figure 5C), while Cd4 was also detected at reasonable levels. Several clusters could be identified based on the expression of genes with previously described functions. Cluster 7 expressed mRNAs characteristic of naïve CD4+ T cells such as Sell and Ccr7. Cluster 4 expressed Foxp3, Ctla4 and other genes typical of Treg cells. Clusters 1 and 5 could be identified as TH17 and TH2 cells, respectively, based on the expression of cytokines, transcription factors and surface markers (Figure 5C,D,F and Table S1). For instance, cells in cluster 1 were highly enriched for the expression of Il17a, Ccr6 and Rorc mRNA (Figure 5C,D). Cluster 1 cells also expressed the gene for the IL17C receptor Il17re. IL‐17C:IL17RE interactions have previously been shown to be required for the development of experimental autoimmune encephalomyelitis and to promote TH17 responses by inducing expression of IκBζ. 37 Other genes highly expressed in cluster 1 included Sdc4, Smox, Aqp3, Ramp1 and Tmem176a (Figure 5D). Gene ontology (GO) analysis identified several enriched molecular processes in TH17 cells related to ribonucleotide metabolic processes and hypoxia (Figure 5E).
FIGURE 5.
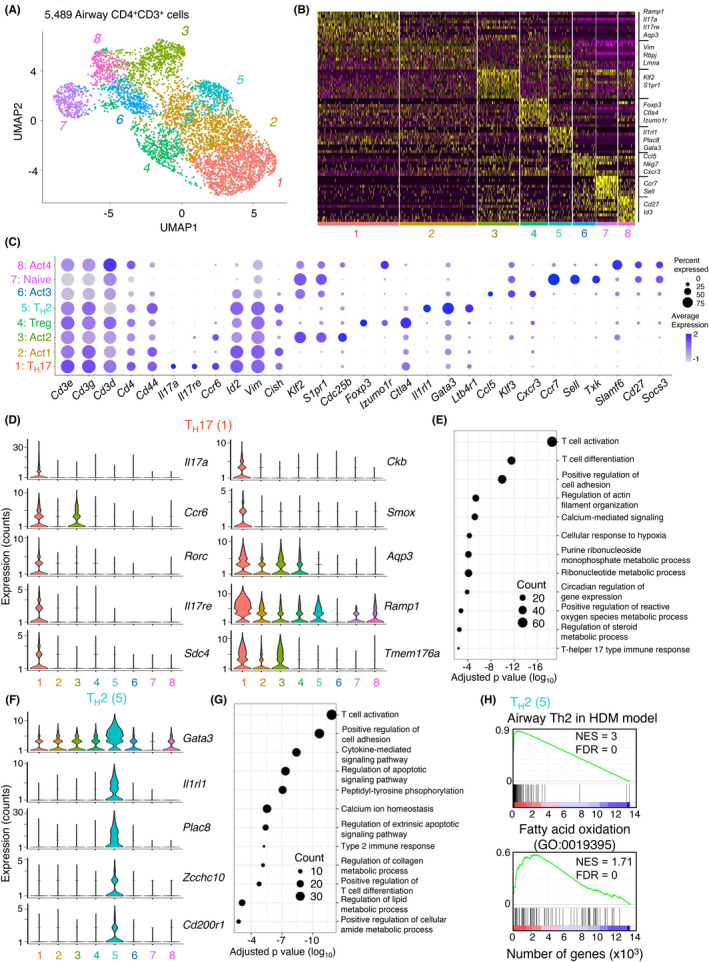
Analysis of T helper cells by scRNA‐Seq identifies several distinct subsets including a prominent TH17 cell cluster. (A) UMAP representation of 5489 single TH cells from the airways of a mouse administered dog allergen extracts. (B) Heatmap of top 10 differentially expressed genes in each cluster. (C) DotPlot of expression of key genes across all clusters. Clusters were assigned the following identities: Cluster 1: TH17, Cluster 2: activated cells (Act)1, Cluster 3: Act2, Cluster 4: Treg, Cluster 5: TH2, Cluster 6: Act3, Cluster 7: naïve, Cluster 8: Act4. (D) Violin plots of significant genes for the TH17 (1) cluster. (E) DotPlot showing gene ontology of biological processes in the TH17 (1) cluster. (F) Violin plots of significant genes for the TH2 (5) cluster. (G) DotPlot showing gene ontology of biological processes in the TH2 (5) cluster. (H) Gene set enrichment analysis of the Th2 cell cluster (5) for genes enriched in Th2 cells in the HDM model 17 and for genes involved in fatty acid oxidation
Cells in cluster 5 resembled TH2 cells and expressed classical TH2 cell‐associated genes including Gata3, Il1rl1, Il5 and Il13 (Figures 5F, S5B and Table S1). Several genes that have recently been reported to be highly expressed in TH2 cells in the HDM model, 17 such as Plac8, Zcchc10, Cd200r1 and Il6 were also found to be enriched in the TH2 cluster in this model (Figure 5F and Table S1). GO analysis identified regulation of apoptotic pathways, type 2 immune response and regulation of lipid metabolism as enriched molecular processes in TH2 cells (Figure 5G), in line with molecular processes enriched in the HDM model. 17 When the gene transcription profile of TH2 cells in this model was compared with that generated to HDM allergens, a high overlap was observed (Figure 5H). Furthermore, TH2 cells in the airways were enriched for the expression of genes related to fatty acid oxidation (Figure 5H), as they were in response to HDM. This suggests that TH2 cells generated in the HDM and dog allergen extract models have a high degree of similarity, despite large differences in the levels of endotoxin between these extracts.
Cluster 8 cells (Act4) expressed genes typically associated with Tfh cells, including Tox2, Il21, Bcl6 and Cxcr5. Gene set enrichment confirmed that these cells were significantly enriched for markers typically associated with germinal centre Tfh cells (Figure S5A).
Cells in cluster 6 (Act3) appeared to express some genes of recently described CD4+ CTL including Ccl5, Gzmk, Ly6c2, Nkg7 and Tbx21 (Figure 5C and Table S1). This cluster was not specifically enriched for Ifng mRNA expression (Figure S5B). Whether these cells have true cytotoxic potential is unclear.
Expression of Ifng, Il13 and Foxp3 was also observed in the TH17 cell cluster to some extent (Figure S5B). We thus analysed the co‐expression of TH1, TH2, TH17 and Treg cell‐associated cytokines/transcription factors by flow cytometry. This depicted a significantly higher percentage of IL‐17+ IFN‐γ+ expressing T helper effector cells in mice administered dog allergen extracts compared with those administered HDM (Figure S5C), although IL‐17+ cells co‐expressing Foxp3, IL‐5 or IL‐13 were not significantly increased in these mice. Thus, T helper cells from mice administered dog allergens exhibit greater breadth in cytokine production compared with cells from mice administered HDM.
Taken together, scRNA‐Seq reveals exquisite gene expression profiles for several T helper cell populations responding to dog allergen extracts. This depicts a highly conserved transcriptional profile for TH2 cells between the HDM and dog allergen extract models, but also pinpoints several cell clusters that are enriched in the airways of mice exposed to dog allergen extracts, including TH17, Tfh and a putative CD4+ CTL population.
3.6. T‐cell antigen receptor (TCR) analysis depicts a high frequency of shared clones between TH17, TH2 and Treg cells
TCRs were also sequenced from single cells isolated from the airways of mice administered dog allergen extracts. Productive α/β chains were called in most single cells analysed and 1716 different clonotypes could be identified. The most common TCR detected was found in around 4% of cells (Figure 6A). There was no indication that the response was biased toward specific TCR α or β chains since the most common TRAV was detected in 3% of cells and the most common TRBV in around 6% of cells (Figure 6B). We plotted the clonotype size distribution onto the UMAP, 38 which depicted obvious clonal outgrowths in several clusters including the TH17, TH2, Treg clusters and in clusters Act1 and Act2 (Figure 6C). The cluster of naïve CD4+ T cells, which makes up around 5% of airway infiltrating cells, was the most diverse, with each cell expressing a distinct TCR. In the Th17, Th2 and Act1 clusters, large clonal outgrowths were observed with 20% of clones accounting for approximately 65% of the total cells in the cluster (Figure 6D). Correlation analysis showed that the clonal composition of most clusters overlapped, with the exception of naïve cells, which was composed entirely of unique clones, and Act3 (putative CD4+ CTL), which also appeared clonally distinct (Figure 6E). This suggests that CD4+ CTL may follow a distinct differentiation trajectory to cells in other effector clusters. Intriguingly, considerable clonal overlap was observed between the Treg cell cluster and other effector clusters. This indicates that many Treg cells may be ‘induced’ from naïve CD4+ T cells (so‐called iTreg) in response to dog allergens, or that thymic Treg cells transdifferentiate into other effector subsets after activation. A high degree of overlap was also observed between Th2 cells, Th17, Act1, Act2 and Act4 cells, indicative of a shared differentiation trajectory. Thus, analysis of TCR clonality indicates patterns of shared and restricted clonality between transcriptionally distinct subsets.
3.7. Prophylactic administration of recombinant multimeric dog allergens ameliorates airway hyperresponsiveness and TH2 cell responses to dog allergen extracts
Several clinical trials have explored the use of dog allergen extracts in AIT. These trials have had mixed results, partially attributed to varying quality and composition of allergen extracts. 39 The use of recombinant allergen proteins has been proposed as an alternative to whole allergen extracts. 10 , 40 We analysed whether sublingual administration of multimeric Can f 1‐2‐4‐6 protein could induce tolerance to dog allergen extracts if administered prior to sensitization. We administered mice three sublingual challenges per week for four weeks of the recombinant Can f 1‐2‐4‐6 protein and thereafter, subjected mice to the dog allergen model over 15 days (Figure 7A). Can f 1‐2‐4‐6 significantly reduced the total number of cells infiltrating the airways, in particular of eosinophils, whereas the number of T helper cells in the lavage was not significantly reduced (Figure 7B–D). Mice administered Can f 1‐2‐4‐6 had a higher frequency of Foxp3+ Treg cells in the lung, although the mean fluorescence intensity of Foxp3 in Treg was not significantly different across conditions (Figure 7E). Recombinant Can f 1‐2‐4‐6 strongly reduced proportions of TH2 cytokine‐producing cells in the airways and lung tissue and concomitantly increased the proportions of TH1 and TH17 cytokine‐producing cells (Figure 7F). Can f 1‐2‐4‐6, however, appeared to have little impact on the frequency of IL‐17‐producing CD4+ T cells and even significantly increased the frequency of IFN‐γ+ CD4+ T cells (Figure 7F). Culture of lymphocytes from mediastinal lymph nodes showed that mice administered Can f 1‐2‐4‐6 multimer produced less IL‐5, IL‐13 and IL‐10 in response to whole dog allergen extracts (Figure S6). Lymphocytes from mice administered only dog allergen extracts produced significant quantities of IL‐5 in response to Can f 1, but this was reduced when mice were administered Can f 1‐2‐4‐6 multimer prior to sensitization (Figure S6B). In contrast, IL‐17 and IFN‐γ secretion was significantly enhanced in cells from mice administered Can f 1‐2‐4‐6 (Figure S6D,E).
FIGURE 7.
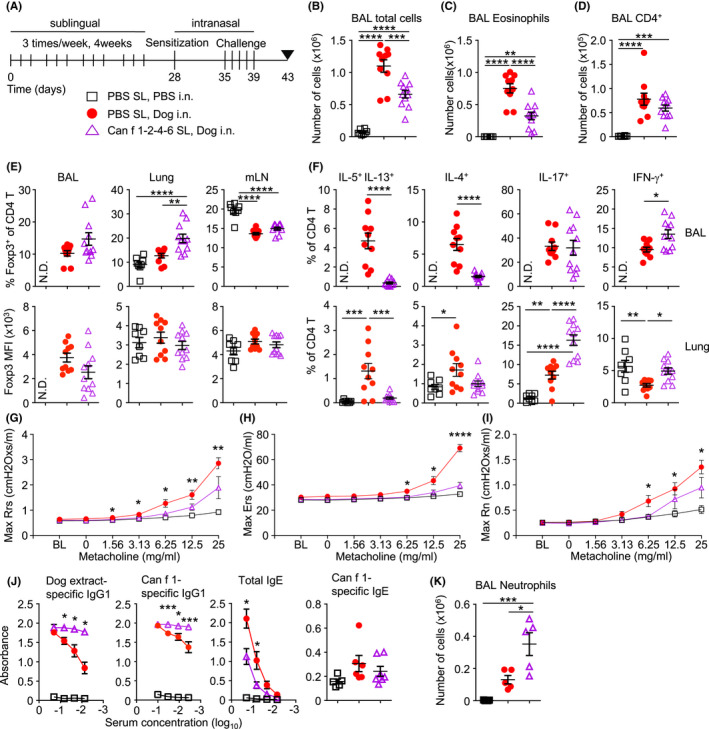
Sublingual administration of Can f 1‐2‐4‐6 ameliorates AHR and TH2 but not TH17 cell responses to dog allergen extracts. (A) Mice were sublingually administered either PBS or Can f 1‐2‐4‐6 protein 3 times per week for four weeks followed by i.n. sensitization with either PBS or dog allergen extracts (1 μg) on day 28 and 5 i.n. challenges (10 μg total) on days 35–39, mice were sacrificed on day 43; PBS sublingual, PBS i.n. (open square, B–F n = 8), PBS sublingual, dog allergen extracts (red circle, B–F n = 10), Can f 1‐2‐4‐6 sublingual, dog allergen extracts (open purple triangle, B–F n = 11). (B) Total number of cells in the BAL. (C) Number of eosinophils in the BAL. (D) Number of T cells in the BAL. (E) Frequency of Foxp3+ cells among total CD4+ cells and mean fluorescence intensity (MFI) of Foxp3 expression on gated Treg cells from the BAL, lung and mLN. (F) Frequency of IL‐5+ IL‐13+,IL‐4+, IL‐17+ and IFN‐γ+ cells among total CD4+ cells from the BAL and lung tissue. (G–I) Airway resistance to increasing doses of methacholine as measured by FlexiVent (PBS sublingual, PBS i.n. n = 6, PBS sublingual, dog allergen extracts n = 10, Can f 1‐2‐4‐6 sublingual, dog allergen extracts n = 10). (G) Overall resistance Rrs. (H) Elastance Ers. (I) Newtonian resistance Rn. (J) Dog allergen‐specific serum IgG1 and total serum IgE ELISA representative of 3 experiments (PBS sublingual, PBS i.n. n = 3, PBS sublingual, dog allergen extracts = 5, Can f 1‐2‐4‐6 sublingual, dog allergen extracts n = 4). Can f 1‐specific IgG1 (various dilutions) and IgE (1:3 dilution) was also measured (PBS sublingual, PBS i.n. n = 5, PBS sublingual, dog allergen extracts = 6, Can f 1‐2‐4‐6 sublingual, dog allergen extracts n = 7). (K) Number of neutrophils in the BAL (PBS sublingual, PBS i.n. n = 5, PBS sublingual, dog allergen extracts n = 5, Can f 1‐2‐4‐6 sublingual, dog allergen extracts n = 5). N.D. Denotes not determined. One‐way ANOVA with Bonferroni's post‐test was performed to adjust for multiple comparisons. *p < .05, p < .01, ***p < .001, ****p < .0001
We next tested whether Can f 1‐2‐4‐6 could reduce airway hyperresponsiveness in mice sensitized and challenged with dog allergen extracts. Mice administered Can f 1‐2‐4‐6 exhibited reduced overall airway resistance, elastance and Newtonian resistance compared with mice receiving sublingual PBS (Figure 7G–I). Mice administered sublingual recombinant Can f 1‐2‐4‐6 also exhibited much higher titres of dog allergen extract‐ and Can f 1‐specific IgG1 in serum, compared to mice that received sublingual PBS (Figure 7J). A significant reduction in total serum IgE was observed in mice administered sublingual Can f 1‐2‐4‐6 protein, although Can f 1‐specific IgE was not significantly reduced (Figure 7J). Moreover, mice administered Can f 1‐2‐4‐6 developed an even stronger infiltration of neutrophils into the airways (Figure 7K) compared with mice that had received PBS.
Taken together, this study demonstrates that whole dog allergen extracts induce a robust TH2/TH17‐associated inflammation of the airways where Can f 1 in particular induces robust T‐ and B‐cell responses. In addition, prophylactic administration of recombinant multimeric Can f 1‐2‐4‐6 reduced AHR and TH2 cell‐driven responses to dog allergen extracts but did not reduce TH17 cell‐driven neutrophilia.
4. DISCUSSION
This study demonstrates that dog allergen extracts induce airway hyperresponsiveness and inflammation marked by a mixed TH2/TH17 cell response, eosinophilia and neutrophilia in a mouse model of asthma. A similarly mixed TH cell profile in response to dog allergens was also recently observed in one other study. 41 A model using OVA in conjunction with Chlamydia muridarum infection demonstrated a strong TH1/TH17 response and airway neutrophilia, but did not result in high levels of airway eosinophilia. 42 Another model using HDM and β‐glucan reported high levels of both eosinophils and neutrophils in the BAL and a mixed TH2/TH17 response, demonstrating how fungal components can skew the immune response even without previous exposure. 18 Whether fungal components are present in the dog allergen extracts will need to be explored. We previously reported that dog allergen composition differed greatly in extracts from different vendors. 43 In an analysis of HDM extracts, the composition of allergens, proteolytic activity and endotoxin content differed substantially between vendors and impacted on the type of inflammation induced in mice. 44 Allergen composition also affects the ability of extracts to compromise epithelial barrier integrity. 45 The precise components responsible for the inflammatory response triggered by dog allergen extracts should be analysed in future experiments.
This model of airway inflammation induced by dog allergen extracts may more closely resemble severe types of asthma, in which patients have been shown to have higher levels of TH1/TH17 cells and increased airway neutrophilia. 6 Indeed, neutrophilia in response to dog allergen extract instillation appeared to be dependent on IL‐17. While some studies suggest that TH17 cells are resistant to corticosteroids and thus, can continue to promote neutrophilic inflammation in the face of corticosteroid treatment, 46 dexamethasone significantly dampened TH17 and gamma delta cell responses to dog allergen extracts, without greatly impairing the neutrophilic response. This could reflect the ability of corticosteroids to prolong the lifespan of neutrophils directly. 47
A central tenet of allergic models of airway inflammation and atopic asthma is the induction of IgE. Administration of dog allergen extracts induced total serum IgE levels similar to HDM, and serum IgE specific to Can f 1 was detected in mice administered dog allergen extracts. As observed previously, allergen‐specific IgE was detected at much lower levels than IgG1, a frequent observation in mouse models. 36 Depletion of IgG1 may be a solution that promotes the detection of IgE in more robust assays, although the magnitude of IgG1 responses makes this a difficult task.
In line with what we and others have shown previously, scRNA‐Seq can be used to profile diverse T helper cell responses. 17 scRNA‐Seq defined distinct cell clusters including TH2 and Treg cells, whose gene expression profiles largely matched previously published data from the HDM model. 17 Several clusters pinpointed in this analysis contrasted from our previous analysis of the HDM model. In particular, a cluster of TH17 cells, which was not elucidated in response to HDM was apparent in response to dog allergens. Many known and potentially novel regulators of TH17 cells were identified, which could serve as targets to block TH17 cell‐associated inflammation. The expression of these genes will need to be confirmed by analysis of TH17 cells in human asthma. One intriguing target may be the IL‐17C receptor, IL‐17RE. Both IL‐17C and IL‐17RE have been described to be expressed by epithelial cells, regulating the epithelial immune response in an autocrine manner. 48 An antibody binding to IL‐17C and thereby inhibiting receptor binding has been shown to ameliorate disease in mouse models of psoriasis and atopic dermatitis (AD). 49
The elucidation of a cell cluster that appeared to resemble Tfh cells was also in contrast to results from the HDM model. Although IL‐21‐producing cells in the lung tissue and airways have been shown to be a feature of HDM‐induced inflammation, 5 those cells did not appear to express canonical Tfh cell markers such as Bcl6 and Cxcr5. Given that the cells profiled herein come from the airways, this indicates that administration of dog allergen extracts may induce bronchus‐associated lymphoid tissue (BALT). 41 , 50 BALT formation may be due to presence of higher levels of endotoxin in dog allergen extracts. Since pets are known to contribute significantly to household endotoxin levels, 51 , 52 Tfh cells and BALT may be a feature of pet‐allergic asthmatics.
scRNA‐seq also highlighted the plasticity of T helper cells by showing co‐expression of key cytokines and/or Foxp3 between TH17, TH2, TH1 and Treg cells. This plasticity was also evident in the frequency of TCR clones shared between TH17, TH2 cells and Treg cells. TH2/TH17 dual positive cells have been found in BAL fluid of patients with severe asthma. 53 , 54 While the model herein model used whole allergen extracts as opposed to purified proteins, there was still a strong clonal response in T helper cells. This indicates that even when mice are exposed to whole extracts, T cells only react to a limited number of antigenic peptides. We noted that of the recombinant allergens tested in lymphocyte restimulation cultures, only Can f 1 appeared to induce cytokine production from T cells, but this was considerably less than that induced by whole dog allergen extracts. It is possible that non‐lipocalin allergens including Can f 5, Can f 7 or Can f 8 induce strong T and B cell responses in mice, but this remains to be tested.
A goal of this study was to create a model in which to test the efficacy of recombinant dog allergens to modulate allergic immune responses, since extract‐based immune therapies often contain varying levels of allergen content between different batches and suppliers. 43 Prophylactic administration of recombinant Can f 1‐2‐4‐6 reduced AHR and the TH2 cell response, but had little impact on the TH17 cell response, suggesting that this recombinant protein may not effectively target TH17 cell‐mediated neutrophilic inflammation. Whether this recombinant multimeric protein can suppress allergic inflammation when administered post‐sensitization will have to be assessed in subsequent studies.
In all, we present a model of airway inflammation with dog allergen extracts that is characterized by a mixed TH2/TH17 cell‐mediated airway inflammation and which may be useful in understanding adult‐onset asthma. We provide comprehensive gene and TCR profiling of T helper cells reacting to dog allergens in the airways and demonstrate that recombinant Can f family allergens have the capacity to reduce airway hyperresponsiveness and TH2 cytokine production.
AUTHOR CONTRIBUTIONS
Julian M. Stark, Anna Wintersand, Hans Grönlund, Guro Gafvelin, and Jonathan M. Coquet conceived the study. Julian M. Stark, Jielu Liu, Christopher A. Tibbitt, Junjie Ma, Anna Wintersand and Josefine Dunst conducted experiments. Julian M. Stark, Jielu Liu, Murray Christian, Ben Murrell, Anna Wintersand, Josefine Dunst and Taras Kreslavsky analysed data. Mikael Adner provided critical tools and expertise.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
This project was funded by the Swedish Research Council and the Cancer Foundation. The authors would like to acknowledge Gunilla Karlsson Hedestam, Egon Urgard and the Morphological Phenotype Analysis (FENO) Unit for support with infrastructure and experimentation.
Stark JM, Liu J, Tibbitt CA, et al. Recombinant multimeric dog allergen prevents airway hyperresponsiveness in a model of asthma marked by vigorous TH2 and TH17 cell responses. Allergy. 2022;77:2987‐3001. doi: 10.1111/all.15399
REFERENCES
- 1. Disease GBD, Injury I, Prevalence C . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the global burden of Disease study 2016. Lancet. 2017;390(10100):1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lundback B, Backman H, Lotvall J, Ronmark E. Is asthma prevalence still increasing? Expert Rev Respir Med. 2016;10(1):39‐51. [DOI] [PubMed] [Google Scholar]
- 3. Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20(1):4‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 5. Coquet JM, Schuijs MJ, Smyth MJ, et al. Interleukin‐21‐producing CD4(+) T cells promote type 2 immunity to house dust mites. Immunity. 2015;43(2):318‐330. [DOI] [PubMed] [Google Scholar]
- 6. Domvri K, Tzimagiorgis G, Papakosta D. The Th2/Th17 pathway in asthma and the relevant clinical significance. Pneumonologie. 2018;31(3):174‐182. [Google Scholar]
- 7. Akdis CA, Akdis M. Mechanisms of allergen‐specific immunotherapy. J Allergy Clin Immunol. 2011;127(1):18‐27. [DOI] [PubMed] [Google Scholar]
- 8. Sahin E, Dizdar D, Dinc M, Cirik A. Long‐term effects of allergen‐specific subcutaneous immunotherapy for house dust mite induced allergic rhinitis. J Laryngol Otol. 2017;131(11):997‐1001. [DOI] [PubMed] [Google Scholar]
- 9. Tulaeva I, Kratzer B, Campana R, et al. Preventive allergen‐specific vaccination against allergy: mission possible? Front Immunol. 2020;11:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valenta R, Campana R, Marth K, van Hage M. Allergen‐specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272(2):144‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haspeslagh E, Vanheerswynghels M, Deswarte K, et al. Prophylactic allergen immunotherapy with Der p 2 prevents murine asthma by regulating lung GM‐CSF. J Allergy Clin Immunol. 2019;143(6):2307‐2311 e2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton OT, Stranks AJ, Tamayo JM, Koleoglou KJ, Schwartz LB, Oettgen HC. A humanized mouse model of anaphylactic peanut allergy. J Allergy Clin Immunol. 2017;139(1):314‐322 e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cates EC, Fattouh R, Wattie J, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM‐CSF‐mediated mechanism. J Immunol. 2004;173(10):6384‐6392. [DOI] [PubMed] [Google Scholar]
- 14. Havaux X, Zeine A, Dits A, Denis O. A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum molds. Clin Exp Immunol. 2005;139(2):179‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neimert‐Andersson T, Thunberg S, Swedin L, et al. Carbohydrate‐based particles reduce allergic inflammation in a mouse model for cat allergy. Allergy. 2008;63(5):518‐526. [DOI] [PubMed] [Google Scholar]
- 16. Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003;168(8):959‐967. [DOI] [PubMed] [Google Scholar]
- 17. Tibbitt CA, Stark JM, Martens L, et al. Single‐cell RNA sequencing of the T helper cell response to house dust mites defines a distinct gene expression signature in airway Th2 cells. Immunity. 2019;51(1):169‐184 e165. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z, Myers JMB, Brandt EB, et al. β‐Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid‐resistant TH2/TH17 responses. J Allergy Clin Immunol 2017;139(1):54–65. e58, 65.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. Jama. 2002;288(8):963‐972. [DOI] [PubMed] [Google Scholar]
- 20. Polovic N, Waden K, Binnmyr J, et al. Dog saliva ‐ an important source of dog allergens. Allergy. 2013;68(5):585‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konieczny A, Morgenstern J, Bizinkauskas C, et al. The major dog allergens, can f 1 and can f 2, are salivary lipocalin proteins: cloning and immunological characterization of the recombinant forms. Immunology. 1997;92(4):577‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rytkönen‐Nissinen M, Saarelainen S, Randell J, Häyrinen J, Kalkkinen N, Virtanen T. IgE reactivity of the dog lipocalin allergen can f 4 and the development of a sandwich ELISA for its quantification. Allergy Asthma Immunol Res. 2015;7(4):384‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mattsson L, Lundgren T, Olsson P, Sundberg M, Lidholm J. Molecular and immunological characterization of can f 4: a dog dander allergen cross‐reactive with a 23 kDa odorant‐binding protein in cow dander. Clin Exp Allergy. 2010;40(8):1276‐1287. [DOI] [PubMed] [Google Scholar]
- 24. Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol 2009;123(2):362–368. e363, 368.e3. [DOI] [PubMed] [Google Scholar]
- 25. Nilsson O, Binnmyr J, Zoltowska A, Saarne T, Van Hage M, Grönlund H. Characterization of the dog lipocalin allergen C an f 6: the role in cross‐reactivity with cat and horse. Allergy. 2012;67(6):751‐757. [DOI] [PubMed] [Google Scholar]
- 26. Uriarte S, Sastre J. Clinical relevance of molecular diagnosis in pet allergy. Allergy. 2016;71(7):1066‐1068. [DOI] [PubMed] [Google Scholar]
- 27. Klaver D, Posch B, Geisler A, Hermann M, Reider N, Heufler C. Peptides from allergenic lipocalins bind to formyl peptide receptor 3 in human dendritic cells to mediate TH2 immunity. J Allergy Clin Immunol. 2020;145(2):654‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nilsson OB, Neimert‐Andersson T, Bronge M, et al. Designing a multimer allergen for diagnosis and immunotherapy of dog allergic patients. PLoS One. 2014;9(10):e111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tibbitt C, Coquet JM. House dust mite extract and cytokine instillation of mouse airways and subsequent cellular analysis. Bio‐Protocol. 2016;6(14):e1875. [Google Scholar]
- 30. Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single‐cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single‐cell data. Cell. 2019;177(7):1888‐1902.e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen T, Tibbitt CA, Feng X, et al. PPAR‐gamma promotes type 2 immune responses in allergy and nematode infection. Sci Immunol. 2017;2(9):eaal5196. [DOI] [PubMed] [Google Scholar]
- 33. Caucheteux SM, Hu‐Li J, Mohammed R, Ager A, Paul WE. Cytokine regulation of lung Th17 response to airway immunization using LPS adjuvant. Mucosal Immunol. 2017;10(2):361‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Starkhammar M, Georen SK, Swedin L, Dahlen S‐E, Adner M, Cardell LO. Intranasal administration of poly (I: C) and LPS in BALB/c mice induces airway hyperresponsiveness and inflammation via different pathways. PloS One. 2012;7(2):e32110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao S, Jiang Y, Yang X, et al. Lipopolysaccharides promote a shift from Th2‐derived airway eosinophilic inflammation to Th17‐derived neutrophilic inflammation in an ovalbumin‐sensitized murine asthma model. JAsthma. 2017;54(5):447‐455. [DOI] [PubMed] [Google Scholar]
- 36. Lehrer S, Reish R, Fernandes J, Gaudry P, Dai G, Reese G. Enhancement of murine IgE antibody detection by IgG removal. J Immunol Methods. 2004;284(1–2):1‐6. [DOI] [PubMed] [Google Scholar]
- 37. Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin‐17C promotes Th17 cell responses and autoimmune disease via interleukin‐17 receptor E. Immunity. 2011;35(4):611‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma J, Tibbitt CA, Georen SK, et al. Single‐cell analysis pinpoints distinct populations of cytotoxic CD4(+) T cells and an IL‐10(+)CD109(+) TH2 cell population in nasal polyps. Sci Immunol. 2021;6(62):eabg6356. [DOI] [PubMed] [Google Scholar]
- 39. Smith DM, Coop CA. Dog allergen immunotherapy: past, present, and future. Ann Allergy Asthma Immunol. 2016;116(3):188‐193. [DOI] [PubMed] [Google Scholar]
- 40. Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen‐specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66(6):775‐783. [DOI] [PubMed] [Google Scholar]
- 41. Boute M, Ait Yahia S, Nanou J, et al. Direct activation of the aryl hydrocarbon receptor by dog allergen participates in airway neutrophilic inflammation. Allergy. 2021;76:2245‐2249. [DOI] [PubMed] [Google Scholar]
- 42. Horvat JC, Starkey MR, Kim RY, et al. Chlamydial respiratory infection during allergen sensitization drives neutrophilic allergic airways disease. J Immunol. 2010;184(8):4159‐4169. [DOI] [PubMed] [Google Scholar]
- 43. Wintersand A, Asplund K, Binnmyr J, et al. Allergens in dog extracts: implication for diagnosis and treatment. Allergy. 2019;74(8):1472‐1479. [DOI] [PubMed] [Google Scholar]
- 44. Post S, Nawijn MC, Hackett TL, et al. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax. 2012;67(6):488‐495. [DOI] [PubMed] [Google Scholar]
- 45. Steelant B, Farre R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite‐induced allergic rhinitis is accompanied by decreased occludin and zonula occludens‐1 expression. J Allergy Clin Immunol. 2016;137(4):1043‐1053 e1045. [DOI] [PubMed] [Google Scholar]
- 46. Banuelos J, Lu NZ. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. 2016;31:27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ronchetti S, Ricci E, Migliorati G, Gentili M, Riccardi C. How glucocorticoids affect the neutrophil life. Int J Mol Sci. 2018;19(12):4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramirez‐Carrozzi V, Sambandam A, Luis E, et al. IL‐17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159‐1166. [DOI] [PubMed] [Google Scholar]
- 49. Vandeghinste N, Klattig J, Jagerschmidt C, et al. Neutralization of IL‐17C reduces skin inflammation in mouse models of psoriasis and atopic dermatitis. J Investig Dermatol. 2018;138(7):1555‐1563. [DOI] [PubMed] [Google Scholar]
- 50. Hwang JY, Randall TD, Silva‐Sanchez A. Inducible bronchus‐associated lymphoid tissue: taming inflammation in the lung. Front Immunol. 2016;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park J‐H, Spiegelman DL, Gold DR, Burge HA, Milton DK. Predictors of airborne endotoxin in the home. Environ Health Perspect. 2001;109(8):859‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mendy A, Wilkerson J, Salo PM, Cohn RD, Zeldin DC, Thorne PS. Exposure and sensitization to pets modify endotoxin association with asthma and wheeze. J Allergy Clin Immunol Pract. 2018;6(6):2006‐2013 e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R Increased frequency of dual‐positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 2014;134(5):1175–1186. e1177, 1186.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y‐H, Voo KS, Liu B, et al. A novel subset of CD4+ TH2 memory/effector cells that produce inflammatory IL‐17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


