Abstract
Background
Biologics are the cornerstone of treatment of patients with moderate‐to‐severe plaque psoriasis and switches between biologics are frequently needed to maintain clinical improvement over time.
Objectives
The main purpose of this study was to describe precisely switches between biologics and how their pattern changed over time with the recent availability of new biologic agents.
Methods
We included patients receiving a first biologic agent in the Psobioteq multicenter cohort of adults with moderate‐to‐severe psoriasis receiving systemic treatment. We described switches between biologics with chronograms, Sankey and Sunburst diagrams, assessed cumulative incidence of first switch by competing risks survival analysis and reasons for switching. We assessed the factors associated with the type of switch (intra‐class – i.e. within the same therapeutic class ‐ vs. inter‐class) in patients switching from a TNF‐alpha inhibitor using multivariate logistic regression.
Results
A total of 2153 patients was included. The cumulative incidence of switches from first biologic was 34% at 3 years. Adalimumab and ustekinumab were the most prescribed biologic agents as first and second lines of treatment. The main reason for switching was loss of efficacy (72%), followed by adverse events (11%). Patients receiving a TNF‐alpha inhibitor before 2016 mostly switched to ustekinumab, whereas those switching in 2016 or after mostly switched to an IL‐17 inhibitor. Patients switching from a first‐line TNF‐alpha inhibitor before 2016 were more likely to switch to another TNF‐alpha inhibitor compared with patients switching since 2018. Patients switching from etanercept were more likely to receive another TNF‐alpha inhibitor rather than another therapeutic class of bDMARD compared with patients switching from adalimumab.
Conclusion
This study described the switching patterns of biologic treatments and showed how they changed over time, due to the availability of the new biologic agents primarily IL‐17 inhibitors.
Introduction
Psoriasis is a chronic inflammatory disease that affects 2%–4% of the population in Western Europe. 1 , 2 Patients with moderate to severe forms have an impaired quality of life and usually require the use of systemic treatments such as phototherapy, conventional treatments (methotrexate, cyclosporine, acitretin), biological agents and apremilast. 3
Biological agents are recommended in the event of failure of other systemic treatments. 4 Different therapeutic classes of biologics are available in France since the 2000s. Most common prescribed biologics include tumour necrosis factor (TNF)‐alpha inhibitors (adalimumab, infliximab, etanercept and certolizumab), interleukin (IL) 12–23 inhibitor (ustekinumab) and, more recently, IL‐17 (secukinumab, ixekizumab and brodalumab) and IL‐23 inhibitors (guselkumab and risankizumab).
Drug changes, called switches, are frequent among patients with moderate‐to‐severe psoriasis. 5 , 6 , 7 , 8 , 9 , 10 Several studies have assessed the factors associated with the switch of biologics. 5 , 11 However, we have little data on the switching patterns of biological agents and how they were affected by the recent commercialization of IL‐17 and IL‐23 inhibitors.
Moreover, switches in published studies are often described in tables which is not appropriate in the presence of many different alternatives while graphical methods of representation of flows could allow a better visualization of biological switches. 12 , 13
The primary objective of our study was to describe, using graphical representations, the patterns of biologic switches in patients with moderate to severe psoriasis and how they changed over time.
Material and methods
Psobioteq cohort
Psobioteq is a prospective multicenter French psoriasis cohort that enrols adults diagnosed with moderate‐to‐severe psoriasis receiving systemic treatment from 41 French dermatology centers since July 2012. Sociodemographic data, characteristics of psoriasis and treatments are collected using a case report form during routine clinical assessments. Given the real‐life setting of the cohort, patient's care and choices of treatment are not influenced by the participation in Psobioteq. 14 , 15
The study protocol was initially approved by the « Comité d'Evaluation de l'Ethique des projets de Recherche Biomédicale (CEERB) du GHU Nord » (authorization n° JMD/MDM/177–11) and was approved as non‐interventional research involving human subjects in January 2021. Clinical Trials.gov Identifier is NCT01617018. Written informed consent was obtained from all patients before inclusion in the cohort.
Population and data collection
Patients from the Psobioteq cohort were included in this study if they were naïve of any biologic when entering the cohort and started a biologic before 31 December, 2019. Patients enrolled in a clinical trial evaluating biologics before starting their first biological agent were not eligible. Patients were followed from the initiation date of their first biologic to 1 January, 2020.
Biological treatments
All biologics available in France at the time of the study were considered. The first biologics commercialized in France for psoriasis were the TNF‐alpha inhibitors etanercept and infliximab in 2005, followed by adalimumab in 2008, then IL‐12‐23 inhibitor ustekinumab in 2010. IL‐17 inhibitors secukinumab and ixekizumab arrived on the French market in 2016 and brodalumab in 2018. More recent biologics, IL‐23 inhibitor guselkumab and TNF‐alpha inhibitor certolizumab are available for psoriasis treatment since 2019 and IL‐23 inhibitor risankizumab since 2020.
Definitions
Discontinuation of biologic was defined as an interruption of the biological agent for 180 days or more. Any interruption of less than 180 days with rechallenge of the same biological agent was not considered a discontinuation because it may have been due to a temporary suspension or administrative issues.
A switch was defined as a change of treatment with less than 180 days between the two treatments. Because psoriasis often progresses through phases of relapses and remissions, 16 this gap avoids considering as a switch the initiation of a biologic after a period of remission, for whom the factors associated with the choice of biologic would differ. The switch date was defined as the starting date of the new treatment.
Reasons for drug discontinuation were analysed as reported by the investigator: lack of efficacy, adverse events, lack of adherence, occurrence of a contraindication, patient decision, non‐medical reason or clearance.
Statistical analysis
All patients included in this study were analysed for the primary objective. Patients were censored at the date of participation in a clinical trial evaluating biologics as treatment choices can be driven by the experimental nature of the trials.
Different graphical representations were explored to describe biological switches: Sankey diagrams, Sunburst diagrams and chronograms.
Sankey diagrams represent flows between successive steps and their frequency. 12 , 13 The first four lines of biological therapy where considered. We represented switches between biologics, switches from a biologic to a systemic conventional treatment and discontinuations of biologics.
To address the influence of the recent availability of IL‐17 and IL‐23 inhibitors on the switches modalities, we represented (1) switches occurring within the 2012–2015 and 2016–2020 periods in the subset of patients initiating a first biologic within the period and (2) first biologic switches occurring within the 2012–2015, 2016–2017 and 2018–2020 periods in the subset of patients switching their first biological agent within the period. We also represented switches occurring after less than a year of treatment and those occurring after a year or more to visualize the influence of the duration of treatment on the switching patterns.
Sunburst diagrams display successive steps within each patient in a circular representation and allow visualizing therapeutic sequences at an individual level. 13 The first three lines of biological therapy where considered. We also represented treatment sequences occurring within the 2012–2015 and 2016–2020 periods in the subset of patients initiating a first biotherapy within the period.
Chronograms were used to represent the evolution of the proportion of patients on a particular biologic agent over time. Two chronograms, representing the proportion of first‐line biologics and of second‐line biologics over the study period, were made.
We estimated the cumulative incidence of first switch between biologics by competing risks survival analysis with a non‐parametric estimation function. The competing events were biologic discontinuation and inclusion in a clinical trial for psoriasis.
The reasons for switching were reported using number and percentages, for the first switch and for all switches in the study cohort globally and distinguishing intra‐class and inter‐class switches.
All patients receiving a TNF‐alpha inhibitor as a first biologic and switching to another biologic were included in the analysis of factors associated with the choice of intra‐class vs. inter‐class switches. We restricted our analysis to patients receiving TNF‐alpha inhibitors since they are by far the most prescribed first‐line biologic class with IL 12–23i, for whom an intra‐class switch is impossible (only one drug available in this therapeutic class).
Factors associated with the choice of intra‐class vs. inter‐class switches were identified by univariate analysis followed by multivariate logistic regression with forward stepwise selection based on likelihood ratio test. We considered the following potential factors: sociodemographic characteristics, clinical features of psoriasis, treatment characteristics, calendar period of switch, reasons for switching and comorbidities. Costs of therapies were not considered as a potential factor associated with the type of switch as biologics are totally reimbursed by the French Health Insurance when prescribed for patients with severe psoriasis with at least two treatment failures of systemic agents.
Statistical analyses were performed using R v3.6.1 considering p values <0.05 statistically significant (two‐tailed test). We did not impute missing data.
Results
Study population
From July 2012 to 31 December, 2019, 2153 biologic naïve patients were included in the Psobioteq cohort and received a first biological agent (Fig. 1). Tables 1 and 2 describe the characteristics of all patients at biologic initiation and of the 499 patients switching from a TNF‐alpha inhibitor at the time of the first switch, respectively.
Figure 1.
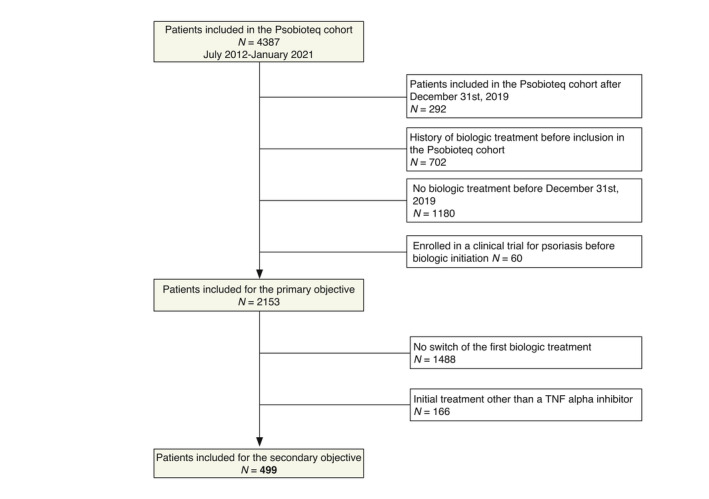
Flow chart of the study population. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 1.
Characteristics of the 2153 patients at the initiation of the biologic
| Patients' characteristics | ||
| Median age, years (n = 2153) | 46.0 (35.0–57.0) | |
| Female sex (n = 2153) | 850 (39.5) | |
| Obesity (BMI ≥ 30 kg/m2) (n = 1914) | 554 (28.9) | |
| Alcohol consumption (n = 1476) | 1004 (68.0) | |
| Liver disease (n = 2120) | 43 (2.0) | |
| Psoriasis | ||
| Median disease duration, years (n = 1960) | 15.0 (7.0–25.0) | |
| Median PASI score (n = 1622) | 11.4 (7.1–13.3) | |
| Median DLQI score (n = 1273) | 10.0 (5.0–16.0) | |
| Psoriasis clinical form (n = 2113) | ||
| Plaque psoriasis | 1905 (90.2) | |
| Guttate | 435 (20.6) | |
| Erythrodermic | 45 (2.1) | |
| Onychopathy | 168 (8.0) | |
| Localized pustular | 83 (3.9) | |
| Generalized pustular | 9 (0.4) | |
| Treatments | ||
| Concomitant treatment with methotrexate (n = 2153) | 72 (3.3) | |
| First biologic (n = 2153) | ||
| TNF‐alpha inhibitors | Adalimumab | 832 (38.6) |
| Etanercept | 340 (15.8) | |
| Infliximab | 44 (2.0) | |
| Certolizumab | 6 (0.3) | |
| IL‐12 23 inhibitor | Ustekinumab | 732 (34.0) |
| IL‐17 inhibitors | Secukinumab | 78 (3.6) |
| Ixekizumab | 63 (2.9) | |
| Brodalumab | 13 (0.6) | |
| IL‐23 inhibitor | Guselkumab | 45 (2.1) |
Median and interquartile range for continuous variables, n (%) for categorical variables
DLQI, Disease Quality of Life Index; PASI, Psoriasis Activity Skin Index.
Table 2.
Major characteristics of the 499 patients switching from a TNF‐ alpha inhibitor (first biologic agent) to another biologic at the time of the switch
| Total (n = 499) | Patients switching from: | ||||
|---|---|---|---|---|---|
| Adalimumab (n = 305, 61.1%) | Etanercept (n = 172, 34.5%) | Infliximab (n = 19, 3.8%) | Certolizumab (n = 3, 0.6%) | ||
| Patients' characteristics | |||||
| Median age, years (n = 499) | 49.0 (37.0–58.0) | 47.0 (35.0–56.0) | 53.0 (41.0–62.0) | 50.0 (41.0–62.0) | 34.0 (31.5–35.0) |
| Female sex (n = 499) | 220 (49.0) | 140 (45.9) | 70 (40.7) | 7 (36.8) | 3 (100.0) |
| Obesity (BMI ≥30 kg/m2) (n = 453) | 167 (36.9) | 98 (35.3) | 59 (38.1) | 10 (58.8) | 0.0 (0.0) |
| Alcohol consumption (n = 386) | 241 (62.4) | 140 (62.8) | 93 (62.4) | 7 (53.9) | 1 (100.0) |
| Liver disease (n = 491) | 17 (3.5) | 4 (1.3) | 12 (7.1) | 1 (5.3) | 0 (0.0) |
| Psoriasis | |||||
| Median disease duration, years (n = 499) | 18.0 (9.9–27.3) | 16.1 (9.0–25.7) | 21.4 (11.5–29.5) | 18.7 (8.4–32.4) | 17.6 (13.7–18.2) |
| Median PASI score (n = 283) | 8.2 (4.7–13.4) | 9.2 (5.2–15.0) | 7.4 (4.4–12.2) | 4.9 (1.0–9.6) | 9.9 (9.9–9.9) |
| Median DLQI score (n = 159) | 8.0 (4.0–16.0) | 9.5 (5.0–17.0) | 7.5 (4.0–12.8) | 2.0 (1.0–2.5) | NA |
| Psoriasis clinical form (n = 495) | |||||
| Plaque psoriasis | 446 (98.0) | 270 (89.4) | 158 (92.4) | 16 (84.2) | 2 (66.7) |
| Guttate | 96 (19.4) | 60 (19.9) | 28 (16.4) | 7 (36.8) | 1 (33.3) |
| Erythrodermic | 15 (3.0) | 8 (2.7) | 6 (3.5) | 1 (5.3) | 0 (0.0) |
| Onychopathy | 22 (4.4) | 17 (5.6) | 3 (1.8) | 2 (10.5) | 0 (0.0) |
| Localized pustular | 22 (4.4) | 16 (5.3) | 3 (1.8) | 3 (15.8) | 0 (0.0) |
| Generalized pustular | 2 (0.4) | 1 (0.3) | 1 (0.6) | 0.0 (0.0) | 0 (0.0) |
| Treatment | |||||
| Concomitant treatment with methotrexate (n = 499) | 23 (4.6) | 11 (3.6) | 9 (5.2) | 3 (15.8) | 0 (0.0) |
Median and interquartile range for continuous variables, n (%) for categorical variables.
DLQI, Disease Quality of Life Index; PASI, Psoriasis Activity Skin Index.
Description of biologic switches
Patients initiating a first biologic were 39.5% female, and the median age was 46.0 (IQR [35.0–56.0]). The median duration of psoriasis was 15 years (IQR [7.0–25.0]). The most prescribed first biologic treatment was adalimumab (n = 832, 38.6%) followed by ustekinumab (n = 732 patients, 34.0%). The majority of patients had plaque psoriasis (n = 1905, 90.2%).
Sankey diagrams
Biologic switches are represented with a Sankey diagram in Fig. 2. Adalimumab and ustekinumab were the most prescribed biologics as first and second lines. IL‐23 and IL‐17 inhibitors were also frequently prescribed as second lines and were predominant as third lines. For the first switch, patients switching from adalimumab mostly switched to another drug class such as IL 12–23i or IL‐17i, whereas patients switching from etanercept mostly switched to another TNF‐alpha inhibitor, mainly adalimumab. A proportion of 14.5% of patients discontinued their first biologic without switching; 7.4% switched to a conventional systemic therapy (half of them because of occurrence of adverse events or a contraindication).
Figure 2.
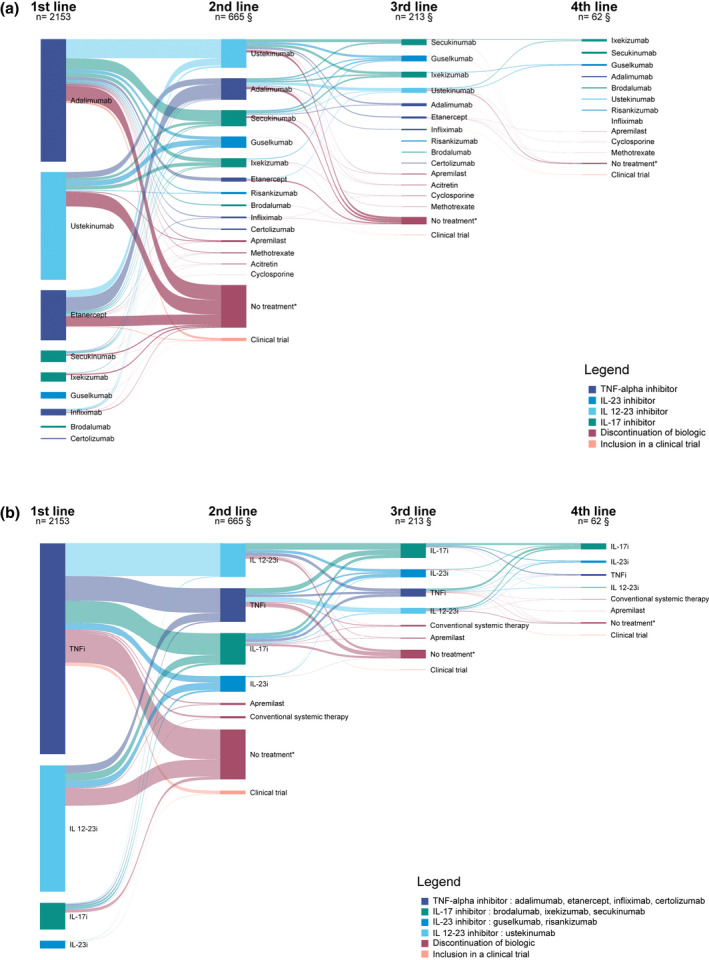
(a) Sankey diagram of switches from a biologic and treatment discontinuation. *No other systemic treatment for 180 days or more. §Number of patients having switched to a biologic. Does not include patients who discontinued their biologic or participated in a clinical trial. Only switches occurring at a frequency of three or more were represented to better visualize the most common switches. Each column represents a biological line of treatment. Treatments are ordered according to frequency, the uppermost biologic in each line of treatment being the most frequent. Following lines of treatment once patients had discontinued their treatment or had been included in a clinical trial were not represented. Tables providing corresponding numbers and percentages are available in the supplementary material (Tables S1–S3). (b) Sankey diagram of switches from a biologic and treatment discontinuation by biologic drug class. *No other systemic treatment for 180 days or more. §Number of patients having switched to a biologic. Does not include patients who discontinued their biologic or participated in a clinical trial. Treatments are ordered according to frequency, the uppermost biologic in each line of treatment being the most frequent. Following lines of treatment once patients had discontinued their treatment or had been included in a clinical trial were not represented. [Colour figure can be viewed at wileyonlinelibrary.com]
Biologic switches occurring before 2016 and those occurring since 2016 are represented in Fig. 3a,b, respectively. TNF‐alpha inhibitors represented the most prescribed first‐line biologic class in those two periods, but a larger proportion of patients received IL‐12‐23 inhibitor as their first biologic after 2016. Furthermore, IL‐17 inhibitors became the third more frequent initial biologic prescribed after 2016 and the most frequently prescribed biologic drug class in second line after 2016 instead of TNF‐alpha and IL 12–23 inhibitors before 2016. Patients switching from a TNF‐alpha inhibitor before 2016 mostly switched to an IL‐12‐23 inhibitor, whereas those switching in 2016 or after mostly switched to an IL‐17 inhibitor. Among patients switching from a TNF‐alpha inhibitor, the proportion of patients receiving another TNF‐alpha inhibitor was 47.4% before 2016 compared with 21.4% from 2016 onwards.
Figure 3.
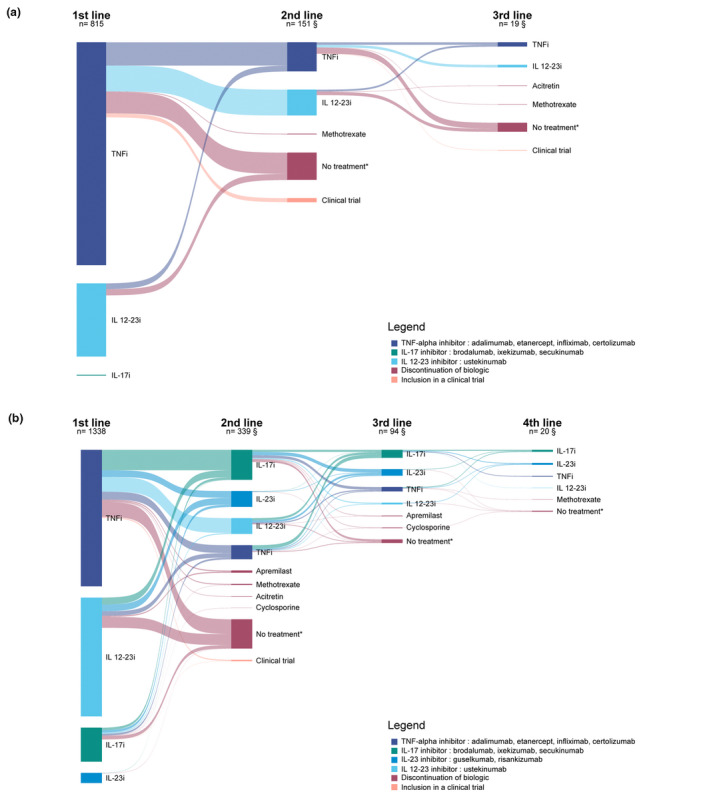
(a) Sankey diagram of switches from a biologic and treatment discontinuation from 2012 to 2015. *No other systemic treatment for 180 days or more. §Number of patients having switched to a biologic. Does not include patients who discontinued their biologic or participated in a clinical trial. Treatments are ordered according to frequency, the uppermost biologic in each line of treatment being the most frequent. Following lines of treatment once patients had discontinued their treatment or had been included in a clinical trial were not represented. (b) Sankey diagram of switches from a biologic and treatment discontinuation from 2016 to 2020. *No other systemic treatment for 180 days or more. §Number of patients having switched to a biologic. Does not include patients who discontinued their biologic or participated in a clinical trial. [Colour figure can be viewed at wileyonlinelibrary.com]
Sankey diagrams of the first and second lines of biologics in patients switching their biologic stratified by period of switch are available in Fig. S1. Fig. S2 represents biologic switches stratified by duration of first treatment. We did not identify major differences in switching patterns between patients switching after less than a year of first biologic treatment and those switching after a year or more.
Sunburst diagrams
Sunburst diagrams of the first three lines of treatment are represented in Fig. 4a,b. Fig. 4a shows that the therapeutic sequences are very diverse. However, some switching sequences appear more frequently. Most patients who switched from etanercept to adalimumab and who required a second switch received ustekinumab. Patients who switched from adalimumab to ustekinumab mostly received an IL‐17 inhibitors (secukinumab or ixekizumab) or guselkumab as a third‐line biologic. Fig. 4b represents therapeutic sequences of biologic drug classes. Patients switched from an IL 12–23i as a first‐line agent to an IL‐17, IL‐23 or TNF‐alpha inhibitors in similar proportions.
Figure 4.
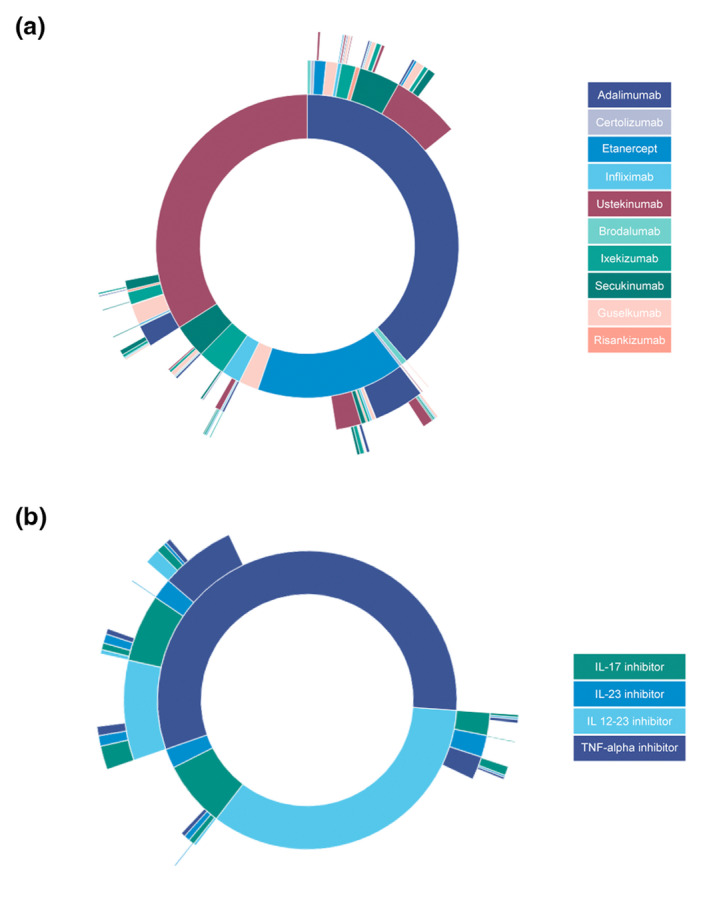
(a) Sunburst diagram of biologic switches by biologic agent. Each circle represents a line of treatment, the inner circle being the first line. (b) Sunburst diagram of biologic switches by biologic drug class. Each circle represents a line of treatment, the inner circle being the first line. [Colour figure can be viewed at wileyonlinelibrary.com]
Sunburst diagrams of the treatment sequences stratified by period of switch are available in Fig. S3.
Chronograms
Chronograms representing the evolution of the proportion of patients on biologic as first and second line are shown in Fig. 5a,b, respectively. These figures further assess the temporal evolution of the prescription of biologics. The proportion of patients receiving etanercept as a first‐line agent greatly decreased over time (Fig. 5a). Furthermore, the proportion of patients receiving secukinumab and ixekixumab increase notably after 2018. There is also a significant evolution of the distribution of second‐line biologics as represented in Fig. 5b. The proportion of patients receiving adalimumab and ustekinumab notably decreased related to the increase of prescription of secukinumab and ixekizumab since 2016 and prescription of guselkumab since 2019.
Figure 5.
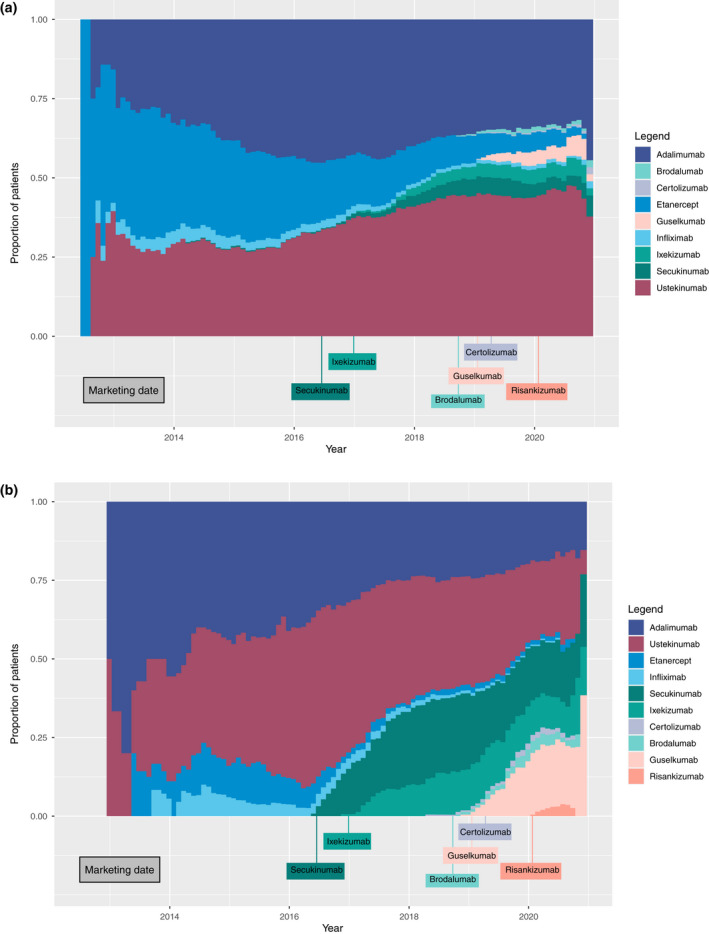
(a) Chronogram of the distribution of first‐line biologics over the study period (July 2012–December 2020). (b) Chronogram of the distribution of second‐line biologics over the study period (July 2012–December 2020). [Colour figure can be viewed at wileyonlinelibrary.com]
Cumulative incidence of switch
Cumulative incidence of first biologic switch is represented in Fig. 6. Cumulative incidence of first biologic switch was 16.2% (95% CI 14.6–17.9) 1 year after the biologic initiation and 33.9% (95% CI 31.6–36.3) 3 years after the initiation.
Figure 6.
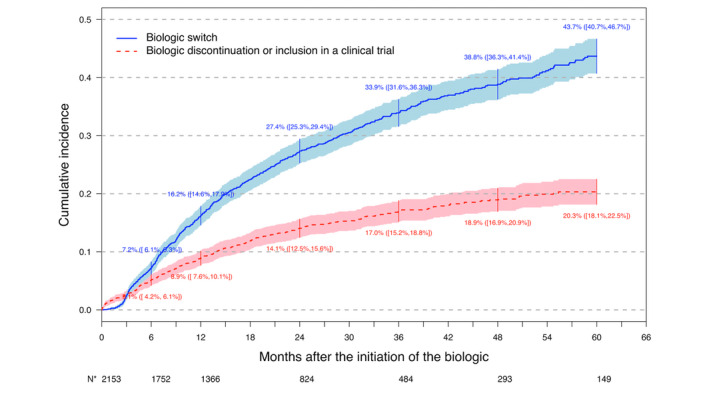
Cumulative incidence of first biologic switch with competing risks of biologic discontinuation or inclusion in a clinical trial. *Number of patients free from any event. [Colour figure can be viewed at wileyonlinelibrary.com]
Reasons for switching
Table 3 describes the reasons for switching for all switches that occurred during the follow‐up. Out of the 1028 switches, 740 (72.0%) were due to inefficacy and 111 (10.8%) to the presence of adverse events. Adverse events lead to an inter‐class switch for 73.0% of switches. Occurrence of a contraindication lead to an inter‐class switch for 83.6% of switches. Similar results were obtained for the reasons of first biologic switches as described in Table S4.
Table 3.
Reasons for switching for all biologic switches the study cohort
| Loss of efficacy | Adverse events | Occurrence of a contraindication | Patient decision | Non medical reason | Clearance | Loss of adherence | Unknown | Total (nb of switches) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (%)* | 740 (72.0%) | 111 (10.8%) | 55 (5.4%) | 36 (3.5%) | 11 (1.1%) | 14 (1.4%) | 8 (0.8%) | 121 (11.8%) | 1028 | |
| Intra‐class switch | 151 (20.4%) | 30 (27.0%) | 9 (16.4%) | 7 (19.4%) | 3 (27.3%) | 1 (7.1%) | 1 (12.5%) | 21 (17.4%) | 211 (20.5%) | |
| TNFi | 120 (79.5%) | 20 (66.7%) | 7 (77.8%) | 6 (85.7%) | 3 (100.0%) | 1 (100.0%) | 1 (100.0%) | 12 (57.1%) | 161 | |
| IL‐17i | 28 (18.5%) | 9 (30.0%) | 2 (22.2%) | 1 (14.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 9 (42.9%) | 46 | |
| IL‐23i | 3 (2.0%) | 1 (3.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 | |
| Inter‐class switch | 589 (79.6%) | 81 (73.0%) | 46 (83.6%) | 29 (80.6%) | 8 (72.7%) | 13 (92.9%) | 7 (87.5%) | 100 (82.6%) | 817 (79.5%) | |
| TNFi to | IL‐17i | 152 (25.8%) | 14 (17.3%) | 4 (8.7%) | 5 (17.2%) | 0 (0.0%) | 1 (14.3%) | 2 (15.4%) | 16 (16.0%) | 184 |
| IL‐12 23i | 154 (26.2%) | 27 (33.3%) | 14 (30.4%) | 10 (34.5%) | 4 (50.0%) | 4 (57.1%) | 1 (7.7%) | 30 (30.0%) | 230 | |
| IL‐23i | 40 (6.8%) | 5 (6.2%) | 3 (6.5%) | 2 (6.9%) | 0 (0.0%) | 1 (14.3%) | 4 (30.8%) | 7 (7.0%) | 58 | |
| IL‐17i to | TNFi | 24 (4.1%) | 3 (3.7%) | 4 (8.7%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 1 (7.7%) | 5 (5.0%) | 36 |
| IL‐12 23i | 10 (1.7%) | 3 (3.7%) | 5 (10.9%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 17 | |
| IL‐23i | 40 (6.8%) | 9 (11.1%) | 3 (6.5%) | 4 (13.8%) | 0 (0.0%) | 0 (0.0%) | 1 (7.7%) | 4 (4.0%) | 50 | |
| IL‐23i to | TNFi | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 |
| IL‐17i | 6 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (2.0%) | 8 | |
| IL‐12 23i | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 | |
| IL12‐23i to | TNFi | 41 (7.0%) | 7 (8.6%) | 7 (15.2%) | 2 (6.9%) | 1 (12.5%) | 0 (0.0%) | 2 (15.4%) | 9 (9.0%) | 65 |
| IL‐17i | 74 (12.6%) | 9 (11.1%) | 5 (10.9%) | 1 (3.5%) | 1 (12.5%) | 1 (14.3%) | 0 (0.0%) | 15 (15.0%) | 99 | |
| IL‐23i | 47 (8.0%) | 4 (4.9%) | 1 (2.2%) | 3 (10.3%) | 2 (25.0%) | 0 (0.0%) | 2 (15.4%) | 12 (12.0%) | 69 | |
Total not equal to 100% since patients could have more than one reason for switching.
Factors associated with the type of biologic switch (intra‐class or inter‐class) in patients treated with a first‐line TNF‐alpha inhibitor
Patients switching their first TNF‐alpha inhibitor in the study cohort were 49% female and their median age was 49.0 (37.0–58.0) at the time of the switch (Table 2). The median duration of disease was 18 years (IQR [9.9–27.3]). Most prescribed initial treatment before switching was adalimumab (n = 305, 61.1%), followed by etanercept (n = 172, 34.5%). Almost all patients had plaque psoriasis (n = 466, 98.0%).
Results of the univariate and multivariate analysis are presented in Tables S5 and S6, respectively. In the multivariate analysis, patients switching from a first‐line TNF‐alpha inhibitor to another biologic before 2016 were more likely to have an intra‐class switch compared with patients switching since 2018, with an adjusted OR of 3.66 (95% CI 1.99–6.82). Patients switching from etanercept were more likely to switch to another TNF‐alpha inhibitor rather than another biologic drug compared with patients receiving adalimumab (aOR 9.40, 95% CI 5.71–15.80).
Discussion
In this study, we thoroughly described and represented the biologic switches occurring in patients with moderate to severe psoriasis. We also identified factors associated with the type of switch in the context of the first switch. Biologics switches are frequent in this population with a cumulative incidence of first biologic switch of 34% after 3 years and mostly happen because of inefficacy of the previous biologic.
With Sankey and Sunburst diagrams, we identified the most common switching patterns among biologics. These diagrams have shown that the first biological treatment affects the choice of the second‐line biologic. Patients receiving etanercept mostly switch to adalimumab, whereas those receiving adalimumab mostly switch to ustekinumab or secukinumab. Patients switching from etanercept were more likely to switch to another TNF‐alpha inhibitor compared with patients switching from adalimumab. This could be explained by the fact that etanercept is considered less efficient than adalimumab and thus a switch from etanercept to adalimumab may be seen as an intensification of TNF‐alpha inhibitor treatment. 17
Chronograms and Sankey diagrams with stratification on the period of switch have clearly shown that the calendar period of switch have greatly affected the choice of biotherapy, for the first‐ and second‐line biologics. Furthermore, in patients switching from a TNF‐alpha inhibitor, the period of switch was statistically associated with the type of switch, with patients switching more frequently within the same class of biologics before 2016 compared with patients switching since 2018. The effect of the calendar period of switch reflects the availability of new treatment alternatives.
In 2018, the French Society of Dermatology published guidelines on the choice of systemic treatments for adults with moderate‐to‐severe psoriasis according to their characteristics. 4 In case of obesity, ustekinumab and ixekizumab are the recommended biologics. TNF‐alpha inhibitor, in particular etanercept, are recommended in the presence of chronic infection as HIV or hepatitis B and C. Nonetheless, we did not find that these clinical characteristics were associated with the type of switch for the first switch. However, these recommendations are not specific to biologic switches. We can hypothesize that the absence of response of a particular biologic and the available alternatives, and thus the period of switch, are the two elements guiding the clinician in choosing the biologic that is most likely to work for a given patient in the context of a switch.
In our study, adalimumab and ustekinumab were the most prescribed biologics as in the Danish cohort DERMBIO 18 and in a US claims database study. 6 The cumulative incidence of switches after the first biologic is consistent with other cohort studies (at 1 year, 16% in the Psobioteq cohort, 17.5% in the BADBIR cohort). 19
We explored different graphical representations of switches. Sankey diagrams represent flows between successive steps but do not depict therapeutic sequences at an individual level. Sunburst diagrams, on the other hand, display treatment sequences but the circular shape of these diagrams limits the number of represented steps. Chronograms allow assessing the evolution of use of biologic agents over time and the impact of the arrival on the market of new biologics. Nonetheless, chronograms do not represent flows at an individual level and cannot differentiate the initiation of a biologic from its persistence. These graphical methods are therefore complementary and combining them has allowed a better and more detailed description and interpretation of biologic prescription and switching patterns. To the best of our knowledge, to date these diagrams have not been used to report biologic switches in psoriasis. The strengths of our study also include the multicenter design with patients from 41 dermatology centers across France.
Our study has some limitations. Some clinically pertinent variables, as PASI and PGA scores, could not be tested in the statistical models because of missing data. Finally, we did not account for center effects in our analyses, as we believed that the effect was probably most significant at the prescriber's level and could vary over time.
Conclusion
This study highlights the switching patterns among biologics and their evolution over time with the recent availability of new biologics. The calendar period of the switch and the initial biologic agent were identified as factors associated with the type of switch in patients switching from their first TNF‐alpha inhibitor. The choice of biologic for the switch is a complex, multifactorial decision, affected by the availability of new effective biologics. These findings point out the importance of using adapted graphical methods to report switches that can reveal the complexity of the different types of switches and their evolution over time.
Supporting information
Appendix S1 Supporting Information.
Table S1 Description of treatment patterns of first‐line biologic.
Table S2 Description of treatment patterns of second‐line biologic.
Table S3 Description of treatment patterns of third‐line biologics.
Table S4 Reasons for switching for first biologic switches in the study cohort.
Table S5 Univariate analysis of factors associated with the type of switch.
Table S6 Factors associated with an intra‐class switch for the first biologic switch (multivariate analysis, n = 444).
Figure S1 First biologic switch among patients switching their biologic stratified by period.
Figure S2 (a) First biologic switch of patients switching after less than a year of initial treatment. (b) First biologic switch of patients switching after a year or more of initial treatment.
Figure S3 (a) Sunburst diagram of biologic switches occurring from 2012 to 2015 by biologic agent. (b) Sunburst diagram of biologic switches occurring from 2012 to 2015 by drug class. (c) Sunburst diagram of biologic switches occurring from 2016 to 2020 by drug class. (d) Sunburst diagram of biologic switches occurring from 2016 to 2020 by biologic agent.
Acknowledgments
We acknowledge, from the Centre de pharmaco‐épidémiologie de l'AP‐HP, Nessima Yelles, Sarra Pochon and Hadia Hafirassou for the implementation, management and monitoring of the PsoBioTeq cohort study, Diakhou Ndao for its datamanagement and Hugo Arlégui for its statistical analysis coordination.
Funding sources
The PsoBioTeq cohort study is supported by unrestricted research grants from the French Ministry of Health (PHRC AOM 09195), the French National Drug and Healthcare Products Safety Agency (ANSM) and private funders (Abbvie, Janssen, Pfizer, MSD France, Lilly France, Novartis Pharma, Celgene, Amgen and Leo Pharma). None of the private funders had any role in the design of this ancillary study, data management, analysis and interpretation of the data, preparation or approval of this manuscript, or the decision to submit it for publication. They received the manuscript for information purposes before submission. The study was sponsored by the Direction of Clinical Research and Innovation (DRCI, AP‐HP, Direction de la Recherche Clinique et de l'Innovation, Assistance Publique–Hôpitaux de Paris). The PsoBioTeq cohort was set up under the auspices of the French Society of Dermatology (SFD) and its Psoriasis Research Group. R Curmin was supported by a grant from Institut Pierre Louis D'Epidemiologie et de Santé Publique (IPLESP, INSERM and Sorbonne Université).
Conflicts of interest
R. Curmin, Y. De Rycke, S. Guillo, O. Chosidow and E. Sbidian have no conflicts of interest to disclose. H. Bachelez has undertaken activities as a paid consultant, advisor or speaker for Abbvie, Almirall, Anaptysbio, Biocad, Boehringer Ingelheim, Dermavant, Janssen, Kyowa Kirin, Leo Pharma, Lilly, Novartis and UCB. He has also received grant funding from Boehringer Ingelheim, Bristol Myers Squibb and Pfizer. M. Beylot‐Barry offers a consultancy service and is an investigator for Abbvie, Amgen, Janssen, Leo Pharma, Lilly and Novartis; N. Beneton has undertaken activities as a paid consultant, advisor or speaker for Abbvie, Almirall, Janssen, Lilly and Novartis. A. Dupuy has undertaken activities as a paid consultant for Sanofi. P. Joly is a consultant for Abbvie, Akari, Amgen, Argenx, Astra Zeneca, Chugai, GSK, Janssen, Kezar Life Science, Lilly, Novartis, Principabio, Regeneron, Roche, Sanofi Aventis, Servier, Thermofisher and UCB. D. Jullien is a consultant for AbbVie, Almirall, Amgen, Boehringer ingelheim, Bristol Myers Squibb, Celgene, Janssen‐Cilag, Lilly, MEDAC, Novartis and UCB. M.A. Richard is a consultant for Pfizer, Leo Pharma, Janssen, Galderma, AbbVie, Novartis, Pierre Fabre, Merck and BMS. M. Viguier has undertaken activities as a paid consultant, advisor or speaker for Abbvie, Almirall, BMS, Janssen, Pfizer, Leo Pharma, Medac, Boehringer Ingelheim, Novartis and Lilly. C. Paul has been an investigator and consultant for Almirall, AbbVie, Amgen, Boehringer Ingelheim, Celgene, GSK, Janssen, LEO Pharma, Lilly, Merck, Novartis, Pfizer, Sanofi and UCB. E. Mahe is a consultant for Abbvie, Janssen, Leo Pharma, Lilly and Novartis, has also been an investigator for Abbvie, Amgen, AstraZeneca, Boehringer, Celgene, Janssen, and Novartis, and has received speaker remuneration from Abbvie, Janssen, Lilly, Leo Pharma and Novartis. F. Tubach is head of the Pharmacoepidemiology Centre of the AP‐HP and of the Clinical Research Unit of Pitié‐Salpêtrière Hospitals; both these structures have received unrestricted research funding, grants and consultancy fees from a large number of pharmaceutical companies that have contributed indiscriminately to the salaries of its employees. F. Tubach did not receive any personal remuneration from these companies.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Parisi R, Iskandar IYK, Kontopantelis E et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 2020; 28: m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richard MA, Corgibet F, Beylot‐Barry M et al. Sex‐ and age‐adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the « OBJECTIFS PEAU » study. J Eur Acad Dermatol Venereol JEADV 2018; 32: 1967–1971. [DOI] [PubMed] [Google Scholar]
- 3. Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med 2014; 4(8): a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amatore F, Villani A‐P, Tauber M, Viguier M, Guillot B. Psoriasis research Group of the French Society of dermatology (Groupe de Recherche Sur le psoriasis de la Société Française de Dermatologie). French guidelines on the use of systemic treatments for moderate‐to‐severe psoriasis in adults. J Eur Acad Dermatol Venereol JEADV. 2019; 33: 464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Özkur E, Kıvanç Altunay İ, Oğuz Topal İ et al. Switching biologics in the treatment of psoriasis: a multicenter experience. Dermatol Basel Switz 2021; 237: 22–30. [DOI] [PubMed] [Google Scholar]
- 6. Feldman SR, Zhang J, Martinez DJ et al. Real‐world treatment patterns and healthcare costs of biologics and apremilast among patients with moderate‐to‐severe plaque psoriasis by metabolic condition status. J Dermatol Treat 2021; 32: 203–211. [DOI] [PubMed] [Google Scholar]
- 7. Hu Y, Chen Z, Gong Y, Shi Y. A review of switching biologic agents in the treatment of moderate‐to‐severe plaque psoriasis. Clin Drug Investig 2018; 38: 191–199. [DOI] [PubMed] [Google Scholar]
- 8. Aravamuthan R, Vadivelu S, Arumugam S. Sustainability and switching of biologics for psoriasis. Int J Res Dermatol 2020; 6: 398. [Google Scholar]
- 9. Blauvelt A, Shi N, Somani N et al. Comparison of two‐year treatment adherence, persistence, discontinuation, reinitiation, and switching between psoriasis patients treated with ixekizumab or secukinumab in real‐world settings. J Am Acad Dermatol 2021; 86(3): 581–589. [DOI] [PubMed] [Google Scholar]
- 10. Sbidian E, Mezzarobba M, Shourick J et al. Choice of systemic drugs for the Management of Moderate‐to‐severe Psoriasis: a cross‐country comparison based on National Health Insurance Data. Acta Derm Venereol 2021; 101(6): adv00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Honda H, Umezawa Y, Kikuchi S et al. Switching of biologics in psoriasis: reasons and results. J Dermatol 2017; 44: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 12. Lamer A, Laurent G, Pelayo S, El Amrani M, Chazard E, Marcilly R. Exploring patient path through Sankey diagram: a proof of concept. Stud Health Technol Inform 2020; 270: 218–222. [DOI] [PubMed] [Google Scholar]
- 13. Roux J. Care pathways of persons with multiple sclerosis in France using administrative data [Internet] 2018. Available from: https://tel.archives‐ouvertes.fr/tel‐02379451
- 14. Sbidian E, Giboin C, Bachelez H et al. Factors associated with the choice of the first biologic in psoriasis: real‐life analysis from the Psobioteq cohort. J Eur Acad Dermatol Venereol JEADV 2017; 31: 2046–2054. [DOI] [PubMed] [Google Scholar]
- 15. Cohorte multicentrique de patients recevant un traitement systémique (conventionnel ou biothérapie) pour un psoriasis cutané modéré à sévère / Portail Epidemiologie ‐ France ¦ Health Databases [Internet]. [cited 2021 Aug 23]. Available from: https://epidemiologie‐france.aviesan.fr/epidemiologie‐france/fiches/cohorte‐multicentrique‐de‐patients‐recevant‐un‐traitement‐systemique‐conventionnel‐ou‐biotherapie‐pour‐un‐psoriasis‐cutane‐modere‐a‐severe
- 16. Ayala‐Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis Auckl NZ 2016; 6: 7–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sbidian E, Chaimani A, Garcia‐Doval I et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Cochrane Database Syst Rev 2021; 4: CD011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egeberg A, Ottosen MB, Gniadecki R et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate‐to‐severe plaque psoriasis. Br J Dermatol 2018; 178: 509–519. [DOI] [PubMed] [Google Scholar]
- 19. Iskandar IYK, Ashcroft DM, Warren RB et al. Patterns of biologic therapy use in the management of psoriasis: cohort study from the British Association of Dermatologists biologic interventions register (BADBIR). Br J Dermatol 2017; 176: 1297–1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Table S1 Description of treatment patterns of first‐line biologic.
Table S2 Description of treatment patterns of second‐line biologic.
Table S3 Description of treatment patterns of third‐line biologics.
Table S4 Reasons for switching for first biologic switches in the study cohort.
Table S5 Univariate analysis of factors associated with the type of switch.
Table S6 Factors associated with an intra‐class switch for the first biologic switch (multivariate analysis, n = 444).
Figure S1 First biologic switch among patients switching their biologic stratified by period.
Figure S2 (a) First biologic switch of patients switching after less than a year of initial treatment. (b) First biologic switch of patients switching after a year or more of initial treatment.
Figure S3 (a) Sunburst diagram of biologic switches occurring from 2012 to 2015 by biologic agent. (b) Sunburst diagram of biologic switches occurring from 2012 to 2015 by drug class. (c) Sunburst diagram of biologic switches occurring from 2016 to 2020 by drug class. (d) Sunburst diagram of biologic switches occurring from 2016 to 2020 by biologic agent.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


