Abstract
We describe a cluster of COVID-19 breakthrough infections after vaccination in Kyamulibwa, Kalungu District, Uganda. All but 1 infection were from SARS-CoV-2 Omicron strain BA.5.2.1. We identified 6 distinct genotypes by genome sequencing. Infections were mild, suggesting vaccination is not protective against infection but may limit disease severity.
Keywords: COVID-19, SARS-CoV-2, Omicron, infections, Uganda, respiratory infections, zoonoses, coronavirus disease, severe acute respiratory syndrome coronavirus 2, viruses
The SARS-CoV-2 Omicron variant BA.5 was initially reported in South Africa in late February 2022 (1). The BA.5 variant, and especially the subvariant BA.5.2.1, has now spread to at least 104 countries globally; 197,425 genomes had been reported in GISAID (http://www.gisaid.org) as of September 16, 2022. The BA.5 spike protein shares substitutions with earlier Omicron variants but includes some of the Delta variant immune evasion changes. The BA.4/BA.5 viruses are reported to escape earlier Omicron immune responses, and vaccination does not fully block infection but may limit severity of disease (2–5). Infection of vaccinated persons (breakthrough infections) with SARS-CoV-2 strains is known, and such infections were reported recently among a highly vaccinated community within the US Embassy in Uganda (6). The frequency and outcomes of BA.5 vaccine breakthrough infections, both in Uganda and globally, are yet to be determined.
The Medical Research Council Unit in Uganda maintains a rural population cohort in Kyamulibwa, Kalungu District, southwestern Uganda (7). Unit staff were vaccinated as soon as vaccines were available in the country (March 2021), and most received at least 2 doses of COVID-19 vaccine by June 2021, with ongoing efforts for booster vaccination rolling out in the country. Staff members who had any symptoms indicating respiratory infections, including COVID-19, were routinely tested using Abbott’s Panbio COVID-19 antigen rapid tests (Abbott, https://www.abbott.com). If cases of COVID-19 were detected, all staff were tested to detect asymptomatic cases. During such routine testing of staff members, a cluster of SARS-CoV-2 infections among vaccinated staff was detected. Test positivity during this period of infection was 18.5% (12 positive from 65 staff members tested), which was in the range of previous infection waves (January 3–10, 2022: 11.7%; June 6–14, 2021: 32.5%; November 30−December 1, 2020: 19.3%). Most infected staff members had mild symptoms, and all cases were quickly resolved (Appendix Table).
We performed sequencing by methods previously described (8). Nine cases yielded full genome SARS-CoV-2 sequences that we lineage-typed using Nextclade (9) and Pangolin (10) software; 8 of the 9 genomes were from the BA.5 lineage and 1 was BA.2.31, all variants within the Omicron variant-of-concern lineage. Although all 9 genomes belonged to the Omicron lineage, we detected 6 distinct subvariants (Figure). Genomes from cases 1, 2, 7, and 10 (all BA.5.2.1) were identical, suggesting a common infection source for these 4 cases. However, genomes for cases 4, 6, 11, and 12 genomes (also BA.5.2.1) were distinct from cases 1, 2, 7, and 10 and from each other, differing by 2–5 nt changes. The case 5 genome (BA.2.31) represents a 6th virus source for the cluster of breakthrough infections.
Figure.
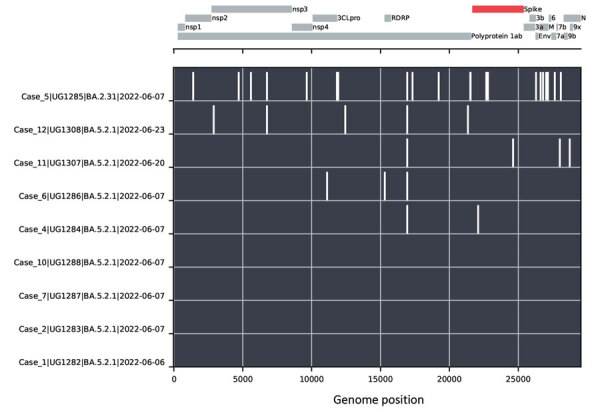
Nucleotide changes between SARS-CoV-2 genomes from a cluster of COVID-19–positive persons in Kyamulibwa, Kalungu District, rural Uganda. The lower portion of the chart shows nucleotide differences from the case 1 genome, plotted as white bars. The absence of bars in the BA.5.2.1 genomes from cases 1, 2, 7, and 10 indicates identical sequences. Case 5 was determined to be lineage BA.2.31, and cases 4, 6, 11, and 12 genomes demonstrated virus variants of lineage BA.5.2.1 distinct from the genomes from cases 1, 2, 7, and 10. A schematic of the SARS-CoV-2 genome is shown in the top portion of the chart, with protein coding regions marked.
The detection of 5 distinct BA.5.2.1 sublineages found in Kalungu District in a short time period indicates multiple BA.5 sublineages were already circulating in other parts of Uganda and demonstrates the speed of movement of SARS-CoV-2. Uganda reported an increase in COVID-19 cases during this period, and both BA.5.2.1 and BA.2.31 virus strains potentially contributed to this increase in infections. Of note, 9 of the 12 COVID-19–positive staff members in this report routinely traveled on shared unit vehicles to and from Masaka or Kampala, which might account for the virus spread. In addition, the unit travel records show shared vehicle usage, suggesting a likely but not confirmed source of infection for cases 2, 7, and 10. The 3 infected staff members whose testing results did not yield sufficient PCR products for sequencing were asymptomatic, suggesting low viral loads (Appendix Table).
Many countries have reported increasing COVID-19 cases with BA.4 or BA.5 and derivatives as a major identified lineage. The global trend toward relaxed travel and quarantine restrictions and the mild infections in vaccinated and previously infected individuals might help enable global movement of these variants. This probably is evidenced by the timing of BA.5 appearance in rural Uganda within weeks of the variant being initially reported in other parts of the world (South Africa in late February 2022, Germany in mid-March 2022, the United States in late March 2022, Portugal in early April 2022, and Uganda in early June 2022).
In conclusion, the detection of 6 distinct sublineages of SARS-CoV-2 (5 of BA.5.2.1 and 1 of BA.2.31) in Kyamulibwa, Kalungu District, Uganda, within a short period indicates substantial diversity of and rapid movement of these viruses into and within Uganda. Combined with recent increases in reported SARS-CoV-2 infections throughout the country, our findings emphasize the need for vigilance, surveillance, and continued testing in this rural community and throughout the country. The mild nature of symptoms in these 12 cases, and in many vaccinated persons, reinforces the importance of community vaccination efforts.
Additional information on SARS-CoV-2 Omicron BA.5 infections in vaccinated persons, rural Uganda.
Acknowledgments
We thank the staff and technicians working at the Kyamulibwa, Kalungu District field station that help make this work possible.
This work was supported by the UK Medical Research Council (MRC/UK Research and Innovation) and the UK Department for International Development under the MRC/DFID Concordat agreement (grant agreement no. MC_PC_20010) and Wellcome Trust, UK FCDO—Wellcome Epidemic Preparedness—Coronavirus (grant agreement no. 220977/Z/20/Z). The COVID-19 surveillance project in Kalungu District was supported with additional funding from the UK MRC (grant agreement no. MC_PC_20011).
This study was approved by the Uganda Virus Research Institute-Research and Ethics Committee (UVRI-REC Federalwide Assurance [FWA] FWA No. 00001354, study reference GC/127/20/04/771). Sequences described here are available from GISAID under accession numbers EPI_ISL_13332769–75, 15005215 and 15005216.
Biography
Dr Mugisha is a medical scientist working at the Uganda Research Unit of the Medical Research Council/Uganda Virus Research Institute and London School of Hygiene & Tropical Medicine. During COVID-19, he has led surveillance activity in the unit’s General Population Cohort in southwestern Uganda. His research interests are primarily on the health and social issues affecting older people in low- and middle-income countries.
Footnotes
Suggested citation for this article: Mugisha J, Mpairwe B, Newton R, Cotten M, Phan MVT. SARS-CoV-2 Omicron BA.5 infections in vaccinated persons, rural Uganda. Emerg Infect Dis. 2023 Jan [date cited]. https://doi.org/10.3201/eid2901.220981
References
- 1.Tegally H, Moir M, Everatt J, Giovanetti M, Scheepers C, Wilkinson E, et al. ; NGS-SA consortium. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022;28:1785–90. 10.1038/s41591-022-01911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura I, Yamasoba D, Tamura T, Nao N, Oda Y, Mitoma S, et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5. Cell, 2022;185(21):3992-4007.e16. ISSN 0092-8674 [DOI] [PMC free article] [PubMed]
- 3.Khan K, Karim F, Ganga Y, Bernstein M, Jule Z, Reedoy K, et al. ; COMMIT-KZN Team. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. [Erratum in: Nat Commun. 2022 Oct 13;13] [1]. Nat Commun. 2022;13:4686. 10.1038/s41467-022-32396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–8. 10.1038/s41586-022-05053-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, Zhou D, Ginn HM, Selvaraj M, et al. ; OPTIC Consortium; ISARIC4C Consortium. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433.e13. 10.1016/j.cell.2022.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JR, Owusu D, O’Laughlin K, Cohen AL, Ben Hamida A, Patel JC, et al. SARS-CoV-2 breakthrough infections among US Embassy staff members, Uganda, May–June 2021 [cited 2022 Jun 12]. Emerg Infect Dis. 2022;28:1279–80. 10.3201/eid2806.220427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asiki G, Murphy G, Nakiyingi-Miiro J, Seeley J, Nsubuga RN, Karabarinde A, et al. ; GPC team. The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. [cited 2020 Mar 3]. Int J Epidemiol. 2013;42:129–41. 10.1093/ije/dys234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten M, Bugembe DL, Kaleebu P, Phan MVT. Alternate primers for whole-genome SARS-CoV-2 sequencing. Virus Evol. 2021. Feb 4 [cited 2021 Mar 7]. https://academic.oup.com/ve/advance-article/doi/10.1093/ve/veab006/6128533 [DOI] [PMC free article] [PubMed]
- 9.Aksamentov I, Roemer C, Hodcroft E, Neher R. Nextclade: clade assignment, mutation calling and quality control for viral genomes [cited 2022 Jun 13]. J Open Source Softw. 2021;6:3773. 10.21105/joss.03773 [DOI] [Google Scholar]
- 10.O’Toole Á, Scher E, Underwood A, Jackson B, Hill V, McCrone JT, et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021;7:veab064. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on SARS-CoV-2 Omicron BA.5 infections in vaccinated persons, rural Uganda.


