Abstract
Background and purpose
Recent application of the mild cognitive impairment concept to Parkinson disease (PD) has proven valuable in identifying patients at risk of dementia. However, it has sparked controversy regarding the existence of cognitive subtypes. The present review evaluates the current literature pertaining to data‐driven subtypes of cognition in PD.
Methods
Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines, systematic literature searches for peer‐reviewed articles on the topic of cognitive subtyping in PD were performed.
Results
Twenty‐two relevant articles were identified in the systematic search. Subtype structures showed either a spectrum of severity or specific domains of impairment. Domain‐specific subtypes included amnestic/nonamnestic, memory/executive, and frontal/posterior dichotomies, as well as more complex structures with less definitive groupings. Preliminary longitudinal evidence showed some differences in cognitive progression among subtypes. Neuroimaging evidence provided insight into distinct patterns of brain alterations among subtypes.
Conclusions
Recurring phenotypes in the literature suggest strong clinical relevance of certain cognitive subtypes in PD. Although the current literature is limited, it raises critical questions about the utility of data‐driven methods in cognitive research. The results encourage further integration of neuroimaging research to define the latent neural mechanisms behind divergent subtypes. Although there is no consensus, there appears to be growing consistency and inherent value in identifying cognitive subtypes in PD.
Keywords: cluster analysis, cognitive impairment, dementia, neuroimaging, Parkinson disease
INTRODUCTION
Cognitive impairment is an intrinsic feature of Parkinson disease (PD), with a cumulatively high risk of developing dementia [1]. The desire to prevent this cognitive decline at its earliest stages has led to the characterization of mild cognitive impairment in PD (PD‐MCI). Comprehensive criteria for a diagnosis of PD‐MCI were introduced by the Movement Disorder Society (MDS) in 2012 [2]. These criteria have helped standardize the definition of PD‐MCI, but have also highlighted the heterogeneity of cognitive symptoms experienced by people with PD. Thus, to improve diagnosis, prognosis, and treatment of PD‐MCI, research has led to the development of cognitive subtypes, a concept that has been met with healthy discourse.
Currently, the MDS criteria suggest single‐ versus multiple‐domain subtyping, which distinguishes between patients with impairments in one versus more than one of the five cognitive domains (memory, executive, attention/working memory, language, visuospatial). Emerging research has demonstrated that more specific subtyping may have clinical significance, for example, the amnestic subtype (i.e., includes memory impairment) has been shown to be associated with greater functional disability and more rapid cognitive decline [3, 4]. Moreover, the dual syndrome hypothesis suggests there are two cognitive syndromes within PD; a posterior‐cortical syndrome with impairments reflecting damage to the posterior regions of the brain (i.e., memory and visuospatial dysfunction) and a frontal syndrome with impairments reflecting damage to the frontal regions (i.e., executive and attentional dysfunction) [5]. The posterior syndrome is thought to exhibit more rapid decline toward dementia and respond more positively to cholinergic treatment than the frontal syndrome, which benefits from dopaminergic treatment [5, 6].
Machine learning has increasingly been adopted to explore cognitive subtypes of PD. Machine learning clustering techniques use iterative algorithms to group patients based on data trends, limiting a priori assumptions and reducing risk of bias from investigator‐made decisions (e.g., cutoffs for a “failed” test). These techniques are not only prominent in PD cognitive research, but have also been applied to biological subtyping in depression [7], motor subtyping in PD [8], and cognitive subtyping in psychosis [9]. The present review aimed to consolidate current literature pertaining to data‐driven cognitive subtypes in PD in terms of subtype characteristics (i.e., based on impairment severity or based on domain[s] of impairment) and accompanying neuroimaging evidence.
METHOD
Eligibility criteria
Original research articles published in peer‐reviewed journals that used data‐driven machine learning techniques to explore cognitive subtypes in PD were included. Studies that included PD‐related samples (e.g., dementia with Lewy bodies, secondary parkinsonism) were excluded. Studies that defined subtypes of PD using other clinical variables (e.g., motor, psychiatric, physiological measures) or did not focus on cognitive subtyping were excluded. Studies that did not use data‐driven machine‐learning algorithms to delineate subtypes (e.g., manual grouping by the researcher) were excluded. Studies that explored subtypes within only one cognitive domain (e.g., only memory or only executive function) were excluded.
Databases and search terms
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines were adhered to [10]. Four literature searches were performed using a combination of the following systematic search terms: (Parkinson* disease) AND (“cognitive subtypes” OR “cognitive sub‐types” OR “cognitive subgroups” OR “cognitive sub‐groups” OR “cognitive phenotype” OR “cluster analysis”). Where available, MeSH terms were used. Results included publications up to October 2020 from Web of Science, PsycINFO, PubMed, and Cumulative Index to Nursing and Allied Health Literature. Additional publications were also identified through manual searches of reference lists cited in eligible articles. An updated search was performed in January 2022.
Study selection
Two independent reviewers (D.P., J.Y.) evaluated search results. Duplicates and abstracts of incorrect format (e.g., conference abstracts, reviews, meta‐analyses, case studies) were removed. Initial exclusions were made based on abstract content, after which full‐text articles were evaluated for inclusion. Discrepancies in article selection were resolved by discussion with a third arbitrator (N.N.D.).
Data extraction
The following data were extracted from the reviewed articles: (i) publication details (authors, year, journal), (ii) sample characteristics (sample size, mean age, education, global cognition, disease duration, and gender [% female]), (iii) neurocognitive measures, (iv) subtyping methodology, (v) number of subtypes, and (vi) subtype characteristics (number of participants, cognitive profile). Additional data were extracted for the neuroimaging studies: (i) imaging methodology and (ii) neuroimaging results.
Quality assessment
A modified version of the Newcastle‐Ottawa Scale was used to assess the quality of cross‐sectional studies [11], whereas longitudinal studies were assessed using the cohort version of the Newcastle‐Ottawa Scale [12]. Specific criteria used by the two assessors (D.P., J.Y.) in the present review are provided in Supplementary Material 1.
RESULTS
Systematic searches produced 538 articles (Figure 1), 22 of which were eligible for review. To aid discussion, results will be stratified into studies that defined subtypes based on impairment severity and those that based subtypes on impairment domain(s). Summaries of the severity‐ and domain‐based evidence are provided in Tables 1 and 2, respectively. All 22 of the reviewed studies were of moderate to high quality (quality range: 5–8, Supplementary Material 2). Nine studies defined subtypes based on impairment severity [13, 14, 15, 16, 17, 18, 19, 20, 21], and 11 studies revealed subtypes based on domains of impairment [22, 23, 24, 25, 26, 27]. Two longitudinal studies were identified (Table 3) [28, 29].
FIGURE 1.
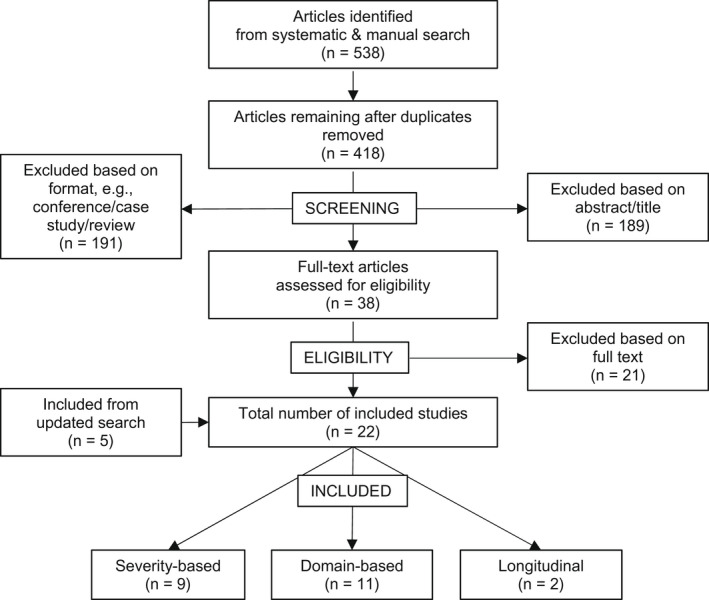
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) study selection flowchart
TABLE 1.
Severity‐based subtypes
| Citation | Sample characteristics, mean ± SD | Cluster method | Cluster variables | Imaging method | Description of clusters (% prevalence) | Neuroimaging results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age, years | Education, years | Global cognition | Gender, % female | Disease duration, years | ||||||
| Barvas et al. (2021) | 65 PD | 67.9 ± 7.5 | 10.8 ± 4.8 | MoCA: 21.8 ± 2.8 | 35% | 7.4 ± 5.3 | Latent profile analysis |
Attention: attentional matrices Memory: RAVLT (delayed), ROCF (delayed) Executive: phonemic fluency, Stroop (errors & time), social cognition assessment, Ekman 60‐faces test Language: semantic fluency, object picture naming, action verb picture naming Visuospatial: JLO |
NA |
|
NA |
| Dujardin et al. (2013) | 489 PD, 69 PDD | 63.4 ± 9.2 | 11.2 ± 3.3 | MMSE: 27.1 ± 2.5 | 40% | 8.2 ± 6.4 | k‐means cluster analysis |
Global efficiency: MMSE Attention/working memory: digit span (forward and backward) Verbal episodic memory: Grober and Buschke SRT or RAVLT (immediate & delayed) Executive: Stroop (interference & errors), TMT (B/A), phonemic and semantic fluency |
NA |
|
NA |
|
Measures specific to Maastricht Site Verbal episodic memory: RAVLT (immediate & delayed) Processing speed: WAIS symbol substitution test Visuospatial: visual and object spatial perception test | |||||||||||
|
Measures specific to Lille site Verbal episodic memory: Buschke SRT (immediate & delayed) Processing speed: SDMT Visuospatial: construction subscale of MDRS, MMSE pentagons | |||||||||||
| Dujardin et al. (2015) | 156 PD | 67 ± 7 | 11.4 ± 2.9 | MMSE: 26.5 ± 2.5 | 30% | 9.0 ± 5.4 | k‐means cluster analysis |
Global efficiency: MMSE, MDRS Attention/working memory: digit span (forward & backward), SDMT Executive: TMT (B/A), Stroop (interference & errors), phonemic fluency (single & alternating) Verbal episodic memory: HVLT‐R (learn1, immediate, delayed, recognition, intrusions) Language: BNT, semantic fluency Visuospatial: JLO |
NA |
|
NA |
|
Hassan et al. (2017), using Dujardin et al. (2015) clusters |
124 PD | 67.0 ± 7.2 | 11.4 ± 3.2 | MMSE: 26.6 ± 2.4 | 30% | 9.2 ± 5.8 | EEG: dense‐EEG source connectivity | See Dujardin et al. (2015)
|
Disruptions in functional connectivity in the alpha 1 & 2, beta, and gamma frequency bands, with Group 1 > Group 2 > Group 3 in power spectral density. In the delta and theta frequency bands, power spectral density increased with cognitive impairment (Group 1 < Group 2 < Group 3). Functional alterations in frontotemporal connectivity observed between Group 1 and Group 2, and functional alterations in frontoparietal and frontocentral connectivity observed in Group 3 | ||
|
Lopes et al. (2017), using Dujardin et al. (2015) clusters |
119 PD | 65.3 ± 7.2 | 48.0 ± 3.3 | MMSE: 27 ± 2.2 | 32% | 8.7 ± 5.9 | 3‐T rs‐fMRI: graph theory and network‐based statistic | See: Dujardin et al. (2015)
|
Functional segregation of the brain decreased with greater cognitive deficit (Group 1 > Group 2 > Group 3 > Group 4). Group 1 & Group 2 demonstrated greater hub connections in the associative frontal, temporal, and occipital areas as well as limbic, sensorimotor, and insular areas. Group 3 demonstrated greater hub connections than Group 4 in associative frontal, temporal, occipital, limbic, primary sensorimotor, and cingulate areas | ||
|
Wolters et al. (2020), using Dujardin et al. (2015) clusters |
124 PD | 66.1 ± 7.0 | 12.0 ± 3.3 | MMSE: 27.3 ± 2.1 | 31% | 8.8 ± 5.4 | 3‐T MRI: Voxel‐ and vertex‐based morphometry | Reduced grey matter in left medial temporal pole in Group 4 compared to Group 1. Group 4 also showed reduced cortical thickness compared to Group 1 in the right inferior temporal gyrus. Reduced cortical folding in Group 4 compared to Group 1 in right temporal regions. No difference in white matter lesions across groups | |||
| Kenney et al. (2022) | 494 PD | 64.7 ± 9.0 | 15.0 ± 2.8 | DRS‐2: 137.0 ± 4.5 | 28% | 9.6 ± 5.3 | Hierarchical & k‐means cluster analysis |
Composite scores used for each domain Executive: Stroop (interference), TMT (B), phonemic fluency Verbal memory: HVLT‐R (delayed); WMS‐III logical memory (delayed) Language: BNT, semantic fluency Visuospatial: JLO, facial recognition test Attention/working memory: digit span (forward and backward) |
NA |
|
NA |
| McKinlay et al. (2009) | 40 PD | 66.5 ± 6.5 | 13.6 ± 1.0 | MMSE: 28.5 ± 1.2 | NR | 6.5 ± 4.9 | k‐means cluster analysis |
Executive: phonemic and sematic fluency, clock drawing, key search, zoo map, Stroop (interference) Problem solving: card sorting, matrix reasoning, stockings of Cambridge, tower test Attention/working memory: digits span (forward & backward), LNS, reading span, spatial span Processing speed: Stroop (word & colour naming) Memory: logical memory (immediate & delayed), paired associates (immediate & delayed), auditory recall index Visuospatial: JLO, clock copying |
NA |
|
NA |
| Souza et al. (2016) | 40 PDD, 39 PD‐MCI, and 21 PD‐NC | 61.3 ± NA | 4.5 ± NA | MMSE: 24.3 ± NA | 42% | 8.2 ± NA | Hierarchical & k‐means cluster analysis | MMSE, clock drawing, digit span (forward & backward), CERAD word list (immediate, delayed, recognition), FAB, and semantic fluency | NA |
|
NA |
Abbreviations: BNT, Boston Naming Test; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; DRS‐2, Dementia Rating Scale 2; EEG, electroencephalography; FAB, Frontal Assessment Battery; HVLT‐R, Hopkin's Verbal Learning Test Revised; JLO, Judgment of Line Orientation; LNS, Letter Number Sequencing; MDRS, Mattis Dementia Rating Scale; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; NA, not applicable; NR, Not reported; PD, Parkinson disease; PDD, PD dementia; PD‐MCI, PD with mild cognitive impairment; PD‐NC/PD‐NCI, PD with normal cognition; PD‐UCI, PD with uncertain cognition; RAVLT, Rey Auditory Verbal Learning Test; ROCF, Rey–Osterrieth Complex Figure; rs‐fMRI, resting‐state functional magnetic resonance imaging; SDMT, Symbol Digit Modality Test; SRT, Selective Reminding Test; TMT, Trail Making Test; WMS, Weschler Memory Scale.
TABLE 2.
Domain‐based subtypes
| Citation | Sample characteristics, mean ± SD | Cluster method | Cluster variables | Imaging method | Description of clusters, % prevalence | Neuroimaging results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age, years | Education, years | Global cognition | Gender, % female | Disease duration, years | ||||||
| Alonso‐Recio et al. (2018) | 71 PD | 63.9 ± 7.7 | 15.1 ± 6.1 | MMSE: 28.8 ± 1.3 | 48% | 7.0 ± 4.1 | Latent profile analysis |
Memory: Test de Aprendizaje Verbal España‐Complutense (immediate & delayed) 7/24 spatial recall (immediate & delayed), semantic fluency, BNT Executive: phonemic fluency, TMT (A & B‐A), digit span (backward) |
NA |
|
NA |
| Brennan et al. (2017) | 199 PD | 70.6 ± 7.5 | 16.2 ± 2.3 | DRS‐2: 136.8 ± 5.6 | 33% | 6.9 ± 5.2 | Latent class analysis |
Executive: LNS, phonemic fluency Language: BNT, semantic fluency Verbal episodic memory: HVLT‐R (delayed & recognition) Visuospatial: JLO, clock drawing |
NA |
|
NA |
| Crowley et al. (2021) | 116 PD | 78.7 ± 6.2 | 16.3 ± 2.5 | 28.4 ± 1.4 | 28% | 7.1 ± 4.8 | k‐means cluster analysis |
Composite scores used for each domain Executive: SDMT, LNS, TMT‐B, Stroop Memory: Logical memory, HVLT (delayed & recognition) |
3‐T structural MRI: voxel‐based morphometry and fractional anisotropy |
|
PD executive: lower total brain volume and higher ventricular volume compared to PD cognitively well and healthy controls. Reduced putamen volume and right entorhinal‐hippocampal connectivity compared to healthy controls. Reduced thalamus volume compared to PD cognitively well. Reduced right dorsolateral‐prefrontal cortex to caudate nucleus connections connectivity compared to all groups PD memory: bilaterally reduced entorhinal‐hippocampal connections compared to healthy controls PD cognitively well: only reduced putamen volume and right entorhinal‐hippocampal connectivity compared to healthy controls |
| Inguanzo et al. (2021) | 62 PD | NA | NA | NA | 26% | NA | Hierarchical clustering with Ward's method on grey matter and fractional anisotropy data |
Attention/working memory: TMT (A & B), digit span (forward & backward), Stroop, SDMT Executive: phonemic fluency, semantic fluency Language: BNT Memory: RAVLT (immediate, delayed, recognition) Visuospatial: JLO, VFDT, FRT |
3‐T structural MRI: voxel‐based morphometry and fractional anisotropy |
|
PD1: showed characteristic posterior‐cortical atrophy. Reduced grey matter in occipital, medial frontal, orbital and temporal cortices compared to controls. Reduced grey matter of subcortical regions (bilateral putamen, caudate, thalamus, nucleus accumbens, hippocampus) compared to controls and PD3. Reduced grey matter of subcortical regions (thalamus, amygdala, putamen, hippocampus) compared to PD2. Reduced fractional anisotropy compared to controls mainly in fronto‐occipital tracts PD2: cortical atrophy only (bilateral orbital, medial prefrontal, and temporal) compared to controls. Reduced grey matter in right middle temporal gyrus compared to PD3. Greater grey matter volume of the cerebellum compared to controls and PD3 PD3: no detectable atrophy compared to HC |
| Kawabata et al. (2018) | 72 PD | 68.5 ± 7.8 | 13.8 ± 3.2 |
MMSE: 28.7 ± 0.7 ACE‐R: 88.5 ± 4.1 |
54% | 5.6 ± 3.3 | Hierarchical clustering with Ward's method |
The five sub‐scores of the ACE‐R: Attention/Orientation Memory Fluency Language Visuospatial |
3‐T rs‐fMRI: group‐ICA dual regression, and regional FC analyses |
|
Compared to PD‐NC and PD‐NA, PD‐A had reduced FC in precuneus and posterior cingulate cortex within the ventral DMN. PD‐A also showed lower FC compared to HC in left cuneus within the visuospatial network. Compared with the HC and PD‐NC, PD‐NA demonstrated reduced FC in lingual gyrus within the primary visual network and in lingual gyrus and calcarine gyrus within the medial visual network. This difference was larger between PD‐NA and HC than PD‐NA and PD‐NC. PD‐NA showed reduced FC in bilateral cerebellar lobule within the cerebellum–brainstem network relative to PD‐A. PD‐A displayed lower mean regional FC in the ventral DMN than PD‐NA and PD‐NC. PD‐A and PD‐NA were likely to show lower mean regional FC in the visuospatial network, primary visual network, and medial visual network ROIs compared to HC |
| 3‐T MRI: voxel‐based morphometry | PD‐A group showed reduced grey matter volume in left amygdala, right rectal gyrus, and right middle occipital gyrus compared to age‐ and gender‐ matched HC. No differences between PD‐A, PD‐NC, and PD‐NA | ||||||||||
| LaBelle et al. (2017) | 424 de novo PD | 61.7 ± 9.7 | 15.5 ± 3 | NR | 34% | 0.5 ± 0.5 | Latent class analysis |
Learning and memory: HVLT‐R (immediate & delayed) Visuospatial: JLO Working memory: LNS Processing speed: SDMT Language: semantic fluency |
NA |
|
NA |
| Bayram et al. (2019), using LaBelle et al. (2017) clusters | 122 de novo PD | 63.5 ± 7.3 | 14.8 ± 2.6 | MoCA: 27.0 ± 2.2 | 28% | 0.5 ± 0.5 | 3‐T structural MRI: deformation‐based morphometry |
Weak: most widespread pattern of atrophy compared to typical class (lateral and inferior temporal regions, posteromedial and lateral frontal regions, insula and motor cortex) Weak‐visuospatial/strong‐memory: atrophy in prefrontal, lateral temporal, parietal, insular, motor cortex, and subcortical regions compared to typical class. Atrophy in left frontotemporal, precentral gyrus, right frontal, and putamen compared to typical Weak‐visuospatial: atrophy in left rolandic operculum including the precentral gyrus and the insula compared to typical Amnestic: atrophy in lateral temporal, parietal, occipital, insular, and motor cortex compared to typical Strong: no atrophy patterns compared to typical |
|||
| Liepelt‐Scarfone et al. (2012) | 97 PD, 24 PDD | 68.7 ± 6.9 | NR | MMSE: 26.6 ± 2.6 | 33% | 6.6 ± 5.1 | Hierarchical cluster analysis |
19 neurocognitive variables (Tower of London, TMT (A & B), digit span (forward & backward), figure test, word‐list memory (false positives, recall, recognition), BNT, semantic & phonemic fluency, CERAD (praxis & delay), WMS‐R logical memory, VOSP, BAXT, alertness, Go‐Nogo) reduced down to six factors through PCA: Factor 1: frontal lobe function Factor 2: word‐list memory and recall Factor 3: attention Factor 4: logical memory Factor 5: praxis and visual perception Factor 6: fluency and naming ability |
NA |
|
NA |
| Pourzinal et al. (2020) | 85 PD | 68.5 ± 8.5 | 13.0 ± 3.5 | MoCA: 25.2 ± 2.8 | 42% | 5.9 ± 5.8 | k‐means cluster analysis |
Posterior‐cortical: BVMT and HVLT (immediate & delayed), category fluency, BNT Frontal: TMT (A & B‐A), phonemic fluency, working memory subscale from the PD‐CRS |
NA |
|
NA |
| Uribe et al. (2016) | 88 PD | 60.4 ± 9.3 | 10.6 ± 4.8 | MMSE: 29.0 ± 1.0 | 42% | 8.0 ± 5.6 | Hierarchical clustering with Ward's method on cortical thickness data |
Executive: phonemic and semantic fluency Memory: RAVLT (total & recall) Attention/working memory: digit span (forward & backward), Stroop (word, colour, & word‐colour), SDMT, TMT (A, B, & A‐B) Language: BNT Visuospatial: VFDT, JLO |
3‐T structural MRI |
|
Pattern 1: cortical thinning in posterior cingulate/isthmus of the cingulate gyrus, precuneus, precentral gyrus compared to Pattern 2. Significant cortical thinning in lateral and medial regions bilaterally, including precentral gyrus, inferior and superior parietal areas, cuneus, posterior cingulate gyrus, and parahippocampal gyrus compared to Pattern 3. Reduced cortical thickness in lateral and medial regions bilaterally, including the precentral gyrus, inferior and superior parietal areas, cuneus, posterior cingulate gyrus, and parahippocampal gyrus compared to healthy controls Pattern 2: cortical thinning in dorsolateral and orbital frontal regions compared to Pattern 1, and cortical thinning in superior parietal and occipital areas and left dorsolateral frontal cortex compared to Pattern 3. Also showed cortical atrophy in bilateral superior parietal and occipital areas and bilateral frontal regions such as middle frontal, orbitofrontal, and right anterior superior frontal regions compared to controls Pattern 3: cortical thinning in the left medial orbitofrontal cortex compared to Pattern 1 and no cortical thinning compared to controls |
| Uribe et al. (2018) | 77 de novo PD | 63.1 ± 8.1 | 16.0 ± 6.0 | MoCA: median = 27.5, IQR = 3.0 | 38% | 1 ± 1.9 | Hierarchical clustering with Ward's method on cortical thickness data |
Memory: HVLT‐R (total, delayed, recognition) Visuospatial: JLO Attention/working memory: SDMT and LNS Executive: phonemic (letter "f") and semantic (animal) fluency |
3‐T structural MRI |
|
Pattern 1: cortical thinning in bilateral orbitofrontal, anterior cingulate, and lateral and medial anterior temporal gyri Pattern 2: cortical thinning in bilateral occipital gyrus, cuneus, superior parietal gyrus, and left postcentral gyrus |
Abbreviations: ACE‐R, Addenbrooke's Cognitive Examination‐Revised; BAXT, Berlin Apraxia Test; BNT, Boston Naming Test; BVMT, Brief Visuospatial Memory Test; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; DMN, default mode network; DRS‐2, Dementia Rating Scale 2; FC, functional connectivity; FRT, forced response test; HC, healthy controls; HVLT‐R, Hopkin's Verbal Learning Test Revised; ICA, independent component analysis; IQR, interquartile range; JLO, Judgment of Line Orientation; LNS, Letter Number Sequencing; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; NA, not applicable; NR, not reported; PCA, principal component analysis; PD, Parkinson disease; PD‐A, PD‐amnestic; PD‐CRS, Parkinson's Disease Cognitive Rating Scale; PDD, PD dementia; PD‐NA, PD‐nonamnestic; PD‐NC, PD‐normal cognition; RAVLT, Rey Auditory Verbal Learning Test; ROI, region of interest; rs‐fMRI, resting‐state functional MRI; SDMT, Symbol Digit Modality Test; TMT, Trail Making Test; VFDT, Visual Form Discrimination Test; VOSP, Visual Object and Space Perception; WMS‐R, Weschler Memory Scale Revised.
TABLE 3.
Longitudinal studies
| Citation | Samples characteristics, mean ± SD | Follow‐up period | Subtyping method | Cluster variables | Imaging method | Description of clusters (% prevalence) | Neuroimaging results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age, years | Education, years | Global cognition | Gender, % female | Disease duration, years | |||||||
| Andersson et al. (2021) | 349 PD | 63.6 ± 7.5 | 15.5 ± 3.0 | NA | 35% | 0.5 ± 1.1 | Annually for 5 years | Multivariate latent class linear mixed model |
Composite scores used for each domain Memory: HVLT (immediate & delayed) Executive: SDMT, LNS, semantic fluency Visuospatial: JLO |
NA |
|
NA |
|
Uribe et al. (2019), using Uribe et al. (2016) clusters |
88 PD | 60.4 ± 9.3 | 10.6 ± 4.8 | MMSE: 29.0 ± 1.0 | 42% | 8.0 ± 5.6 | 3.8 ± 0.4 years | Hierarchical clustering with Ward's method on cortical thickness data |
Executive: phonemic and semantic fluency Memory: RAVLT (total & recall) Attention/working memory: digit span (forward & backward), Stroop (word, colour & word‐colour), SDMT, TMT (A, B, & A‐B) Language: BNT Visuospatial function: VFDT, JLO |
3‐T structural MRI |
See Uribe et al. (2016)
|
High attrition rates (78%) for Pattern 1 prevented analysis on the neuroimaging or cognitive data Pattern 2 (55% attrition): subcortical grey matter atrophy over time. Reductions in left parahippocampal gyrus and precuneus and right inferior parietal and temporal gyri, fusiform and lateral occipital gyri thickness over time. No difference in cortical thickness decline compared to controls Pattern 3 (25% attrition): cortical grey matter atrophy over time. Cortical thinning of lateral and medial regions of temporal and parietal lobes, lateral occipital and extending to frontal regions such as the precentral and postcentral gyri and left pars opercularis. Increased cortical thinning in the left pars opercularis and precentral gyri over time compared to healthy controls |
Abbreviations: BNT, Boston Naming Test; HVLT, Hopkin's Verbal Learning Test; JLO, Judgment of Line Orientation; LNS, Letter Number Sequencing; MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging; NA, not applicable; PD, Parkinson disease; RAVLT, Rey Auditory Verbal Learning Test; SDMT, Symbol Digit Modality Test; TMT, Trail Making Test; VFDT, Visual Form Discrimination Test.
DISCUSSION
This is the first systematic review of data‐driven evidence for cognitive subtypes in PD. Severity‐based and domain‐based subtype structures were identified, with a gradient of global brain alterations among the severity‐based subtypes, and unique patterns of brain alterations revealed among the domain‐based subtypes. Although the number of subtypes varied across domain‐based models, some recurring phenotypes were revealed. Studies clustering structural brain imaging data and employing longitudinal methods provided additional information from a novel perspective.
Severity‐based models
All of the severity‐based models clustered cognitive data, the majority of which revealed three subtypes ranging from cognitively intact to severely impaired [13, 16, 17, 18]. Dujardin et al. [14, 15], using two distinct cohorts, revealed five subtypes ranging from cognitively intact to severely impaired, with high prevalence of PD dementia in the more severe clusters. Three subsequent studies investigated the neural correlates of these clusters [19, 20, 21]. However, all three studies combined the two most severely impaired clusters for their analyses, and Hassan et al. [20] also combined the two least impaired clusters due to their small sample sizes. Across their three groups, Hassan et al. [20] employed electroencephalography. They reported reduced frontotemporal alpha band connectivity in the moderately impaired group compared to the cognitively intact, and reduced frontocentral, frontotemporal, frontofrontal, and occipitocentral alpha connectivity in the severely impaired group compared to the moderately impaired. This suggests disintegration of frontotemporal connectivity may occur first in the cognitive progression toward dementia, followed by more pervasive connectivity deficits as impairment advances. Power spectral density in the delta and theta bands also increased with global impairment, further confirming the spread of functional dysconnectivity.
Using resting‐state functional magnetic resonance imaging (rs‐fMRI), Lopes et al. [21] found reduced brain segregation with increasing global impairment, reflected by lower local efficiency and higher global efficiency of resting‐state networks with each level of impairment. This was speculated to reflect increased randomness of brain networks as cognitive ability decreased. Finally, Wolters et al. [19] employed voxel‐ and vertex‐based morphometry techniques to explore structural MRI differences between the clusters. They found reductions in grey matter and cortical folding of temporal regions in the severely impaired group compared to the cognitively intact group, which is expected given this group encompasses patients with mild dementia. Taken together, all three neuroimaging studies revealed how alterations in functional and structural integrity of the brain contribute to global cognitive decline [19, 20, 21].
Domain‐based models
Most domain‐based models clustered cognitive data to reveal subtypes; however, there was more variability in the resulting phenotypes. Alonso‐Recio et al. [22] revealed four subtypes: executive dysfunction, memory dysfunction, memory and executive dysfunction, and cognitively intact. Using similar measures, Pourzinal et al. [27] also revealed four subtypes from a series of frontal‐ and posterior‐based measures: posterior‐cortical impairment, frontal impairment, global impairment, and cognitively intact. Brennan et al. [23] revealed three subtypes: cognitively intact, amnestic (i.e., memory impairment), and mixed deficit. In contrast, Liepelt‐Scarfone et al. [25] performed clustering on a series of cognitive factors derived from an exploratory factor analysis. This methodology revealed a subtype with global cognitive deficits across all factors, and a subtype with attention, memory, and visuospatial deficits.
Kawabata et al. [26] derived amnestic and nonamnestic subtypes by clustering subscores of the Addenbrooke's Cognitive Examination‐Revised. Subsequent rs‐fMRI analysis revealed that, compared to the nonamnestic subtype and a group of cognitively intact PD patients, the amnestic subtype demonstrated reduced functional connectivity in various posterior regions of the brain related to the default mode, primary visual, and medial visual networks. Relative to amnestic patients, nonamnestic patients exhibited reduced functional connectivity in the cerebellum within the cerebellum–brainstem network. Voxel‐based morphometry also showed reduced grey matter volume of the amygdala, middle occipital gyrus, and rectal gyrus in the amnestic group compared to healthy controls, but no structural differences between the three PD groups. Altogether, the results implicate posterior brain regions in PD‐related memory impairment and suggest a more profound role of the cerebellum in nonamnestic deficits in PD.
LaBelle et al. [24] revealed six subtypes: a weak‐overall group, two similar high‐performing groups (typical‐overall and strong‐overall), and three subtypes specific to domain (amnestic, strong‐memory, and weak‐visuospatial). A subsequent study compared structural MRI data of the six subtypes [30]. Compared to a “typical‐performing” subtype, they found unique patterns of atrophy in all subtypes except for the cognitively intact “strong” group, which remained structurally intact. Interestingly, the authors recognized that the amnestic subtype, with primarily posterior (temporoparieto‐occipital) regions of atrophy, and the weak subtype, with widespread atrophy, most closely resembled a PD‐MCI profile.
Crowley et al. [31] derived memory‐impaired, executive‐impaired, and cognitively intact subtypes and compared their grey and white matter structural MRI features. The executive‐impaired subtype demonstrated reduced subcortical grey and white matter relative to healthy controls, particularly in the putamen. Right entorhinal‐hippocampal (ERC‐HIPP) connectivity and right dorsolateral‐prefrontal cortex to caudate nucleus (DLPFC‐CN) connectivity were also reduced compared to controls. The memory‐impaired subtype showed only reduced bilateral ERC‐HIPP connectivity compared to controls, and the cognitively intact subtype showed reduced putamen volume and right ERC‐HIPP relative to controls. The results suggest that executive impairments are associated with widespread atrophy, and although reduced integrity of the right ERC‐HIPP may be intrinsic to PD, bilateral degeneration is indicative of amnestic impairments.
Three studies used the inverse approach of the aforementioned studies, performing cluster analyses on structural MRI data and comparing the resulting cognitive profiles to identify domain‐based subtypes [32, 33, 34]. Uribe et al. [32, 33] clustered whole‐brain cortical thickness data to report two general patterns of atrophy relating to frontal and posterior regions of the brain. Their first study [32] revealed distinct differences between patterns in cognitive performance, despite some overlap. Atrophy primarily in the orbitofrontal region was associated with deficits in processing speed and attention/working memory, and posterior (temporoparietal) atrophy was associated with additional deficits in semantic and episodic memory. They also revealed a third, cognitively intact, phenotype with no detectable atrophy compared to healthy controls. In their subsequent study, however, Uribe et al. [33] revealed less distinctive phenotypes, with the posterior (parieto‐occipital) pattern of atrophy corresponding to episodic and working memory impairments, and the orbitofrontal pattern of atrophy not demonstrating any deficits. Finally, Inguanzo et al. [34] clustered grey and white matter features to reveal three subtypes: (i) a cognitively intact subtype with no atrophy relative to controls; (ii) a subtype exhibiting cortical (orbital, medial prefrontal, and temporal) atrophy, increased grey matter volume of the cerebellum, and executive and attentional deficits; and (iii) a subtype with widespread cortical and subcortical atrophy, reduced integrity of fronto‐occipital white matter tracts, and poor performance across all cognitive domains. Interestingly, these results again indicate an association between executive/attentional function and cerebellum integrity.
Longitudinal studies
Uribe et al. [28] explored changes in cognitive performance and brain structure of the subtypes from Uribe et al. [32] at 4‐year follow‐up. They reported that Pattern 1, defined by posterior atrophy at baseline and older age of onset, was excluded due to high attrition rates (78%). Noncompleters from the globally impaired Pattern 1 subtype demonstrated greater functional disability, intellectual disability, and dementia symptoms than noncompleters from Pattern 2 and Pattern 3, alluding to a more rapid disease progression and cognitive decline in this group. Pattern 2, defined by orbitofrontal atrophy at baseline, exhibited decreases in semantic fluency and attention and processing speed, and increased posterior atrophy over time, comparable to that of healthy controls. Moreover, their stable global cognitive ability over time suggests that the Pattern 2 subtype may have a gradual cognitive progression despite domain‐specific declines. Pattern 3, defined by intact brain structure at baseline and stable global cognition over time, exhibited widespread atrophy over time comparable to controls. They only showed greater atrophy than controls in the frontal lobe, accompanied by reduced processing speed and attention. Importantly, the three subtypes did not differ in terms of disease duration or severity at baseline, suggesting that their different trajectories are independent of disease evolution.
Taking a different approach altogether, Andersson et al. [29] clustered longitudinal cognitive data to define subtypes by their rate of cognitive decline over 5 years. They revealed a majority group with relatively stable cognition over time and a much smaller group with rapid cognitive decline. Interestingly, rapid decliners showed mild impairments across the memory and executive composite measures at baseline, whereas the cognitively stable group did not. The rapid decliners were also older than those who maintained stable cognition, and had lower levels of cerebrospinal fluid amyloid‐ß42, a prominent Alzheimer disease (AD) biomarker. As composite scores were used for each cognitive domain, it is unclear whether any standalone neurocognitive test was a predictor of rapid decline. Regardless, the findings question the role of AD pathology in PD cognition.
Clinical and neuroimaging correlates of subtypes
Severity‐based subtypes
Considering the variety of cognitive symptoms at any given level of global impairment, severity‐based subtyping does not address the heterogeneity of cognition in PD. However, it successfully highlights the clinical correlates of cognitive decline. For example, older age [14, 15, 17] and lower education [13, 14, 15, 16, 17] were repeatedly associated with severe impairment. The less impaired clusters had lower disease duration [14, 17], disease severity [14, 18], functional disability [13, 14], and levodopa intake [14] than the most impaired clusters, and the more impaired clusters expressed greater depression [14, 15, 18], apathy [14, 15, 18], and anxiety [15, 18] symptoms than the less impaired clusters. These findings align with well‐established associations between neuropsychiatric symptoms and global cognition in PD [35, 36]. Neurobiologically, subtypes with more severe cognitive deficits typically demonstrated more widespread global alterations in functional and structural integrity of the brain [19, 20, 21].
Amnestic phenotype
Most notably, a subtype characterised by memory deficit was revealed in all but three studies [25, 32, 33]. The amnestic [23, 24, 26], memory dysfunction [22, 31], Pattern 1 [32], and posterior‐cortical [27] subtypes were all defined by impairments in memory recall. The prevalence ranged from 12% [24] to 42% [27]. These groups were generally older (mean age range = 64–72 years), composed of more males than cognitively intact groups [23, 24, 27], and had later age at onset compared to executive [32] and cognitively intact subtypes [26]. Education did not differ from other phenotypes in all studies but one, in which education was lower than the executive subtype [32]. Two studies revealed higher disease severity in amnestic compared to cognitively intact clusters [23, 24]. This was independent of disease duration and education, suggesting a more rapid and severe disease progression for this subtype from diagnosis. Reduced functional connectivity within posterior regions of the default mode network and visuospatial network [26], volumetric grey matter alterations in the amygdala, right rectal gyrus, and right middle occipital gyrus [26], unique temporoparieto‐occipital atrophy [30], cortical thinning in precentral, posterior cingulate, and parahippocampal gyri, cuneus, and inferior and superior parietal areas [32], and reduced bilateral ERC‐HIPP white matter connectivity were characteristic of the amnestic subtype relative to other subtypes [31]. Despite involvement of some frontal regions, the amnestic subtype appears to be associated primarily with dysfunction and atrophy of temporal and posterior‐cortical regions of the brain.
Executive phenotype
Subtypes with prominent executive deficits also appeared several times across the domain‐based studies [22, 27, 32]. The executive dysfunction [22, 31], frontal [27], Pattern 2 [32], and PD2 [34] subtypes were all defined by executive function or attention/working memory deficits. Prevalence varied greatly between studies (8% [27], 33% [22]), and these subtypes were associated with relatively younger age (mean age range = 61–69 years) [22, 27, 32], earlier age at onset than amnestic [32], and higher education than amnestic [32] and globally impaired [27] subtypes. In the executive subtypes ‐ relative to other subtypes or healthy controls‐neural changes included prominent frontal (dorsolateral prefrontal, orbital frontal) cortical thinning [32], lower total brain, putamen, and thalamus volume, reduced right ERC‐HIPP and DLPFC‐CN connectivity [31] and cortical (bilateral orbital, medial prefrontal, and temporal) atrophy, and increased cerebellum grey matter volume [34]. Although these findings demonstrate the greater involvement of "frontal" regions in the executive‐impaired phenotype, particularly compared to the amnestic phenotype, alterations in some posterior and subcortical regions remain a core feature of the subtype.
Globally impaired phenotype
Another commonality across domain‐based studies was the global subtype, which was present in all cluster structures except for two [26, 31]. Below‐average performance across most or all of the cluster variables defined this subtype, and it was associated with older age (mean age range = 66–75) [22, 24, 27, 34], lower education [13, 24, 27], later age at onset [34], and greater motor symptoms [18, 24, 27], disease severity [18, 23, 27], and disease duration [23]. The prevalence of the global subtype ranged from 5% [24] to 56% [25], with the lower rate likely due to de novo samples. Neurodegeneration in this subtype was rampant, with widespread atrophy across frontal, posterior, and subcortical regions of the brain characteristic of this subtype [30, 32, 34].
Cognitively intact phenotype
Cognitively intact subtypes, defined by average or above‐average performance on all cognitive measures, were present in all but one of the domain‐based studies [25]. In terms of clinical characteristics, this subtype was generally associated with younger age (mean age range = 56–62) [22, 24, 27, 34], higher education [13, 24, 27], and less severe motor symptoms [18, 24, 26, 27], disease severity [18, 23, 27], and disease duration [23]. Its prevalence ranged from 15% [22] to 67% [24], with the higher rate likely due to de novo samples. This subtype had relatively high functional and structural integrity of the brain [26, 30, 31, 32, 34]. This integrity was generally maintained over time, with some decline in processing speed, semantic fluency, and attention related to cortical thinning of the inferior frontal and precentral gyri above and beyond normal ageing [28].
Methodological considerations
Why do we see severity‐ versus domain‐based models?
Several methodological factors may explain the derivation of severity‐based over domain‐based phenotypes. Studies that included global cognitive measures as cluster variables may have fostered clusters based on cognitive severity due to the direct contribution of global cognitive performance to the algorithm [14, 15, 17]. Furthermore, although there are no strict parameters for cluster analyses, the ratio of variables to participants should be restricted to limit dimensionality of the dataset [37]. Extradimensionality of the data may therefore explain the moderate “uncertain” grouping in McKinlay et al. [16] (variables = 29; N = 40), which was neither clinically nor theoretically significant. Finally, as k‐means clustering is sensitive to outliers [38], the studies [14, 15, 17, 18] that used untransformed raw scores or z‐scores may have produced clusters disproportionately based on outliers. This effect is particularly relevant for studies that did not exclude dementia [13, 14, 17], as severe global impairments may weigh heavily on the cluster structure to create groupings based on magnitude rather than type of impairment.
Sample characteristics
Differences in sample characteristics would contribute to the varied prevalence of specific domain‐based subtypes. For example, LaBelle et al. [24] studied newly diagnosed participants from the Parkinson's Progressive Marker Initiative (PPMI) [39], explaining their low prevalence (12%) of the amnestic subtype, which is typically associated with longer disease duration [40]. Discrepancy between the Uribe et al. [32, 33] studies may also be due to their initial use of the de novo PPMI [39] sample (disease duration, mean ≈ 2 years) [33], which contrasts with the more advanced disease duration (mean ≈ 8 years) of the subsequent Uribe et al. [32] sample. Milder cognitive symptoms and atrophy are expected in the less evolved disease state, potentially explaining the differences in clustering and atrophy patterns between the two cohorts.
Cluster variables
The choice and number of cluster variables also largely influences interpretation of the resulting domain‐based cluster structure. Limited executive and attention/working memory measures in the PPMI protocol may have therefore resulted in inadequate assessment of these functions, and thus poor characterization of the executive or frontal subtype. This reasoning is used by Uribe et al. [33] to justify the marked cortical thinning in orbitofrontal regions of the Pattern 1 subtype despite an apparent absence of executive impairment. Bayram et al. [30] also did not reveal an executive subtype using PPMI data, with only one of six variables assessing executive/attentional ability. Similarly, only two of the eight variables employed within Brennan et al. [23] were executive/attentional measures, and an executive subtype was not revealed.
Implications
Cautious selection of measures within domains
The assignment of measures to cognitive domains is limited by the intricate interconnectedness of cognitive processes [41]. However, measures that are selected within domains should be as distinct as possible and remain consistent for the purposes of interpreting data. In this sense, the present review highlights major flaws in the current literature. For example, semantic fluency was considered either a test of memory, executive functioning, or language among the reviewed studies and thus supported different interpretations across studies. The combining of scores across measures, either through factor analysis or by creating composite z‐scores for a given domain, was also troublesome. Take, for example, the executive composite from Andersson et al. [29], which averaged across three measures (letter‐number sequencing, symbol digit modalities task, and semantic fluency) that could also be considered attention/working memory, or language, or memory tasks. In the same vein, Liepelt‐Scarfone et al. [25] derived clusters from six cognitive factor scores identified through factor analysis. Although this method is strongly data‐driven, the Trail Making Test Part A fell under the “fluency and naming ability” factor, of which it is neither, and the “word‐list memory and recall” factor encompassed recognition and intrusion subscales, which are distinct memory processes. Researchers should select measures within domains with caution, and be aware that combining scores across measures, although useful for statistical purposes, conflates distinct cognitive processes and hinders interpretation of results.
Which measures and domains are relevant to PD‐MCI?
The present review also calls into question the relevance of certain cognitive measures and domains to PD cognitive subtypes. For instance, the Judgment of Line Orientation task was employed in almost every study to measure visuospatial function, yet this measure failed to discriminate subtypes in most instances. Similarly, the Boston Naming Test showed consistently poor discriminative ability, only discerning the cognitively intact subtype from the rest of the sample in all studies that utilized it [22, 23, 27]. On the whole, evidence from the Uribe et al. [28, 32, 33] studies suggests that memory and executive/attention measures are sensitive to unique patterns of atrophy in PD, whereas language and visuospatial measures do not appear to discriminate underlying neural changes occurring in PD cognitive subtypes. Some work has been done to map cognitive measures onto specific brain regions in PD [42], but further research is needed to confirm clinically significant regions and corresponding measures to implement in PD‐MCI criteria to better discriminate subtypes and advance discovery of neural drug targets for treatment.
Future of machine learning in PD research
The reviewed evidence highlights the importance of delineating data‐driven cognitive subtypes within PD. Neuroimaging evidence provided some support for the dual syndrome dichotomy [5], which is consistent with recent findings showing a dissociation in the resting‐state networks of frontal “dysexecutive” and posterior‐cortical cognitive subtypes in PD [43]. Other subtyping structures revealed in the review include an amnestic/nonamnestic or memory/executive dichotomy. Despite the lack of consensus, the present review demonstrates the emerging role of machine learning in PD cognitive subtyping and highlights the need for further revision of PD‐MCI criteria. The data‐driven approach reduces the number of a priori assumptions needed for analysis, allowing identification of subtypes based on trends in the data rather than parameters imposed by the researcher. This method of subtyping has facilitated the much‐needed stratification of PD patients, which is vital for clinical trials and biomarker studies that require more homogenous patient groups to reveal their effects [44].
Limitations and suggestions for future research
The exploratory nature of the studies reduces the likelihood that this review was affected by a reporting bias skewed toward significant results. However, a notable limitation is the scarcity of longitudinal data, which are essential for determining the prognosis of subtypes. Although the dual syndrome hypothesis suggests that patients with posterior impairments will progress toward dementia more rapidly than the other phenotypes [5], this remains to be addressed using data‐driven methodology. Moreover, there was considerable variability in subtype structures. This may be attributed to a multitude of factors related to clustering methodology (e.g., type and number of cognitive measures, sample size, clustering algorithm). However, it is also possible that certain subtypes simply were not prevalent within some samples due to convenience sampling. Additionally, inherent differences in sample characteristics related to cognitive ability such as age, proportion of males, disease duration, and motor severity undeniably account for some of the conflicting results.
Data‐driven analyses may also be limited in their clinical application, as they provide information about trends at the group level. Although this is useful for creating generalizable diagnostic criteria, information at the individual level is required to evaluate suitability and relevance of subtypes for patients to assist personalized precision medicine approaches. Cluster structures are also limited in that they do not translate directly to cutoff scores on neurocognitive measures. More research is required to define phenotypes in terms of standardized neurocognitive test scores and create applicable diagnostic methodology. Finally, the various neuroimaging modalities employed hinder comparison of results across studies.
CONCLUSIONS
The present review aimed to provide a concise and critical summary of the data‐driven evidence for cognitive subtypes in PD. A systematic search revealed two general subtype structures: those based on global cognitive severity and those based on domains of impairment. Severity subtypes provided insight into the clinical features of cognitive decline in PD yet lacked specific cognitive profiling, whereas domain‐based studies revealed support for subtyping based on distinct cognitive processes. Similarly, studies exploring subtypes based on impairment severity found a gradient of global brain alterations, whereas studies exploring subtypes based on impairment domain revealed unique patterns of brain alterations. Preliminary longitudinal evidence for diverging neuroanatomical prognoses in PD cognitive subtypes encourages further research in this area.
AUTHOR CONTRIBUTIONS
Dana Pourzinal: Conceptualization (equal); data curation (lead); investigation (lead); methodology (lead); project administration (lead); writing – original draft (lead); writing – review and editing (equal). Jihyun Yang: Methodology (supporting); supervision (supporting); writing – review and editing (equal). Rachael A. Lawson: Writing – review and editing (equal). Katie L. McMahon: Supervision (equal); writing – review and editing (equal). Gerard J. Byrne: Funding acquisition (equal); supervision (equal); writing – review and editing (equal). Nadeeka N. W. Dissanayaka: Conceptualization (equal); resources (lead); supervision (lead); writing – review and editing (lead).
FUNDING INFORMATION
This work was supported by the NHMRC fellowship to N.N.D. and the Australian Government Research Training Program scholarship to D.P.
CONFLICT OF INTEREST
The authors declare that there was no potential conflict of interest.
Supporting information
TABLES S1–S2
TABLES S3–S4
ACKNOWLEDGMENTS
N.N.D. is supported by the National Health and Medical Research Boosting Dementia Research Leadership Fellowship (APP1137339). Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Pourzinal D, Yang J, Lawson RA, McMahon KL, Byrne GJ, Dissanayaka NN. Systematic review of data‐driven cognitive subtypes in Parkinson disease. Eur J Neurol. 2022;29:3395‐3417. doi: 10.1111/ene.15481
DATA AVAILABILITY STATEMENT
n/a.
REFERENCES
- 1. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837‐844. doi: 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- 2. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349‐356. doi: 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsiouris KM, Konitsiotis S, Koutsouris DD, Fotiadis DI. Prognostic factors of rapid symptoms progression in patients with newly diagnosed Parkinson's disease. Artif Intell Med. 2020;103:101807. doi: 10.1016/j.artmed.2020.101807 [DOI] [PubMed] [Google Scholar]
- 4. Vasconcellos LFR, Pereira JS, Charchat‐Fichman H, et al. Mild cognitive impairment in Parkinson's disease: characterization and impact on quality of life according to subtype. Geriatr Gerontol Int. 2019;19(6):497‐502. doi: 10.1111/ggi.13649 [DOI] [PubMed] [Google Scholar]
- 5. Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener Dis. 2012;11(2):79‐92. doi: 10.1159/000341998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams‐Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson's disease: 10‐year outlook in an incident population‐based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258‐1264. doi: 10.1136/jnnp-2013-305277 [DOI] [PubMed] [Google Scholar]
- 7. Beijers L, Wardenaar KJ, van Loo HM, Schoevers RA. Data‐driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol Psychiatry. 2019;24(6):888‐900. doi: 10.1038/s41380-019-0385-5 [DOI] [PubMed] [Google Scholar]
- 8. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 2015;72(8):863‐873. doi: 10.1001/jamaneurol.2015.0703 [DOI] [PubMed] [Google Scholar]
- 9. Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quidé Y. A systematic review of studies reporting data‐driven cognitive subtypes across the psychosis spectrum. Neuropsychol Rev. 2019;30(4):446‐460. doi: 10.1007/s11065-019-09422-7 [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta‐analysis. PLoS One. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells G, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. 2009.
- 13. Barvas E, Mattavelli G, Zappini F, Giardina F, Ottaviani D, Papagno C. Cognitive phenotypes in Parkinson's disease: a latent profile analysis. Neuropsychol. 2021;35(4):451‐459. doi: 10.1037/neu0000737 [DOI] [PubMed] [Google Scholar]
- 14. Dujardin K, Leentjens AFG, Langlois C, et al. The spectrum of cognitive disorders in Parkinson's disease: a data‐driven approach. Mov Disord. 2013;28(2):183‐189. doi: 10.1002/mds.25311 [DOI] [PubMed] [Google Scholar]
- 15. Dujardin K, Moonen AJH, Behal H, et al. Cognitive disorders in Parkinson's disease: confirmation of a spectrum of severity. Parkinsonism Relat Disord. 2015;21(11):1299‐1305. doi: 10.1016/j.parkreldis.2015.08.032 [DOI] [PubMed] [Google Scholar]
- 16. McKinlay A, Grace RC, Dalrymple‐Alford JC, Roger D. Cognitive characteristics associated with mild cognitive impairment in Parkinson's disease. Dement Geriatr Cogn Disord. 2009;28(2):121‐129. doi: 10.1159/000235247 [DOI] [PubMed] [Google Scholar]
- 17. Souza CP, Oliveira GN, Foss MP, Tumas V. Cluster analysis of cognitive performance in a sample of patients with Parkinson's disease. Dement Neuropsychol. 2016;10(4):315‐319. doi: 10.1590/s1980-5764-2016dn1004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenney LE, Ratajska AM, Lopez FV, Price CC, Armstrong MJ, Bowers D. Mapping actuarial criteria for Parkinson's disease‐mild cognitive impairment onto data‐driven cognitive phenotypes. Brain Sci. 2022;12(1). doi: 10.3390/brainsci12010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolters AF, Moonen AJH, Lopes R, et al. Grey matter abnormalities are associated only with severe cognitive decline in early stages of Parkinson's disease. Cortex. 2020;123:1‐11. doi: 10.1016/j.cortex.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 20. Hassan M, Chaton L, Benquet P, et al. Functional connectivity disruptions correlate with cognitive phenotypes in Parkinson's disease. Neuroimage Clin. 2017;14:591‐601. doi: 10.1016/j.nicl.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopes R, Delmaire C, Defebvre L, et al. Cognitive phenotypes in Parkinson's disease differ in terms of brain‐network organization and connectivity. Hum Brain Mapp. 2017;38(3):1604‐1621. doi: 10.1002/hbm.23474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alonso‐Recio L, Martin‐Plasencia P, Ruiz M, Serrano JM. Differences in cognitive performance in nondemented Parkinson's disease: a latent profile analysis of cognitive subtypes. J Clin Exp Neuropsychol. 2018;40(8):777‐789. doi: 10.1080/13803395.2018.1432570 [DOI] [PubMed] [Google Scholar]
- 23. Brennan L, Devlin KM, Xie SX, et al. Neuropsychological subgroups in non‐demented Parkinson's disease: a latent class analysis. J Parkinsons Dis. 2017;7(2):385‐395. doi: 10.3233/jpd-171081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LaBelle DR, Walsh RR, Banks SJ. Latent cognitive phenotypes in de novo Parkinson's disease: a person‐centered approach. J Int Neuropsychol Soc. 2017;23(7):551‐563. doi: 10.1017/s1355617717000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liepelt‐Scarfone I, Gräber S, Berger MF, et al. Cognitive profiles in Parkinson's disease and their relation to dementia: a data‐driven approach. Int J Alzheimers Dis. 2012;2012:1‐11. doi: 10.1155/2012/910757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawabata K, Watanabe H, Hara K, et al. Distinct manifestation of cognitive deficits associate with different resting‐state network disruptions in non‐demented patients with Parkinson's disease. J Neurol. 2018;265(3):688‐700. doi: 10.1007/s00415-018-8755-5 [DOI] [PubMed] [Google Scholar]
- 27. Pourzinal D, Yang JHJ, Byrne GJ, et al. Identifying subtypes of mild cognitive impairment in Parkinson's disease using cluster analysis. J Neurol. 2020;267(11):3213‐3222. doi: 10.1007/s00415-020-09977-z [DOI] [PubMed] [Google Scholar]
- 28. Uribe C, Segura B, Baggio HC, et al. Progression of Parkinson's disease patients' subtypes based on cortical thinning: 4‐year follow‐up. Parkinsonism Relat Disord. 2019;64:286‐292. doi: 10.1016/j.parkreldis.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 29. Andersson S, Josefsson M, Stiernman LJ, Rieckmann A. Cognitive decline in Parkinson's disease: a subgroup of extreme decliners revealed by a data‐driven analysis of longitudinal progression. Front Psychol. 2021;12. doi: 10.3389/fpsyg.2021.729755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bayram E, Bluett B, Zhuang XW, Cordes D, LaBelle DR, Banks SJ. Neural correlates of distinct cognitive phenotypes in early Parkinson's disease. J Neurol Sci. 2019;399:22‐29. doi: 10.1016/j.jns.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crowley SJ, Banan G, Amin M, et al. Statistically defined Parkinson's disease executive and memory cognitive phenotypes: demographic, behavioral, and structural neuroimaging comparisons. J Parkinsons Dis. 2021;11(1):283‐297. doi: 10.3233/jpd-202166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uribe C, Segura B, Baggio HC, et al. Patterns of cortical thinning in nondemented Parkinson's disease patients. Mov Disord. 2016;31(5):699‐708. doi: 10.1002/mds.26590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uribe C, Segura B, Baggio HC, et al. Cortical atrophy patterns in early Parkinson's disease patients using hierarchical cluster analysis. Parkinsonism Relat Disord. 2018;50:3‐9. doi: 10.1016/j.parkreldis.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 34. Inguanzo A, Sala‐Llonch R, Segura B, et al. Hierarchical cluster analysis of multimodal imaging data identifies brain atrophy and cognitive patterns in Parkinson's disease. Parkinsonism Relat Disord. 2021;82:16‐23. doi: 10.1016/j.parkreldis.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 35. Alzahrani H, Venneri A. Cognitive and neuroanatomical correlates of neuropsychiatric symptoms in Parkinson's disease: a systematic review. J Neurol Sci. 2015;356(1):32‐44. doi: 10.1016/j.jns.2015.06.037 [DOI] [PubMed] [Google Scholar]
- 36. Trojano L, Papagno C. Cognitive and behavioral disorders in Parkinson's disease: an update. II: behavioral disorders. Neurol Sci. 2018;39(1):53‐61. doi: 10.1007/s10072-017-3155-7 [DOI] [PubMed] [Google Scholar]
- 37. Steinbach M, Ertöz L, Kumar V. The challenges of clustering high dimensional data. In: Wille LT, ed. New Directions in Statistical Physics: Econophysics, Bioinformatics, and Pattern Recognition. Springer Berlin Heidelberg; 2004:273‐309. [Google Scholar]
- 38. Everitt BS, Landau S, Leese M, Stahl D. Cluster Analysis. 5th ed. John Wiley & Sons Ltd.; 2011. [Google Scholar]
- 39. Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI) – establishing a PD biomarker cohort. Ann Clin Transl Neurol. 2018;5(12):1460‐1477. doi: 10.1002/acn3.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das T, Hwang JJ, Poston KL. Episodic recognition memory and the hippocampus in Parkinson's disease: a review. Cortex. 2019;113:191‐209. doi: 10.1016/j.cortex.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neurosci. 2019;21(3):227‐237. doi: 10.31887/DCNS.2019.21.3/pharvey [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ibarretxe‐Bilbao N, Junque C, Marti MJ, Tolosa E. Brain structural MRI correlates of cognitive dysfunctions in Parkinson's disease. J Neurol Sci. 2011;310(1):70‐74. doi: 10.1016/j.jns.2011.07.054 [DOI] [PubMed] [Google Scholar]
- 43. Lang S, Hanganu A, Gan LS, et al. Network basis of the dysexecutive and posterior cortical cognitive profiles in Parkinson's disease. Mov Disord. 2019;34(6):893‐902. doi: 10.1002/mds.27674 [DOI] [PubMed] [Google Scholar]
- 44. Greenland JC, Williams‐Gray CH, Barker RA. The clinical heterogeneity of Parkinson's disease and its therapeutic implications. Eur J Neurosci. 2019;49(3):328‐338. doi: 10.1111/ejn.14094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLES S1–S2
TABLES S3–S4
Data Availability Statement
n/a.


