Abstract
Background
The use of eliciting doses (EDs) for food allergens is necessary to inform individual dietary advice and food allergen risk‐management. The Eliciting Dose 01 (ED01) for milk and egg, calculated from populations of allergic subjects undergoing oral food challenges (OFCs), are 0.2 mg total protein. The respective Eliciting Dose 05 (ED05) is 2.4 mg for milk and 2.3 mg for egg. As about 70% children allergic to such foods may tolerate them when baked, we sought to verify the EDs of that subpopulation of milk and egg‐allergic children.
Methods
We retrospectively assessed consecutive OFC for fresh milk and egg between January 2018 and December 2020 in a population of baked food‐tolerant children.
Results
Among 288 children (median age 56 ‐ IQR 36–92.5 months, 67.1% male) included, 87 (30.2%) returned positive OFC results, 38 with milk and 49 with egg. The most conservative ED01 was 0.3 mg total protein (IQR 0.03–2.9) for milk and 14.4 mg total protein (IQR 3.6–56.9) for egg. The respective ED05 was 4.2 (IQR 0.9–19.6) mg for milk and 87.7 (IQR 43–179) mg for egg. Such thresholds are, respectively, 1.5 (milk ED01), 1.75 (milk ED05), 72 (egg ED01), and 38.35 (egg ED05) times higher than the currently used thresholds.
Conclusions
The subpopulation of children allergic to milk and egg, but tolerant to baked proteins, displays higher reactivity thresholds than the general population of children allergic to milk and egg. Their risk stratification, in both individual and population terms, should consider this difference. In baked milk‐tolerant children, milk causes reactions at lower doses than egg in our group of egg‐tolerant children. This could be associated with the relative harmlessness of egg compared with milk in the determinism of fatal anaphylactic reactions in children.
Keywords: challenge tests, food allergy, nutrition, pediatrics
We assessed 288 children tolerant to beef, baked milk, and baked egg. 87 children returned positive OFC results, 38/146 with fresh milk and 49/142 with raw egg. We analyzed patients' LOAEL and NOAEL distributions by ICSA approach. The most conservative ED01 and ED05 were, respectively, 0.3 and 4.2 mg for milk and 14.4 and 87.7 mg for egg.Abbreviations: ED01 and ED05, doses predicted to elicit allergic reactions in 1% and 5% of patients; ICSA, Interval‐Censoring Survival Analysis; LOAEL, Low Observed Adverse Effect Level; NOAEL, No Observed Adverse Effect Level; OFC, Oral Food Challenge; sIgE, specific IgE; SPT, Skin Prick Test
Abbreviations
- ED01 and ED05
doses predicted to elicit allergic reactions in 1% and 5% of patients
- ICSA
Interval‐Censoring Survival Analysis
- LOAEL
Low Observed Adverse Effect Level
- NOAEL
No Observed Adverse Effect Level
- OFC
Oral Food Challenge
- sIgE
specific IgE
- SPT
Skin Prick Test
1. INTRODUCTION
Allergic reactions to milk and egg proteins are the most prevalent food allergies in European children. 1 , 2 They are a common cause of severe life‐threatening reactions in pediatric allergic patients and could persist throughout life, placing a heavy burden on the quality of life, the development of other allergic diseases, and growth. 3 , 4 , 5
In the most sensitive individuals, trace amounts of milk and egg allergens may provoke allergic reactions, even from incidental contacts as opening packages, inhaling vapors from cooking, use of shared utensils, and kissing the lips of someone who has eaten the offending food. 6 , 7 Such reactions may be life‐threatening, in particular for cow's milk. This food triggers more than 25% cases of fatal anaphylaxis in children, while egg has only exceptionally been associated with mortality for food allergy. 8
The primary and safest strategy for managing milk/egg allergy is strict avoidance of the causal food. To implement it, clinicians personalize their advices to patients informing the suggestions to their threshold of reactivity at oral food challenges (OFCs) among other clinical considerations. The population of milk‐ and egg‐allergic children includes a spectrum of more and less sensitive individuals. The former may also react to milk and egg in baked products as muffins or biscuits; less sensitive children tolerate baked foods. 9 In addition, the 10–20 percent milk‐allergic children reacting to beef are considered having a more severe and persistent Cow's Milk Allergy (CMA). 10 , 11 When OFC‐negative to baked milk and egg, children are not requested to avoid such foods. 12 Food‐allergic children with a high reactivity threshold may not be requested to avoid precautionary‐labelled prepackaged foods. 13 , 14
The use of precautionary allergen labelling (PAL) is based on population‐based threshold dose distributions for the different food allergens. 15 , 16 To this end, data from cohorts of subjects assessed in oral food challenges (OFCs) are used to calculate eliciting doses (EDs) and to specify the relation between EDs and symptoms. 17 , 18
Based on such thresholds, the Voluntary Incidental Trace Allergen Labelling (VITAL), a scheme developed by the Australian and New Zealand food industries, established reference doses of 0.2 mg total protein (Eliciting Dose 01) for milk and egg, 16 , 19 and an Eliciting Dose 05 between 0.5 and 2.4 mg protein for both foods. 16 , 20 , 21 Such thresholds have been calculated on populations of patients allergic to both raw and baked foods. As raw and baked food are not likely equivalent, we aimed to explore the EDs of the subset of children allergic to milk and egg, who tolerate beef and baked proteins. We posed the hypothesis that children allergic to native products, but not to baked foods, may have a higher reactivity threshold that may exempt them from compliance with the current PAL.
2. CASELOAD AND METHODS
2.1. Study design
At the Bambino Gesù Allergy Department, patients with suspected milk/egg allergy are periodically exposed to oral food challenges (OFCs) until they get tolerance. For safety reasons, patients are excluded from OFC if an anaphylactic reaction had occurred in the last 6 months for children of 0.5–5 years, 12 months for children of 6–12 years, and 2 years for patients aged over 13 years. 22 Also not admitted to the fresh milk/egg OFC are those reacting, respectively, to baked milk (or beef) and baked egg, which are considered fresh milk/egg allergic by default. If challenges with beef or baked food return negative, the native food is tested at OFC. 23
In this setting, we retrospectively assessed consecutive children who underwent OFC for fresh milk and egg between January 2018 and December 2020. For those who underwent repeated OFCs to the same food, we included only the first procedure for each type of food.
The clinical suspicion was placed based on the clinical history, and the entry symptoms were classified on parents' descriptions reported in the personal medical records. We only considered children with a personal history of acute IgE‐mediated allergic reactions (developing within 1–2 h after food intake) from mild‐to‐severe systemic reactions (anaphylaxis). Before the OFC, parents were exhaustively informed about the risks of the procedure and gave their written consent. On the day, we administered the OFC, the sensitivity to the respective food was tested at skin prick test (SPT) and specific IgE (sIgE) determination. Patients who had no sensitization to milk or egg demonstrated by skin test (wheal Ø ≥ 3 mm; Lofarma) or sIgE determination (≥0.35 kU/L; ImmunoCAP Thermo Fisher) were excluded.
We performed a seven steps OFC, open or blinded (double‐blind, placebo‐controlled food challenges; DBPCFC), 23 using pasteurized low‐fat milk or pasteurized whole egg (a fluid mixture of egg white and yolk). Specifically, for milk, we set the lowest dose to 3.43 mg of protein and the total amount of protein to 4955.8 mg, corresponding to 0.1 and 144.4 ml, respectively; for egg, we set the lowest dose to 12.36 mg and the total amount of protein at 8577.8 mg of proteins (about a 50 g egg and a half) (Table 1). We derived the protein content of the foods from the U.S. Department of Agriculture. 24 Patients remained under observation for at least 2 h after the last dose. The local ethics committee gave ethical approval to the use of such clinical data for this specific study.
TABLE 1.
Equivalent amount of milk and egg used in DBPCFCs
| Step dose | Pasteurized whisked hen's egg | Egg protein | Pasteurized cow's milk | Cow's milk protein |
|---|---|---|---|---|
| 1 | 100 mg | 12.36 mg | 0.1 ml | 3.43 mg |
| 2 | 300 mg | 37.08 mg | 0.3 ml | 10.29 mg |
| 3 | 1000 mg | 123.6 mg | 1 mL | 34.32 mg |
| 4 | 3000 mg | 370.8 mg | 3 ml | 102.96 mg |
| 5 | 10,000 mg | 1236 mg | 10 ml | 343.2 mg |
| 6 | 20,000 mg | 2472 mg | 30 ml | 1029.6 mg |
| 7 | 35,000 mg | 4326 mg | 100 ml | 3432 mg |
| Cumulative dose | 69,400 mg | 8577.84 mg | 144.4 ml | 4955.80 mg |
2.2. Symptom grading and threshold data
Our trained staff performed OFCs in hospital with close supervision and immediate availability of emergency treatment, according to guidelines. The same clinicians observe and value the oral food challenges' result, and the doses were administered until objective symptoms appeared. 25 , 26 Briefly, the reactive symptoms classified patients in five groups, from subjective symptoms (nausea, abdominal pain, pruritus, oral allergy syndrome) to systemic reactions (Table 2). 27 For this study, we designated the initial point at which objective symptoms occur at a specific dose as the Lowest Observed Adverse Effect Level (LOAEL) and the highest dose that does not lead to objective symptoms as the No Observed Adverse Effect Level (NOAEL). According to previous studies, we choose an Interval‐Censoring Survival Analysis (ICSA) approach to our data. This method is necessary when the exact dose that provokes a reaction in a patient is unknown, but it falls into a particular interval. Specifically, as previously described, 28 if a patient had an objective reaction at the first dose, left censoring occurred, and the NOAEL was set to zero with the LOAEL set as that first dose. If a patient did not experience an objective reaction after the 7th dose, the cumulative given dose was designated as NOAEL. In this case, we considered the data to be right‐censored, and the LOAEL set to infinity. If the patient was unable or unwilling to continue the challenge because of the subjective symptoms, we discontinued the challenge and considered the final administered dose right censored. In all other cases, interval‐censoring occurs bounded by the NOAEL and LOAEL.
TABLE 2.
Group based on grading of symptoms developed during oral food challenge (OFC) 26
| Group | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Skin | Localized pruritus, flushing, urticaria, angioedema | Generalized pruritus, flushing, urticaria, angioedema | Any of the previous | Any of the previous | Any of the previous |
| GI tract | Oral pruritus, oral “tingling,” mild lip swelling | Any of the previous, nausea and/or emesis | Any of the previous plus repetitive vomiting | Any of the previous plus diarrhea | Any of the previous loss of bowel control |
| Respiratory tract | ‐ | Nasal congestion and/or sneezing | Rhinorrea, marked congestion, sensation of throat pruritus or tightness | Any of the previous, hoarseness, “barky” cough, difficulty swallowing, dyspnea, wheezing, cyanosis | Any of the previous, respiratory arrest |
| Cardiovascular | ‐ | ‐ | Tachycardia | Any of the previous, dysrhythmia and/or mild hypotension | Severe bradycardia and/or hypotension or cardiac arrest |
| Neurological | ‐ | Change in activity | Change in activity level plus anxiety | “Light headedness,” feeling of “pending doom” | Loss of consciousness |
Note: Boldface symptoms are indications for the use of epinephrine.
2.3. Statistical analysis
We correlated the individual threshold to age and sex of patients, history of clinical manifestations, sIgE level results, or SPTs wheal size using multiple logistic regression (SPSS package, BM Corporation). A p‐value of <.05 was considered statistically significant; sIgE values >100 kU/L received a designated value of 101 kU/L.
We also used SAS 15.1 (SAS Institute) and its LIFEREG procedure to fit parametric models to the interval‐censored data. We considered Log‐normal, log‐logistic, and Weibull models to fit these data and to extrapolate EDs (ED01, ED05, ED10, ED25, and ED50), which are the doses predicted to elicit allergic reactions in 1%, 5%, 10%, 25%, and 50% of milk and egg‐allergic patient, respectively. 29 The goodness‐of‐fit was assessed by the Anderson‐Darling test. Confidence intervals (CI) were calculated and added.
3. RESULTS
3.1. Patient characteristics
Of the 304 consecutive patients evaluated, 146 completed the milk and 142 the egg OFC protocol. The mean age of the 288 patients who completed OFCs was 69.5 months (SD 46.5), the median age, together with their clinical characteristics, appears in Table 3. Ninety‐eight percent of the challenges were open, four (1.4%) were DBPCFC. Hundred‐eleven patients were reported with possible allergic reactions to both milk and egg.
TABLE 3.
Characteristics of 288 patients who completed OFC protocol
| Milk | Egg | Total | |
|---|---|---|---|
| Age (months), Median (25th,75th percentile) | 54 (32.2, 91.7) | 56 (38, 93) | 56 (36, 92.5) |
| Male, n (%) | 98 (67.1) | 96 (67.6) | 194 (67.3) |
| Atopic dermatitis, n (%) | 43 (29.4) | 61 (42.9) | 104 (36.1) |
| Respiratory allergy, n (%) | 26 (17.8) | 17 (12) | 43 (14.9) |
| Challenges performed, n | 146 | 142 | 288 |
| OFC, n (%) | 143 (97.9) | 141 (99.3) | 284 (98.6) |
| DBPCFC, n (%) | 3 (2.1) | 1 (0.7) | 4 (1.4) |
| Positive outcome, n (% of performed challenges) | 38 (26.1) | 49 (34.5) | 87 (30.2) |
| First dose responders, n (% of positive challenges) | 1 (2.6) | 1 (2) | 2 (2.3) |
| LOAEL, mg | 3.43 | 12.36 | ‐ |
Abbreviations: DBPCFC, double‐blinded placebo‐controlled food challenge; LOAEL, lower observed adverse effect level; OFC, open food challenge.
Thirty‐eight (26.1%) of the 146 patients who completed the OFC with milk had a positive result. Sixteen of them had been reported with a severe systemic reaction at clinical history, according to the established clinical criteria. 21 Fifteen patients reported allergic reactions to other foods (8 to egg) and twelve a personal history of atopic dermatitis. After food challenge, 28 (73.6%) patients developed urticarial eruptions and were classified in Group 2; three developed Group 3 reactions, and seven patients developed a Group 4 systemic reaction (four with urticaria and dyspnea, one with emesis and dyspnea, one with urticaria and mild hypotension, and one with emesis with dyspnea).
Of the 142 patients who completed OFC with egg, 49 (34.5%) were positive. Based on symptoms developed during the food challenge, we classified 26 of them (53.1%) in Group 2 (generalized urticaria). Nine had Group 3 reactions, and 11 patients experienced a systemic reaction during OFC: six with urticaria and dyspnea (one with lip edema), two with urticaria dyspnea and emesis, two with dyspnea and mild hypotension, and one with emesis with dyspnea.
Only one patient from each of the two groups developed objective symptoms on the first dose (left‐censored), with a LOAEL of 3.43 mg of milk proteins and 12.36 mg of egg proteins: both had Group 4 reactions (emesis with dyspnea). We observed at least one positive OFC for every single step‐dose for both allergens, and 10.5% and 24.5% of reactions after the 7th dose of milk and egg, respectively (Figure 1).
FIGURE 1.
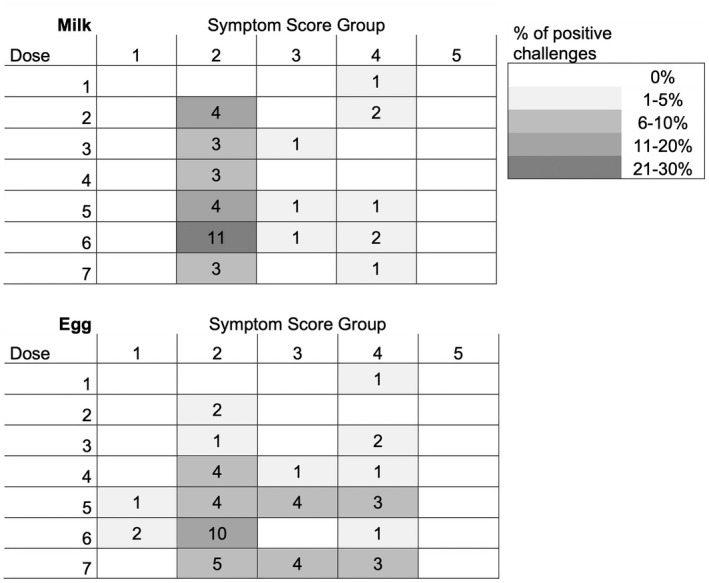
Symptom Score Group observed during milk and egg positive food challenges. The number of patients given to a particular dose is indicated in the plots. The intensity of gray shading denotes the percentage of challenged patients responding with a given symptom score (see Table 2)
Logistic regression analysis showed no significant correlation between the individual threshold dose and the following clinical characteristics of patients or in vivo/in vitro test results: age, sex, atopic diseases, manifestation referred, SPTs wheal size, sIgE level, type of symptoms developed at OFC (see Table S1 in this article's Online Repository).
3.2. Eliciting doses
We fitted the data with log‐normal, log‐logistic, and Weibull distributions models (Figures 2A,B) and extrapolated EDs from these models. No significant differences were found between the three models. The milk EDs ranged from 0.3 to 1.4 mg proteins (ED01), from 4.2 to 6 mg (ED05), from 12.8 to 15.1 (ED10), from 45.5 to 66 (ED25), and from 185.6 to 268.6 (ED50). The egg EDs ranged from 14.4 to 29.7 mg (ED01), from 87.7 to 115.1 (ED05), from 156.2 to 219.6 mg (ED10), from 409.6 to 600.1 (ED25), and from 1195.5 to 1595.5 mg (ED50) (Table 4).
FIGURE 2.
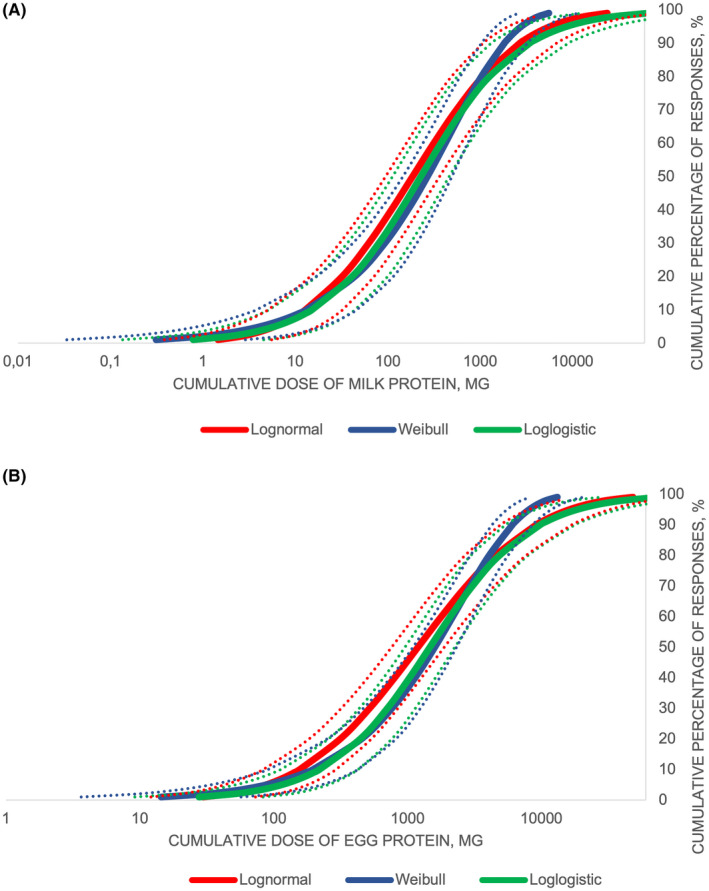
Probability distribution curves of thresholds for cow's milk (A) and hen's egg (B) based on Log‐normal, Log‐logistic, and Weibull distribution models. The dashed lines indicate the 95% confidence interval
TABLE 4.
ED1, ED5, ED10, ED25, and ED50 (mg protein) of milk and egg
| Model | ED01 mg protein (95% CI) | ED05 mg protein (95% CI) | ED10 mg protein (95% CI) | ED25 mg protein (95% CI) | ED50 mg protein (95% CI) | |
|---|---|---|---|---|---|---|
| Milk | Log‐normal | 1.4 (0.4–5.5) | 6 (2.1–17.4) | 12.8 (5.1–32.5) | 45.5 (21.4–96.8) | 185.6 (94.7–363.7) |
| Log‐logistic | 0.8 (0.1–4.5) | 6 (1.7–21.4) | 15.1 (5.2–44.2) | 58.8 (25.6–134.7) | 228.2 (112.7–462) | |
| Weibull | 0.3 (0.03–2.9) | 4.2 (0.9–19.6) | 13.3 (3.8–46.2) | 66 (27.6–157.4) | 268.6 (146–494.1) | |
| Egg | Log‐normal | 29.7 (12.1–73) | 87.7 (43–179) | 156.2 (83.6–291.9) | 409.6 (246.4–680.7) | 1195.5 (760.5–1879.2) |
| Log‐logistic | 27.6 (9.2–83.1) | 115.1 (52.1–254.2) | 219.6 (112.9–426.8) | 567.7 (341.3–944.2) | 1467.7 (956–2253.1) | |
| Weibull | 14.4 (3.6–56.9) | 88.2 (34.1–228.5) | 196.4 (90.9–424.7) | 600.1 (351.1–1026) | 1595.5 (1097.5–2319.4) |
Note: ED01, ED05, ED10, ED50: cumulative amount of food protein predicted to cause an allergic reaction in 1%, 5%, 10%, or 50% of the food allergic population, respectively.
Abbreviations: CI, Confidence interval; ED, eliciting dose.
4. DISCUSSION
This is the first study aimed to evaluate the thresholds of children allergic to native egg and milk only. Our patients were tolerant to beef, baked milk, and baked egg. This population, which can be identified by specific challenges to baked foods, 30 represents the majority of milk/egg allergy sufferers. As they display a shorter duration of the condition, and present with less complex clinical pictures, they are considered those with the less severe forms of milk and egg allergy. 31
The prevalence of positive OFC in our center was 26.1% and 34.5% for milk and egg, respectively. While a similar prevalence was reported in children with OFCs positive to egg (33%) and milk (32%), 32 other caseloads showed higher prevalence rates, both for milk and egg. 33 , 34 , 35 , 36 , 37
Our threshold values are similar to those found in VITAL for milk ED01 and ED05, considerably higher for egg ED01 and ED05. To explain this finding, we first examined the hypothesis that the high amount of egg administrated on our OFCs (one hen egg and a half) may have determined this difference. The majority of previous studies administered a total dose of half egg, with a starting dose lower than our 12.36 mg protein. 32 , 38 , 39 , 40 , 41 , 42 However, this is unlikely to have influenced the results, for two reasons: first, many previous studies used a first dose similar 43 , 44 or even higher 45 , 46 , 47 than ours; second, in our experience, only one of the 49 patients reacted to this first dose. Conversely, one in four patients reacted after the seventh dose. Had the higher doses of our egg OFC influenced the reactivity, we would have recorded a high number of left‐censored children. Since this is not the case, we infer that our caseload of egg‐allergic children displays higher ED01 and ED05 threshold values than that reported in VITAL, consistently with the fact that they are allergic to native egg only and that no statistical correlation between patients' individual threshold and their age, gender, sIgE, and/or SPT levels was found.
Our milk allergic children also showed a higher reactivity threshold than that of VITAL both at level 01 and at level 05, but differences were much smaller. Again, this is unlikely to be due to different food doses at challenge. Studies with milk OFCs in general use inferior cumulative amounts of milk, with starting doses similar 48 , 49 , 50 or higher, 51 , 52 , 53 to our 3 mg milk protein.
These findings echo those of other studies for milk, but not for egg. For instance, in a single‐center pediatric experience using a cumulative dose amount of protein of 2190 mg, corresponding to about 63 ml of milk and 1/3 egg, the ED05 were set at 1.07 for milk and at 1.51 for egg. 54 The only study comparing thresholds of baked‐tolerant vs. baked‐sensitive egg‐ and milk‐allergic patients found ED10 and ED50 values similar to ours for milk, but much lower for egg 55 (Table 4). We recently published tolerance threshold data of children with severe baked milk and baked egg allergy, using a different OFC scheme than the one used in this study. The mean thresholds of reactivity we found in that context were 116.3 (±107.6) and 128.3 (±96.7) mg protein for milk and egg, respectively; the EDs were not calculated. 22 A further study found much lower levels ED05 and ED10 for milk. 56 These differences could be due to different selected populations, difference in mean age, and difference in the method of administration of the OFC.
The interpretation of such data has limitations. First, interpreting the patient's reaction during a food challenge may depend on the experience of the clinical investigator and the patients' attitude, especially for those who had undergone over one reaction prior to food challenge. 57 For the purpose of a correct interpretation of the tests, clear stopping rules must be used, and a diagnosis of reaction must be based on objective symptoms. 21 This is not always the case across studies, including those used in the calculation of the VITAL thresholds. Second, for appropriate statistical power, it is essential to get a representative sample of sufficient size to allow accurate estimation of population‐based threshold dose distributions for each allergen. 58 Again, this is not the case in several studies. Third, the population in OFCs must be clearly characterized from a clinical point of view to avoid misinterpretations. Finally yet importantly, it would be ideal to get an intra‐individual validation of the data by comparing the reproducibility of the eliciting doses at least in the subgroup of patients to whom repeated OFCs have been performed 54 : this has not been possible in this study due to the small number of them.
In any case, should our results be confirmed by other studies they could explain why, in spite of a similar prevalence in the population, milk continues to be more represented than egg in the caseloads of mortality due to food anaphylaxis in children. 8 , 59 It is licit to infer that lower thresholds may induce parallel higher probability of severe reactions. Despite their low ED01, we found a low frequency of first‐dose responders in milk challenges: only one (2.6%) out of 38 positive patients developed an urticarial eruption after 0.1 ml administration (left‐censored), while the frequency reported in these studies for milk‐allergic children irrespective of their tolerance to baked milk ranged from 8% to 21%, considering positive challenges with objective symptoms.
As in other studies, 20 we found that cutaneous reactions were the most frequent manifestations: 66 of 87 positive patients (75.9%) developed a skin symptom (mainly urticarial eruptions) during OFC. In our study, no patient developed a Group 5 systemic reaction, probably for the trained staff prompt intervention at the first onset of objective symptoms. No relationship was found between the threshold dose and the development of more severe reactions. Therefore, as in other studies, 60 , 61 , 62 patients with anaphylaxis do not present with a lower threshold.
In most studies, EDs for cow's milk and egg proteins have been associated to the age of patients, the severity of allergic reactions or the OFC protocol used, such as the number of doses and the total amount of food allergen administered. 32 , 33 , 63 , 64 Therefore, risk analysis for specific groups of patients (i.e., children vs. adults, comparison between various countries) had statistical limits which may influence results when comparing retrospective data from single studies or single protocol‐specific datasets. 16 , 54
In conclusion, our study found similar milk EDs, but higher egg EDs compared with those previously found in other studies. Our data suggest that children with baked egg tolerance may be less exposed to the risk of severe reactions than children with baked milk tolerance. They could be tolerating to egg traces and perhaps they could assume egg‐PAL pre‐packaged foods. Should our results be confirmed by studies comparing EDs differences between raw and baked food in pediatric population, the clinical attitude toward the two allergies could be differentiated. To confirm these data, we will evaluate if such difference in thresholds is present also in children allergic to baked egg/milk. For the moment, these data add to the knowledge about thresholds of reactivity for these foods, an essential characteristic of the hazard that allergens present to the food‐allergic population. 65
AUTHOR CONTRIBUTIONS
RLV developed the concept, prepared the infrastructure of the study, elaborated the data, performed the statistical analysis, and contributed made the draft. AF initiated the concept and contributed made the first draft. CR, VC, LD, VF, MM, and AS administered the OFCs, evaluated the clinical data, included them in the database and contributed to the discussion. ALP collaborated to the inclusion of data in the database, collaborated to the statistical analysis, and contributed to the discussion. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest to disclose.
Supporting information
Table S1
5. ACKNOWLEDGMENTS
Open access funding provided by Ospedale Pediatrico Bambino Gesu.
Valluzzi RL, Riccardi C, Arasi S, et al. Cow's milk and egg protein threshold dose distributions in children tolerant to beef, baked milk, and baked egg. Allergy. 2022;77:3052‐3060. doi: 10.1111/all.15397
Funding information
None.
REFERENCES
- 1. Schoemaker AA, Sprikkelman AB, Grimshaw KE, et al. Incidence and natural history of challenge‐proven cow's milk allergy in European children‐‐EuroPrevall birth cohort. Allergy. 2015;70:963‐972. [DOI] [PubMed] [Google Scholar]
- 2. Xepapadaki P, Fiocchi A, Grabenhenrich L, et al. Incidence and natural history of hen's egg allergy in the first 2 years of life‐the EuroPrevall birth cohort study. Allergy. 2016;71:350‐357. [DOI] [PubMed] [Google Scholar]
- 3. Jansen PR, Petrus NCM, Venema A, et al. Higher polygenetic predisposition for asthma in cow's milk allergic children. Nutrients. 2018;10:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koike Y, Sato S, Yanagida N, et al. Predictors of persistent milk allergy in children: a retrospective cohort study. Int Arch Allergy Immunol. 2018;175:177‐180. [DOI] [PubMed] [Google Scholar]
- 5. Robbins KA, Wood RA, Keet CA. Persistent cow's milk allergy is associated with decreased childhood growth: a longitudinal study. J Allergy Clin Immunol. 2020;145:713‐716. [DOI] [PubMed] [Google Scholar]
- 6. Eriksson NE, Möller C, Werner S, Magnusson J, Bengtsson U. The hazards of kissing when you are food allergic. A survey on the occurrence of kiss‐induced allergic reactions among 1139 patients with self‐reported food hypersensitivity. J Investig Allergol Clin Immunol. 2003;13:149‐154. [PubMed] [Google Scholar]
- 7. Porcaro F, Caminiti L, Crisafulli G, Guglielmo F, Pajno GB. Anaphylaxis to cutaneous exposure to bovine colostrum based cream. Asian Pac J Allergy Immunol. 2019;37:9‐11. [DOI] [PubMed] [Google Scholar]
- 8. Baseggio Conrado A, Ierodiakonou D, Gowland MH, Boyle RJ, Turner PJ. Food anaphylaxis in the United Kingdom: analysis of national data, 1998‐2018. BMJ. 2021;17(372):n251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Upton J, Nowak‐Wegrzyn A. The impact of baked egg and baked Milk diets on IgE‐ and non‐IgE‐mediated allergy. Clin Rev Allergy Immunol. 2018;55:118‐138. [DOI] [PubMed] [Google Scholar]
- 10. Restani P, Fiocchi A, Velonà T, Bruni P, Giovannini M, Galli CL. Meat allergy: proteins involved and cross‐reactivity between different animal species. J Am Coll Nutr. 1997;16:565‐570. [DOI] [PubMed] [Google Scholar]
- 11. Martelli A, De Chiara A, Corvo M, Restani P, Fiocchi A. Beef allergy in children with cow's milk allergy; cow's milk allergy in children with beef allergy. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):38‐43. [DOI] [PubMed] [Google Scholar]
- 12. Karaman S, Erdem SB, Nacaroğlu HT, Karkiner CŞ, Can D. The quantity of unheated milk tolerated as a predictor of tolerance to baked milk. Asian Pac J Allergy Immunol. 2019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13. Graham F, Caubet JC, Eigenmann PA. Can my child with IgE‐mediated peanut allergy introduce foods labeled with "may contain traces"? Pediatr Allergy Immunol. 2020;31:601‐607. [DOI] [PubMed] [Google Scholar]
- 14. Zuberbier T, Dörr T, Aberer W, et al. Proposal of 0.5 mg of protein/100 g of processed food as threshold for voluntary declaration of food allergen traces in processed food‐a first step in an initiative to better inform patients and avoid fatal allergic reactions: a GA2LEN position paper. Allergy. 2021;77(6):1736‐1750. [DOI] [PubMed] [Google Scholar]
- 15. Fierro V, Di Girolamo F, Marzano V, Dahdah L, Mennini M. Food labeling issues in patients with severe food allergies: solving a hamlet‐like doubt. Curr Opin Allergy Clin Immunol. 2017;17:204‐211. [DOI] [PubMed] [Google Scholar]
- 16. Remington BC, Westerhout J, Meima MY, et al. Updated population minimal eliciting dose distributions for use in risk assessment of 14 priority food allergens. Food Chem Toxicol. 2020;139:111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fiocchi A, Risso D, DunnGalvin A, et al. Food labeling issues for severe food allergic patients. World Allergy Organ J. 2021;14:100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen KJ, Turner PJ, Pawankar R, et al. Precautionary labelling of foods for allergen content: are we ready for a global framework? World Allergy Organ J. 2014;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Houben GF, Baumert JL, Blom WM, et al. Full range of population eliciting dose values for 14 priority allergenic foods and recommendations for use in risk characterization. Food Chem Toxicol. 2020;146:111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wheeler MW, Westerhout J, Baumert JL, Remington BC. Bayesian stacked parametric survival with frailty components and interval‐censored failure times: an application to food allergy risk. Risk Anal. 2021;41:56‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner PJ, d'Art YM, Duca B, et al. Single‐dose oral challenges to validate eliciting doses in children with cow's milk allergy. Pediatr Allergy Immunol. 2021;32:1056‐1065. [DOI] [PubMed] [Google Scholar]
- 22. Fierro V, Marzano V, Monaci L, et al. Threshold of reactivity and tolerance to precautionary allergen‐labelled biscuits of baked milk‐ and egg‐allergic children. Nutrients. 2021;13:4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiocchi A, Artesani MC, Riccardi C, et al. Impact of omalizumab on food allergy in patients treated for asthma: a real‐life study. J Allergy Clin Immunol Pract. 2019;7:1901‐1909. [DOI] [PubMed] [Google Scholar]
- 24. https://fdc.nal.usda.gov/index.html. Accessed June 20, 2021.
- 25. Nowak‐Wegrzyn A, Assa'ad AH, Bahna SL, et al. Work group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123(6 Suppl):S365‐S383. [DOI] [PubMed] [Google Scholar]
- 26. Keil T, McBride D, Grimshaw K, et al. The multinational birth cohort of EuroPrevall: background, aims and methods. Allergy. 2010;65:482‐490. [DOI] [PubMed] [Google Scholar]
- 27. Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111(6 Pt 3):1601‐1608. [PubMed] [Google Scholar]
- 28. Taylor SL, Moneret‐Vautrin DA, Crevel RW, et al. Threshold dose for peanut: risk characterization based upon diagnostic oral challenge of a series of 286 peanut‐allergic individuals. Food Chem Toxicol. 2010;48:814‐819. [DOI] [PubMed] [Google Scholar]
- 29. Spanjersberg MQ, Kruizinga AG, Rennen MA, Houben GF. Risk assessment and food allergy: the probabilistic model applied to allergens. Food Chem Toxicol. 2007;45:49‐54. [DOI] [PubMed] [Google Scholar]
- 30. Dantzer JA, Dunlop JH, Wood RA. Standard testing fails to identify patients who tolerate baked milk. J Allergy Clin Immunol. 2020;146:1434‐1437. [DOI] [PubMed] [Google Scholar]
- 31. Fiocchi A, Restani P. Adverse reactions to bovine proteins‐‐then and now. Ann Allergy, Asthma & Immunology. 2002;89:S1‐S2. [DOI] [PubMed] [Google Scholar]
- 32. Dang AT, Chundi PK, Mousa NA, et al. The effect of age, sex, race/ethnicity, health insurance, and food specific serum immunoglobulin E on outcomes of oral food challenges. World Allergy Organ J. 2020;13:100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benhamou AH, Zamora SA, Eigenmann PA. Correlation between specific immunoglobulin E levels and the severity of reactions in egg allergic patients. Pediatr Allergy Immunol. 2008;19:173‐179. [DOI] [PubMed] [Google Scholar]
- 34. Blom WM, Vlieg‐Boerstra BJ, Kruizinga AG, van der Heide S, Houben GF, Dubois AEJ. Threshold dose distributions for 5 major allergenic foods in children. J Allergy Clin Immunol. 2013;131:172‐179. [DOI] [PubMed] [Google Scholar]
- 35. Eller E, Hansen TK, Bindslev‐Jensen C. Clinical thresholds to egg, hazelnut, milk and peanut: results from a single‐center study using standardized challenges. Ann Allergy Asthma Immunol. 2012;108:332‐336. [DOI] [PubMed] [Google Scholar]
- 36. Gupta M, Grossmann L, Spergel J, Cianferoni A. Egg food challenges are associated with more gastrointestinal reactions. Children. 2015;2:371‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rolinck‐Werninghaus C, Niggemann B, Grabenhenrich L, Wahn U, Beyer K. Outcome of oral food challenges in children in relation to symptom‐eliciting allergen dose and allergen‐specific IgE. Allergy. 2012;67:951‐957. [DOI] [PubMed] [Google Scholar]
- 38. Bellach J, Schwarz V, Ahrens B, et al. Randomized placebo‐controlled trial of hen's egg consumption for primary prevention in infants. J Allergy Clin Immunol. 2017;139:1591‐1599. [DOI] [PubMed] [Google Scholar]
- 39. Pérez‐Rangel I, del Río PR, Escudero C, Sánchez‐García S, Sánchez‐Hernández JJ, Ibáñez MD. Efficacy and safety of high‐dose rush oral immunotherapy in persistent egg allergic children: a randomized clinical trial. Ann Allergy Asthma Immunol. 2017;118:356‐364. [DOI] [PubMed] [Google Scholar]
- 40. Staden U, Rolinck‐Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261‐1269. [DOI] [PubMed] [Google Scholar]
- 41. Morisset M, Moneret‐Vautrin DA, Guenard L, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. Eur Ann allergy Clin Immunol. 2007;39:12‐19. [PubMed] [Google Scholar]
- 42. Norgaard A, Bindslev‐Jensen C. Egg and milk allergy in adults. Diagnosis and characterization. Allergy. 1992;47:503‐509. [DOI] [PubMed] [Google Scholar]
- 43. Escudero C, Sánchez‐García S, Rodríguez del Río P, et al. Dehydrated egg white: an allergen source for improving efficacy and safety in the diagnosis and treatment for egg allergy. Pediatr Allergy Immunol. 2013;24:263‐269. [DOI] [PubMed] [Google Scholar]
- 44. Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of immunoglobulin E‐mediated food allergy in 6–9‐year‐old urban schoolchildren in the eastern Black Sea region of Turkey. Clin Exp Allergy. 2009;39:1027‐1035. [DOI] [PubMed] [Google Scholar]
- 45. Vazquez‐Ortiz M, Alvaro M, Piquer M, et al. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg‐allergic children. Clin Exp Allergy. 2014;44:130‐141. [DOI] [PubMed] [Google Scholar]
- 46. Faraj Z, Kim HL. Skin prick testing with extensively heated milk or egg products helps predict the outcome of an oral food challenge: a retrospective analysis. Allergy Asthma Clin Immunol. 2012;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eggesbo M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population‐based study in young children. Allergy. 2001;56:403‐411. [DOI] [PubMed] [Google Scholar]
- 48. Morisset M, Moneret‐Vautrin DA, Kanny G, et al. Thresholds of clinical reactivity to milk, egg, peanut and sesame in immunoglobulin E‐dependent allergies: evaluation by double‐blind or single‐blind placebo‐controlled oral challenges. Clin Exp Allergy. 2003;33:1046‐1051. [DOI] [PubMed] [Google Scholar]
- 49. Devenney I, Norrman G, Oldaeus G, Strömberg L, Fälth‐Magnusson K. A new model for low‐dose food challenge in children with allergy to milk or egg. Acta Paediatr. 2006;95:1133‐1139. [DOI] [PubMed] [Google Scholar]
- 50. Caminiti L, Passalacqua G, Barberi S, et al. A new protocol for specific oral tolerance induction in children with IgE‐mediated cow's milk allergy. Allergy Asthma Proc. 2009;30:443‐448. [DOI] [PubMed] [Google Scholar]
- 51. Dambacher WM, de Kort EH, Blom WM, Houben GF, de Vries E. Double‐blind placebo‐controlled food challenges in children with alleged cow's milk allergy: prevention of unnecessary elimination diets and determination of eliciting doses. Nutr J. 2013;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fiocchi A, Travaini M, D'Auria E, Banderali G, Bernardo L, Riva E. Tolerance to a rice hydrolysate formula in children allergic to cow's milk and soy. Clin Exp Allergy. 2003;33:1576‐1580. [DOI] [PubMed] [Google Scholar]
- 53. Flinterman AE, Hoekstra MO, Meijer Y, et al. Clinical reactivity to hazelnut in children: association with sensitization to birch pollen or nuts? J Allergy Clin Immunol. 2006;118:1186‐1189. [DOI] [PubMed] [Google Scholar]
- 54. Blom WM, Michelsen‐Huisman AD, van Os‐Medendorp H, et al. Accidental food allergy reactions: products and undeclared ingredients. J Allergy Clin Immunol. 2018;142:865‐875. [DOI] [PubMed] [Google Scholar]
- 55. Remington BC, Westerhout J, Campbell DE, Turner PJ. Minimal impact of extensive heating of hen's egg and cow's milk in a food matrix on threshold dose‐distribution curves. Allergy. 2017;72:1816‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manny E, La Vieille S, Dominguez SA, et al. Probabilistic risk assessment for milk in dark chocolate, cookies and other baked goods with PAL sold in Canada. Food Chem Toxicol. 2021;152:112196. [DOI] [PubMed] [Google Scholar]
- 57. 7 Van Erp FC, Knulst AC, Meijer Y, Gabriele C, Van Der Ent CK. Standardized food challenges are subject to variability in interpretation of clinical symptoms. Clin Transl Allergy. 2014;4:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. 8 Klein Entink RH, Remington BC, Blom WM, et al. Food allergy population thresholds: an evaluation of the number of oral food challenges and dosing schemes on the accuracy of threshold dose distribution modeling. Food Chem Toxicol. 2014;70:134‐143. [DOI] [PubMed] [Google Scholar]
- 59. Dorris S. Fatal food anaphylaxis: registering a rare outcome. Ann Allergy Asthma Immunol. 2020;124:445‐446. [DOI] [PubMed] [Google Scholar]
- 60. Eigenmann PA, Ebisawa M, Greenhawt M, et al. Addressing risk management difficulties in children with food allergies. Pediatr Allergy Immunol. 2021;32:658‐666. [DOI] [PubMed] [Google Scholar]
- 61. Santos AF, Du Toit G, O'Rourke C, et al. Biomarkers of severity and threshold of allergic reactions during oral peanut challenges. J Allergy Clin Immunol. 2020;146:344‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arasi S, Nurmatov U, Dunn‐Galvin A, et al. Consensus on DEfinition of food allergy SEverity (DEFASE) an integrated mixed methods systematic review. World Allergy Organ J. 2021;14:100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allen KJ, Remington BC, Baumert JL, et al. Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications. J Allergy Clin Immunol. 2014;133:156‐164. [DOI] [PubMed] [Google Scholar]
- 64. Taylor SL, Baumert JL, Kruizinga AG, et al. Establishment of reference doses for residues of allergenic foods: report of the VITAL expert panel. Food Chem Toxicol. 2014;63:9‐17. [DOI] [PubMed] [Google Scholar]
- 65. Food and Agricultural Organization of the United Nations / World Health Organization . Summary report of the Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Part 2: Review and establish threshold levels in foods of the priority allergens. 20 August 2021. https://cdn.who.int/media/docs/default‐source/food‐381safety/jemra/2nd‐allergen‐summary‐report‐20aug2021.pdf?sfvrsn=915a8417_8. Accessed January 6, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


