Abstract
Autoimmune encephalitis (AE) is a neurological disorder caused by autoimmune attack on cerebral proteins. Experts currently recommend staged immunotherapeutic management, with first‐line immunotherapy followed by second‐line immunotherapy if response to first‐line therapy is inadequate. Meta‐analysis of the evidence base may provide higher quality evidence to support this recommendation. We undertook a systematic review of observational cohort studies reporting AE patients treated with either second‐line immunotherapy or first‐line immunotherapy alone, and outcomes reported using the modified Rankin Scale (mRS; search date: April 22, 2020). We performed several one‐stage multilevel individual patient data (IPD) meta‐analyses to examine the association between second‐line immunotherapy and final mRS scores (PROSPERO ID CRD42020181805). IPD were obtained for 356 patients from 25 studies. Most studies were rated as moderate to high risk of bias. Seventy‐one patients (71/356, 19%) were treated with second‐line immunotherapy. We did not find a statistically significant association between treatment with second‐line immunotherapy and final mRS score for the cohort overall (odds ratio [OR] = 1.74, 95% confidence interval [CI] = .98–3.08, p = .057), or subgroups with anti‐N‐methyl‐D‐aspartate receptor encephalitis (OR = 1.03, 95% CI = .45–2.38, p = .944) or severe AE (maximum mRS score > 2; OR = 1.673, 95% CI = .93–3.00, p = .085). Treatment with second‐line immunotherapy was associated with higher final mRS scores in subgroups with anti‐leucine‐rich glioma‐inactivated 1 AE (OR = 6.70, 95% CI = 1.28–35.1, p = .024) and long‐term (at least 12 months) follow‐up (OR = 3.94, 95% CI = 1.67–9.27, p = .002). We did not observe an association between treatment with second‐line immunotherapy and lower final mRS scores in patients with AE. This result should be interpreted with caution, given the risk of bias, limited adjustment for disease severity, and insensitivity of the mRS in estimating psychiatric and cognitive disability.
Keywords: anti‐N‐methyl‐D‐aspartate receptor encephalitis, cohort studies, immunoglobulins, rituximab
Key Points.
We reviewed cohort studies of AE, treated with first‐line or second‐line immunotherapy, reporting outcomes using the mRS
Individual patient data for 356 patients were obtained from 25 studies
We did not find an association between second‐line immunotherapy and lower final mRS scores in patients with AE
These results should be interpreted with caution due to risk of bias, limited adjustment for severity, and insensitivity of the mRS to cognitive impairment
1. INTRODUCTION
The autoimmune encephalitides (AEs) are a group of inflammatory neurological disorders in which immune responses against central nervous system proteins result in a range of neurological symptoms, including seizures, psychiatric disturbance, movement disorders, and memory impairment. 1 These symptoms persist for years after diagnosis, 2 , 3 impact on patients' independence and employment, 4 and are associated with significant health care costs. 5 Observational studies suggest that outcomes are improved by treatment with immunotherapy, particularly in patients with antibodies against neuronal surface proteins. 6 , 7 Experts currently recommend treatment with "first‐line" immunotherapeutic agents (immunoglobulin, intravenous steroids, plasma exchange), followed by escalation to more intensive "second‐line" agents (rituximab, cyclophosphamide) if first‐line agents are ineffective. 8 , 9
Current treatment recommendations are based on narrative systematic reviews of observational cohort studies, the largest of which report good functional outcomes in 65%–80% of patients treated with escalation to second‐line immunotherapy 7 , 10 ; however, the evidence overall is mixed, particularly for nonseizure outcomes such as cognition. 2 Harmonizing data from these observational studies may provide better quality evidence to inform patient management. We conducted a systematic review and individual patient data (IPD) meta‐analysis to explore whether second‐line immunotherapy is associated with improved functional outcomes among patients with AE, compared to treatment with first‐line therapy alone.
2. MATERIALS AND METHODS
2.1. Standard protocol approvals, registrations, and patient consents
This study utilized existing data in the public domain and nonidentifiable data from existing data collections, and is therefore exempt from ethical review under the Australian National Health and Medical Research Council National Statement on Ethical Conduct in Human Research. This study was registered on PROSPERO (International Prospective Register of Systematic Reviews) prior to commencement (CRD42020181805), and is reported in accordance with the IPD‐specific PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines.
2.2. Eligibility criteria
Eligible studies described adult patients (18 years or older) with AE according to published consensus diagnostic criteria, 11 treated with either second‐line immunotherapy (rituximab or cyclophosphamide) or first‐line immunotherapy alone (plasma exchange, intravenous methylprednisolone, or intravenous immunoglobulin), and reported patient outcomes using the modified Rankin score (mRS), an ordinal functional disability scale commonly used to measure functional outcomes in neurological conditions (scale definitions: 0 = no symptoms; 1 = symptoms but able to carry out all usual activities; 2 = unable to perform all previous activities but able to look after own affairs without assistance; 3 = requires assistance with affairs but able to walk without assistance; 4 = unable to walk or attend to own bodily needs without assistance; 5 = bedridden, incontinent, and requiring constant nursing care and attention; 6 = death). 12 Patients with significant extracerebral or nonneurological involvement were excluded. Eligible studies were observational cohort studies with at least five patients meeting inclusion criteria, and published in English, with no limitation regarding publication type or study date.
2.3. Search strategy
The search strategy was developed in consultation with a research librarian. Studies were identified using searches of Medline, the Cochrane Central Register of Controlled Trials, Embase, and clinicaltrials.gov. The search strategy including search terms are shown in Table 1. The last bibliographic search was performed on April 22, 2020.
TABLE 1.
Search strategy
| Database | Search terms | Limitations |
|---|---|---|
| Ovid Medline (Embase Classic + Embase) |
|
|
| Ovid Medline (Embase Classic + Embase) |
(NMDA or VGKC or LGI1 or CASPR2 or GAD or GABA or AMPA or DPPX or Hu or Ma or mGluR5) and (immunosuppress* or rituximab or cyclophosphamide or mycophenolate or methotrexate or azathioprine or ivig or immunoglobulin or plasma exchange or prednisolone or methylprednisolone) |
Human studies |
| Ovid Medline (Cochrane Central Register of Controlled Trials) | Encephalitis | MeSH term |
| clinicaltrials.gov | Encephalitis |
Abbreviation: MeSH, Medical Subject Headings.
2.4. Study selection
Study selection, quality assessment, and data extraction were performed independently by two reviewers (AH, AD) using Covidence Extraction 2.0 (Veritas Health Innovation, Melbourne, Australia). Titles and abstracts were screened for studies potentially meeting inclusion criteria, followed by full‐text review to identify studies eligible for inclusion. Conflicts were resolved by discussion and consensus.
2.5. Quality assessment
Quality assessment was performed using the Newcastle‐Ottawa Scale (NOS) 13 risk of bias tool for observational cohort studies, modified by study authors for the present review to identify biases related to retrospective outcome and covariate assessment, and studies with one exposure arm (see Appendix S1). Risk of bias was assessed in three domains and scored using the NOS star allocation system with more stars indicating lower risk of bias: (1) selection bias, maximum three stars; (2) bias due to confounding, maximum two stars; and (3) measurement bias, maximum two stars.
2.6. Data extraction
IPD for eligible studies were extracted from the published report where available, and sought via the corresponding author where unavailable. Data extraction was performed using a standardized template. Study data included study design (retrospective, prospective), start and end dates, and country. IPD included age at time of treatment (years), sex, treatment group (first‐line only, second‐line), mRS score at last follow‐up, timing of last follow‐up (months since disease onset), maximum mRS score (peak mRS score during acute illness), and AE subtype (anti‐N‐methyl‐D‐aspartate receptor [NMDA], anti‐gamma‐aminobutyric acid receptor, anti‐leucine‐rich glioma‐inactivated 1 [LGI‐1], anti‐dipeptidyl‐peptidase like protein‐6, anti‐α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor, anti‐contactin‐associated protein‐like 2 receptor, other/sub‐type not specified).
2.7. Data analysis
IPD were used for statistical analysis, performed using Stata (v16.1, StataCorp). Univariate summary statistics are reported as mean (SD) for continuous covariates, or number (percentage) for categorical covariates. Maximum and final mRS scores for the entire cohort were compared using a Wilcoxon signed‐rank test. Covariates in the exposure and comparison groups were analyzed using t‐tests for continuous covariates and chi‐squared test for categorical covariates. The significance level was set at α = .05.
One‐stage generalized multilevel mixed‐effects linear regression models (GLMMs) fitted to final mRS scores were used to examine the association of second‐line therapy with these final mRS scores. 14 , 15 Final mRS score was used as the outcome of interest, as opposed to change in mRS score, which would bias the study toward finding a treatment effect rather than favoring the null hypothesis (patients undergoing second‐line immunotherapy are likely to have higher maximum mRS scores, and patients with worse maximum mRS scores have more potential for improvement in score than patients with smaller maximum mRS scores). Missing values for covariates were imputed by best‐subsets regression imputation on all independent variables and the dependent variable using Stata's impute command 16 prior to modeling. A one‐stage approach was selected to minimize bias due to small study sizes and studies with a single treatment group. Final mRS score was fit to a linear regression model using maximum likelihood estimation, and transformed to fit final mRS as an ordinal categorical scale using logit link function. Multilevel models were used to account for clustering within studies, allowing for random effects with assumed normal distribution on study as the group‐level covariate. Age, sex, maximum mRS score, and time to follow‐up (months) were included as covariates in adjusted models as patient‐level covariates with fixed effects.
Subgroup analysis examined the two most common forms of AE (NMDA, LGI‐1), severe AE (maximum mRS score > 2), 4 , 7 , 17 and patients with >12 months of follow‐up. Patients were selected for inclusion in subgroups based on nonimputed data.
Heterogeneity between studies was not formally estimated, as the IPD GLMM approach accounts for heterogeneity across trials, and methodological approaches to estimation of heterogeneity following a one‐stage approach for nonlinear outcomes are still evolving. 15 , 18
2.8. Data availability
The set of deidentified individual patient data retrieved from published IPD and used for analysis is available on request. Data obtained from other authors is not included in the shared dataset.
3. RESULTS
3.1. Study selection
From the 7244 studies identified by our search, 539 duplicates were removed and 6168 studies were excluded as irrelevant at title and abstract screening. A total of 537 studies underwent full‐text review, of which 52 met eligibility criteria for seeking IPD (Figure 1). IPD were obtained for 25 studies (Table 2), extractable from the published report for 24 studies and obtained from the corresponding author for one additional study. IPD were not obtained for the remaining 27 studies, which were excluded from subsequent analysis.
FIGURE 1.
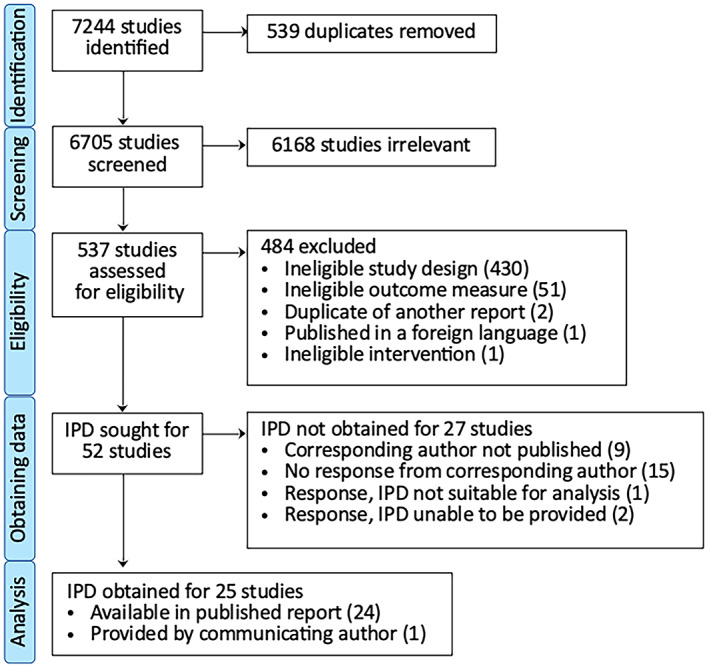
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) individual patient data (IPD) flow diagram.
TABLE 2.
Characteristics of included studies
| Study: first author, year | n a | AE subtype | Study design | Observation period | Country | Missing | Follow‐up, months, mean (range) | Risk of bias | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | First line | Second line | Age | Sex | max mRS | 1 ★★★ | 2 ★★ | 3 ★★★ | ||||||
| Aungsumart 2019 28 | 18 | 15 | 3 | NMDA | Retrospective | 2011–2016 | Thailand | 12 | ★★ | ★★ | ★ | |||
| Cui 2018 29 | 10 | 10 | GABA | Not stated | 2016 | China | 11 (7–15) | ★★ | ★ | ★ | ||||
| Finke 2017 30 | 29 | 24 | 5 | LGI‐1 | Retrospective | 2013–2015 | Germany | + | 11 (10–15) b | ★ | ★ | ★★ | ||
| Gastaldi 2020 31 | 15 | 14 | 1 | Any AE | Retrospective | 2012–2016 | Italy | + | 15 (1–76) b | ★★ | ★★ | ★★ | ||
| Guan 2015 32 | 14 | 14 | GABA | Retrospective | 2012–2014 | China | 8 (1–21) b | ★★ | ★★ | ★ | ||||
| Hara 2017 33 | 9 | 4 | 5 | DPPX | Retrospective | 2013–2016 | Spain, USA | 24 (6–72) | ★ | ★★ | ★★ | |||
| Heine 2016 34 | 10 | 10 | Any AE | Prospective | 2013–2015 | Germany | ★★ | ★★ | ★ | |||||
| Hoftberger 2015 35 | 20 | 15 | 5 | AMPA | Retrospective | 2009–2014 | Multiple | 25 (1–66) | ★★ | ★ | ★★ | |||
| Huang 2015 36 | 25 | 25 | NMDA | Retrospective | 2011–2013 | China | + | 8 (.5–17) | ★ | |||||
| Irani 2014 37 | 5 | 5 | LGI‐1 | Retrospective | 2006–2013 | USA | 26 (10–65) | ★★ | ★★ | ★★ | ||||
| Joubert 2016 38 | 11 | 8 | 3 | CASPR2 | Retrospective | 2009–2015 | France | 38 (11–92) | ★ | ★ | ||||
| Kamble 2015 39 | 6 | 6 | Any AE | Prospective | 2011–2015 | India | 11 (1–37) | ★★ | ★ | |||||
| Liao 2017 40 | 19 | 18 | 1 | Any AE | Retrospective | 2009–2017 | China | 23 (0–84) b | ★★ | ★★ | ★★ | |||
| Melamud 2020 41 | 8 | 6 | 2 | Any AE | Retrospective | 2013–2018 | Argentina | 20 (5–54) | ★★ | ★ | ★★ | |||
| Dogan Onugoren 2016 42 | 13 | 12 | 1 | Any AE | Retrospective | 2011–2015 | Germany | 10 (.3–41) | ★★ | ★ | ★★ | |||
| Shin 2013 43 | 12 | 9 | 3 | LGI‐1 | Retrospective | 2012–2013 | Korea | 7 (1–24) | ★ | ★ | ||||
| Spatola 2018 44 | 5 | 4 | 1 | Any AE | Retrospective | 2005–2017 | Spain | 37 (5–62) | ★★ | ★★ | ★★ | |||
| Viaccoz 2014 45 | 11 | 3 | 8 | NMDA | Retrospective | 2007–2013 | France | 11 (6–12) | ★ | ★★ | ★ | |||
| Wang 2018 46 | 13 | 13 | LGI‐1 | Retrospective | 2015–2017 | China | 2 | ★★ | ★ | ★ | ||||
| Wang 2019 47 | 10 | 2 | 8 | Any AE | Retrospective | 2016–2018 | China | 9.5 (6–47) b | ★★ | ★★ | ★★ | |||
| Wegner 2014 19 | 10 | 5 | 5 | Any AE | Retrospective | 2008–2012 | Germany | + | + | + | 25 (8–40) | ★★ | ★★ | ★ |
| Yang 2019 48 | 23 | 23 | Any AE | Retrospective | 2015–2017 | China | 14 (3–36) b | ★★ | ★ | ★ | ||||
| Yeo 2018 49 | 5 | 5 | Any AE | Retrospective | 2013–2016 | Singapore | 36 (13–108) b | ★★ | ★ | ★ | ||||
| Zhang 2018 50 | 16 | 14 | 2 | NMDA | Retrospective | 2013–2017 | China | + | 6 | ★★ | ★ | ★ | ||
| Zhang 2019 51 | 34 | 26 | 8 | NMDA | Prospective | 2012–2017 | China | 12 | ★★ | ★ | ★★ | |||
Note: Risk of bias domain 1, selection bias; risk of bias domain 2, confounding; risk of bias domain 3, measurement bias. Any AE indicates AE of mixed subtypes.
Abbreviations: AE, autoimmune encephalitis; AMPA, anti‐α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor; CASPR2, anti‐contactin‐associated protein‐like 2 receptor; DPPX, anti‐dipeptidyl‐peptidase‐like protein‐6; GABA, anti‐gamma‐aminobutyric acid receptor; LGI‐1, anti‐leucine‐rich glioma‐inactivated 1; max mRS, maximum modified Rankin Scale score; NMDA, anti‐N‐methyl‐D‐aspartate receptor.
Number of patients from original study extracted for inclusion in current study.
Aggregate follow‐up data extracted from published report.
3.2. Risk of bias
Risk of bias assessments are shown in Table 2. Most studies were of moderate to high risk of selection bias (NOS domain 1), with no study scoring the maximum of three stars. Nine of the 25 included studies reported patients in only one treatment group; eight reported only patients treated with first‐line immunotherapy, and one reported only patients treated with second‐line immunotherapy. No study reported predisease mRS estimates or systematic descriptions of premorbid neurological function. Fourteen studies were at moderate to high risk of bias due to confounding (NOS domain 2). One study did not report age or sex in IPD, although aggregate statistics were presented. 19 Five did not report maximum mRS scores. Selection and reporting of other markers of disease severity were inconsistent between reports overall. Timing of the final mRS assessment was variable both between and within studies. Most studies were at high risk of measurement bias (NOS domain 3) due to inadequate description of the method by which mRS scores were determined in retrospective studies (i.e., whether scores were extracted from mRS scores documented contemporaneously in the medical record, or retrospectively estimated based on clinical descriptions documented in the medical record).
3.3. Individual patient data
IPD were obtained for 356 patients. There were no missing data for outcome, treatment group, or AE subtype. Data were missing for all remaining covariates: maximum mRS score (95/356 missing, 27%), sex (11/356 missing, 3%), age (10/356 missing, 10%), and time to follow‐up mRS assessment (130/356, 36.5%).
Females constituted 46.1% (159/345) of the cohort. Age was bimodally distributed, with an early peak at approximately 20 years of age and a later peak at approximately 65 years of age, with the early peak composed largely of patients with NMDA AE (Figure S1). The most common AE subtypes were NMDA (151/356, 42.4%) and LGI‐1 (98/356, 27.5%). The most frequent maximum mRS score was 5 (mRS = 5, 101/261, 38.7%), and the most frequent final mRS score was 1 (mRS = 1, 111/356, 31.2%), shown in Figure 2. The mean length of follow‐up was 14.6 months (±14.8 months, range = .3–92). Wilcoxon signed‐rank test using complete case analysis (261/356, 73.3%) found final mRS scores were significantly lower than maximum mRS scores across the entire cohort (p < .0001).
FIGURE 2.

Distribution of maximum and final modified Rankin score (mRS) scores in patients treated with first‐line versus second‐line immunotherapy. (A) First‐line immunotherapy only. (B) Second‐line immunotherapy.
Seventy‐one patients (71/356, 19%) were treated with second‐line immunotherapy. Individuals treated with second‐line immunotherapy compared to those treated with first‐line immunotherapy only were similar in terms of sex, maximum mRS score, duration of follow‐up, and proportion with NMDA and LGI‐1 subtypes; however, patients treated with second‐line immunotherapy were significantly younger (42.1 vs. 47.8 years, p = .015; Table 3). Characteristics of patients in each of the NMDA, LGI‐1, severe AE, and long‐term follow‐up subgroups are provided in Table S1.
TABLE 3.
Patient characteristics by treatment group
| Characteristic | First‐line therapy | Second‐line therapy | p |
|---|---|---|---|
| Total | 290 (81.5) | 66 (18.5) | |
| Sex, n (%) | |||
| Male | 154 (53) | 32 (48.5) | .064 |
| Female | 130 (44.8) | 29 (43.9) | |
| Missing | 6 (2.1) | 5 (6.6) | |
| Age, years, mean (SD) | 47.8 (1.1) | 42.1 (2.4) | .015 |
| AE subtype, n (%) | |||
| NMDA | 117 (40.3) | 34 (51.5) | .097 |
| LGI‐1 | 84 (29.0) | 14 (21.2) | .203 |
| Maximum mRS | |||
| Missing, n (%) | 82 (28.3) | 13 (19.7) | .155 |
| Mean (SD) | 3.7 (.1) | 4.3 (.1) | .999 |
| Follow‐up | |||
| Missing, n (%) | 115 (39.7) | 15 (22.7) | .010 |
| Months, mean (SD) | 18.6 (2.1) | 13.4 (1.1) | .987 |
Abbreviations: AE, autoimmune encephalitis; LGI‐1, anti‐leucine‐rich glioma‐inactivated 1; mRS, modified Rankin Scale; NMDA, anti‐N‐methyl‐D‐aspartate receptor.
3.4. Meta‐analysis
Results of one‐stage GLMM statistical modeling fitted to ordinal final mRS scores are shown in Table 4. All models were statistically significant, indicating that the models explain a significant proportion of the variance in final mRS scores, with the exception of the unadjusted model for the cohort overall.
TABLE 4.
Results of statistical modeling
| Overall, n = 356 | NMDA, n = 151 | LGI‐1, n = 98 | Long‐term follow‐up, n = 132 | Severe AE, n = 227 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | s | |
| Model significance | Unadjusted model, p = .0699 | Adjusted model, p = .0008 | p = .0188 | p = .0121 | p = .0006 | p = .0094 | ||||||||||||
| Treatment effect | 1.66 | .95–2.88 | .070 | 1.74 | .98–3.08 | .057 | 1.03 | .45–2.38 | .944 | 6.71 | 1.28–35.1 | .024 | 3.94 | 1.67–9.27 | .002 | 1.71 | .82–3.55 | .153 |
| Age | 1.02 | 1.01–1.04 | .001 | 1.05 | 1.02–1.07 | .001 | 1.01 | .97–1.05 | .694 | 1.05 | 1.03–1.08 | .001 | 1.03 | 1.01–1.05 | .002 | |||
| Sex | 1.51 | .77–1.73 | .498 | 1.30 | .68–2.49 | .426 | .80 | .34–1.89 | .612 | 1.46 | .73–2.91 | .285 | 1.29 | .78–2.14 | .321 | |||
| Maximum mRS | 1.59 | 1.21–2.09 | .001 | 1.57 | .94–2.63 | .085 | 2.74 | 1.32–5.69 | .007 | 1.64 | 1.08–2.49 | .021 | 1.75 | 1.19–2.55 | .004 | |||
| Length of follow‐up | .99 | .97–1.01 | .248 | .96 | .90–1.02 | .144 | 1.00 | .94–1.07 | .942 | 1.01 | .98–1.03 | .675 | 1.00 | .98–1.02 | .653 | |||
Abbreviations: AE, autoimmune encephalitis; CI, confidence interval; LGI‐1, anti‐leucine‐rich glioma‐inactivated 1; mRS, modified Rankin Scale score; NMDA, anti‐N‐methyl‐D‐aspartate receptor; OR, odds ratio.
The unadjusted model for the cohort overall did not find a statistically significant association between treatment with second‐line immunotherapy and final mRS score (odds ratio [OR] = 1.67, 95% confidence interval [CI] = .96–2.88, p = .070), although the lower boundary of the 95% CI was close to 1, with the second‐line‐treated cohort having 67% increased odds of being in a higher final mRS category. The adjusted model also did not find a statistically significant association between treatment with second‐line immunotherapy and final mRS score (OR = 1.74, 95% CI = .98–3.08, p = .057), although again the lower boundary of the CI was close to 1, with the second‐line‐treated cohort having 74% increased odds of being in a higher final mRS category.
The adjusted model for the severe AE subgroup (OR = 1.67, 95% CI = .93–3.00, p = .085) also did not find a statistically significant association between treatment with second‐line immunotherapy and final mRS score, although the lower boundary of the CI was approaching 1, with second‐line‐treated patients having 67% increased odds of being in a higher final mRS category.
The model for the NMDA AE subgroup did not find an association between treatment with second‐line immunotherapy and final mRS score (OR = 1.03, 95% CI = .45–2.38, p = .944). In contrast, the model for the LGI‐1 AE subgroup found treatment second‐line immunotherapy was associated with higher final mRS scores, although with wide CIs (OR = 6.70, 95% CI = 1.28–35.1, p = .024). The model for the long‐term follow‐up subgroup also found an association between treatment with second‐line immunotherapy and higher final mRS scores, again with wide CIs (OR = 3.94, 95% CI = 1.67–9.27, p = .002).
Covariates consistently associated with higher final mRS scores in the above models were increasing age and higher maximum mRS scores (see Table 4). Increasing age was associated with being in a higher final mRS category for all subgroups except LGI‐1 AE, with narrow CIs but small ORs. High maximum mRS score was associated with being in a higher final mRS category for all subgroups except NMDA AE, with narrow CIs, and ORs in the various subgroups ranging from 1.57 to 2.74.
4. DISCUSSION
This systematic review and one‐stage IPD meta‐analysis describes one of the largest cohorts of patients with AE reported to date. We examined the association between treatment with second‐line immunotherapy and functional outcomes estimated using the mRS score. Although we found mRS scores to be significantly lower at the end of follow‐up than prior to treatment overall, we did not find a relationship between treatment with second‐line immunotherapy and lower final mRS scores. Specifically, there was no association between second‐line immunotherapy treatment and final mRS scores for the overall cohort, or subgroups with NMDA or severe AE. However, treatment with second‐line immunotherapy was associated with higher final mRS scores in subgroups with LGI‐1 AE and patients with at least 12 months of follow‐up. Moderate to high risk of bias in most studies as well as limited statistical adjustment for disease severity and treatment delay limits definitive conclusions. The findings may also reflect the insensitivity of the mRS to key AE sequelae such as cognitive impairment and mood disturbance.
Our findings add to those of a recent study that examined the association of second‐line immunotherapy with mRS scores in patients with NMDA AE. 20 This single‐level meta‐analysis found second‐line agents were not associated with improved functional outcomes in this AE subgroup. Our study found similar results in a broader AE population, with more generalizable findings due to stricter eligibility criteria and use of multilevel meta‐analytic models. These findings contrast, however, with those of several observational studies demonstrating an association between exposure to second‐line immunotherapy and improved functional outcomes. The largest studies report good functional outcomes (mRS = 0–2) in 65%–78% of patients treated with second‐line therapy. 7 , 10 , 21 Cohorts used in these studies examined a subgroup of patients who had failed to respond to first‐line immunotherapy. This is described in expert recommendations as an indication for escalation to second‐line immunotherapy; however, first‐line treatment failure is currently subjectively defined, and therefore specific measures are rarely reported in IPD. Without the ability to account for first‐line immunotherapy response, our negative results may reflect severity bias, with severe AE patients more likely to be administered second‐line immunotherapy, and suboptimal outcomes related to severe disease masking a potential treatment response.
Another important potential confounder unmeasured in the present study was delay to initiation and escalation of immunotherapy. Our analysis included predominantly older studies published in the 5 years following the seminal paper recommending the tiered treatment approach, 7 and are likely representative of more conservative treatment practices with associated delays to second‐line immunotherapy in particular. This is compared to current practices, which urge early assessment of response to first‐line immunotherapy and a low threshold for escalation to second‐line immunotherapy, 22 with evidence indicating this proactive treatment approach results in a greater probability and degree of clinical improvement. 20 It is possible the suboptimal treatment response suggested by the present study is due to historical delays to therapy in these early reports, rather than ineffectiveness of second‐line agents in AE. This is an area of active research, with international consortia currently investigating the benefit of early second‐line immunotherapy in prospective AE studies. 23
In addition to these confounders, the use of the mRS as a measure of AE outcomes and disease severity also potentially contributes to the negative findings. The mRS is the most reported outcome measure in the literature for patients with AE. 24 However, the mRS is biased toward detection of physical disability and is relatively insensitive to cognitive and psychiatric impairments. 25 , 26 The correlation of the mRS with other important functional outcomes such as independent living and returning to work, study, or driving have not yet been reported. Similar limitations apply to the use of maximum mRS as a surrogate estimate of AE severity. We extracted the maximum mRS to estimate AE severity, as disease‐specific markers of severe AE such as status epilepticus, respiratory failure, and admission to an intensive care unit were inconsistently available for analysis; however, high mRS scores are not specific for severe AE, and patients with an mRS score of 5 (bedridden and requiring constant nursing care) display a wide range of AE symptoms. 27 The results of the current study may therefore reflect the insensitivity of the mRS to cognitive disability at follow‐up, or its poor specificity for severe AE. Further research is needed to determine the most appropriate tools for estimating disease severity and reporting patient outcomes in AE research.
We found treatment with second‐line immunotherapy was associated with higher final mRS scores in patients with LGI‐1 AE. Although this needs to be interpreted with caution given the wide CIs, the result is in keeping with reports in the literature demonstrating some degree of persisting cognitive disability after immunotherapy in most patients with LGI‐1 AE. 4 These findings may reflect the increased age of this patient subgroup, with less capacity for rehabilitative plasticity and less cognitive reserve. These findings also may be biased by treatment delay, as LGI‐1 has a gradual rather than abrupt onset associated with diagnostic and therapeutic delays, 1 an important factor influencing response to treatment in patients with AE. 24 We were unable to adjust for this covariate due to its infrequent reporting in the available IPD.
The results of this study also need to be interpreted in the context of several other methodological limitations. The included IPD were largely sourced from published reports, biasing the analysis toward smaller, earlier studies, with potential selection and publication bias limiting generalizability of the results, particularly in the modern era of early initiation and escalation of immunotherapy as discussed above. The small number of patients in the second‐line immunotherapy group also means the study was likely underpowered to detect small treatment effects.
The paucity of prospective controlled studies published in this field means that meta‐analyses based on observational data are currently the best available level of evidence to inform management of these patients. However, given their limitations, these studies are far from an optimal guide. Prospective controlled studies have historically been challenging to conduct and complete, as patients are dispersed geographically and across a range of treatment specialties, making recruitment challenging. These hurdles are not insurmountable, and the establishment of international consortia in recent years has led to the commencement of several prospective controlled trials, the outcomes of which will provide high‐quality evidence to guide management and support access to treatment for patients in the coming years.
5. CONCLUSIONS
We found no association between treatment with second‐line immunotherapy and lower final mRS scores in patients with AE. This finding may relate to selection and publication bias due to the high proportion of published IPD used in our analysis, and unmeasured confounders such as AE severity, treatment delay, and first‐line treatment response. The findings may also reflect the insensitivity of the mRS to cognitive impairment at follow‐up in patients with AE, and poor discriminatory capacity for severe AE. These findings suggest that second‐line immunotherapy may not be of benefit in all patients with AE, although they cannot exclude a benefit in certain subgroups and clinical scenarios and should be interpreted with caution given the methodological limitations. Further research is required to better define the subgroups of patients in whom second‐line therapy is of clinical benefit.
AUTHOR CONTRIBUTIONS
A.H. and A.D. performed the systematic review and data extraction. M.A. provided data. R.C.B. and M.C. provided advice regarding statistical analysis. A.H. undertook statistical analysis and prepared the manuscript. W.D. and X.C. contributed substantively to study design and manuscript editing. S.B. contributed substantively to study design. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
FIGURE S1
APPENDIX S1
TABLE S1
ACKNOWLEDGMENTS
The authors report no targeted funding for this study. The authors thank Jim Berryman, liaison librarian at Brownless Biomedical Library, University of Melbourne, for his recommendations and expertise in the literature search. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians. [Correction added on 05 July, 2022, after first online publication: CAUL funding statement has been added.]
Halliday A, Duncan A, Cheung M, Boston RC, Apiwattanakul M, Camacho X, Second‐line immunotherapy and functional outcomes in autoimmune encephalitis: A systematic review and individual patient data meta‐analysis. Epilepsia. 2022;63:2214–2224. 10.1111/epi.17327
REFERENCES
- 1. Dalmau J, Graus F. Antibody‐mediated encephalitis. N Engl J Med. 2018;378(9):840–51. 10.1056/NEJMra1708712 [DOI] [PubMed] [Google Scholar]
- 2. Gibson LL, McKeever A, Coutinho E, Finke C, Pollak TA. Cognitive impact of neuronal antibodies: encephalitis and beyond. Transl Psychiatry. 2020;10(1):1–17. 10.1038/s41398-020-00989-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30(3):345–53. 10.1097/WCO.0000000000000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arino H, Petit‐Pedrol M, Armangue T, Saiz A, Dalmau J, Graus F. Anti‐LGI1‐associated cognitive impairment: clinical profiles and long‐term outcome. Neurology. 2016;86(16 Suppl 1):759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li A, Gong X, Guo K, Lin J, Zhou D, Hong Z. Direct economic burden of patients with autoimmune encephalitis in western China. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e891. 10.1212/NXI.0000000000000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol. 2014;16(6):771–8. 10.1093/neuonc/nou030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–65. 10.1016/S1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. 2015;15(12):1391–419. 10.1586/14737175.2015.1115720 [DOI] [PubMed] [Google Scholar]
- 9. Abboud H, Probasco JC, Irani S, Ances B, Benavides DR, Bradshaw M, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. 2021;92(7):757–68. 10.1136/jnnp-2020-325300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalmau J, Lancaster E, Martinez‐Hernandez E, Rosenfeld MR, Balice‐Gordon R. Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. 10.1016/S1474-4422(10)70253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin Scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–6. 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Accessed August 11, 2021]
- 14. Burke DL, Ensor J, Riley RD. Meta‐analysis using individual participant data: one‐stage and two‐stage approaches, and why they may differ. Stat Med. 2017;36(5):855–75. 10.1002/sim.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riley RD, Debray TPA. The one‐stage approach to IPD meta‐analysis. Individual participant data meta‐analysis. Chichester, UK: John Wiley & Sons Ltd; 2021. p. 127–62. 10.1002/9781119333784.ch6 [DOI] [Google Scholar]
- 16. StataCorp . Stata Statistical Software. 2019. https://www.stata.com/support/faqs/resources/citing‐software‐documentation‐faqs/. [Accessed Aug 23, 2021]
- 17. Nepal G, Shing YK, Yadav JK, Rehrig JH, Ojha R, Huang DY, et al. Efficacy and safety of rituximab in autoimmune encephalitis: a meta‐analysis. Acta Neurol Scand. 2020;142(5):449–59. 10.1111/ane.13291 [DOI] [PubMed] [Google Scholar]
- 18. Thomas D, Radji S, Benedetti A. Systematic review of methods for individual patient data meta‐analysis with binary outcomes. BMC Med Res Methodol. 2014;14:79. 10.1186/1471-2288-14-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wegner F, Wilke F, Raab P, Tayeb SB, Boeck AL, Haense C, et al. Anti‐leucine rich glioma inactivated 1 protein and anti‐N‐methyl‐D‐aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F‐fluoro‐2‐deoxy‐d‐glucose positron emission tomography. BMC Neurol. 2014;14:136. 10.1186/1471-2377-14-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nosadini M, Eyre M, Molteni E, Thomas T, Irani SR, Dalmau J, et al. Use and safety of immunotherapeutic management of N‐methyl‐d‐aspartate receptor antibody encephalitis: a meta‐analysis. JAMA Neurol. 2021;78(11):1333–44. 10.1001/jamaneurol.2021.3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee WJ, Lee ST, Byun JI, Sunwoo JS, Kim TJ, Lim JA, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86(18):1683–91. 10.1212/WNL.0000000000002635 [DOI] [PubMed] [Google Scholar]
- 22. Nosadini M, Thomas T, Eyre M, Anlar B, Armangue T, Benseler SM, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1052. 10.1212/NXI.0000000000001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clardy S. A phase‐2b, double‐blind, randomized controlled trial to evaluate the activity and safety of inebilizumab in anti‐NMDA receptor encephalitis and assess markers of disease. 2022. https://clinicaltrials.gov/ct2/show/NCT04372615. [Accessed May 19, 2022]
- 24. Broadley J, Seneviratne U, Beech P, Buzzard K, Butzkueven H, O'Brien T, et al. Prognosticating autoimmune encephalitis: a systematic review. J Autoimmun. 2019;96:24–34. 10.1016/j.jaut.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 25. Rethnam V, Bernhardt J, Johns H, Hayward KS, Collier JM, Ellery F, et al. Look closer: the multidimensional patterns of post‐stroke burden behind the modified Rankin Scale. Int J Stroke. 2021;16(4):420–8. 10.1177/1747493020951941 [DOI] [PubMed] [Google Scholar]
- 26. Yeshokumar AK, Gordon‐Lipkin E, Arenivas A, Cohen J, Venkatesan A, Saylor D, et al. Neurobehavioral outcomes in autoimmune encephalitis. J Neuroimmunol. 2017;312:8–14. 10.1016/j.jneuroim.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 27. Lim JA, Lee ST, Moon J, Jun JS, Kim TJ, Shin YW, et al. Development of the clinical assessment scale in autoimmune encephalitis. Ann Neurol. 2019;85(3):352–8. 10.1002/ana.25421 [DOI] [PubMed] [Google Scholar]
- 28. Aungsumart S, Ha A, Apiwattanakul M. Abnormal level of consciousness predicts outcomes of patients with anti‐NMDA encephalitis. J Clin Neurosci. 2019;62:184–7. 10.1016/j.jocn.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 29. Cui J, Bu H, He J, Zhao Z, Han W, Gao R, et al. The gamma‐aminobutyric acid‐B receptor (GABAB) encephalitis: clinical manifestations and response to immunotherapy. Int J Neurosci. 2018;128(7):627–33. 10.1080/00207454.2017.1408618 [DOI] [PubMed] [Google Scholar]
- 30. Finke C, Prüss H, Heine J, Reuter S, Kopp UA, Wegner F, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine‐rich, glioma‐inactivated 1 antibodies. JAMA Neurol. 2017;74(1):50–9. 10.1001/jamaneurol.2016.4226 [DOI] [PubMed] [Google Scholar]
- 31. Gastaldi M, Mariotto S, Giannoccaro MP, Iorio R, Zoccarato M, Nosadini M, et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: a multicenter retrospective study. Eur J Neurol. 2020;27:633–43. [DOI] [PubMed] [Google Scholar]
- 32. Guan HZ, Ren HT, Yang XZ, Lu Q, Peng B, Zhu YC, et al. Limbic encephalitis associated with anti‐γ‐aminobutyric acid B receptor antibodies: a case series from China. Chin Med J (Engl). 2015;128(22):3023–8. 10.4103/0366-6999.168989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hara M, Ariño H, Petit‐Pedrol M, Sabater L, Titulaer MJ, Martinez‐Hernandez E, et al. DPPX antibody–associated encephalitis: main syndrome and antibody effects. Neurology. 2017;88(14):1340–8. 10.1212/WNL.0000000000003796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heine J, Ly LT, Lieker I, Slowinski T, Finke C, Prüss H, et al. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: a pilot study. J Neurol. 2016;263(12):2395–402. 10.1007/s00415-016-8277-y [DOI] [PubMed] [Google Scholar]
- 35. Hoftberger R, van Sonderen A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, et al. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology. 2015;84(24):2403–12. 10.1212/WNL.0000000000001682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang X, Fan C, Wu J, Ye J, Zhan S, Song H, et al. Clinical analysis on anti‐N‐methyl‐D‐aspartate receptor encephalitis cases: Chinese experience. Int J Clin Exp Med. 2015;8(10):18927–35. [PMC free article] [PubMed] [Google Scholar]
- 37. Irani SR, Gelfand JM, Bettcher BM, Singhal NS, Geschwind MD. Effect of rituximab in patients with leucine‐rich, glioma‐inactivated 1 antibody‐associated encephalopathy. JAMA Neurol. 2014;71(7):896–900. 10.1001/jamaneurol.2014.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joubert B, Saint‐Martin M, Noraz N, Picard G, Rogemond V, Ducray F, et al. Characterization of a subtype of autoimmune encephalitis with anti‐contactin‐associated protein‐like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol. 2016;73(9):1115–24. 10.1001/jamaneurol.2016.1585 [DOI] [PubMed] [Google Scholar]
- 39. Kamble N, Netravathi M, Saini J, Mahadevan A, Yadav R, Nalini A, et al. Clinical and imaging characteristics of 16 patients with autoimmune neuronal synaptic encephalitis. Neurol India. 2015;63(5):687–96. 10.4103/0028-3886.166532 [DOI] [PubMed] [Google Scholar]
- 40. Liao S, Qian Y, Hu H, Niu B, Guo H, Wang X, et al. Clinical characteristics and predictors of outcome for onconeural antibody‐associated disorders: a retrospective analysis. Front Neurol. 2017;8:584. 10.3389/fneur.2017.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Melamud LI, Fernández VC, Manin A, Villa AM. Autoimmune encephalitis and immune therapy: lessons from Argentina. Acta Neurol Belg. 2020;120(3):565–72. 10.1007/s13760-018-1013-x [DOI] [PubMed] [Google Scholar]
- 42. Dogan Onugoren M, Golombeck KS, Bien C, Abu‐Tair M, Brand M, Bulla‐Hellwig M, et al. Immunoadsorption therapy in autoimmune encephalitides. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e207. 10.1212/NXI.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shin YW, Lee ST, Shin JW, Moon J, Lim JA, Byun JI, et al. VGKC‐complex/LGI1‐antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol. 2013;265(1):75–81. 10.1016/j.jneuroim.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 44. Spatola M, Sabater L, Planagumà J, Martínez‐Hernandez E, Armangué T, Prüss H, et al. Encephalitis with mGluR5 antibodies. Neurology. 2018;90(22):e1964–72. 10.1212/WNL.0000000000005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Viaccoz A, Desestret V, Ducray F, Picard G, Cavillon G, Rogemond V, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82(7):556–63. 10.1212/WNL.0000000000000126 [DOI] [PubMed] [Google Scholar]
- 46. Wang D, Hao Q, He L, He L, Wang Q. LGI1 antibody encephalitis—detailed clinical, laboratory and radiological description of 13 cases in China. Compr Psychiatry. 2018;81:18–21. 10.1016/j.comppsych.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 47. Wang K, Chen Z, Wu D, Ding Q, Zheng X, Wang J, et al. Early second‐line therapy is associated with improved episodic memory in anti‐NMDA receptor encephalitis. Ann Clin Transl Neurol. 2019;6(7):1202–13. 10.1002/acn3.50798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X, Li AN, Zhao XH, Liu XW, Wang SJ. Clinical features of patients with anti‐leucine‐rich glioma inactivated‐1 protein associated encephalitis: a Chinese case series. Int J Neurosci. 2019;129(8):754–61. 10.1080/00207454.2019.1567507 [DOI] [PubMed] [Google Scholar]
- 49. Yeo T, Chen Z, Yong KP, Wong PYW, Chai JYH, Tan K. Distinction between anti‐VGKC‐complex seropositive patients with and without anti‐LGI1/CASPR2 antibodies. J Neurol Sci. 2018;391:64–71. 10.1016/j.jns.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 50. Zhang W, Cui L, Wang W, Jiao Y, Zhang Y, Jiao J. Early identification of anti‐NMDA receptor encephalitis presenting cerebral lesions in unconventional locations on magnetic resonance imaging. J Neuroimmunol. 2018;320:101–6. 10.1016/j.jneuroim.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Liu G, Jiang M, Chen W, Su Y. Efficacy of therapeutic plasma exchange in patients with severe refractory anti‐NMDA receptor encephalitis. Neurotherapeutics. 2019;16(3):828–37. 10.1007/s13311-019-00725-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1
APPENDIX S1
TABLE S1
Data Availability Statement
The set of deidentified individual patient data retrieved from published IPD and used for analysis is available on request. Data obtained from other authors is not included in the shared dataset.


