Abstract
Aim
There are discrepancies in the guidelines on preparation for colorectal surgery. While intravenous antibiotics (IV) are usually administered, the use of mechanical bowel preparation (MBP) and/or oral antibiotics (OA) is controversial. A recent network meta‐analysis (NMA) demonstrated that the addition of OA reduced incisional surgical site infections (iSSIs) by more than 50%. We aimed to perform a NMA including only the highest quality randomized clinical trials (RCTs) in order to determine the ranking of different treatment strategies and assess these RCTs for methodological problems that may affect the conclusions of the NMAs.
Method
A NMA was performed according to PRISMA guidelines. RCTs of adult patients undergoing elective colorectal surgery with appropriate antibiotic cover and with at least 250 participants recruited, clear definition of endpoints and duration of follow‐up extending beyond discharge from hospital were included. The search included Medline, Embase, Cochrane and SCOPUS databases. Primary outcomes were iSSI and anastomotic leak (AL). Statistical analysis was performed in Stata v.15.1 using frequentist routines.
Results
Ten RCTs including 5107 patients were identified. Treatments compared IV (2218 patients), IV + OA (460 patients), MBP + IV (1405 patients), MBP + IV + OA (538 patients) and OA (486 patients). The likelihood of iSSI was significantly lower for IV + OA (rank 1) and MBP + IVA + OA (rank 2), reducing iSSIs by more than 50%. There were no differences between treatments for AL. Methodological issues included differences in definition, assessment and frequency of primary endpoint infections and the limited number of participants included in some treatment options.
Conclusion
While this NMA supports the addition of OA to IV to reduce iSSI it also highlights unanswered questions and the need for well‐designed pragmatic RCTs.
What does this paper add to the literature?
Assessment of the highest quality randomized clinical trials (RCTs) in a network meta‐analysis confirmed that combining oral and intravenous antibiotics reduced incisional surgical site infections by more than 50%. There were significant methodological issues related to definition, assessment and frequency of endpoint infections, and the limited sample size. Further pragmatic RCTs assessing the impact of adding oral antibiotics should be performed.
INTRODUCTION
Anastomotic leak (AL) and incisional surgical site infection (iSSI) continue to be frustratingly prevalent complications in elective colorectal surgery [1], resulting in significant morbidity for patients and cost to health care providers. Ongoing research focusing on the prevention of these complications has not yet resulted in a consensus on the importance of different bowel preparation regimens. This is reflected in differences in practices and guidelines in America [2] and Europe [3] and of international societies such as the ERAS Society [4].
One method for assessing outcomes where there are differences in practice is large database reviews. Studies reviewing the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database report that the addition of oral antibiotics (OA) significantly reduces iSSI, AL, ileus and hospital stay [5, 6, 7]. While these results reflect real life practice, they also raise questions. What is the quality of evidence in databases compared with randomized clinical trials (RCTs)? In terms of controlling for differences in risk factors, American Society of Anesthesologists grade, disseminated cancer, laparoscopic surgery and other risk factors favour the OA group [5, 6]. Accurately adjusting for these as well as other potential differences is difficult. Also, no data are available on the adequacy of the combined antibiotic cover against aerobic and anaerobic bacteria between the groups being compared.
Another method is to systematically assess high‐quality studies, in this case RCTs, by using meta‐analysis or network meta‐analysis (NMA). Meta‐analyses compare two options using pairwise comparisons. These have demonstrated no important differences between mechanical bowel preparation (MBP) and MBP with intravenous antibiotics (MBP + IV) and intravenous antibiotics alone (IV). In contrast, other meta‐analyses have shown advantages for IV + OA compared with IV [8, 9, 10]. Unfortunately, many included RCTs compare IV regimens that provide incomplete aerobic and anaerobic cover with IV + OA regimens providing good aerobic and anaerobic antibiotic cover, making it unclear if this difference is due to better antibiotic cover or the additional use of OA.
NMA has the advantage of integrating data assessing multiple options into a network where direct evidence from head‐to‐head comparisons and indirect evidence of comparisons linked within the network are assessed. A NMA of RCTs comparing methods of bowel preparation that only included RCTs with good aerobic and anaerobic cover in all groups being compared has recently been published [11]. This demonstrated that the addition of OA to IV reduced the incidence of iSSI by more than 50%. This was the case both with and without the use of MBP. There were no differences in AL or in other clinical outcomes. Has this NMA resolved the controversy, or are additional studies still required?
Systematic reviews depend on the quality of the included studies and the methodological quality of the review [1]. While many quality issues are recognized and assessed in the Cochrane Collaboration's risk of bias tool, others are less well‐recognized. For example, meta‐analyses combining small studies with different methods for diagnosing endpoints and inadequate blinding have reported results which conflict with subsequent, high‐quality RCTs. Examples in the colorectal literature include meta‐analyses looking at the use of wound protectors to prevent iSSI [12, 13] and prophylactic mesh placement to prevent parastomal hernias [14, 15]. We therefore wanted to assess the impact of ‘lower quality’ studies on our NMA results by performing a NMA including only the highest quality studies, in‐line with predefined criteria. We will then examine the limitations of these NMAs to identify any outstanding questions about bowel preparation in elective colorectal surgery.
METHOD
We performed a systematic review of RCTs comparing methods of bowel preparation in elective colorectal surgery. This included the use of IV, OA, MBP, enema (E) and combinations of these. The use of E with MBP was counted as part of the MBP. Studies had to compare at least two bowel preparation options. Outcomes assessed were iSSI and AL. AL was defined as clinical disruption of the anastomosis. A radiological diagnosis of AL without a clinical problem, or a space SSI without a clinical AL, were not counted. An iSSI required a wound problem consistent with the Centers for Disease Control (CDC) definitions of superficial incisional or deep incisional SSI.
Predefined criteria for selecting the ‘best quality studies’ were RCTs, good aerobic and anaerobic cover in all groups being compared, at least 250 participants recruited, clear definitions of the endpoints, and the duration of endpoint follow‐up extending to after discharge from hospital. Exclusion criteria included studies which were not RCTs, were in paediatric patients, where results for different bowel preparation interventions were combined [16] and where the ‘best study’ criteria were not met. Effective aerobic cover was defined in line with Clinical and Laboratory Standards Institute guidelines [11], and effective anaerobic cover was defined as a MIC90 <16 for the majority of anaerobic pathogens and/or an overall resistance to anaerobic bacteria of less than 20%. Direct comparisons of all colorectal resections, all left‐sided colon resections and all colon resections were included.
Medline, Embase, the Cochrane Library (CENTRAL) and SCOPUS databases and trial registries (clinicaltrials.gov and WHO International Clinical Trials Registry) were searched. The bibliographies of included studies, clinical practice guidelines and systematic reviews were hand searched for other relevant articles. There were no limitations on language or publication period. Two researchers independently screened all citations, reviewed identified abstracts for eligibility, extracted data and summarized the methodological quality of studies using the Cochrane Collaboration's risk of bias tool for RCTs. Discrepancies were resolved by the senior author. Corresponding authors were contacted to clarify information as required.
Network diagrams illustrated the direct comparisons between the bowel preparation treatments. Random effects NMA was performed, including direct and indirect comparisons, to determine the pooled relative effect of each treatment compared with every other treatment for the outcomes of interest. Analyses were performed using a frequentist framework in Stata v.15.1 [17]. Categorical data were summarized as odds ratios (95% confidence intervals) and presented in league tables. The relative ranking of different bowel preparations was estimated for each outcome using the distribution of ranking probabilities and surface under cumulative ranking curves [18]. Between‐study heterogeneity was evaluated using the tau‐square statistic and each model was assessed for global and local inconsistency. A more detailed description of the methods including the search strategy and statistics is published elsewhere [11].
RESULTS
From 6834 titles, 472 abstracts and 175 identified studies, 10 eligible RCTs including 5107 patients were identified. Identified bowel preparation options were: MBP + IV six studies, IV with no bowel preparation with or without E (IV) eight studies, IV and OA with or without E (IV + OA) two studies, MBP with IV with good aerobic and anaerobic cover and additional OA (MBP + IV + OA) three studies, and OA with no bowel preparation with or without E (OA) one study.
The PRISMA flow chart is summarized in Figure 1. The network is illustrated in Figure 2, and the details of the included RCTs are presented in Table 1.
FIGURE 1.
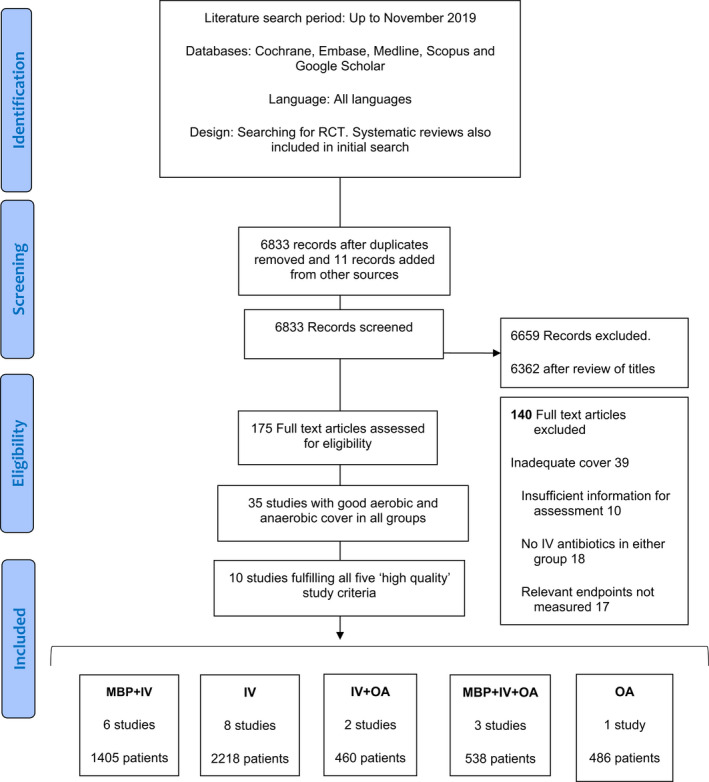
Prisma flow diagram for data collection. IV, intravenous antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics; RCT, randomized clinical trial
FIGURE 2.
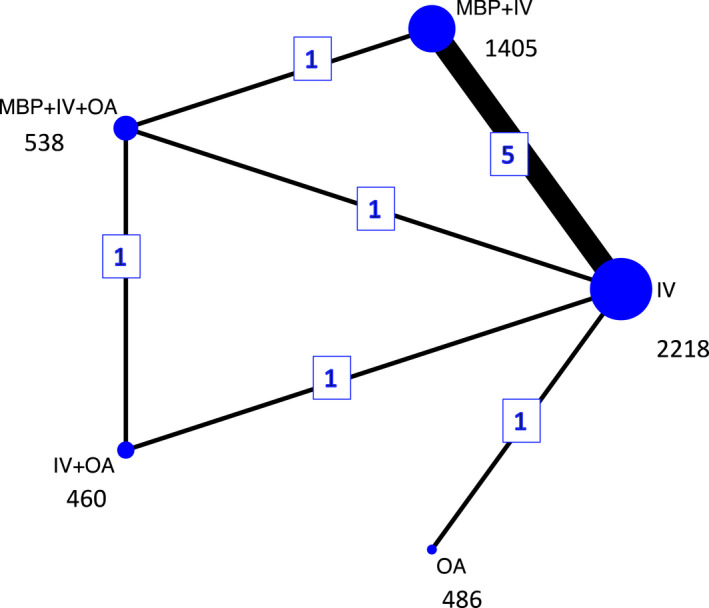
Network plot of direct comparisons for surgical site infection. The size of the individual nodes represents the number of patients studied for each bowel preparation option, and the thickness of the lines is proportional to the number of studies directly comparing the different nodes. IV, intravenous antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics
TABLE 1.
Summary table of included studies
| Study | Country | Sample size | Mean age (years) | Sex (%F) | Group and number | IV antibiotics | Oral antibiotics | MBP | Definition of iSSI | iSSI primary endpoint? | Definition of AL | AL primary endpoint? | Time for diagnosis | Blinding | No. of iSSIs | No. of ALs | Issues with paper |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abis (2019) [41] a | Netherlands | 455 | 67.8 | 72 | MBP + IV + OA (155) | 1 g cefazolin, 0.5 g metronidazole | 0.5 g ampotericin B, 100 mg colistin sulphate, 80 mg tobramycin | MBP Given | According to CDC guidelines | No | Clinical suspicion and requiring a radiological or surgical intervention | Yes | Within 30 days | Open label | 4/155 (2.5%) | 12/155 (7.7%) |
No blinding Similar infection rates for iSSI and AL Only left‐sided groups included because of transitivity assumption for AL |
| MBP + IV (161) | 1 g cefazolin, 0.5 g metronidazole | MBP given | 20/161 (12.4%) | 19/161 (11.8%) | |||||||||||||
| Contant (2007) [50] | Netherlands | 1354 | 67 | 50 | MBP + IV (670) | Broad‐spectrum anaerobic options and metronidazole | Hospital protocol | Mild‐erythema or discharge of seroma | No | Clinical suspicion confirmation by radiology or surgery | Yes | Up to 2/52 postdischarge | Unblinded | 90/670 (13.4%) | 32/670 (4.8%) | Unblinded but similar infection rates in both groups | |
| IV (684) | Broad‐spectrum anaerobic options and metronidazole | Severe‐ discharge of pus, necrosis or wound dehiscence | 96/684 (14.0%) | 37/684 (5.4%) | |||||||||||||
| Espin Basany (2020) [40] | Spain | 536 | 71 | 45 | IV (269) | 1.5 g cefuroxime and 1 g metronidazole | According to CDC guidelines | Yes | Not clearly stated, although investigations with CT and surgery discussed | No | 4/52 postdischarge | Assessor blinded | 22/269 (8.2%) | 0/269 (0%) | |||
| IV + OA (267) | 1.5 g cefuroxime and 1 g metronidazole | Ciprofloxacin 750 mg ×4 and metronidazole 250 mg ×9 | 5/267 (1.9%) | 0/267 (0%) | |||||||||||||
| Fa‐Si‐Oen (2005) [51] | Netherlands | 250 | 70 | 54 | MBP + IV (125) | Cefazolin or gentamicin and 1.5 g metronidazole | 4 l PEG | Clinically significant infection of the skin requiring wound evacuation | Yes | Clinical diagnosis. Major if laparotomy, minor if radiological intervention | Yes | Up to 3 months | Unclear | 9/125 (7.2%) | 7/125 (5.6%) |
Blinding not clear, but similar infection rates in both groups Wound and AL rates similar |
|
| IV (125) | Cefazolin or gentamicin and 1.5 g metronidazole | 7/125 (5.6%) | 6/125 (4.8%) | ||||||||||||||
| Hjalmarsson (2015) [52] | Sweden | 985 | 70 | 47 | OA (486) | Cotrimoxazole and 1.2 g metronidazole | According to CDC guidelines | Yes | Clinically confirmed anastomotic insufficiency | No | Assessed daily for 28 days | Assessor blinded | 34/486 (7.0%) | 17/486 (3.5%) | Wound and AL rates similar in IV group | ||
| IV (499) | 1.5 g cefuroxime, 1.5 g metronidazole | 18/499 (3.6%) | 17/499 (3.4%) | ||||||||||||||
| Koskenvuo (2019) [53] | Finland | 396 | 70.1 | 49 | MPB + IV + OA (196) | 1.5 g cefuroxime, 0.5 g metronidazole | 2 g neomycin, 2 g metronidazole | 2 l PEG | According to CDC guidelines | Yes | Clinical suspicion and confirmation of anastomotic dehiscence within 30 days after surgery | No | 30 days | Assessor blinding | 13/196 (7%) | 7/196 (3.6%) | |
| IV (200) | 1.5 g cefuroxime, 0.5 g metronidazole | 2 l PEG | 21/200 (10.5%) | 8/200 (4.0%) | |||||||||||||
| Miettinen (2000) [54] | Finland | 267 | 63 | 51 | MBP + IV (138) | 2 g ceftriaxone, 1 g metronidazole | PEG | Presence of pus or discharge with microbiological culture | NS, but first listed | Brief description. Implies clinical suspicion confirmed by radiology | NS, third outcome listed | 1–2 months | Unclear | 5/138 (3.6%) | 5/138 (3.6%) |
Blinding unclear Wound infection and AL infection rates similar in both groups |
|
| IV (129) | 2 g ceftriaxone, 1 g metronidazole | 3/129 (2.3%) | 3/129 (2.3%) | ||||||||||||||
| Platell (2006) [48] | Australia | 294 | 66 | 65 | MBP + IV (147) | Timentin or gentamicin and 0.5 g metronidazole | 3 l PEG | Discharge with microbiological confirmation | No | Clinical suspicion confirmed with radiology or surgery | Yes | 30 days | Unclear | 21/147 (14.3%) | 7/147 (4.8%) | Blinding unclear but similar infection rates in both groups | |
| IV + E (147) | Timentin or gentamicin and 0.5 g metronidazole | Phosphate E | 19/147 (13.3%) | 3/147 (2.0%) | |||||||||||||
| Ram (2005) [55] | Israel | 329 | 68 | 39 | MBP + IV (164) | 1 g ceftriaxone, 0.5 g metronidazole | 3.3 g Na phosphate | Discharge with microbiological confirmation | NS, but first listed | Clinical suspicion confirmed with radiology (water‐soluble contrast) | NS, fourth (last) listed | 1, 3 and 6 weeks postdischarge | Not stated | 10/165 (6.1%) | 2/165 (1.2%) | Blinding unclear | |
| IV (165) | 1 g ceftriaxone, 0.5 g metronidazole | 16/164 (9.8%) | 1/164 (0.6%) | ||||||||||||||
| Zmora (2003) [44] | Israel | 380 | 68 | 48 | MBP + IV + OA (187) | Broad‐spectrum aerobic and anaerobic | Neomycin and erythromycin ×3 | 4.5 l PEG | Erythema needing antibiotics or requiring opening of wound | Infectious complications, including WI | Clinical suspicion confirmed by imaging or surgery or faecal material in drain | Infectious complications including AL | Up to 1 month | Unblinded | 12/187 (6.4%) | 7/187 (3.7%) | No blinding but similar infection rates in both groups |
| IV + OA (193) | Broad‐spectrum aerobic and anaerobic | Neomycin and erythromycin ×3 | 11/193 (6.0%) | 4/193 (2.1%) |
Abbreviations: AL, anastomotic leak; CDC, Centers for Disease Control; IV, intravenous antibiotics; MBP, mechanical bowel preparation; NS, not stated; OA, oral antibiotics; PEG, polyethylene glycol; WI, wound infection.
Details provided by corresponding author.
Even in the ‘best studies’, quality issues were identified. For iSSI, there were differences in the definition, assessment, follow‐up and frequency of infection. Definitions were according to CDC guidelines on four occasions and were individually defined on six occasions; in two, erythema was sufficient and in four, discharge from the wound was required. iSSI was the primary endpoint on four occasions, was possibly the primary endpoint on three occasions and was not the primary endpoint on three occasions. Duration of follow‐up was until 2 weeks after discharge on one occasion, up to 4 weeks (1 month or 30 days) after surgery on six occasions and for longer on three occasions. Only one RCT detailed the effort made to diagnose an iSSI. With respect to frequency of iSSI, in four RCTs the rates of iSSI and AL were similar for at least one of the bowel preparation options. The rate of iSSI varied from 1.9% to 14.3%. This varied from 3.6% to 14.3% for MBP + IV from 2.3% to 14% for IV and from 2.5% to 7% for MBP + IV + OA. In three studies there was assessor blinding, in four studies details were unclear and three studies were ‘open label’ or unblinded. Similar observations were made for AL (Table 1).
All patients were assessed for iSSI and AL. Analysis of iSSI demonstrated consistency between direct and indirect measurements (χ2 = 1.03, p = 0.59), local consistency with all loops having an inconsistency factor (IF) < 1.0 and no evidence of publication bias (Figure S1). The best ranking (Figure 3) was achieved with IV + OA (71% probability of being the best treatment) followed by MBP + IV + OA (71% probability of being the second‐best treatment). In order of ranking, these were followed by IV, MBP + IV and OA. Patients treated with either IV + OA or MBP + IV + OA had significantly fewer iSSIs when compared with any of the other treatment options (p < 0.001; Table 2; Figure 4). MBP + IV had significantly fewer associated iSSIs than OA (p = 0.045). There was no significant difference between IV + OA (460 patients) and MBP + IV + OA (538 patients).
FIGURE 3.
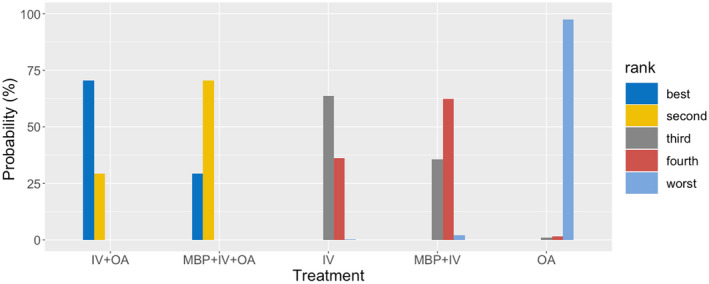
Rankogram for incisional surgical site infection. The rankogram shows the probability of each preparation option being ranked best to worst. For example, IV + OA has a 70.6% probability of being ranked best, 29.4% probability of being ranked second and a <1% probability for the other options. In comparison, IV has a 63.6% probability of being ranked third, a 36.2% probability of being ranked fourth and <1% probability for the other options. IV, intravenous antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics
TABLE 2.
Odds ratios (95% CI) for comparisons of treatments for incisional surgical site infection and anastomotic leak a
| IV | OA | IV + OA | MBP + IV + OA | MBP + IV | |
|---|---|---|---|---|---|
| Incisional surgical site infection | |||||
| IV | 2.01 (1.12,3.61) | 0.23 (0.11,0.49) | 0.27 (0.14,0.54) | 1.05 (0.82,1.34) | |
| OA | 0.50 (0.28,0.89) | 0.11 (0.04,0.30) | 0.14 (0.05,0.34) | 0.52 (0.28,0.98) | |
| IV + OA | 4.37 (2.05,9.31) | 8.79 (3.38,22.87) | 1.19 (0.59,2.41) | 4.58 (2.10,9.97) | |
| IV + MBP + OA | 3.68 (1.84,7.35) | 7.39 (2.98,18.31) | 0.84 (0.42,1.70) | 3.85 (1.91,7.78) | |
| MBP + IV | 0.95 (0.74,1.22) | 1.92 (1.02,3.63) | 0.22 (0.10,0.48) | 0.26 (0.13,0.52) | |
| Anastomotic leak | |||||
| IV | 1.03 (0.52,2.04) | 0.41 (0.11,1.54) | 0.69 (0.36,1.32) | 0.92 (0.63,1.35) | |
| OA | 0.97 (0.49,1.93) | 0.40 (0.09,1.77) | 0.67 (0.26,1.72) | 0.90 (0.41,1.97) | |
| IV + OA | 2.44 (0.65,9.20) | 2.51 (0.56,11.16) | 1.68 (0.51,5.51) | 2.25 (0.60,8.42) | |
| IV + MBP + OA | 1.45 (0.76,2.79) | 1.49 (0.58,3.85) | 0.60 (0.18,1.96) | 1.34 (0.72,2.50) | |
| MBP + IV | 1.08 (0.74,1.59) | 1.11 (0.51,2.44) | 0.44 (0.12,1.66) | 0.74 (0.40,1.39) | |
OR > 1: the outcome is more likely after treatment in the corresponding cell in the top row when compared with treatment indicated in the corresponding cell in the left column. OR < 1: the outcome is less likely after treatment in the corresponding cell in the top row when compared with treatment indicated in the corresponding cell in the left column.
FIGURE 4.
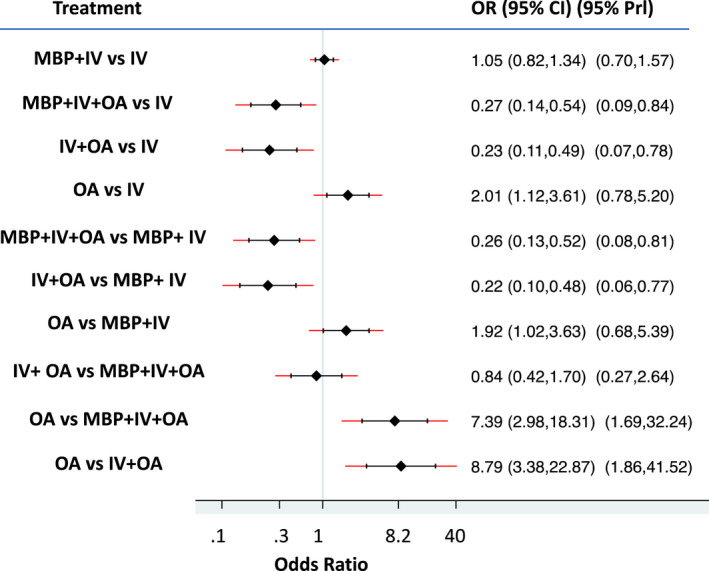
Forest plot comparing odds ratios (ORs) for treatments and their effect on the occurrence of incisional surgical site infection (iSSI). OR > 1: iSSI is more likely after treatment listed on the left when compared with treatment indicated on the right. OR < 1: iSSI is more likely after treatment listed on the right when compared with treatment indicated on the left. CI, confidence interval; IV, intravenous antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics; Prl, predicted interval
Analysis of AL demonstrated consistency between direct and indirect measurements (χ2 = 0.71, p = 0.70), local consistency with all loops having an IF < 1.0 and no evidence of publication bias (Figure S2). The best ranking was achieved with IV + OA (77% probability of being the best treatment), followed by MBP + IV + OA (58% probability of being the second‐best treatment) (Figure S3). While the odds of AL were lower in IV + OA and MBP + IV + OA, this did not reach statistical significance (Table 2; Figure 5).
FIGURE 5.

Forest plot comparing odds ratios for treatments and their effect on the occurrence of anastomotic leak. OR > 1: iSSI is more likely after treatment listed on the left when compared with treatment indicated on the right. OR < 1: iSSI is more likely after treatment listed on the right when compared with treatment indicated on the left. CI, confidence interval; IV, intravenous antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics; Prl, predicted interval
DISCUSSION
The main findings for ‘the ten best studies’ were very similar to our larger NMA which included 35 RCTs [11]. In both NMAs, IV + OA and MBP + IV + OA were significantly better than other options, reducing iSSI by more than 50%. There were no significant differences in AL. Our current analyses, which only includes high‐quality RCTs, with good statistical consistency and no publication bias, suggest that the addition of OA to IV, both with and without MBP, should become standard practice in elective colorectal surgery. However, a more careful analysis demonstrates methodological problems with even the best RCTs, as well as limitations within the NMAs, which require further examination.
Incisional SSI within 30 days is one of eight clinical indicators for measuring quality, safety and improvement in perioperative care [19]. In this NMA, the frequency of iSSI ranged from 1.9% to 14.3%, with a concerning three‐ to four‐fold variation for the same method of bowel preparation. One of the domains in the PRECIS‐2 toolkit is the intensity and measurement of follow‐up, including both the duration and frequency of measurement. When diagnosing iSSI an important factor is the ability of patients to report back to the medical team (incorporating the patient's perspective). The majority of iSSIs develop after discharge from hospital [20, 21], with 50%–80% of iSSIs being identified by the 16th postoperative day [22, 23, 24]. While RCTs in this NMA followed patients for approximately 30 days or more, infections developing postdischarge may be overlooked without a targeted identification strategy. Diagnosis of iSSI is also more frequent when patients are formally contacted after discharge [13, 25]. For example, when followed up by phone interview 30 days after surgery, 57% of iSSIs had started after hospital discharge [26] and even more iSSIs were identified when using a validated, patient‐centred questionnaire 4 weeks after discharge [27]. The Bluebelle study developed a wound scoring questionnaire for iSSI with an area under the receiver operating characteristic curve of 0.9056 (0.82–0.98) when assessed against CDC criteria [28]. The ROSSINI study, with an iSSI rate of 25% after open abdominal surgery, included the clinical training of wound assessors, clinical reviews at days 7 and 30 and a patient self‐reported questionnaire to identify iSSI. This high level of scrutiny is likely to identify an iSSI rate of greater than 10%–15% in studies combining elective laparoscopic and open colorectal surgery.
Another domain in the PRECIS‐2 toolkit is the importance of primary outcome. It is especially important to distinguish between primary and secondary endpoints when performing meta‐analyses. Matthews et al. [29] demonstrated that iSSI rates were 12.6% when studied as the primary endpoint and 5.1% as a secondary endpoint. In this NMA, iSSI was the primary endpoint on four occasions, was unclear on three occasions and was a secondary endpoint on three occasions. While this introduces heterogeneity, we do note that there were similar rates of iSSI in all categories, including two of the three secondary endpoint studies which identified iSSI rates of more than 10%. Another issue is the potential impact of lack of blinding on iSSI results. A review of blinding in surgical RCTs comparing laparoscopic and open surgery showed a significant reduction in the differences in length of stay and complications when participant or assessor blinding was achieved [30]. While patient blinding is not possible with MBP, assessor blinding can still be achieved. In this NMA, assessor blinding was confirmed in only three RCTs. It is difficult to control for the cumulative impact of small differences introduced by variations in definitions, primary endpoints and follow‐up, only a proportion of iSSIs being identified, and a lack of blinding. However, this combination does increase the risk of inaccuracy, such as exaggerated differences between treatments.
In this NMA, when we compare IV + OA or MBP + IV + OA with IV or MBP + IV, there was an approximate four‐fold reduction in iSSI. Are these results pathophysiologically credible? When OA were introduced, RCTs in the 1970s comparing MBP with OA against MBP alone (with no IV being used) demonstrated a reduction in iSSIs from 42% to 17% [31] and from 31% to 9% [32]. When using effective IV [33] we would expect the impact of adding OA to IV to be less. It is therefore likely that the advantages of adding OA in our NMA are exaggerated. In terms of pathophysiology, MBP + IV + OA reduces bacterial colonization within the colon [34]. The mechanism of using IV + OA without MBP is not due to this mechanism and is likely to be more nuanced. This may involve host–gut flora interactions and changes in the microbiome including adjustments in levels of commensal and pathogenic bacteria. Avoiding the detrimental impact of MBP on the microbiome (which includes reducing Bifidobacterium and Lactobacillus [35]) and on the colonic mucosa [36] may be an important factor. Understanding the physiology will aid our understanding of the benefits of IV + OA without MBP. This will also help with clinical decision‐making as correct conclusions are most likely when clinical outcomes, pathophysiology and statistical results are consistent with each other, as illustrated in the differences in meta‐analyses assessing the impact of single‐ and double‐ring wound protectors on iSSI [12, 37].
The main weakness of our larger NMA is the limited number of RCTs and patients included in some treatment options. Similarly, in this NMA of high‐quality studies approximately 500 patients were included in the IV + OA, MBP + IV + OA and OA only groups. The limitation is demonstrated by a sample size of 434 patients being required in each group to demonstrate a reduction in a clinical outcome from 10% to 5% with 80% power, assuming a well‐designed RCT with 1:1 randomization. There also needs to be caution interpreting results when a treatment examined by few studies is ranked at either extreme of the network. For example, IV + OA (two RCTs) performed as the best option for iSSI, and OA alone (one RCT) as the worst option. The relatively limited data in the IV + OA group was expected, as this has only been assessed in a few studies. However, with the long history of using MBP + IV + OA and the amount of literature discussing this, the limited number of patients in the MBP + IV + OA group (in both our previous larger NMA and in this NMA) was less than expected and is the main reason for controversy around the advantages of MBP + IV + OA. Another limitation of our NMAs was insufficient numbers of RCTs reporting results separately for colon and rectal surgery. For example, most of the IV + OA cases underwent colon surgery, and this option has not been adequately assessed in rectal surgery. Other potential limitations of our main NMA, including issues of study complexity, the antibiotic cover of included studies and the impact of changes in practice over time have been discussed previously [11].
For some, the results of our NMAs incorporating all RCTs with good antibiotic cover, combined with the international literature [38, 39, 40, 41, 42, 43, 44, 45], will provide sufficient information to routinely add OA to IV, with or without MBP, in preparation for colorectal surgery. However, in this discussion we have highlighted problems with limited sample size for some bowel preparation options, including both IV + OA and MBP + IV + OA, ‘greater than expected’ differences in iSSI when OA are added to IV and methodological problems with the frequency of diagnosis of iSSI and lack of assessor blinding. In this context, the NMA should be viewed as an opportunity to design and implement large, rigorous studies to test the main findings [1]. It is important that such studies are pragmatic [46], designed to answer current questions, and not repeating the limitations of previous RCTs. Studies need to be powered to assess for differences in iSSI and AL as primary endpoints, have a clearly agreed method for diagnosing iSSI (consistent with the ROSSINI study or including another validated, patient‐focused questionnaire), include blinding of assessors and include an assessment of the impact of IV + OA on the microbiome. For iSSI, RCTs examining the role of IV + OA and MBP + IVA + OA in both colon and rectal surgery [47] are indicated. For AL, larger RCTs assessing the impact of combining OA with IV, with good antibiotic cover in all groups, will clarify if OA significantly reduces rates of AL or not. The severity of AL [48] should also be assessed. Research should also assess the impact of OA with or without MBP on the microbiome, including on commensal and pathogenic bacteria, and the preferential colonization of collagenase‐producing bacteria such as Pseudomonas aeruginosa and Enterococcus faecalis [49]. In contrast, sufficient data exist comparing MBP + IV and intravenous antibiotics alone, and no further studies comparing these options are necessary.
In conclusion, in this NMA including only high‐quality RCTs, some treatment options were underpowered and methodological issues were still present. To address ongoing questions, large, well‐designed and pragmatic studies, as described above, assessing the impact of additional OA in colon and rectal surgery are required.
AUTHOR CONTRIBUTIONS
John C. Woodfield: Conceptualization, methodology, data review, writing (original draft and review). Kari Clifford: Methodology, data collection, data analysis and visualisation, writing (review). Barry Schmidt: Methodology, data collection. Mark Thompson‐Fawcett: Supervision, writing (original draft and review).
CONFLICT OF INTEREST
We declare no competing interests.
Supporting information
Figures S1–S3
Woodfield JC, Clifford K, Schmidt B, Thompson‐Fawcett M. Has network meta‐analysis resolved the controversies related to bowel preparation in elective colorectal surgery?. Colorectal Dis. 2022;24:1117–1127. 10.1111/codi.16194
[Correction added on 12 July 2022, after first online publication : CAUL Funding statement has been added.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Barie PS. Does a well‐done analysis of poor‐quality data constitute evidence of benefit? Ann Surg. 2012;255:1030–1. [DOI] [PubMed] [Google Scholar]
- 2. Migaly J, Bafford AC, Francone TD, Gaertner WB, Eskicioglu C, Bordeianou L, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis Colon Rectum. 2019;62:3–8. [DOI] [PubMed] [Google Scholar]
- 3. Moran B, Gollins S, Adams R, et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017) – Multidisciplinary Management. Colorectal Dis. 2017;19:37–66. [DOI] [PubMed] [Google Scholar]
- 4. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:259–84. [DOI] [PubMed] [Google Scholar]
- 5. Kiran RP, Murray ACA, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262:416–23. [DOI] [PubMed] [Google Scholar]
- 6. Garfinkle R, Abou‐Khalil J, Morin N, Ghitulescu G, Vasilevsky CA, Gordon P, et al. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS‐NSQIP analysis by coarsened exact matching. Dis Colon Rectum. 2017;60:729–37. [DOI] [PubMed] [Google Scholar]
- 7. Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg. 2015;261:1034–40. [DOI] [PubMed] [Google Scholar]
- 8. Bellows CF, Mills KT, Kelly TN, Gagliardi G. Combination of oral non‐absorbable and intravenous antibiotics versus intravenous antibiotics alone in the prevention of surgical site infections after colorectal surgery: a meta‐analysis of randomized controlled trials. Tech Coloproctol. 2011;15:385–95. [DOI] [PubMed] [Google Scholar]
- 9. Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta‐analysis of randomized controlled clinical trials. Dis Colon Rectum. 2016;59:70–8. [DOI] [PubMed] [Google Scholar]
- 10. Koullouros M, Khan N, Aly EH. The role of oral antibiotics prophylaxis in prevention of surgical site infection in colorectal surgery. Int J Colorectal Dis. 2017;32:1–18. [DOI] [PubMed] [Google Scholar]
- 11. Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for antibiotic administration for bowel preparation among patients undergoing elective colorectal surgery: a network meta‐analysis. JAMA Surg. 2022;157:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gheorghe A, Calvert M, Pinkney TD, Fletcher BR, Bartlett DC, Hawkins WJ, et al. Systematic review of the clinical effectiveness of wound‐edge protection devices in reducing surgical site infection in patients undergoing open abdominal surgery. Ann Surg. 2012;255:1017–29. [DOI] [PubMed] [Google Scholar]
- 13. Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, Dowswell G, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomized controlled trial (ROSSINI Trial). BMJ. 2013;347:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cross AJ, Buchwald PL, Frizelle FA, Eglinton TW. Meta‐analysis of prophylactic mesh to prevent parastomal hernia. Br J Surg. 2016;104:179–86. [DOI] [PubMed] [Google Scholar]
- 15. Odensten C, Strigård K, Rutegård J, Dahlberg M, Ståhle U, Gunnarsson U, et al. Use of prophylactic mesh when creating a colostomy does not prevent parastomal hernia: a randomized controlled trial – STOMAMESH. Ann Surg. 2019;269:427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung B, Lannerstad O, Påhlman L, Arodell M, Unosson M, Nilsson E. Preoperative mechanical preparation of the colon: the patient's experience. BMC Surg. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. StataCorp . STATA Statistical Software, release 15.
- 18. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One. 2013;8:76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haller G, Bampoe S, Cook T, Fleisher LA, Grocott MPW, Neuman M, et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine initiative: clinical indicators. Br J Anaesth. 2019;123:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell DH, Swift G, Gilbert GL. Surgical wound infection surveillance: the importance of infections that develop after hospital discharge. Aust NZ J Surg. 1999;69:117–20. [DOI] [PubMed] [Google Scholar]
- 21. Law DJW, Mishriki SF, Jeffery PJ. The importance of surveillance after discharge from hospital in the diagnosis of postoperative wound infection. Ann R Coll Surg Engl. 1990;72:207–9. [PMC free article] [PubMed] [Google Scholar]
- 22. Hall JC, Willsher PC, Hall JL. Randomized clinical trial of single‐dose antibiotic prophylaxis for non‐reconstructive breast surgery. Br J Surg. 2006;93:1342–6. [DOI] [PubMed] [Google Scholar]
- 23. Stockley JM, Allen RM, Thomlinson DF, Constantine CE. A district general hospital's method of post‐operative infection surveillance including post‐discharge follow‐up, developed over a five‐year period. J Hosp Infect. 2001;49:48–54. [DOI] [PubMed] [Google Scholar]
- 24. Ferraz EM, Ferraz AA, Coelho HS, Pereira Viana VP, Sobral SM, Vasconcelos MD, et al. Postdischarge surveillance for nosocomial wound infection: does judicious monitoring find cases? Am J Infect Control. 1995;23:290–4. [DOI] [PubMed] [Google Scholar]
- 25. Taylor EW, Duffy K, Lee K, Noone A, Leanord A, King PM, et al. Telephone call contact for post‐discharge surveillance of surgical site infections. A pilot, methodological study. J Hosp Infect. 2003;55:8–13. [DOI] [PubMed] [Google Scholar]
- 26. Woodfield JC, Jamil W, Sagar PM. Incidence and significance of postoperative complications occurring between discharge and 30 days: a prospective cohort study. J Surg Res. 2016;206:77–82. [DOI] [PubMed] [Google Scholar]
- 27. Woodfield J, Deo P, Davidson A, Chen TYT, van Rij A. Patient reporting of complications after surgery: what impact does documenting postoperative problems from the perspective of the patient using telephone interview and postal questionnaires have on the identification of complications after surgery? BMJ Open. 2019;9:28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reeves BC, Rooshenas L, Macefield RC, et al. Three wound‐dressing strategies to reduce surgical site infection after abdominal surgery: the bluebelle feasibility study and pilot RCT. Health Technol Assess. 2019;23:vii–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matthews JH, Bhanderi S, Chapman SJ, Nepogodiev D, Pinkney T, Bhangu A. Underreporting of secondary endpoints in randomized trials: cross‐sectional, observational study. Ann Surg. 2016;264:982–6. [DOI] [PubMed] [Google Scholar]
- 30. Amer MA, Herbison GP, Grainger SH, Khoo CH, Smith MD, McCall JL. A meta‐epidemiological study of bias in randomized clinical trials of open and laparoscopic surgery. Br J Surg. 2021;108:477–83. [DOI] [PubMed] [Google Scholar]
- 31. Matheson DM, Arabi Y, Baxter‐Smith D, Alexander‐Williams J, Keighley MRB. Randomized multicentre trial of oral bowel preparation and antimicrobials for elective colorectal operations. Br J Surg. 1978;65:597–600. [DOI] [PubMed] [Google Scholar]
- 32. Clarke J, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double‐blind clinical study. Ann Surg. 1977;186:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keighley MR, Alexander‐Williams J, Arabi Y, et al. Comparison between systemic and oral antimicrobial prophylaxis in colorectal surgery. Lancet. 1979;313:894–7. [DOI] [PubMed] [Google Scholar]
- 34. Nichols RL, Broido P, Condon RE, Gorbach SL, Nyhus LM. Effect of preoperative neomycin–erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Surg Infect. 2000;1:133–41. [DOI] [PubMed] [Google Scholar]
- 35. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2017;14:43–54. [DOI] [PubMed] [Google Scholar]
- 36. Golder AM, Steele CW, Conn D, MacKay GJ, McMillan DC, Horgan PG, et al. Effect of preoperative oral antibiotics in combination with mechanical bowel preparation on inflammatory response and short‐term outcomes following left‐sided colonic and rectal resections. BJS Open. 2019;3:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards JP, Ho AL, Tee MC, Dixon E, Ball CG. Wound protectors reduce surgical site infection: a meta‐analysis of randomized controlled trials. Ann Surg. 2012;256:53–9. [DOI] [PubMed] [Google Scholar]
- 38. Alcantara Moral M, Serra Aracil X, Bombardó Juncá J, Mora López L, Hernando Tavira R, Ayguavives Garnica I, et al. A prospective, randomised, controlled study on the need to mechanically prepare the colon in scheduled colorectal surgery. Cir Esp. 2009;85:20–5. [DOI] [PubMed] [Google Scholar]
- 39. Toh JWT, Phan K, Hitos K, Pathma‐Nathan N, el‐Khoury T, Richardson AJ, et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection. JAMA Netw Open. 2018;1:e183226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Espin Basany E, Solís‐Peña A, Pellino G, Kreisler E, Fraccalvieri D, Muinelo‐Lorenzo M, et al. Preoperative oral antibiotics and surgical‐site infections in colon surgery (ORALEV): a multicentre, single‐blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;1253:1–10. [DOI] [PubMed] [Google Scholar]
- 41. Abis GSA, Stockmann HBAC, Bonjer HJ, van Veenendaal N, van Doorn‐Schepens MLM, Budding AE, et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg. 2019;106:355–63. [DOI] [PubMed] [Google Scholar]
- 42. Cannon JA, Altom LK, Deierhoi RJ, Morris M, Richman JS, Vick CC, et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum. 2012;55:1160–6. [DOI] [PubMed] [Google Scholar]
- 43. Verma G, Pareek S, Singh R. Mechanical bowel preparation in elective colorectal surgery: a practice to purge or promote? Indian J Gastroenterol. 2007;26:142–3. [PubMed] [Google Scholar]
- 44. Zmora O, Mahajna A, Bar‐Zakai B, Rosin D, Hershko D, Shabtai M, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg. 2003;237:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anjum N, Ren J, Wang G, Li G, Wu X, Dong H, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum. 2017;60:1291–8. [DOI] [PubMed] [Google Scholar]
- 46. Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials. 2009;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frontali A, Panis Y. Bowel preparation in colorectal surgery: back to the future? Updates Surg. 2019;71:205–7. [DOI] [PubMed] [Google Scholar]
- 48. Platell C, Barwood N, Makin G. Randomized clinical trial of bowel preparation with a single phosphate enema or polyethylene glycol before elective colorectal surgery. Br J Surg. 2006;93:427–33. [DOI] [PubMed] [Google Scholar]
- 49. Jaferi MD, Nfonsam V, Shogan B, Hyman N. Why do anastomoses leak? J Gastrointest Surg. 2021;25:2728–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Contant CM, Hop WC, van't Sant HP, Oostvogel HJ, Smeets HJ, Stassen LP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. 2007;370:2112–7. [DOI] [PubMed] [Google Scholar]
- 51. Fa‐Si‐Oen P, Roumen R, Buitenweg J, Van De Velde C, Van Geldere D, Putter H, et al. Mechanical bowel preparation or not? Outcome of a multicenter, randomized trial in elective open colon surgery. Dis Colon Rectum. 2005;48:1509–16. [DOI] [PubMed] [Google Scholar]
- 52. Hjalmarsson C, Karlberg J, Törnqvist P, Arbman G, Frisk B, Modin M. Orally administered trimethoprim‐sulfamethoxazole and metronidazole as infection prophylaxis in elective colorectal surgery. Surg Infect (Larchmt). 2015;16:604–10. [DOI] [PubMed] [Google Scholar]
- 53. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single‐blinded trial. Lancet. 2019;394:840–8. [DOI] [PubMed] [Google Scholar]
- 54. Miettinen RPJ, Laitinen ST, Mäkelä JT, Pääkkönen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum. 2000;43:669–75. [DOI] [PubMed] [Google Scholar]
- 55. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Dis Colon Rectum. 2005;140:129–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


