Abstract
Telitacicept, an injectable recombinant human B‐lymphocyte stimulating factor receptor‐antibody fusion protein, is a new dual B lymphocyte stimulator (BLyS)/APRIL (a proliferation‐inducing ligand) inhibitor that effectively blocks proliferation of B lymphocytes. This study evaluates the pharmacokinetic characteristics, tolerability, and safety of a single subcutaneous injection of various doses (80, 160, and 240 mg) of telitacicept in healthy Chinese subjects. This trial is a single‐center, randomized, open‐label phase I clinical study that includes three dose groups (80, 160, and 240 mg) with 12 subjects in each dose group. The subjects were randomly assigned to different dose groups in a 1:1:1 ratio for a single subcutaneous administration trial. After a single dose, the maximum serum concentration (Cmax) of total and free telitacicept was reached within 0.5‐1 days. The elimination half‐lives of total and free telitacicept at doses of 80–240 mg were 10.9–11.9 days and 11–12.5 days, respectively. The formation and elimination of the BLyS‐telitacicept complex were much slower; the median time to Cmax was 15–57 days and was significantly prolonged with increasing dose. Only two of the 36 healthy subjects had positive antidrug antibodies with antibody titers of 1:15. The severity of adverse events was mild or moderate, and no higher treatment‐emergent adverse events were reported. In conclusion, total telitacicept within a dose range of 80–240 mg and free telitacicept within a dose range of 160–240 mg had linear pharmacokinetic characteristics.
Keywords: BLyS/APRIL, pharmacokinetics, safety, telitacicept, tolerability
Autoimmune disease (AD), a type of chronic disease that causes multiple‐organ and multiple‐system damage due to dysfunction of the body's immune system, causes great suffering to patients. Common ADs include systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjogren's syndrome. 1 Glucocorticoids and conventional nonsteroidal anti‐inflammatory drugs have nonspecific anti‐inflammatory effects and inhibit immune function, and long‐term use of these drugs can lead to many adverse reactions. 2 In recent years, targeted biologics that interact with cytokines, receptors, and signaling molecules have been developed and have significantly improved AD patients’ quality of life. 3 , 4
Biological agents that act in AD exert their therapeutic effects by blocking key inflammatory cytokines or cell surface molecules. Some examples of these biological agents are monoclonal antibodies (mAbs), targeting tumor necrosis factor (TNF) and interleukin 1, B lymphocyte stimulator (BLyS) inhibitors, T‐cell inhibitors, integrin mAbs, and other agents. 1 , 5 Among them, belimumab (Benlysta), a single‐target mAb that binds soluble BLyS, was approved by the US FDA in 2011 for the treatment of SLE. 6 , 7
Recombinant human B‐lymphocyte stimulating factor receptor‐antibody fusion protein for injection (telitacicept, Tai'ai) is a dual inhibitor of BLyS/APRIL (a proliferation‐inducing ligand). 8 BLyS, a member of the TNF family, mediates the survival of immature peripheral B lymphocytes and their differentiation into mature B cells. APRIL is a homolog of the TNF superfamily and is involved in mature B‐cell activation and plasma cell antibody secretion. BLyS and APRIL receptors include the BLyS receptor (also referred to as transmembrane activator and calcium‐modulating cyclophylin ligand interactor [TACI]), B‐cell maturation antigen, and B‐cell‐activating factor‐receptor. 8 , 9 , 10 The extracellular soluble portion of telitacicept TACI can neutralize BLyS and APRIL and effectively block the interaction between BLyS and APRIL and their receptors, thereby effectively blocking the proliferation of B lymphocytes and the maturation of T lymphocytes. 11 , 12 Previous single‐dose phase I clinical trials have shown that the safety of single doses of telitacicept ranging from 1.2 to 540 mg in RA patients is good. 13 Good safety and tolerability of multiple‐dose telitacicept in RA and SLE patients has also been observed. 14 , 15 Moreover, a randomized, double‐blind, placebo‐controlled phase IIb clinical trial in patients with SLE showed that compared with the placebo group, the group that received telitacicept at 240 mg had a higher SLE Responder Index (SRI) at week 48 (79.2% vs 32.0%, P < 0.001), the study's primary endpoint. 8 Healthy subjects have no potential concomitant medication effects and they are an ideal homogeneous population. However, telitacicept has not been studied in clinical trials in healthy Chinese subjects. Therefore, in this trial, three doses (80, 160, and 240 mg) of telitacicept were evaluated for pharmacokinetic (PK) characteristics, safety, and tolerability in healthy Chinese subjects.
Methods
Study Design
This trial was a single‐center, randomized, open‐label, phase I clinical study. The research protocol and its revision were conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Clinical Medical Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. The study is registered in the China Clinical Trial Registry, registration number CTR20192033.
Eligible subjects were healthy volunteers 18–50 years of age, 50% males and 50% females with body mass index ranging from 19 to 26, males weighing no less than 50 kg and females weighing no less than 45 kg. All subjects signed an informed consent form, and the first subject was enrolled on September 17, 2019. This trial complied with the privacy rights of human subjects. Given the exploratory nature of this study, no estimation was performed for sample size. The sample size was determined according to the requirement of the National Medical Products Administration (MNPA) for phase 1 PK studies. 16 In this trial, three doses (80, 160, and 240 mg) were selected for PK analysis in healthy people, with 12 subjects in each dose group. A table was randomly generated by SAS 9.4 (SAS Institute Inc., Cary, North Carolina) using stratified blocks. Men and women were stratified, and the participants were randomly assigned to one of three doses (80, 160, or 240 mg) at a ratio of 1:1:1 for a single subcutaneous injection trial. The three dosing regimens were administered in parallel.
Pharmacokinetic Assessments
Blood samples for PK analysis of total telitacicept, free telitacicept, and BLyS‐telitacicept complex were collected before administration and at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 5, 8, 15, 22, 29, 43, and 57 days after administration. The serum concentrations of total telitacicept, free telitacicept, and BLyS‐telitacicept complex were measured by ELISAs. 13
The coating antibody and the detection antibody used in the measurement of the serum concentration of total telitacicept were a mouse antibody against human TACI and a biotinylated goat antibody against human TACI, respectively (both from R&D Systems, Minneapolis, Minnesota). The lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ) were 15.0 and 500.0 ng/mL, respectively. The accuracy was −22.9%–19.6% and the precision was 0.0%–19.6%. The coating antibody and the secondary antibody used in determination of the serum concentration of free telitacicept by ELISA were a mouse antibody against human TACI (GenScript, Piscataway, New Jersey) and a biotinylated goat antibody against human TACI/TNFRSF13B (R&D Systems). The LLOQ and ULOQ were 15.0 and 500.0 ng/mL, respectively. The accuracy was −18.0%–24.3%, the precision was −13.6%–2.0%, and the total error was 4.6%–21.9%. The coating antibody and the secondary antibody used in determination of the serum concentration of the BLyS‐telitacicept complex were a goat antibody against human BAFF/BlyS/TNFSF13B and a biotinylated goat antibody against human TACI/TNFRSF13B, respectively (both from R&D Systems). The LLOQ and ULOQ were 20.0 and 1280.0 U/mL, respectively. The accuracy was −15.7%–14.4%, the precision was 2.2%–15.7%, and the total error was 3.1%–21.8%.
Pharmacodynamics Assessments
Blood samples for immunological analysis were collected before administration of the drug and at 8, 15, 22, 29, 43, and 57 days after administration. The concentrations of immunoglobulin M (IgM), immunoglobulin A (IgA), immunoglobulin G (IgG), complement component 3 (C3), and complement component 4 (C4) in the blood were measured by immunonephelometry; the counts of B cells were analyzed by flow cytometry using absolute B lymphocyte counts in peripheral blood. 15
Biomarker Assessments
Blood samples for biomarker analysis were collected before administration of the drug and at 29 and 57 days after administration. Serum concentrations of free BlyS and APRIL were measured by ELISA. The Human BAFF/Blys/TNFSF13B kit produced by R&D Systems was used to measure the concentration of free Blys; the coating antibody and the enzyme‐linked antibody were a mouse mAb against BAFF/Blys and a mouse antibody against the BAFF/Blys conjugate, respectively. The LLOQ and ULOQ were 62.5 and 4000.0 pg/mL, respectively. The accuracy was −20.6%–14.6%, the precision was 0.0%–17.1%, and the total error was 1.9%–22.6%. The coating antibody and the detection antibody used to measure the free APRIL concentration were polyclonal antibodies against human APRIL; the detection antibody was biotinylated and both were purchased from Abcam. The LLOQ and the ULOQ were 0.78 and 50.0 ng/mL, respectively. The accuracy was −19.3%–21.5%, the precision was 0.1%–17.9%, and the total error was 1.3%–21.9%.
Immunogenicity Assessments
Blood samples for analysis of immunogenicity were collected before administration of the drug and at 29 and 57 days after administration. Using the antibody titer method, the serum samples to be tested were placed in precoated 96‐well plates, diluted to various concentrations and fully bound after incubation at 37°C. Goat anti‐human IgG Fc‐HRP (Bethyl, Montgomery, Texas) was added to the validation wells and goat anti‐human IgG F(ab)′2‐HRP (Abcam, Cambridge, UK) was added to the other wells; the optical density (OD) values of the solution in each well at a specific wavelength were then measured. If the ratio of the OD value of the serum sample to that of the negative control sample was greater than 2.0 at a specific dilution, the concentration at that dilution was defined as the antibody titer at that time point and the ADA test was considered positive. Otherwise, the ADA test was considered negative. 13
Analysis
Pharmacokinetics
The PK parameters measured were the maximum serum concentration (Cmax), the time to Cmax (Tmax), the blood drug concentration at the last collection time at which the blood drug concentration could be determined (Clast), the time to Clast (Tlast), the elimination rate constant (λz), the terminal half‐life (t1/2), the area under the serum concentration‐time curve from 0 hours to the last detectable concentration (AUC0‐t), the AUC from time 0 extrapolated to infinity (AUC0‐∞), the apparent volume of distribution (Vz/F), and the apparent clearance (CLz/F). The PK parameters were calculated using a noncompartment model (WinNonlin, version 8.1, Pharsight Corporation, Mountain View, California) based on the serum concentrations of total and free telitacicept and the telitacicept‐BLyS complex of each subject. The arithmetic mean, standard deviation, coefficient of variation, quantile, maximum value, minimum value, geometric mean, and geometric coefficient of variation for the parameters in each dose group were calculated and subjected to descriptive statistics.
The mean concentration–time (c–t) profiles for individuals and for each group were drawn using a PK concentration set. In the analysis of dose proportionality, the relationships between telitacicept dose and drug exposure parameters (Cmax and AUC) after administration were analyzed using a power model (ln[PK parameter] = intercept [α] + slope [β] × ln[dose] + error). The linear discriminant criterion was that the 95% confidence intervals (CIs) for β included the value 1.
Pharmacodynamics
The measured values of immunological parameters (IgG, IgA, IgM, C3, C4, and B cells), the change in the values from baseline, and the rate of change from baseline were statistically analyzed. Rate of change (%) = (postdose value − baseline value)/baseline value × 100%.
Biomarker Evaluation
The measured concentrations of BLyS and APRIL and the changes from baseline were statistically analyzed.
Immunogenicity
The positivity rate of antidrug antibodies (ADAs) is reported and the antibody titers for subjects with ADA‐positive results are provided.
Safety
Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 22.0) and classified using system organ classification and preferred term (SOC/PT). 17 Laboratory examinations, vital signs, electrocardiograms, and physical examinations were recorded and analyzed.
Results
Subjects
A total of 173 healthy subjects were screened for participation in this study; 137 failed the screening and a total of 36 subjects were enrolled. Twelve subjects were enrolled in each of the 80, 160, and 240 mg groups. Thirty‐five of the subjects completed the trial (Figure S1 and Table S1). Subject no. 214, who received a subcutaneous injection of 160 mg of telitacicept on September 26, 2019, informed the investigators by phone call on November 13, 2019, that she was pregnant. On November 14, 2019, her blood pregnancy test was positive and this subject withdrew from the test early; her withdrawal did not affect the collection or testing of blood sample points. On November 19, 2019, the B‐ultrasound results showed the presence of an intrauterine pregnancy and on November 21, 2019, a painless abortion was performed. The blood of the pregnant subject was reviewed on December 23, 2019, and no related AEs were reported through the end of the study.
Pharmacokinetics
The mean serum c–t profiles of total telitacicept, free telitacicept, and the BLyS‐telitacicept complex after single subcutaneous injections of 80, 160, and 240 mg telitacicept are shown in Figures 1, 2, 3. In the individuals who received 80–240 mg telitacicept, the median time to the peak concentration of total telitacicept was 0.5–1 days, and the low‐dose group showed an earlier peak time. The elimination half‐life of was roughly the same for the three doses, with t1/2 ranging from 10.9 to 11.9 days. The median time to the peak concentration of free telitacicept was 1 day, and the t1/2 increased slightly as the dose was increased. The median time to the peak concentration of the BLyS‐telitacicept complex was 15–57 days, and the time to the peak concentration increased significantly as the dose was increased. In some subjects in the 240 mg group, the final concentration of the BLyS‐telitacicept complex was greater than half of the peak concentration, therefore parameters such as t1/2 and AUC0‐∞ could not be estimated. The PK parameters are shown in Table 1. The half‐widths of the CIs of Cmax, AUC0‐t, and AUC0‐∞ for total telitacicept were 0.843, 0.201, and 0.200, respectively. The 95% CIs of β for the Cmax, AUC0‐t, and AUC0‐∞ of total telitacicept included the value 1, indicating that total telitacicept within a dose range of 80–240 mg had linear PK characteristics. The combination of antigen and antibody in biological analogs leads to incomplete linearity of PK characteristics at low doses, therefore the dose proportionality of free telitacicept was analyzed only for the two higher‐dose groups. The half‐widths of the CIs of Cmax, AUC0‐t, and AUC0‐∞ for free telitacicept were 0.764, 0.336, and 0.345, respectively. The 95% CIs of β for Cmax, AUC0‐t, and AUC0‐∞ of free telitacicept included the value 1, indicating that free telitacicept within a dose range of 160–240 mg exhibited linear PK characteristics (Table 2).
Figure 1.
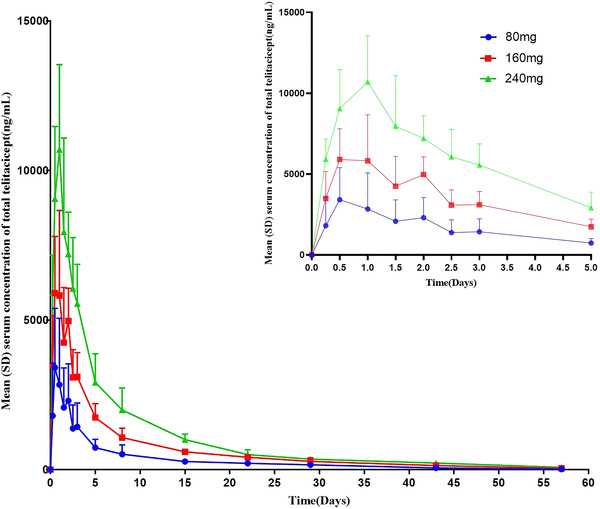
The mean serum concentration of total telitacicept. The error bars are SD.
Figure 2.
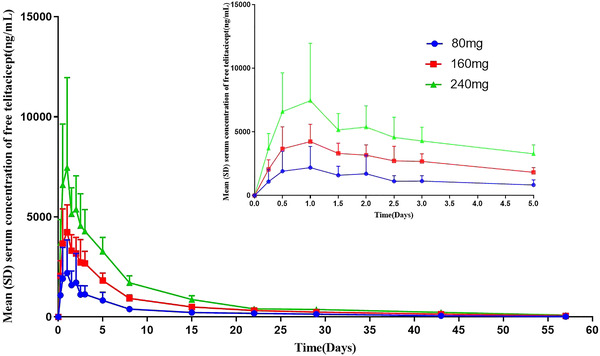
The mean serum concentration of free telitacicept. The error bars are SD.
Figure 3.
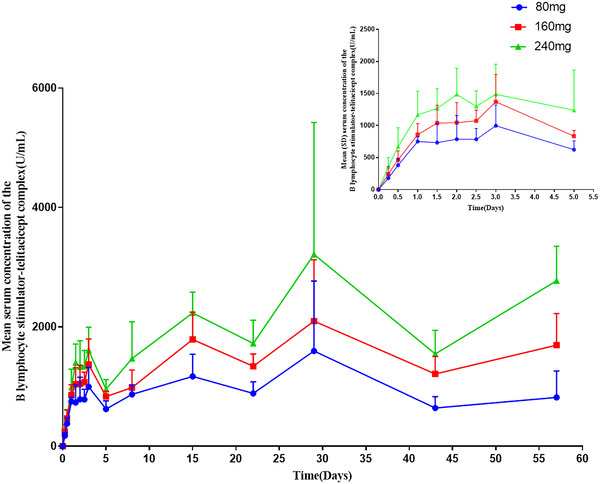
The mean serum concentration of the B lymphocyte stimulator‐telitacicept complex. The error bars are SD.
Table 1.
Summary of the Pharmacokinetic Parameters of Total Telitacicept, Free Telitacicept, and BLyS‐Telitacicept Complexes in Each Dose Group Following a Single Subcutaneous Injection of 80, 160, or 240 mg of Telitacicept
| Total Telitacicept | Free Telitacicept | BLyS–Telitacicept Complex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 80 mg | 160 mg | 240 mg | 80 mg | 160 mg | 240 mg | 80 mg | 160 mg | 240 mg | ||
| PK Parameters | (N = 12) | (N = 12) | (N = 12) | (N = 12) | (N = 12) | (N = 12) | PK Parameters | (N = 12) | (N = 12) | (N = 12) |
| Cmax (μg/mL) | 3.6 ± 2.0 | 6.7 ± 2.5 | 11.0 ± 2.8 | 2.3 ± 1.7 | 4.7 ± 1.5 | 8.9 ± 4.4 | Cmax (U/μL) | 1.8 ± 1.1 | 2.4 ± 1.0 | 3.5 ± 2.1 |
| AUC0‐t (days*μg/mL) | 17.9 ± 8.4 | 37.1 ± 8.9 | 61.7 ± 13.0 | 14.9 ± 6.7 | 31.9 ± 5.0 | 53.5 ± 9.3 | AUC0‐t (days*U/μL) | 54.7 ± 17.2 | 84.2 ± 16.6 | 117.0 ± 35.5 |
| AUC0‐∞ (days*μg/mL) | 18.7 ± 8.7 | 38.9 ± 9.5 | 64.6 ± 12.7 | 15.3 ± 6.8 | 32.8 ± 5.3 | 55.2 ± 9.8 | AUC0‐∞ (days*U/μL) | NE | NE | NE |
| Tmax (days) | 0.5 (0.5–2.0) | 1.0 (0.5–2.0) | 1.0 (0.5–1.0) | 1.0 (0.5–2.0) | 1.0 (0.5–1.0) | 1.0 (0.5–2.0) | Tmax (days) | 15.0 (1.5–57.0) | 29.0 (15.0–57.0) | 57.0 (15.0–57.0) |
| t1/2 (days) | 10.9 ± 1.4 | 11.6 ± 1.5 | 11.9 ± 4.5 | 11.0 ± 1.7 | 11.8 ± 1.8 | 12.5 ± 2.7 | t1/2 (days) | NE | NE | NE |
| Tlast (days) | 43.0 (43.0–57.0) | 43.0 (43.0–61.0) | 43.0 (43.0–57.0) | 57.0 (43.0–57.0) | 57.0 (57.0–61.0) | 57.0 (57.0–57.0) | Tlast (days) | 57.0 (57.0–57.0) | 57.0 (57.0–61.0) | 57.0 (57.0–57.0) |
| Clast (ng/mL) | 52.1 ± 24.3 | 104.0 ± 53.8 | 160.4 ± 69.6 | 28.6 ± 10.9 | 50.7 ± 16.3 | 92.3 ± 24.1 | Clast (U/mL) | 817.6 ± 441.3 | 1694.4 ± 528.4 | 2772.8 ± 577.3 |
| Vz/F (L) | 75.6 ± 23.2 | 72.3 ± 17.0 | 65.8 ± 24.2 | 93.8 ± 39.1 | 84.2 ± 12.0 | 79.7 ± 20.5 | Vz/F(L*mg/U) | NE | NE | NE |
| CLz/F (mL/days) | 8.4 ± 2.8 | 7.5 ± 1.7 | 6.7 ± 1.2 | 10.1 ± 2.9 | 8.7 ± 1.4 | 7.7 ± 1.2 | CLz/F (L*mg/(days*U)) | NE | NE | NE |
AUC0‐t, area under the serum concentration–time curve from time zero to the last detectable concentration; AUC0‐∞, area under the serum concentration–time curve from time zero extrapolated to infinity; Clast, last blood drug concentration at collection time at which the blood drug concentration can be determined; Cmax, maximum serum concentration; CLz/F, apparent clearance; NE, not evaluable; Tlast, time to Clast; Tmax, time to Cmax; t1/2, terminal half‐life; Vz/F, apparent volume of distribution.
Data are mean ± SD, except Tmax and Tlast represent the median (minimum − maximum).
Table 2.
The Relationship Among Total Telitacicept Cmax, AUC0‐t, AUC0‐∞, and Dose Following a Single Subcutaneous Injection of 80–240 mg of Telitacicept and the Relationship Among Free Telitacicept Cmax, AUC0‐t, AUC0‐∞, and Dose Following a Single Subcutaneous Injection of 160 or 240 mg of Telitacicept
| PK Parameter | Parameter | Estimated Value | SE | 95%CI | R 2 | |
|---|---|---|---|---|---|---|
| Total telitacicept | ||||||
| Cmax (μg/mL) | α | −3.7 | 0.7 | −5.1 to −2.3 | 0.7 | |
| β | 1.1 | 0.1 | 0.8–1.4 | |||
| AUC0‐t (h*μg/mL) | α | 0.9 | 0.5 | −0.2 to 1.9 | 0.8 | |
| β | 1.2 | 0.1 | 1.0–1.4 | |||
| AUC0‐∞ (h*μg/mL) | α | 0.9 | 0.5 | −0.1 to 1.9 | 0.8 | |
| β | 1.2 | 0.1 | 1.0–1.4 | |||
| Free telitacicept | ||||||
| Cmax (μg/mL) | α | −6.1 | 1.9 | −10.1 to −2.1 | 0.4 | |
| β | 1.5 | 0.4 | 0.7–2.3 | |||
| AUC0‐t (h*μg/mL) | α | 0.2 | 0.9 | −1.6 to 2.0 | 0.7 | |
| β | 1.3 | 0.2 | 0.9–1.6 | |||
| AUC0‐∞ (h*μg/mL) | α | 0.2 | 0.9 | −1.7 to 2.0 | 0.7 | |
| β | 1.3 | 0.2 | 0.9–1.6 | |||
α, intercept; AUC0‐∞, area under the serum concentration–time curve from time zero extrapolated to infinity; AUC0‐t, area under the serum concentration–time curve from time zero to the last detectable concentration; β, slope; Cmax, maximum serum concentration; CI, confidence interval; SE, standard error.
Pharmacodynamics
The data on the rate of change in immunological indexes in each dose group of healthy subjects after a single subcutaneous injection of telitacicept, compared with the baseline, are shown in Figure 4. Compared with the baseline, the rate of change in IgG concentration decreased after treatment in each dose group. The 80 mg dose group reached −8.3% at 8 days and then recovered slowly following the 15‐day visit. Compared with the baseline, the rate of change in C3 concentration was most obvious in the 240 mg group, reaching 15.8% at 8 days, while the changes in the 80 and 160 mg groups reached 5.3% and 6.9%, respectively. The B‐cell count began to decrease after a brief increase 8 days after administration and gradually decreased over time.
Figure 4.
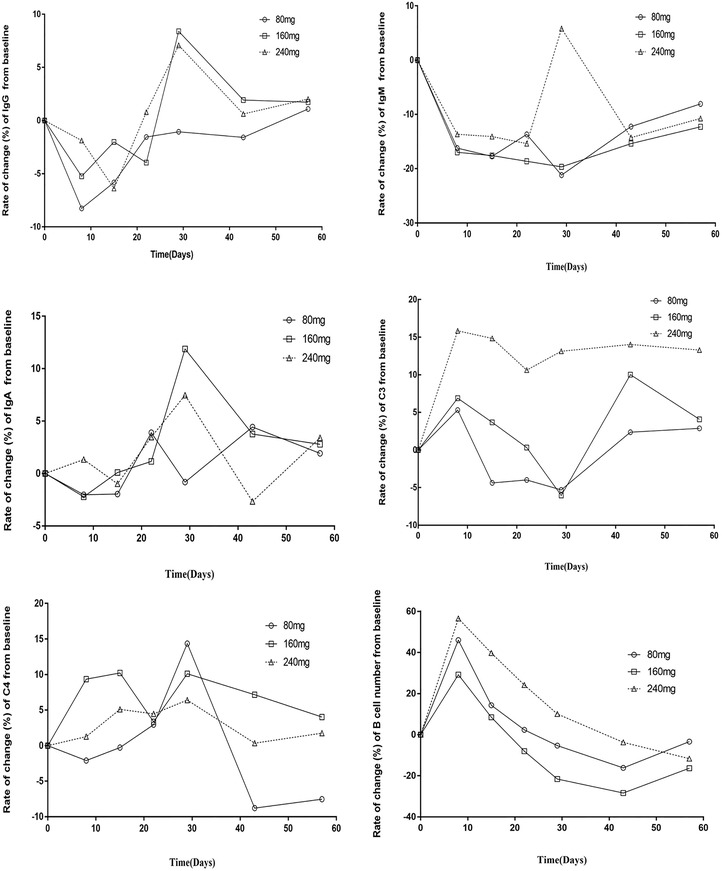
Analysis of the rate of change (%) in the levels of immunological indicators (IgG, IgA, IgM, C3, C4, and B cells) from baseline after medication. Rate of change (%) = (postdose value − baseline value)/baseline value × 100%.
The concentration of free BLyS increased significantly as the dose increased. The serum concentration of free BLyS was significantly higher than the baseline concentration in all groups at 29 and 57 days after administration, but at 57 days it was almost half of that at 29 days. This may be due to the formation of a BLyS‐telitacicept complex. APRIL is weakly expressed in healthy humans and no corresponding results were detected (Table 3).
Table 3.
The Changes from Baseline of BLyS and APRIL
| Check Index Visit | Statistics | 80 mg Group (N = 12) | 160 mg Group (N = 12) | 240 mg Group (N = 12) | Total (N = 36) |
|---|---|---|---|---|---|
| BLyS (pg/mL) | |||||
| Day 29 | Mean ± SD | 73736.6 ± 18724.2 | 117440.7 ± 14059.2 | 158662.8 ± 25707.2 | 116613.3 ± 40209.2 |
| Median | 70208.3 | 116465.4 | 165223.1 | 115683.8 | |
| Day 57 | Mean ± SD | 33156.0 ± 14757.2 | 62312.2 ± 21308.8 | 95978.2 ± 16855.1 | 63815.5 ± 31275.6 |
| Median | 30348.5 | 56976.6 | 90596.5 | 61344.5 | |
| APRIL (ng/mL) | |||||
| Day 29 | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Median | 0 | 0 | 0 | 0 | |
| Day 57 | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Median | 0 | 0 | 0 | 0 |
Change value = postdose value − baseline value.
Immunogenicity
In the 80 mg group, ADA positivity was observed at 29 days in subject no. 208 and at 57 days in subject no. 217; the titer ratios were 1:15, suggesting that telitacicept‐binding antibodies were produced in the two subjects. All other subjects were negative for ADA (Table 4).
Table 4.
The Positivity Rate of Antidrug Antibodies
| Check Index Visit | 80 mg (N = 12) | 160 mg (N = 12) | 240 mg (N = 12) | Total (N = 36) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Day 29 | 1 (8.3) | 0 (0) | 0 (0) | 1 (2.8) |
| Day 57 | 1 (8.3) | 0 (0) | 0 (0) | 1 (2.8) |
| Total a | 2 (16.7) | 0 (0) | 0 (0) | 2 (5.6) |
A positive test result at any time point was considered positive.
Safety
Of the 36 subjects in the trial, 18 reported a total of 42 AEs; all of the reported AEs occurred after administration and thus were treatment‐emergent adverse events (TEAEs). A total of 15 subjects reported 29 drug‐related adverse reactions. Table 5 shows the incidence of AEs and adverse reactions in each dose group. The severity of AEs was mild or moderate, and most of the AEs were moderate. There were no higher TEAEs, and there were no serious adverse events or AEs that led to withdrawal of participants from the trial. The most frequent TEAEs were elevated blood triglyceride levels (five cases) and increased numbers of urinary white blood cells (four cases) (Table S2). Three of the 18 individuals in this study who reported TEAEs received medication for adverse events, and none of the others were treated. The outcome of 41 of the cases of TEAEs was ‘‘cure’’, and the outcome of one case was ‘‘remission’’. There were no clinically significant abnormalities in vital signs, physical examination results, electrocardiograms, or laboratory examination results in any of the dose groups.
Table 5.
The Incidence of Adverse Events and Adverse Reactions in Different Dose Groups
| 80 mg (N = 12) | 160 mg (N = 12) | 240 mg (N = 12) | Total (N = 36) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Adverse events | 6 (50.0) | 5 (41.7) | 7 (58.3) | 18 (50.0) |
| Adverse reactions | 5 (41.7) | 5 (41.7) | 5 (41.7) | 15 (41.7) |
| Serious adverse events | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Serious adverse reactions | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Adverse events that led to withdrawal from the trial | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Adverse reactions that led to withdrawal from the trial | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
The relationship of the study drug is definitely related, very likely related, and possibly related, which is regarded as an adverse reaction of the drug.
Discussion
This is a phase I trial of an injectable recombinant human BLyS receptor‐antibody fusion protein in healthy Chinese subjects. The trial demonstrated that telitacicept in serum has linear PK characteristics and high safety and tolerability over a dose range of 80–240 mg.
The finding that telitacicept has linear PK characteristics is consistent with the results of previous studies in which serum levels of total telitacicept were found to increase as the dose of telitacicept increased. 13 In this study, after a single injection of telitacicept, serum levels of free telitacicept also increased with the dose. However, the AUC and Cmax for free telitacicept were slightly lower than those for total telitacicept in the same dose groups, indicating that some of the telitacicept present in serum may participate in the formation of immune complexes. In all three of the dose groups, the serum concentration of BLyS‐telitacicept complexes increased slowly, with a median time to Cmax of 15–57 days, and tended to remain at the maximum concentration for a long time between days 15 and 57.
As a stimulator of B‐cell proliferation and differentiation, BLyS can induce activated B cells to secrete IgG, IgM, and IgA 9 . Stohl et al found that belimumab‐treated patients experienced significant sustained reductions in IgG and improvement in C3/C4 levels. However, memory B cell and T‐cell populations did not decrease. 18 In this study, analysis of the levels of immunoglobulins and complement components in the three dose groups showed that there were small short‐term fluctuations in these components in each dose group after treatment and that these fluctuations tended to disappear at approximately 57 days, resulting in values that were similar to the baseline value. The pharmacodynamic performance of telitacicept in healthy subjects was consistent with its performance in SLE and RA patients. 13 , 14 , 15 After a brief increase 8 days after administration, the number of B cells decreased gradually with time, similar to the results of previous studies. This initial increase is a phenomenon that has been observed after administration of belimumab and other anti‐BLyS agents, and is likely due to redistribution of memory B cells (and perhaps to the presence of a small number of naive B cells) originally present in the lymphoid organs. The subsequent reduction in the number of B cells is likely dominated by mature B cells. 18 , 19 A previous phase I clinical trial of telitacicept in SLE and RA patients only determined the concentrations of BLyS and APRIL before administration. 13 , 14 In the current trial, changes in free BLyS and APRIL were measured after administration. Compared with the baseline values, the free BLyS concentration in the serum of each dose group was significantly increased at 29 and 57 days after administration, but the free BLyS concentration at 57 days was almost half that at 29 days. APRIL is weakly expressed in healthy people, and no changes in APRIL levels were detected in this study, a finding that is consistent with the finding of Zhou's team that human APRIL is highly expressed in tumor tissues but weakly expressed in normal tissues. 20 , 21
Previous clinical trials have shown that telitacicept is tolerated in patients with SLE and RA. Common AEs after administration of telitacicept included upper respiratory tract infection and injection site reactions. 13 , 14 , 15 In the current trial, which was conducted in a healthy population, the most frequent TEAEs were elevated blood triglyceride levels, urine white blood cell positivity, and upper respiratory tract infections. Subcutaneous administration was well tolerated and no unexpected adverse reactions were detected. Among similar mAb biologics, upper respiratory tract infection and pharyngitis were major AEs considered to be associated with belimumab. 22 Common AEs associated with belimumab in healthy volunteers were reported to include pain and erythema at the injection site and headache. 23 Common AEs associated with atacicept included headache, head cold, and sore throat, and 69% of participants reported pain at the injection site immediately after the injection. 24 In contrast, only one case of rash at the injection site was reported in our trial of telitacicept, and no adverse reactions were observed after injection. The adverse reactions were not related to the dose, indicating that the overall safety of telitacicept administered over a dose range of 80–240 mg is good. Previous data showed that both RA and SLE patients treated with telitacicept tested negative for ADA, whereas only two of the 36 healthy subjects in this trial tested positive for ADA. These data indicate the low immunogenicity of telitacicept.
This study has some limitations. First, it was conducted in healthy subjects, so it is not known if the results translate to the target patient population. Second, the concentrations of B‐cell subsets and T‐cell subsets were not measured. However, the pharmacodynamic performance of telitacicept was consistent with the previously reported pharmacodynamic performance of telitacicept in SLE and RA patients. 13 , 14
Conclusions
Total telitacicept showed linear PK characteristics in the dose range of 80–240 mg. Free telitacicept showed linear PK characteristics in the dose range of 160–240 mg. The take‐home message is that telitacicept is a new dual BLyS/APRIL inhibitor that plays a key role in effectively reducing B cell‐mediated autoimmune responses for the treatment of AD. This work provides a foundation for further exploration and optimization of telitacicept for patient administration.
Conflicts of Interest
The authors declare that they have no conflicts of interest to disclose.
Funding
This study was supported by the 512 Talent Cultivation Program of Bengbu Medical College (by 51201313).
Supporting information
Figure S1. Flow diagram of this trial.
Table S1. Baseline characteristics.
Table S2. Systematic analysis of adverse events.
Acknowledgments
The authors thank all the healthy volunteers and the study center staff who participated in the phase I clinical trial.
References
- 1. Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63‐80. [DOI] [PubMed] [Google Scholar]
- 2. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045‐1051. [DOI] [PubMed] [Google Scholar]
- 3. Smolen, JS , Landewé R, Bijlsma J, et al. EULAR recommendation for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79 (6):685‐699. [DOI] [PubMed] [Google Scholar]
- 4. Takeuchi T. Treatment of rheumatoid arthritis with biological agents ‐ as a typical and common immune‐mediated inflammatory disease. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(8):600‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125(4):814‐820. [DOI] [PubMed] [Google Scholar]
- 6. Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011;377(9767):721‐731. [DOI] [PubMed] [Google Scholar]
- 7. Manzi S, Sánchez‐Guerrero J, Merrill JT, et al. Effects of belimumab, a B lymphocyte stimulator‐specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71(11):1833‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi F, Xue R, Zhou X, Shen P, et al. Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol Immunotoxicol. 2021;1‐8. [DOI] [PubMed] [Google Scholar]
- 9. Batten M, Groom J, Cachero TG, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192(10):1453‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shu HB, Hu WH, Johnson H. TALL‐1 is a novel member of the TNF family that is down‐regulated by mitogens. J Leukoc Biol. 1999;65(5):680‐683. [PubMed] [Google Scholar]
- 11. Yao X, Ren Y, Zhao Q, et al. Pharmacokinetics analysis based on target‐mediated drug distribution for RC18, a novel BLyS/APRIL fusion protein to treat systemic lupus erythematosus and rheumatoid arthritis. Eur J Pharm Sci. 2021;159, 105704. [DOI] [PubMed] [Google Scholar]
- 12. Dhillon S. Telitacicept: first approval. Drugs. 2021;81(14), 1671‐1675. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Hou Y, Jiang J, et al. Pharmacokinetics, pharmacodynamics, and tolerability of single ascending doses of RCT‐18 in Chinese patients with rheumatoid arthritis. Clin Pharmacokinet. 2014;53(11):1033‐1044. [DOI] [PubMed] [Google Scholar]
- 14. Chen X, Zhao Q, Hou Y, et al. Pharmacokinetics, pharmacodynamics, short term efficacy and safety of RCT‐18, a novel BLyS/APRIL fusion protein, in patients with rheumatoid arthritis. Br J Clin Pharmacol. 2016;82(1):41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Q, Chen X, Hou Y, et al. Pharmacokinetics, pharmacodynamics, safety, and clinical activity of multiple doses of rct‐18 in chinese patients with systemic lupus erythematosus. J Clin Pharmacol. 2016;56(8):948‐959. [DOI] [PubMed] [Google Scholar]
- 16. National Medical Products Administration . Guidance for the study of the pharmacokinetics of chemical drugs. https://www.nmpa.gov.cn/wwwroot/gsz05106/07.pdf. Updated March 18, 2005. Accessed July 7, 2019.
- 17. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109‐917. [DOI] [PubMed] [Google Scholar]
- 18. Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64(7):2328‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tak PP, Thurlings RM, Rossier C, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double‐blind, placebo‐controlled, dose‐escalating, single‐ and repeated‐dose study. Arthritis Rheum. 2008;58(1):61‐72. [DOI] [PubMed] [Google Scholar]
- 20. Hahne M, Kataoka T, Schroter M, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188(6):1185‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. J Rennert P, Schneider P, Cachero TG, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med. 2000;192 (11):1677‐1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Wan W, Miao L, et al. Pharmacokinetics, pharmacodynamics and safety of belimumab in chinese patients with systemic lupus erythematosus: a phase I, open‐label study. Rheumatol Ther. 2020;7(1):191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai WW, Fiscella M, Chen C, et al. Bioavailability, pharmacokinetics, and safety of belimumab administered subcutaneously in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(4):349‐357. [DOI] [PubMed] [Google Scholar]
- 24. Munafo A, Priestley A, Nestorov I, et al. Safety, pharmacokinetics and pharmacodynamics of atacicept in healthy volunteers. Eur J Clin Pharmacol. 2007;63(7):647‐56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of this trial.
Table S1. Baseline characteristics.
Table S2. Systematic analysis of adverse events.


