Abstract
Wild reservoirs of Japanese encephalitis virus are under‐studied globally, which presents critical knowledge gaps for JEV epidemiology and infection ecology despite decades of received wisdom regarding this high‐impact mosquito‐borne virus. As a result, ardeid birds, generally understood to be the primary reservoirs for JEV, as well as other waterbirds occupying landscapes at high risk for spillover to humans, are frequently ignored by current surveillance mechanisms and infrastructure. This is particularly true in India, which experiences a high annual burden of human outbreaks. Incorporating wild reservoirs into surveillance of human and livestock populations is therefore essential but will first require a data‐driven approach to target individual host species. The current study sought to identify preliminary waterbird target species for JEV surveillance development based on species’ distributions in high‐risk landscapes. Twenty‐one target species were identified after adjusting species presence and abundance for the biotic constraints of sympatry. Furthermore, ardeid bird species richness demonstrated a strong non‐linear association with the distribution of human JEV outbreaks, which suggested areas with the highest ardeid species richness corresponded to low JEV outbreak risk. No association was identified between JEV outbreaks and anatid or rallid richness. The lack of association between Anatidae and Rallidae family‐level diversity and JEV outbreak risk notwithstanding, this study did identify several individual species among these two bird families in high‐risk landscapes. The findings from this work provide the first data‐driven evidence base to inform wildlife sampling for the monitoring of JEV circulation in outbreak hotspots in India and thus identify good preliminary targets for the development of One Health JEV surveillance.
Keywords: infection ecology, Japanese encephalitis, landscape epidemiology, vector‐borne disease, wildlife–livestock–human interface
1. INTRODUCTION
Japanese encephalitis virus (JEV) is a mosquito‐borne zoonotic virus circulating enzootically in wild waterbirds and domestic pigs, and seasonally spills over to humans causing disease (Japanese encephalitis [JE]) with extensive morbidity and mortality, particularly in children (Mackenzie et al., 2002; World Health Organization, 2015). India experiences high JEV incidence, with large outbreaks clustering in the northeast, and to a lesser extent in the southwest, during the monsoon season (National vectorborne Disease Control Program, Ministry of Health & Family Welfare, 2017). Culex tritaeniorhynchus is the primary JEV vector across Asia and also plays a substantial role in transmission in India (Endy & Nisalak, 2002; Longbottom et al., 2017; Samy et al., 2018). In addition to Cx. tritaeniorhynchus, there are four more vector species (Cx. vishnui, Cx. gelidus, Cx. fuscocephala and Cx. pseudovishnui) widely distributed across India (Samy et al., 2018; Thankachy et al., 2019). The near‐ubiquitous presence of vectors notwithstanding, JEV outbreaks in humans manifest within distinct landscape mosaics. Landscapes of fragmented wetland‐rainfed agricultural mosaics, and exhibiting an extensive distribution of ardeid birds and domestic pigs and chickens, presented the greatest risk of JEV outbreaks across India in a recent report of national JEV surveillance between 2010 and 2020 (Walsh et al., 2022).
Despite the substantive annual burden of disease associated with JEV, there is a surprising dearth of knowledge regarding the epidemiology and infection ecology of this arbovirus, particularly with respect to its circulation in wildlife populations. Bird species in the Ardeidae family have long been recognized as key maintenance hosts of JEV (Buescher et al., 1959; Jamgaonkar et al., 2003; Rodrigues et al., 1981; Soman et al., 1977; Bhattacharya & Basu, 2014), while domestic pigs have been implicated as the primary amplification and bridging hosts for human spillover (Baruah et al., 2018; Borah et al., 2013; Carey et al., 1969; Chen & Beaty, 1982; Ghimire et al., 2014; Kakkar et al., 2017; Komada et al., 1968), although additional evidence has shown that some ardeid species can also amplify JEV circulation sufficiently to facilitate direct spillover to humans via the mosquito vectors (Endy & Nisalak, 2002). There is also some evidence to suggest that chickens may also play a role as amplifying hosts, further expanding the potentially relevant interspecific interactions at the wildlife–livestock interface (Bhattacharya et al., 1988; Ogata et al., 1970; Bhattacharya & Basu, 2014). Despite the accepted state of the knowledge regarding JEV reservoir hosts, much of the wildlife survey data that this is based on is outdated, limited to only a small number of ardeid species, and was not collected in India. The most extensive investigation of human outbreaks in India to date provided strong support for the association between JEV outbreaks and both ardeid species distributions and pig density (Walsh et al., 2022). Importantly, this study also identified mosaics of riparian and freshwater marsh wetlands with fragmented rainfed agriculture as the key landscapes in which these hosts contribute to JEV circulation and the high risk of outbreaks. However, no individual ardeid species associated with these landscapes were explored in this study. While JEV isolation from ardeids has been reported outside of India, only one study has isolated virus from ardeid birds in India despite the completion of several serological surveys (Soman et al., 1977). In addition, there are other waterbird species associated with such landscapes, some of which have also been identified as JEV hosts, but again all but one of these were based on serological surveys. A more thorough evaluation of the landscape suitability of individual waterbird species that occupy these landscapes is therefore warranted in order to identify preliminary target species for the development of much‐needed JEV surveillance that incorporates wildlife monitoring, as well as to determine the potential relative contributions of different waterbird families to outbreak risk. In addition, species diversity, or lack thereof, may further contribute to risk in landscapes experiencing JEV outbreaks by way of the dilution effect, whereby in some systems greater species richness may buffer against community transmission due to the potential presence of more non‐host or inefficient host species (Civitello et al., 2015). If high species richness does correspond to diminished JEV outbreak risk, this could have important implications for public health as wetland habitat is rapidly fragmented due to development.
The objectives of the current study were twofold. First, this investigation sought to estimate the landscape suitability of the waterbird species extant in India and to formally adjust the presence and abundance of each species for the potential biotic constraints of sympatry at the community level, and subsequently estimate overall and family‐specific species richness. Second, this study compared these adjusted species distribution and diversity metrics to the distribution of JEV outbreaks to identify optimal target species for wildlife surveillance in high‐risk landscapes at the community level, and to interrogate the extent to which species diversity is associated with landscape risk.
2. MATERIALS AND METHODS
2.1. Data sources
Seventy‐six species in the families Ardeidae, the herons (including egrets) and bitterns (n = 19), Anatidae, the ducks, geese and swans (n = 41) and Rallidae, the rails, coots, crakes and gallinules (n = 16) are documented as extant in India (Lepage et al., 2014). These families comprise the waterbird species that occupy the wetland habitat previously identified as high risk for JEV outbreaks (Walsh et al., 2022). Each of these families has at least one species with documented seroconversion or infection competence. Infection competence is defined here as a viral titre in a host species of sufficient magnitude to infect vector mosquitoes and thus pass on the infection to other hosts (Downs et al., 2019). Seroconversion alone cannot designate a host's infection competence. Among the Ardeidae, Egretta garzetta (Jamgaonkar et al., 2003; Ogata et al., 1970; Scherer, 1959), Ardeola grayii (Jamgaonkar et al., 2003; Rodrigues et al., 1981; Soman et al., 1977), Nycticorax nycticorax (Buescher et al., 1959; Ogata et al., 1970; Scherer, 1959), Ardea intermedia (Egretta intermedia) (Buescher et al., 1959; Ogata et al., 1970; Scherer, 1959), Ardea alba (Nemeth et al., 2012) and Bubulcus ibis (Bubulcus coromandus) (Nemeth et al., 2012; Rodrigues et al., 1981) all have demonstrated infection competence. Among the Anatidae, Anas platyrhynchos (Nemeth et al., 2012; Yang et al., 2011), A. poecilorhyncha (Yang et al., 2011), A. crecca (Yang et al., 2011), A. acuta (Yang et al., 2011), A. strepera (Ghosh et al., 1978), A. penelope (Yang et al., 2011), A. clypeata (Ghosh et al., 1978), A. formosa (Yang et al., 2011), Aythya fuligula (Ghosh et al., 1978) and Aix galericulata (Yang et al., 2011), have documented JEV seroconversion, but only one (A. platyrhynchos) has demonstrated infection competence (Nemeth et al., 2012). Among the Rallidae, Gallinula chloropus (Rodrigues et al., 1981) and Fulica atra (Yang et al., 2011) have documented JEV seroconversion, but neither have documented infection competence.
All observations of Ardeidae species (1,016,733 individual observations of 16 species; Global Biodiversity Information Facility, 2021), Anatidae species (494,863 individual observations of 28 species; GBIF, 2021a) and Rallidae species (377,672 individual observations of 15 species; GBIF, 2021b) between 1 January 2010 and 31 December 2020 across India were acquired from the Global Biodiversity Information Facility (GBIF) to model each species’ distribution, as well as overall species richness and species richness within family. After removing duplicate observations at the same geographic location and those species with an insufficient number of observations available for modelling (n < 100), there remained 241,784 observations of 15 Ardeidae species, 113,427 observations of 21 Anatidae species and 78,372 observations of 12 Rallidae species for the landscape suitability models described below. Bubulcus coromandus was until recently considered a subspecies of B. ibis (B. ibis coromandus), and is represented in the GBIF database as both B. ibis and B. coromandus. Given the importance of domesticated pigs as amplifying hosts for JEV, we further acquired all observations of wild boars (Sus scrofa) to model this species’ landscape suitability and thus evaluate its association with JEV outbreaks.
Because of the potential for differential accessibility, and thus differential reporting of bird occurrence, the background points used to model all species distributions were selected proportional to the human footprint (HFP) (see modelling description below) as a proxy for accessibility, thus correcting for potential spatial sampling bias. The HFP raster was obtained from the Socioeconomic Data & Applications Center (SEDAC) registry (Socioeconomic Data & Applications Center | SEDAC, n.d.) and quantified according to a 2‐stage classification system that has been described in detail (Sanderson et al., 2002). Briefly, a metric for human influence was first quantified based on human population density, rural versus urban location, land cover, degree of night‐time light pollution, and proximity to roads, rail lines, navigable rivers and coastline. The domains were scored and summed to generate the human influence index (HII), and then HFP was calculated as the ratio of the range of minimum and maximum HII in the local terrestrial biome to the range of minimum and maximum HII across all biomes, expressed as a percentage (Sanderson et al., 2002).
Two‐hundred and 94 laboratory‐confirmed outbreaks of JEV were reported to the National Centre for Disease Control's Integrated Disease Surveillance Programme (IDSP) at a spatial resolution of 1 arc minute between 1 January, 2010 and 31 December, 2020 and have been described previously (Walsh et al., 2022). The IDSP maintains a national JEV surveillance system under the administration of India's Ministry of Health and Family Welfare (National Centre for Disease Control et al., n.d.). These surveillance data were also previously externally validated against an independent, laboratory‐confirmed dataset of community surveys of human and mosquito infection (Walsh et al., 2022).
The WorldClim Global Climate database was the source of the climate data used in this study (WorldClim – Global Climate, n.d.). These comprised mean annual temperature, mean annual precipitation, and isothermality. Proximity to surface water was calculated using the proximity function in the QGIS geographic information system (QGIS Development Team, 2009) and subsequently used to generate a distance raster for the hydrogeography data obtained in the Global Lakes and Wetlands Database (Lehner & Döll, 2004; World Wildlife Fund, n.d.). The pixel values of this raster represent the distance in kilometres between surface water and all other pixels within the geographic extent of India.
2.2. Statistical analysis
2.2.1. Species distribution modelling
There were 48 of the 76 extant waterbird species, as well as the one non‐bird species, Sus scrofa, in India with a minimum of 100 unique observations available in the GBIF database (Table S1). This minimum number of observations was a relatively conservative threshold representing an approximately twofold greater minimum sampling threshold than has previously been shown to be the minimum number of observations for best practice in estimating habitat suitability even in large geographic extent (Proosdij et al., 2016). Landscape suitability was estimated using an ensemble approach comprising boosted regression trees (BRT), random forests (RF), and generalized additive models (GAM). Boosted regression trees and RF both partition data space by optimizing homogeneity among predictors and a response (i.e. species presence). The algorithms generate and combine many decision trees, resulting in optimized decision trees that reduce overfitting and can capture complex interactions between the predictors (Breiman, 2001; Elith et al., 2008; Friedman, 2001; James et al., 2000). There are important differences between BRT and RF, however. With RF, only a random subset of predictors is selected from the set of all predictors for the generation of each decision tree. This reduces overfitting by decorrelating the data through the random selection of predictors for each tree. With BRT, overfitting is reduced by growing trees sequentially and learning from the previous iteration rather than decorrelating trees based on the sampling of subsets as with RF. In contrast, the GAM framework fits multiple basis functions for smoothed covariates thus allowing for non‐linear relationships between outcomes and covariates (Wood, 2004, 2017). Each model under BRT, RF and GAM was fit with fivefold cross‐validation. Observation data were thinned so that only one observation per pixel was included in the analysis to avoid artificial spatial clustering (Table S1). Mean annual temperature, mean annual precipitation, isothermality and proximity to surface water were included as landscape features at 30 arc seconds resolution in all models. For each species, each of the three models (BRT, RF and GAM) was evaluated according to model performance, based on the area under the receiver operating characteristic curve (AUC), and model fit, based on the deviance. Landscape suitability for each species was then derived from an ensemble of the three models (BRT, RF, and GAM) using their weighted mean with weights based on AUC (Naimi & Araújo, 2016). To correct for potential spatial sampling bias among the GBIF observations, background points were sampled proportional to the human footprint to serve as a proxy for landscape, and thus bird, accessibility. Each species’ landscape suitability as derived from the ensembles was subsequently summed across all species as a crude estimate of local species richness across the geographic extent of India. This estimate was then adjusted for the biotic constraints of sympatric species at the community level (see Community‐level modelling description below).The sdm package (Naimi & Araújo, 2016) was used for fitting all models and deriving the landscape suitability ensembles.
2.2.2. Community‐level modelling
To compute individual species presence and abundance and species richness, biotic constraints were applied at the scale of the taluk. Taluks are 3rd‐level, subdistrict municipalities that are sufficiently small to reasonably approximate shared space among sympatric species, but which are also sufficiently large enough to demarcate the minimal municipal infrastructure required across most of India for organizing and executing animal and human disease surveillance. The biotic constraints took the form of sympatric species adjustments to the estimates of each species' landscape suitability using the spatially explicit species assemblage modelling (SESAM) framework (D'Amen, Dubuis et al., 2015; Di Cola et al., 2017; Guisan & Rahbek, 2011). First, the landscape suitability for each species was estimated using the ensemble method described above. Second, the individual species distributions were summed to calculate an unadjusted species richness estimate. Third, each species distribution is evaluated with respect to all other species present within each taluk via the probability ranking rule (D'Amen, Dubuis et al., 2015; D'Amen, Pradervand et al., 2015) to determine whether a given species should be retained within, or excluded from, each taluk ‘community’. Under this final step, each species is ranked from highest to lowest based on their suitability estimate obtained in the first step. Those with high suitability are ranked high, while those with low suitability are ranked low. Species are then selected for inclusion in the community starting with the species with the highest suitability estimate and continuing down through the list of ranked species until the sum of selected species is equal to the expected species richness value for each location as represented by the calculation in the second step. Once this threshold sum is reached, the adjusted species richness is achieved and no further species are included in that particular taluk community. This process thus yields an estimate of individual species presence within taluks given the potential biotic constraints of the other species within those same taluks. Under this SESAM framework, for each species retained as present following adjustment for taluk‐level sympatric species, each 1 km2 pixel of their ensemble landscape suitability estimate raster that was greater than or equal to the true skill statistic (TSS) (Allouche et al., 2006) was classified as present (1 = present, 0 = otherwise) and summed across all pixels within the taluk. In this way taluk‐level species abundance was estimated for each species retained in a given taluk. Adjusted estimates of species richness for the Ardeidae, Anatidae, and Rallidae families were also generated by summing the number of species retained within each taluk, for each family, under the SESAM framework. As such this approach provided a framework for taluk‐level community estimation of species richness and individual species' abundance, which was formally adjusted for sympatric species. The SESAM analysis was conducted in R using the ecospat package (Di Cola et al., 2017).
The taluk‐level abundance of each species, adjusted for the biotic constraints of sympatric species, was interrogated to determine which species distributions were associated with high‐risk JEV landscapes. Integrated nested Laplace approximation (INLA) models (Rue et al., 2009) were used to estimate these associations at the community level of the taluk. It is important to note that any such associations identified do not provide any specific insight into species' roles as hosts since species competence for JEV was not measured or evaluated in the current study. Individual species associations were instead explored simply to identify which species may be optimal as sampling targets for implementing new wildlife surveillance in outbreak hotspots across India. The INLA models were fit using the binomial likelihood family and with Besag–York–Mollie priors for the random effects. Taluks were modelled as either outbreak‐positive or outbreak‐negative over the duration of the study, and were thus fit with the binomial family, since most of the taluks across India did not experience outbreaks, while those that did only experienced one or a very small number of outbreaks. The Watanabe–Akaike information criterion (WAIC) was used to assess the fit of all INLA models. The INLA package in R (Rue et al., 2009) was used to fit the INLA models.
3. RESULTS
Twenty‐one of the 48 interrogated waterbird species were positively associated with the distribution of JEV outbreaks after accounting for sympatric species at the taluk level in the estimation of each species’ presence and abundance (Figure 1; Table S2). Wild boars (Sus scrofa) were not associated with JEV outbreaks. Although species in each of the three waterbird families were associated with JEV outbreaks, within‐family species richness was only associated with outbreaks for the Ardeidae family, further supporting this family's dominance in high‐risk landscapes. Importantly, however, this relationship was non‐linear wherein risk increased sharply as species richness increased from 0 to 4 species in the taluk‐level community assemblage, peaked at about 5 species, and then decreased sharply such that the areas of highest species richness were generally associated with low risk (Table 1 and Figure 2).
FIGURE 1.
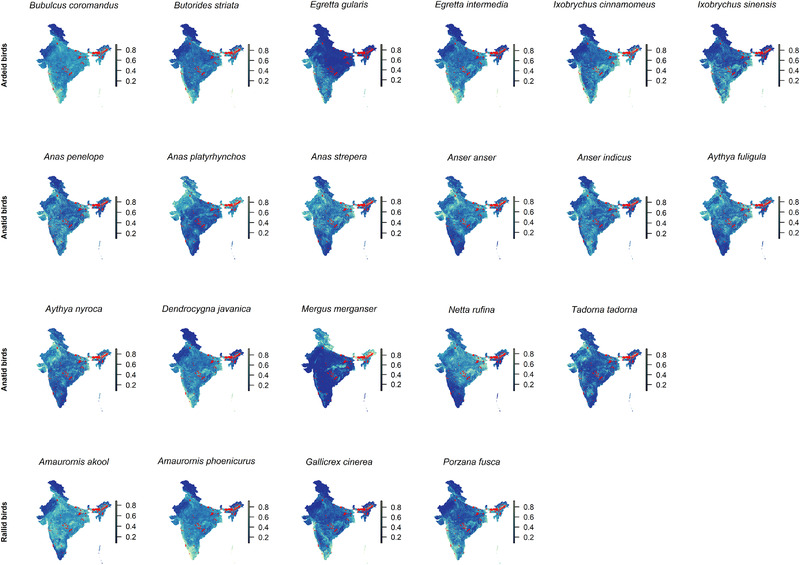
The distribution of the landscape suitability of those Ardeidae, Anatidae and Rallidae species identified as optimal potential targets for wildlife surveillance based on their strong association with the distribution of JEV outbreaks as determined by integrated nested Laplace approximation models after adjusting species presence for the biotic constraints of sympatric species. The overlaid points represent the locations of human JEV outbreaks. Maps are used only for the purposes of depicting species landscape suitability and do not reflect the authors’ assertion of territory or borders of any sovereign country including India
TABLE 1.
Taluk‐level integrated nested Laplace approximation models of Japanese encephalitis virus outbreaks
| Taluk‐level bird family species richness | Coefficient | 95% credible interval | WAIC |
|---|---|---|---|
| Ardeidae | |||
| Model 1 | |||
| Ardeid species richness | 0.161 | 0.051 to 0.272 | 567.16 |
| Model 2 | |||
| Ardeid species richness | 0.482 | 0.161 to 0.819 | 567.39 |
| (Ardeid species richness)2 | −0.025 | −0.050 to −0.002 | |
| Anatidae | |||
| Model 1 | |||
| Anatid species richness | 0.039 | −0.046 to 0.123 | 571.92 |
| Model 2 | |||
| Anatid species richness | −0.117 | −0.390 to 0.169 | 571.13 |
| (Anatid species richness)2 | 0.009 | −0.007 to 0.024 | |
| Rallidae | |||
| Model 1 | |||
| Rallid species richness | 0.111 | −0.081 to 0.306 | 568.65 |
| Model 2 | |||
| Rallid species richness | −0.251 | −0.771 to 0.296 | 569.29 |
| (Rallid species richness)2 | 0.041 | −0.016 to 0.096 | |
| All waterbirds | |||
| Model 1 | |||
| Total species richness | 0.070 | 0.012 to 0.127 | 569.70 |
| Model 2 | |||
| Total species richness | 0.492 | 0.161 to 0.865 | 563.57 |
| (Total species richness)2 | −0.011 | −0.021 to −0.003 |
Note: Within‐family species richness were all adjusted for the biotic constraints of sympatric species using the SESAM framework and fit with two competing models: For each family, Model 1 comprises only within‐family species richness and Model 2 comprises within‐family species richness and a quadratic term for within‐family species richness. Model fit was assessed using the Watanabe–Akaike information criterion (WAIC)
FIGURE 2.
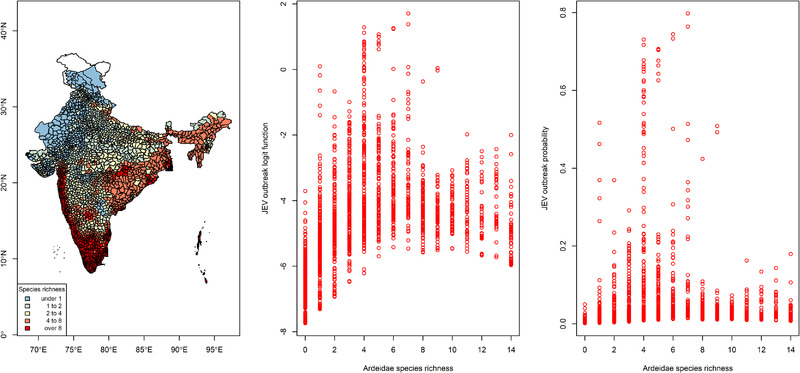
The distribution of taluk‐level community species richness among the ardeid birds (left panel), the distribution of the log odds of taluk‐level JEV outbreaks versus ardeid species richness (middle panel), and the distribution of taluk‐level JEV outbreak probability versus ardeid species richness (right panel) as derived from the integrated nested Laplace approximation model after adjusting species presence for the biotic constraints of sympatric species. Maps are used only for the purposes of depicting species richness and do not reflect the authors’ assertion of territory or borders of any sovereign country including India
4. DISCUSSION
This investigation describes the biogeographical patterns of wild waterbird species and their associations with JEV outbreaks in India. There are several important implications from these findings. First, this work provides the first country‐wide, geographically demarcated estimates of waterbird species richness and abundance in freshwater wetlands across India and adjusted for sympatry. These estimates provided the best approach to identify those species whose distributions most closely followed the distribution of JEV outbreaks, while further allowing for the evaluation of species diversity with respect to outbreak risk. Only ardeid richness was associated with JEV outbreak risk, but crucially, landscapes with high ardeid species richness manifested considerably lower risk than those with low to moderate species richness. While reaffirming the overall importance of Ardeidae species relative to Anatidae and Rallidae species in landscapes of high JEV circulation, this study nevertheless identified several species across all three families that should provide good preliminary targets for the development of improved One Health wildlife JEV surveillance. Moreover, the findings also suggested that landscapes with the highest levels of ardeid diversity were at considerably diminished risk, which may support the potential benefits of leveraging habitat conservation in the interests of public health.
Animal surveillance for JEV in India is minimal in general and virtually non‐existent in wild birds in particular. Because the circulation of JEV in these hosts is fundamental to the virus' infection ecology, there is a critical need to develop and implement surveillance infrastructure that incorporates the monitoring of reservoir birds in landscapes at highest risk for JEV outbreaks, which are delineated by mosaics of wetlands and rainfed agriculture throughout India (Walsh et al., 2022). Unfortunately, given the limited field investigations to inform the selection of optimal targets for waterbird monitoring, a recognized and reliable source of known hosts from which to sample does not currently exist and so any development of wildlife monitoring for JEV must proceed with a minimal evidence base. In order to provide a more evidence‐based list of potential JEV hosts for preliminary surveys, the current study interrogated the waterbird families of high‐risk landscapes in India using community ecology methods that account for sympatry. This should provide a more sound approach than simply sampling birds previously identified as infected, most of which reflect surveys conducted outside of India, many years ago, or relied upon serology alone. Several of the species identified as optimal for sampling, including B. coromandus, E. intermedia and A. platyrhynchos, have been previously demonstrated as competent hosts (Nemeth et al., 2012; Ogata et al., 1970; Scherer, 1959), and therefore reinforce these species as good targets for initial sampling and wildlife monitoring. The value of these results for the development of new wildlife surveillance notwithstanding, it is important to emphasize that these findings provide no insight into host competence or infection status and therefore should not be interpreted as identifying any species as definitive maintenance, amplification or bridging hosts. Rather, this work is intended to inform sampling strategies for the implementation of field investigation and broader wildlife surveillance infrastructure in landscapes of highest outbreak risk (Walsh et al., 2022). Such wildlife surveillance will be critical to understanding the circulation of JEV in wild waterbirds and thereby inform the landscape epidemiology of JEV outbreaks in humans. It is also worth noting that although the focus of the current study was on landscapes of highest JEV risk, these are not necessarily the only landscapes in which JEV circulates or spills over to humans within India or in other countries throughout Asia. For example, compared with the fragmented rainfed agriculture‐wetland mosaics in India targeted in the current study, in Vietnam and Cambodia substantive JEV circulation has been identified in peri‐urban landscapes that comprise different land use structure and animal host occupancy (Di Francesco et al., 2018; Lindahl et al., 2013; Lord et al., 2015; Nguyen‐Tien et al., 2019). As such, we acknowledge that there exist multiple landscapes in which JEV can circulate and may involve different epidemiology and infection ecology and therefore ultimately may require different approaches to control.
Interestingly, this study supported the dominance of ardeid bird presence in landscapes at high risk of JEV outbreaks relative to anatid and rallid bird presence, while also indicating that some individual anatid and rallid species should also be considered for surveillance sampling in these landscapes. Importantly, the strong association between ardeid species richness and JEV outbreaks was non‐linear such that JEV risk increased with increasing richness in landscapes with low to moderate ardeid richness, while risk decreased considerably in areas with the highest species richness. As related above, this study did not isolate virus in any species, nor did it document interspecific interaction. As such, the findings do not provide definitive evidence for the operation of the dilution effect in these landscapes. However, the clear demarcation of JEV risk with corresponding species diversity does suggest that a more careful consideration of the role of species diversity in virus circulation among wildlife hosts should be evaluated wherever possible in future investigation of JEV ecology. Moreover, the results also suggest additional practical approaches to landscape stratification for One Health surveillance development, i.e. by incorporating community structure and composition as well as land use, land cover, and animal husbandry into the architecture of monitoring systems.
In addition to the important caution against over‐interpretation of the results due to the inherent limitations described above, some additional limitations warrant further discussion here. First, it is worth restating that host competence was not assessed in any species in this study and therefore no claims are made with respect to individual or collective species’ host status. Rather this study was concerned with identifying those waterbird species sharing space in the agricultural‐wetland mosaics that have previously been identified as highest risk for JEV outbreaks so that initial wildlife surveillance targets can be located in these ecotones. Second, the findings are not used to make any specific claims about the true nature and influence of interaction (e.g. competitive exclusion or character displacement) among waterbird communities at various scales because, as previously described, direct observation of interspecific interaction and its effects on community composition was not possible under the framework of the current study. As such, these results will again require validation against field studies of directly observed interaction between bird species at multiple scales. Third, despite the exceptionally large number of bird observations obtained for this study, there were insufficient observations for all extant waterbird species in India to model the distribution of each. Therefore, the new estimates presented for species richness and individual species abundances, although the first of their kind for India, are nevertheless based on a sample of bird species (n = 48) and therefore represent a proxy for true species richness and species abundances. Fourth, spatial biases may affect the distribution of species observation data in GBIF due to differential accessibility. Accordingly, background points used in the models were selected proportional to the human footprint to control for these potential biases.
In conclusion, this study has identified optimal preliminary wild waterbird targets for the development of novel One Health JEV surveillance in India. The extent to which the identified species will be validated as important hosts in the infection ecology of JEV in India, and the specific roles individual species may play (e.g. maintenance vs. amplification hosts), must await the data generated from the implementation of these surveillance mechanisms. Nevertheless, we feel the current results provide the best evidence base to date for actioning new surveillance mechanisms that can effectively couple wildlife, livestock, and human health monitoring at the key points of interface in landscapes of wetland‐rainfed agriculture ecotones.
CONFLICT OF INTEREST
The authors declare no conflict of interest exists.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required.
AUTHOR CONTRIBUTIONS
Conceptualization: MW, AP, NV, DS, CW, SS, CM; methodology. MW, AP, NV, SS, CM: formal analysis. MW: investigation. MW, AP, NV, DS, CW, SS, CM: data curation. MW: writing—original draft preparation. MW, AP, NV, DS, CW, SS, CM: writing – review and editing. MW, AP, NV, DS, CW, SS, CM: supervision. All authors have read and agreed to the published version of the manuscript.
Supporting information
TABLE S1. Ardeidae species, Anatidae species, Rallidae species, and Sus scrofa landscape suitability comparisons based on ensemble species distribution models
TABLE S2. Taluk‐level regression coefficients and 95% credible intervals for the associations between Japanese encephalitis virus outbreaks and each species’ abundance (number of species‐present pixels per taluk) as derived from simple integrated nested Laplace approximation models (binomial family)
ACKNOWLEDGEMENTS
The authors received no funding for the conduct of this work.
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Walsh, M. G. , Pattanaik, A. , Vyas, N. , Saxena, D. , Webb, C. , Sawleshwarkar, S. , & Mukhopadhyay, C. (2022). A biogeographical description of the wild waterbird species associated with high‐risk landscapes of Japanese encephalitis virus in India. Transboundary and Emerging Diseases, 69, e3015–e3023. 10.1111/tbed.14656
DATA AVAILABILITY STATEMENT
The data underlying this article are available in https://figshare.com/s/3f9777be3f569bc48ab7.
REFERENCES
- Allouche, O. , Tsoar, A. , & Kadmon, R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology, 43, 1223–1232. 10.1111/j.1365-2664.2006.01214.x [DOI] [Google Scholar]
- Baruah, A. , Hazarika, R. , Barman, N. , Islam, S. , & Gulati, B. (2018). Mosquito abundance and pig seropositivity as a correlate of Japanese encephalitis in human population in Assam, India. Journal of Vector Borne Diseases, 55, 291–296. 10.4103/0972-9062.256564 [DOI] [PubMed] [Google Scholar]
- Bhattacharya, S. , & Basu, P. (2014). Japanese Encephalitis Virus (JEV) infection in different vertebrates and its epidemiological significance: A review. International Journal of Fauna and Biological Studies, 1, 32–37. [Google Scholar]
- Bhattacharya, S. , Chakraborty, S. , Chakraborty, S. , Ghosh, K. , Palit, A. , Mukherjee, K. , Chakraborty, M. , Tandon, N. , & Hati, A. (1988). Density of Culex vishnui and appearance of JE antibody in sentinel chicks and wild birds in relation to Japanese encephalitis cases. Tropical and Geographical Medicine, 38, 46–50. [PubMed] [Google Scholar]
- Borah, J. , Dutta, P. , Khan, S. A. , & Mahanta, J. (2013). Epidemiological concordance of Japanese encephalitis virus infection among mosquito vectors, amplifying hosts and humans in India. Epidemiology and Infection, 141, 74–80. 10.1017/S0950268812000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman, L. (2001). Random forests. Machine Learning, 45, 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Buescher, E. L. , Scherer, W. F. , Rosenberg, M. Z. , & Kutner, L. J. (1959). Immunologic studies of Japanese encephalitis virus in Japan. IV. Maternal antibody in birds. Journal of Immunology, 83, 614–619. [PubMed] [Google Scholar]
- Carey, D. , Reuben, R. , & Myers, R. (1969). Japanese encephalitis studies in Vellore, South India. V. Experimental infection and transmission. Indian Journal of Medical Research, 57, 282–289. [PubMed] [Google Scholar]
- Chen, B. , & Beaty, B. (1982). Japanese encephalitis vaccine (2‐8 strain) and parent (SA 14 strain) viruses in Culex tritaeniorhynchus mosquitoes. American Journal of Tropical Medicine and Hygiene, 31, 403–407. [DOI] [PubMed] [Google Scholar]
- Civitello, D. J. , Cohen, J. , Fatima, H. , Halstead, N. T. , Liriano, J. , McMahon, T. A. , Ortega, C. N. , Sauer, E. L. , Sehgal, T. , Young, S. , & Rohr, J. R. (2015). Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proceedings of the National Academy of Sciences, 112, 8667–8671. 10.1073/pnas.1506279112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amen, M. , Dubuis, A. , Fernandes, R. F. , Pottier, J. , Pellissier, L. , & Guisan, A. (2015). Using species richness and functional traits predictions to constrain assemblage predictions from stacked species distribution models. Journal of Biogeography, 42, 1255–1266. 10.1111/jbi.12485 [DOI] [Google Scholar]
- D'Amen, M. , Pradervand, J. ‐N. , & Guisan, A. (2015). Predicting richness and composition in mountain insect communities at high resolution: A new test of the SESAM framework. Global Ecology and Biogeography, 24, 1443–1453. 10.1111/geb.12357 [DOI] [Google Scholar]
- Di Cola, V. , Broennimann, O. , Petitpierre, B. , Breiner, F. T. , D'Amen, M. , Randin, C. , Engler, R. , Pottier, J. , Pio, D. , Dubuis, A. , Pellissier, L. , Mateo, R. G. , Hordijk, W. , Salamin, N. , & Guisan, A. (2017). ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography, 40, 774–787. 10.1111/ecog.02671 [DOI] [Google Scholar]
- Di Francesco, J. , Choeung, R. , Peng, B. , Pring, L. , Pang, S. , Duboz, R. , Ong, S. , Sorn, S. , Tarantola, A. , Fontenille, D. , Duong, V. , Dussart, P. , Chevalier, V. , & Cappelle, J. (2018). Comparison of the dynamics of Japanese encephalitis virus circulation in sentinel pigs between a rural and a peri‐urban setting in Cambodia. PLOS Neglected Tropical Diseases, 12, 10.1371/journal.pntd.0006644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs, C. J. , Schoenle, L. A. , Han, B. A. , Harrison, J. F. , & Martin, L. B. (2019). Scaling of host competence. Trends in Parasitology, 35, 182–192. 10.1016/j.pt.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Elith, J. , Leathwick, J. R. , & Hastie, T. (2008). A working guide to boosted regression trees. Journal of Animal Ecology, 77, 802–813. 10.1111/j.1365-2656.2008.01390.x [DOI] [PubMed] [Google Scholar]
- Endy, T. P. , & Nisalak, A. (2002). Japanese Encephalitis virus: Ecology and epidemiology. In: J. S. Mackenzie, Barrett A., & Deubel V. (Eds.), Japanese encephalitis and West Nile viruses (pp. 11–48). Berlin: Springer‐Verlag. [DOI] [PubMed] [Google Scholar]
- Friedman, J. (2001). Greedy function approximation: A gradient boosting machine. Annals of Statistics, 29, 1189–1232. [Google Scholar]
- GBIF , (2021a). Global Biodiversity Information Facility, GBIF occurrence download – Anatidae India [Online]. Available at 10.15468/dl.4twgcp [DOI]
- GBIF , (2021b). Global Biodiversity Information Facility, GBIF occurrence download – Rallidae India [Online]. Available at 10.15468/dl.hsqz5p [DOI]
- Ghimire, S. , Dhakal, S. , Ghimire, N. P. , & Joshi, D. D. (2014). Pig sero‐survey and farm level risk factor assessment for Japanese encephalitis in Nepal. International Journal of Applied Sciences and Biotechnology, 2, 311–314. 10.3126/ijasbt.v2i3.10639 [DOI] [Google Scholar]
- Ghosh, S. , Sokhey, J. , Dandawate, C. , Gupta, N. , Obukhova, V. , & Gaidamovich, S. (1978). Arthropod‐borne virus activity in migratory birds, Ghana Bird Sanctuary, Rajasthan State, 1973. Indian Journal of Medical Research, 68, 192–196. [PubMed] [Google Scholar]
- Global Biodiversity Information Facility , (2021). Global Biodiversity Information Facility, GBIF occurrence download – Ardeidae India [Online]. Available at 10.15468/dl.s99zmx [DOI]
- Guisan, A. , & Rahbek, C. (2011). SESAM – A new framework integrating macroecological and species distribution models for predicting spatio‐temporal patterns of species assemblages. Journal of Biogeography, 38, 1433–1444. 10.1111/j.1365-2699.2011.02550.x [DOI] [Google Scholar]
- James, G. , Witten, D. , Hastie, T. , & Tibshirani, R. (2000). An introduction to statistical learning. Current Medicinal Chemistry, 7.
- Jamgaonkar, A. V. , Yergolkar, P. N. , Geevarghese, G. , Joshi, G. D. , Joshi, M. V. , & Mishra, A. C. (2003). Serological evidence for Japanese encephalitis virus and West Nile virus infections in water frequenting and terrestrial wild birds in Kolar District, Karnataka State, India: A retrospective study. Acta Virologica, 47, 185–188. [PubMed] [Google Scholar]
- Kakkar, M. , Chaturvedi, S. , Saxena, V. K. , Dhole, T. N. , Kumar, A. , Rogawski, E. T. , Abbas, S. , Venkataramanan, V. V. , & Chatterjee, P. (2017). Identifying sources, pathways and risk drivers in ecosystems of Japanese Encephalitis in an epidemic‐prone north Indian district. Plos One, 12, 1–17. 10.1371/journal.pone.0175745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada, K. , Sasaki, N. , & Inoue, Y. (1968). Studies of live attenuated Japanese encephalitis vaccine in swine. Journal of Immunology, 100, 194–200. [PubMed] [Google Scholar]
- Lehner, B. , & Döll, P. (2004). Development and validation of a global database of lakes, reservoirs and wetlands. Journal of Hydrology, 296, 1–22. 10.1016/j.jhydrol.2004.03.028 [DOI] [Google Scholar]
- Lepage, D. , Vaidya, G. , & Guralnick, R. (2014). Avibase – A database system for managing and organizing taxonomic concepts. Zookeys, 420, 117–135. 10.3897/zookeys.420.7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, J. F. , Ståhl, K. , Chirico, J. , Boqvist, S. , Thu, H. T. V. , & Magnusson, U. (2013). Circulation of Japanese encephalitis virus in pigs and mosquito vectors within Can Tho City, Vietnam. PLOS Neglected Tropical Diseases, 7, 10.1371/journal.pntd.0002153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom, J. , Browne, A. J. , Pigott, D. M. , Sinka, M. E. , Golding, N. , Hay, S. I. , Moyes, C. L. , & Shearer, F. M. (2017). Mapping the spatial distribution of the Japanese encephalitis vector, Culex tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within areas of Japanese encephalitis risk. Parasites and Vectors, 10, 1–12. 10.1186/s13071-017-2086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, J. S. , Gurley, E. S. , & Pulliam, J. R. C. (2015). Rethinking Japanese encephalitis virus transmission: A framework for implicating host and vector species. PLOS Neglected Tropical Diseases, 9, 10.1371/journal.pntd.0004074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie, J. S. , Barrett, A. , & Deubel, V. (2002). The Japanese encephalitis serological group of flaviviruses: A brief introduction to the group. In: J. S. Mackenzie, Barrett A., and Deubel V. (Eds.), Japanese encephalitis and West Nile viruses (pp. 1–10). Berlin: Springer‐Verlag. [DOI] [PubMed] [Google Scholar]
- Naimi, B. , & Araújo, M. B. (2016). sdm: A reproducible and extensible R platform for species distribution modelling. Ecography, 39, 368–375. 10.1111/ecog.01881 [DOI] [Google Scholar]
- National Centre for Disease Control, Directorate General of Health Services, and Ministry of Health and Family Welfare (n.d.) Integrated Disease Surveillance Programme (IDSP) [Online]. Available at https://idsp.nic.in/ (accessed September 3, 2020)
- National vectorborne Disease Control Program, Ministry of Health and Family Welfare, G. of I ., (2017). Statewise number of AES/JE cases and deaths from 2010–2017.
- Nemeth, N. , Bosco‐Lauth, A. , Oesterle, P. , Kohler, D. , & Bowen, R. (2012). North American birds as potential amplifying hosts of Japanese encephalitis virus. American Journal of Tropical Medicine and Hygiene, 87, 760–767. 10.4269/ajtmh.2012.12-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen‐Tien, T. , Lundkvist, Å. , & Lindahl, J. (2019). Urban transmission of mosquito‐borne flaviviruses – A review of the risk for humans in Vietnam. Infection Ecology & Epidemiology, 9, 10.1080/20008686.2019.1660129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata, M. , Nagao, Y. , Jitsunari, F. , Kitamura, N. , & Okazaki, T. (1970). Infection of herons and domestic fowls with Japanese encephalitis virus with specific reference to maternal antibody of hen (epidemiological study on Japanese encephalitis 26). Acta Medica Okayama, 24, 175–184. [PubMed] [Google Scholar]
- Proosdij, A. S. J. , Sosef, M. S. M. , Wieringa, J. J. , & Raes, N. (2016). Minimum required number of specimen records to develop accurate species distribution models. Ecography, 39, 542–552. 10.1111/ecog.01509 [DOI] [Google Scholar]
- QGIS Development Team , (2009). QGIS Geographic Information System. Open Source Geospatial Foundation.
- Rodrigues, F. M. , Guttikar, S. N. , & Pinto, B. D. (1981). Prevalence of antibodies to Japanese encephalitis and West Nile viruses among wild birds in the Krishna‐Godavari Delta, Andhra Pradesh, India. Transactions of the Royal Society of Tropical Medicine and Hygiene, 75, 258–262. 10.1016/0035-9203(81)90330-8 [DOI] [PubMed] [Google Scholar]
- Rue, H. , Martino, S. , & Chopin, N. (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 71, 319–392. 10.1111/j.1467-9868.2008.00700.x [DOI] [Google Scholar]
- Samy, A. M. , Alkishe, A. A. , Thomas, S. M. , Wang, L. , & Zhang, W. (2018). Mapping the potential distributions of etiological agent, vectors, and reservoirs of Japanese Encephalitis in Asia and Australia. Acta Tropica, 188, 108–117. 10.1016/j.actatropica.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Sanderson, E. W. , Jaiteh, M. , Levy, M. A. , Redford, K. H. , Wannebo, A. V. , & Woolmer, G. (2002). The human footprint and the last of the wild. Bioscience, 52, 891, 10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2 [DOI] [Google Scholar]
- Scherer, W. (1959). Ecological studies of Japanese encephalitis in Japan. Parts I‐IX. American Journal of Tropical Medicine and Hygiene, 8, 644–722. [DOI] [PubMed] [Google Scholar]
- Socioeconomic Data and Applications Center | SEDAC (n.d.). Methods » Last of the Wild, v2 | SEDAC [Online]. Available at http://sedac.ciesin.columbia.edu/data/collection/wildareas‐v2/methods (accessed December 23, 2014)
- Soman, R. , Rodrigues, F. , Guttikar, S. , & Guru, P. (1977). Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in ardeid birds. Indian Journal of Medical Research, 66, 709–718. [PubMed] [Google Scholar]
- Thankachy, S. , Dash, S. , & Sahu, S. S. (2019). Entomological factors in relation to the occurrence of Japanese encephalitis in Malkangiri district, Odisha State, India. Pathogens and Global Health, 113, 246–253. 10.1080/20477724.2019.1670926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, M. G. , Pattanaik, A. , Vyas, N. , Saxena, D. , Webb, C. , Sawleshwarkar, S. , & Mukhopadhyay, C. (2022). High‐risk landscapes of Japanese encephalitis virus outbreaks in India converge on wetlands, rain‐fed agriculture, wild Ardeidae, and domestic pigs and chickens. International Journal of Epidemiology, Online ahead of print. 10.1093/ije/dyac050 [DOI] [PMC free article] [PubMed]
- Wood, S. N. (2004). Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association, 99, 673–686. 10.1198/016214504000000980 [DOI] [Google Scholar]
- Wood, S. N. (2017). Generalized additive models: An introduction with R (2nd edn.). New York: Chapman and Hall/CRC. [Google Scholar]
- World Health Organization . (2015). Japanese encephalitis vaccines: WHO position paper. Weekly Epidemiological Record, 90, 69–87.25726573 [Google Scholar]
- World Wildlife Fund (n.d.). Global Lakes and Wetlands Database [Online]. Available at http://www.worldwildlife.org/pages/global‐lakes‐and‐wetlands‐database
- WorldClim ‐ Global Climate (n.d.). WorldClim – Global Climate Data, Data for current conditions (∼1950‐2000) | WorldClim – Global Climate Data [Online]. Available at http://www.worldclim.org/current (accessed October 23, 2014)
- Yang, D. K. , Oh, Y. I. , Kim, H. R. , Lee, Y. J. , Moon, O. K. , Yoon, H. , Kim, B. , Lee, K. W. , & Song, J. Y. (2011). Serosurveillance for Japanese encephalitis virus in wild birds captured in Korea. Journal of Veterinary Science, 12, 373–377. 10.4142/jvs.2011.12.4.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Ardeidae species, Anatidae species, Rallidae species, and Sus scrofa landscape suitability comparisons based on ensemble species distribution models
TABLE S2. Taluk‐level regression coefficients and 95% credible intervals for the associations between Japanese encephalitis virus outbreaks and each species’ abundance (number of species‐present pixels per taluk) as derived from simple integrated nested Laplace approximation models (binomial family)
Data Availability Statement
The data underlying this article are available in https://figshare.com/s/3f9777be3f569bc48ab7.


