Abstract
Aims
Bariatric surgery remains an effective treatment for the condition of obesity. However it predisposes patients to nutritional deficiencies and related complications. The aim of this study was to identify nutritional abnormalities, weight loss, adherence to supplements, and presence of gastrointestinal symptoms in a cohort of bariatric surgical patients.
Methods
An analysis of the electronic medical records of patients attending a multidisciplinary private clinic in Sydney, Australia from August 2020 to August 2021 was conducted. Data on anthropometric measures, nutritional indices, adherence to supplements and gastrointestinal symptoms preoperatively and then at ≤6 months, 1 and 2 years or more postoperatively were collected.
Results
A total of 231 patients were included in the study. The majority of patients were female (76.2%), with a sleeve gastrectomy (78.8%). Average preoperative BMI was 43.4 ± 7.1 kg/m2. Weight loss ≥2 years postsurgery was 33.5 ± 12.4 kg. The most common abnormalities preoperatively were: C‐reactive protein (47.7%), vitamin D (39%), B12 (31%), parathyroid hormone (27.6%) and ferritin (12.7%). Vitamin B12 (23.2%), parathyroid hormone (23%), vitamin D (17.7%) and ferritin (15.9%) remained common abnormalities postoperatively.
Adherence to multivitamins was 90% in the first year following surgery, declining to 77% at ≥2 years. Gastrointestinal symptoms were predominantly present in the initial stages following surgery, manifesting thiamin deficiency in 6.5% of patients.
Conclusions
Despite achieving durable weight loss, nutritional and related abnormalities remain an ongoing challenge for bariatric surgery. Adherence to nutrient supplements, gastrointestinal symptoms and related complications are important considerations in addressing the problem.
1. INTRODUCTION
Bariatric surgery remains an effective treatment for the condition of obesity, with improvements in quality of life, obesity related co‐morbidities and subsequently mortality. 1 , 2 , 3 , 4 However, bariatric procedures including Roux‐en‐Y gastric bypass, one anastomosis gastric bypass‐mini gastric bypass, sleeve gastrectomy, and laparoscopic adjusted gastric band change the gastrointestinal system, impacting the ingestion, digestion and absorption of nutrients and subsequently result in nutritional deficiencies. 5 , 6 Commonly reported nutritional concerns include vitamins D, B12, B1 (thiamin), folate, iron, ferritin and hyperparathyroidism. 5 , 6 , 7 , 8 Furthermore, nutritional deficiencies have also been documented in patients presenting for obesity surgery, which may be compounded as a consequence of the surgery. 5 , 6
Patients with good nutritional status preoperatively may not show any clinical or subclinical deficiencies in the first few months following surgery. This is predominantly due to the long half‐life and the availability of these micronutrients in body storage. One such deficiency is anaemia, which may be related to vitamin B12, folate and/or iron deficiency and can easily be detected and treated. 9 Other nutritional concerns such as vitamin D, calcium and hyperparathyroidism, have longer‐term and more complex consequences. These include metabolic bone disease, which requires a multifaceted approach, assessment and a specialised treatment. 10
In contrast thiamin can become depleted rapidly following surgery. 11 Thiamin is an essential coenzyme for numerous pathways of the nervous system and despite its availability in major food groups, due to its short half‐life (9–18 days) and low storage levels in the body (30 mg), levels can become depleted within 20 days of poor dietary intake. 11 Hence its deficiency may occur rapidly and result in acute, severe and irreversible side effects. 12 Foregut symptoms, such as vomiting, lead to dietary and multivitamin intolerances. Suboptimal preoperative nutritional status, compounded by these gastrointestinal symptoms, may increase the risk of thiamin deficiency, 9 which can result in different symptoms such as Wet Beriberi, Dry Beriberi or Wernicke's encephalopathy. Wernicke's encephalopathy affects the central nervous system and if untreated may cause irreversible neurological damage, memory loss and coma with a 10–20% reported mortality rate. 12 , 13 , 14 Several case studies following all procedures have reported neuropathy, polyneuropathy and Wernicke's encephalopathy, related to the acute postgastric reduction surgery syndrome and thiamin deficiency. 13 , 15 , 16 , 17 , 18 However, the symptoms of moderate thiamin deficiency are vague and hence may go undiagnosed for some time. 14
The aim of this study was to identify the prevalence of nutritional abnormalities (vitamins B12, Active B12 (holotranscobalamin), thiamin, folate, vitamin D, intact parathyroid hormone (iPTH), haemoglobin (Hb), iron and ferritin, serum albumin, total protein and C‐reactive protein), in a cohort of patients attending a multidisciplinary private bariatric clinic in Australia. Weight loss, adherence to supplementation and the presence of gastrointestinal symptoms were also considered.
2. METHODS
The study was approved by the University of Wollongong/Illawarra Shoalhaven Local Health District Human Research Ethics Committee (HE:2020/172) and formal consent was not required. This study was reported according to the STROBE checklist. 19 The study accessed electronic medical records of patients who attended a multidisciplinary private clinic in Sydney, Australia from August 2020 to August 2021. Data included anthropometric measures, nutritional indices (vitamins B12, Active B12, thiamin, folate, vitamin D [Serum 25 {OH}], iPTH, iron, albumin and total protein), adherence to supplements, and gastrointestinal symptoms. Data on nutritional values were collected prospectively, preoperatively and then at ≤6 months, 1 and 2 years or more postoperatively.
All patients who attended the clinic during the study period and completed the blood tests were included in the study. They had undergone a multidisciplinary assessment and met the international criteria for bariatric surgery. 5 , 20 The nutritional counselling and recommendations for the multivitamin and mineral supplements were provided by the dietitian according to the bariatric guidelines. 6 , 9 , 20 The multivitamin and mineral supplements recommended included a comprehensive bariatric‐specific high‐dose multivitamin and mineral supplement aiming to provide 200% of the recommended dietary intake, calcium and vitamin D (aiming for a total of 1200–1500 mg calcium with 800 IU vitamin D). Supplements of iron, vitamins B12, thiamin, folate and additional vitamin D were recommended to meet the requirements of individual patients as required.
A Wedderburn scale was used for measuring weight and body mass index (BMI) (weight [kg]/Height2 [cm]), weight loss (WL), percentage of total weight loss (%TWL) (weight loss (kg)/Pre op weight × 100) and percentage of Excess Weight Loss (%EWL) were calculated (weight loss (kg)/EW (kg) × 100).
The nutritional and biochemical markers reported were vitamins B12, Active B12, thiamin, folate, D, iPTH, haemoglobin (Hb), iron and ferritin, albumin, total protein and C‐reactive protein. Vitamin B6 levels were not routinely tested, however, levels were reported if available. Levels of nutrients were assessed based on the standard laboratory values (Table 2).
TABLE 2.
Laboratory values and percentage of abnormalities in patients at each assessment point
| Biochemical marker (normal range) | Time | N | Minimum | Maximum | Mean ± SD | Abnormalities N (%) |
|---|---|---|---|---|---|---|
| Thiamin pyrophosphatase (TPP) (66–200 nmol/L) | Pre op | 112 | 75 | 445 | 179.6 ± 54.8 | 0 (0.0) |
| ≤6/12 post op | 88 | 48 | 360 | 165.1 ± 51.6 | 2 (2.3) | |
| 1 year post op | 49 | 55 | 340 | 173.5 ± 65.3 | 1 (2.1) | |
| ≥2 years post op | 63 | 65 | 340 | 177.0 ± 56.9 | 0 (0.0) | |
| CRP (0.0–0.5 mg/L) | Pre op | 109 | 0.5 | 48.6 | 7.7 ± 7.3 | 52 (47.7) |
| ≤6/12 post op | 83 | 0.4 | 24.0 | 4.5 ± 4.6 | 21 (25.3) | |
| 1 year post op | 48 | 0.4 | 85.5 | 5.5 ± 12.6 | 6 (12.5) | |
| ≥2 years post op | 62 | 0.4 | 25.7 | 2.5 ± 3.6 | 5 (8.1) | |
| iPTH (1.6–6.9 pmol/L) | Pre op | 105 | 2.0 | 21.1 | 6.8 ± 3.4 | 29 (27.6) |
| ≤6/12 post op | 84 | 2.0 | 10.5 | 5.4 ± 1.9 | 15 (17.9) | |
| 1 year post op | 47 | 2.7 | 35.0 | 6.4 ± 4.8 | 8 (17.0) | |
| ≥2 years post op | 61 | 2.1 | 13.7 | 6.2 ± 2.4 | 14 (23.0) | |
| Albumin (36–47 g/L) | Pre op | 110 | 35.0 | 49.0 | 42.5 ± 2.8 | 2 (1.8) |
| ≤6/12 post op | 87 | 35.0 | 49.0 | 41.7 ± 3.1 | 0 (0.0) | |
| 1 year post op | 49 | 30.0 | 48.0 | 41.9 ± 3.6 | 2 (4.0) | |
| ≥2 years post op | 62 | 35.0 | 50.0 | 41.8 ± 3.2 | 0 (0.0) | |
| Vitamin D (50–140 nmol/L) Total deficiency: <50 nmol/L | Pre op | 107 | 14.0 | 108.0 | 53.1 ± 19.6 |
Total: 42 (39) Mild: 31 (29.0) Moderate: 11 (10.3) Severe: 0 (0.0) |
| Mild deficiency: 30–49 nmol/L | ≤6/12 post op | 86 | 31.0 | 149.0 | 77.5 ± 22.0 | Total: 8 (9.3) |
| Moderate deficiency: 13–29 nmol/L |
Mild: 8 (9.3) Moderate: 0 (0.0) |
|||||
| Severe deficiency: <13 nmol/L | Severe: 0 (0.0) | |||||
| 1 year post op | 47 | 33.0 | 142.0 | 76.5 ± 23.7 |
Total: 5 (10.6) Mild: 5 (10.6) Moderate: 0 (0.0) Severe: 0 (0.0) |
|
| ≥2 years post op | 62 | 16.0 | 253.0 | 68.8 ± 32.3 |
Total: 11 (17.7) Mild: 11 (17.7) Moderate: 0 (0.0) Severe: 0 (0.0) |
|
| Iron (5–30 μmol/L) | Pre op | 112 | 5.9 | 37.0 | 14.7 ± 5.3 | 1 (0.9) |
| ≤6/12 post op | 87 | 9.0 | 117.0 | 18.6 ± 11.8 | 0 (0.0) | |
| 1 year post op | 49 | 3.0 | 28.2 | 17.3 ± 6.0 | 2 (4.1) | |
| ≥2 years post op | 62 | 9.0 | 43.6 | 20.0 ± 6.6 | 0 (0.0) | |
| Ferritin (15–200 μg/L) | Pre op | 110 | 9.0 | 533.0 | 125.3 ± 119.8 |
Low: 14 (12.7) High: 12 (10.9) |
| ≤6/12 post op | 87 | 14.0 | 559.0 | 136.2 ± 112.9 |
Low: 6 (6.9) High: 3 (3.5) |
|
| 1 year post op | 49 | 7.0 | 358.0 | 107.2 ± 81.8 |
Low: 7 (14.3) High: 5 (10.2) |
|
| ≥2 years post op | 63 | 6.0 | 344.0 | 87.7 ± 69.6 |
Low: 10 (15.9) High: 3 (4.8) |
|
| Vitamin B12 (135–650 pmol/L) <250 pmol/L | Pre op | 97 | 150.0 | 1028.0 | 350.9 ± 155.7 | 30 (31.0) |
| ≤6/12 post op | 59 | 148.0 | 1042.0 | 381.8 ± 185.6 | 16 (27.1) | |
| 1 year post op | 42 | 140.0 | 1352.0 | 398.9 ± 228.6 | 8 (19.0) | |
| ≥2 years post op | 56 | 127.0 | 845.0 | 407.3 ± 179.4 | 13 (23.2) | |
| Active B12 * (>35 pmol/L) | Pre op | 87 | 36.0 | 127.0 | 82.3 ± 26.0 | 1 (1.1) |
| ≤6/12 post op | 71 | 24.0 | 124.0 | 65.5 ± 27.3 | 3 (4.2) | |
| 1 year post op | 38 | 33.0 | 127.0 | 71.4 ± 25.3 | 2 (5.3) | |
| ≥2 years post op | 42 | 26.0 | 125.0 | 71.8 ± 29.3 | 4 (9.5) | |
| Folate (>7.0 nmol/L) | Pre op | 111 | 4.5 | 54.0 | 29.4 ± 10.1 | 1 (0.9) |
| ≤6/12 post op | 85 | 6.7 | 53.0 | 26.3 ± 10.2 | 1 (1.2) | |
| 1 year post op | 51 | 6.7 | 54.0 | 29.9 ± 11.1 | 1 (2.0) | |
| ≥2 years post op | 61 | 12.7 | 54.0 | 30.0 ± 9.6 | 1 (1.6) | |
| Total protein (64–83 g/L) | Pre op | 109 | 41.0 | 144.0 | 71.7 ± 8.6 | 3 (2.7) |
| ≤6/12 post op | 84 | 56.0 | 145.0 | 69.6 ± 9.5 | 5 (5.9) | |
| 1 year post op | 49 | 40.0 | 79.0 | 67.5 ± 6.0 | 6 (12.2) | |
| ≥2 years post op | 62 | 57.0 | 79.0 | 67.9 ± 3.7 | 5 (8.1) | |
| Haemoglobin (M) (130–180g/L) | Pre op | 23 | 122.0 | 166.0 | 147.5 ± 13.3 | 1 (4.3) |
| ≤6/12 post op | 12 | 137.0 | 164.0 | 148.3 ± 8.7 | 0 (0.0) | |
| 1 year post op | 8 | 125.0 | 152.0 | 139.1 ± 8.8 | 1 (12.5) | |
| ≥2 years post op | 15 | 129.0 | 172.0 | 147.4 ± 11.5 | 2 (13.3) | |
| Haemoglobin (F) (119–160) | Pre op | 85 | 106.0 | 158.0 | 135.9 ± 9.8 | 3 (3.5) |
| ≤6/12 post op | 74 | 112.0 | 167.0 | 135.5 ± 11.1 | 2 (2.7) | |
| 1 year post op | 43 | 106.0 | 156.0 | 127.5 ± 9.8 | 4 (9.3) | |
| ≥2 years post op | 48 | 113.0 | 159.0 | 134.2 ± 10.3 | 1 (2.1) |
Abbreviations: CRP, C‐reactive protein; F, female; iPTH, intact Parathyroid hormone; M, male.
Analysed if vitamin B12 150–250 pmol/L.
At the time of each nutritional assessment, adherence to the recommended multivitamin and mineral supplements and reported gastrointestinal symptoms were recorded. Hospital readmissions were noted and data regarding reasons for readmission, course of management, and nutrition management were retrieved.
Data were deidentified prior to analysis. The study was approved by the University of Wollongong/Illawarra Shoalhaven Local Health District Human Research Ethics Committee (HE:2020/172) and for this type of study formal consent was not required.
Descriptive statistics were expressed as mean ± SD for continuous variables (anthropometry and analytical variables) and percentages for categorical data (deficiency or compliance rates). Inferential analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) version 27. Linear mixed models were used to compare baseline and follow‐up data and Bonferroni post‐hoc test to pair‐wise comparisons. A p value <0.05 was considered statistically significant.
3. RESULTS
The baseline characteristics are described in Table 1. A total of 231 patients had a complete nutrition panel and were all included in the study. The mean age at time of surgery was 47.0 ± 11.8 years, the mean preoperative BMI was 43.4 ± 7.1 kg/m2 and the majority of patients were female (76.2%). The majority of the procedures were done as a primary procedure (n = 185, 80%), with the main procedure being sleeve gastrectomy (78.8%), followed by gastric bypass (15.6%; Table 1).
TABLE 1.
Baseline characteristics of bariatric patients included in the study
| n = 231 | |
|---|---|
| Gender ratio F/M, n (%) | 176/55 (76.2/23.8) |
| Age at the time of surgery, years | 47.0 ± 11.8 |
| (Range) | (18–73) |
| Body weight, kg, mean± SD | 122.1 ± 23.6 |
| (Range) | (74.4–220.0) |
| BMI, kg/m2 ± SD | 43.4 ± 7.1 |
| (Range) | (31.0–66.5) |
| Excess weight, kg, mean± SD | 51.5 ± 19.8 |
| Surgery types, n (%) | LSG: 182 (78.8%) |
| RYGB: 36 (15.6%) | |
| OAGB: 9 (3.9%) | |
| LAGB: 3 (1.3%) | |
| Banded SG: 1 (0.4%) | |
| Primary/Revisional surgery, n (%) | 185/46 (80/20) |
| Primary surgery, n (%) | LAGB: 32 (70.0%) |
| LSG: 4 (8.7%) | |
| LAGB and LSG: 8 (17.3%) | |
| GS: 1 (2.2%) | |
| Fixed band: 1 (2.2%) | |
| ESG: 1 (2.2%) |
Abbreviations: ESG, endoscopic sleeve gastrectomy; GS, gastric stapling; LAGB, laparoscopic adjusted gastric banding; LSG, laparoscopic sleeve gastrectomy; OAGB, one anastomosis gastric bypass (mini gastric bypass); RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
The mean weight loss was 30.7 ± 10.6 kg, 37.3 ± 14.1 kg, 33.5 ± 12.4 Kg, at 6, 12 month and 2 or more years following surgery. The weight loss was significantly different at each time point compared to the preoperative weight (p = <0.001), stabilising at 1 year postoperative with no significant difference in measures after 1 year (Figure 1).
FIGURE 1.
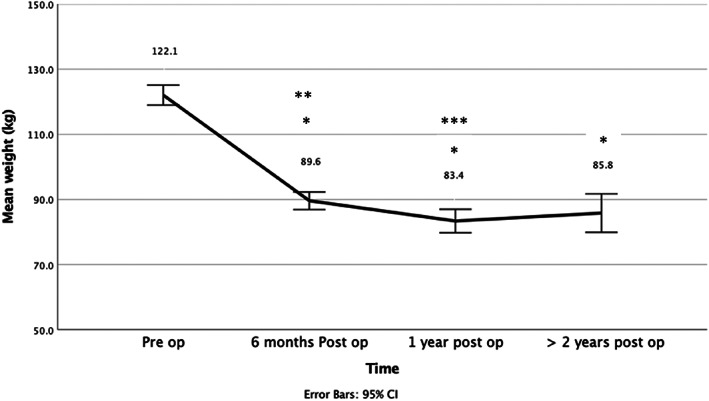
Weight loss over time following bariatric surgeries. *Statistically different to pre op weight p ≤ 0.001. **Statistically different to >2 years post op weight p = 0.009. ***No difference between 1 year and > 2 years post op weight.
Further details on the changes in anthropometrical measures following bariatric procedures are found in Table S1 (online supplementary material).
Prior to bariatric surgery, the most common nutritional disorders, related to vitamins D (39%), B12 (31%), ferritin (12.7%) and hyperparathyroidism (27.6%). Folate, Active B12, iron, Hb, total protein and albumin abnormalities were minimal and there was no thiamin deficiency observed preoperatively in this cohort. C‐reactive protein levels were elevated in 47.7% of patients (Table 2).
The common nutritional disorders at ≥2 years related to vitamin B12 (23.2%), hyperparathyroidism (23%), vitamin D (17.7%) and ferritin (15.9%). Few abnormalities in folate, Active B12, iron, Hb, total protein and albumin were observed but an improvement in C‐reactive protein was observed over time. In the period ≤6 months, thiamin deficiency was present in 2.3% patients (n = 2), both with foregut symptoms, necessitating hospital admission for assessment and management. At 1 year, one patient had a thiamin abnormality with clinically reported symptoms (Table 2).
Vitamin B6 toxicity was an incidental finding in 5 (2.2%) patients, with the range of values being from 1062–6936 nmol/L (normal range 20–190 nmol/L). The reported level of symptoms varied; with some asymptomatic and others reporting neuropathy. The main reason for vitamin B6 toxicity was self‐prescription of supplements such as zinc, magnesium or other high dose multivitamin and mineral supplement. These generally included 30–50 mg of vitamin B6 in the form of pyridoxine hydrochloride and in some cases, they may have been taken for up to 1 year. Apart from one patient (13 years post laparoscopic adjusted gastric banding), who in addition to the B6 toxicity, had vitamin D and total protein abnormalities, all others did not exhibit any additional nutritional abnormalities. All B6 levels were corrected with 3 weeks of stopping the high dose supplements.
Mean vitamin D levels improved over time, with a significant difference at 6 months, 1 year and ≥ 2 years postoperative compared to the preoperative values (Figure S1). iPTH improved at 6 months postoperative, however, no difference was seen beyond that time point (Figure S2, online supplemetary material). Iron levels and C‐reactive protein levels both improved over time. However, at 6 months postoperative a significant reduction was observed in mean values of Active B12, folate and total protein with some (total protein) persisting over time (Figures S3–S5, online supplementary material).
The adherence to the multivitamins was higher than other specific additional supplements. Adherence was 90% in the first year following surgery but declined to 77% at 2 years and beyond. In this cohort, 60% of patients were taking additional calcium and vitamin D supplements, with adherence again declining over time. Iron and vitamin B12 supplements are generally recommended based on patients' requirements. As the recommendation to take these additional supplements increased over time, so did their uptake (Figure 2).
FIGURE 2.
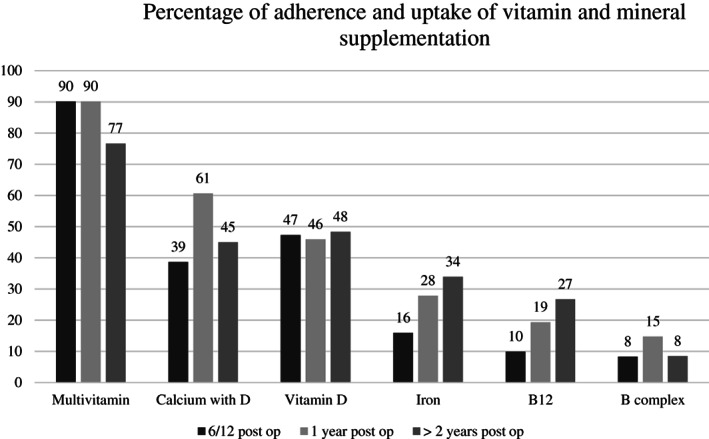
Percentage of adherence and uptake of multivitamin and mineral supplements by patients.
The most common gastrointestinal symptoms reported by patients are described in Figure 3. Reflux, food intolerances, nausea and vomiting were common. There was improvement in the prevalence of some symptoms, however reflux remained a problem longer term, with 44% of patients at 6 months, 25% at 1 year, and 28% at ≥2 years requiring daily proton pump inhibitors for its management.
FIGURE 3.
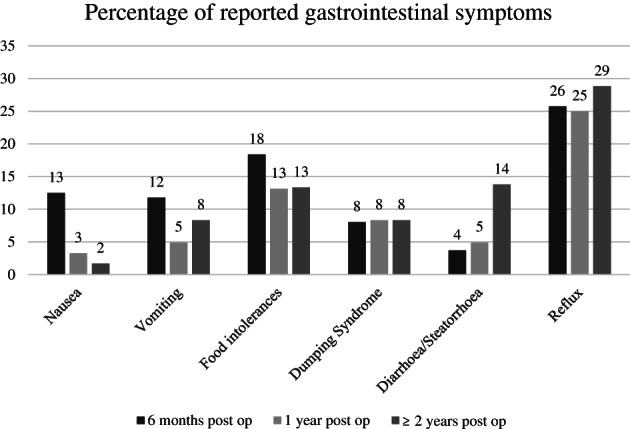
Percentage of reported gastrointestinal symptoms over time in bariatric patients.
From 214 patients, the substantive complication rate was 6.5%, with 12 patients requiring hospital readmissions and 2 endoscopic interventions. Only two patients had thiamin levels assessed during their hospital stay and both did develop thiamin deficiency. One patient with a very complicated medical and mental health history developed refeeding syndrome and required intensive care unit admission, enteral nutrition support, as well psychological support.
4. DISCUSSION
Consistent with other studies, 21 , 22 , 23 , 24 , 25 our research shows that bariatric surgery results in significant weight loss (TWL: 37.3 ± 14.1), which then stabilises and is maintained beyond 2 years following surgery. However nutritional abnormalities remain a concern, requiring close monitoring and adjustment based on individual patients. This is even more crucial in the acute stages postoperatively when patients may be at a higher risk of complications related to their surgery; or are still adjusting to their new gastrointestinal system and hence may experience foregut symptoms and its related acute and severe nutritional consequences.
The main preoperative nutritional abnormalities in this cohort were related to vitamin D, hyperparathyroidism, B12 and ferritin. The prevalence of hypovitaminosis D (39%), was consistent with other Australian studies in the bariatric surgical population, reported as 32–57%, 8 , 26 , 27 and was expectedly higher than in the average population, reported as 23% in the Australian Health Survey. 28 It is important to note that levels of Vitamin D deficiency can widely vary depending on what reference range is used (Table 2 describes the reference values in this study). An inverse relationship between BMI and vitamin D has been suggested in the literature, possibly due to the sequestration of vitamin D, being a fat‐soluble vitamin, in the adipose tissue. 29 Vitamin D is essential for the absorption of calcium and hence optimal musculoskeletal and metabolic bone health. Furthermore hyperparathyroidism was evident in 27.6% of patients in this cohort, which is also a common abnormality in the bariatric surgical population and with a similar impact on bone mineral density and metabolic bone health. 10
Epidemiological studies suggest a protective benefit of obesity against osteoporosis, due to increased bone mineral density as a result of a larger bone size and a higher mechanical loading, but there is more recent evidence of low vitamin D and hyperparathyroidism in this population. 10 A review by Brzozowska et al. suggests a strong inverse relationship between total fat mass and bone mineral density, discussing the consequence of obesity and its related metabolic syndrome in bone formation rate, bone mineral density and increasing fractures. 30 Given the impact of weight loss on metabolic bone health following bariatric surgery, the screening and early management of vitamin D and iPTH has been recommended for this at‐risk population. 6 , 7 , 10
In this cohort, 31% of patients had a vitamin B12 abnormality. Vitamin B12 (or cobalamin) deficiency has been reported in varying degrees in patients seeking bariatric surgery (0–30%), with the higher deficiency rate in those on medications such as proton pump inhibitors, H2 Blockers, or Metformin, all of which reduce its absorption rate. 6 , 7 , 31 As a water‐soluble vitamin, B12 is stored in substantial amounts in the liver and hence deficiency takes some time to develop. B12 deficiency can result in symptoms such as pernicious anaemia, paraesthesia, numbness, changes in reflexes and in extreme cases gait ataxia, dementia, psychosis and neuropathy. 6 Due to resection (in the sleeve gastrectomy and one anastomosis gastric bypass) or the bypass of the gastric fundus (in gastric bypass) and hence reduction in intrinsic factor patients are at risk of developing vitamin B12 deficiency following surgery, and hence early detection and correction is essential for optimal outcome. 9 Measurement of serum vitamin B12 may not be adequate to detect vitamin B12 deficiency and further tests such as Active B12, methylmalonic acid and homocysteine have also been recommended. 6 , 9 In this study, we also measured Active B12 and found very low deficiency rates (1.1%). Folate, iron, Hb, total protein and albumin abnormalities were negligible. Our findings are similar to other studies showing low prevalence of folate deficiency, 8 , 32 however these results contrast with others who report higher prevalence of folate deficiency of 54%. 7 This contrast could be due to a high level of adherence to the multivitamin supplementation. Iron deficiency was also negligible in this cohort with only one patient experiencing low iron levels. This is similar to other findings, 8 whilst others report much higher deficiency rates (13–47%) in the bariatric candidate patients. 6 , 32 , 33
The prevalence of thiamin deficiency has not been fully explored in bariatric surgical candidates. 34 In this study we did not identify any thiamin abnormality in those screened preoperatively (n = 112), although others have reported a prevalence of deficiency of 5.5% 32 and up to 29%. 6 The difference in studies may be related to preoperative nutritional health, the quality of the diet, and possible food fortification. There is disparity in the recommendation for routine preoperative thiamin screening in the bariatric guidelines, with some endorsing routine screening for all patients 6 and others reporting a lack of evidence to do so. 7 Therefore, it is not surprising to observe inconsistencies in clinical practices. In a survey of Australia and New Zealand bariatric clinicians only 35.7% of the respondents routinely screened for thiamin levels preoperatively. 35 Considering the short half‐life of thiamin and its low storage level in the body, ensuring adequate thiamin status through routine preoperative assessment may be helpful in managing high‐risk patients in the acute stages following surgery.
After 2 years post surgery, the situation may change. Consistent with the literature, 6 , 7 , 9 , 32 , 36 in this cohort nutritional disorders such as abnormal vitamins B12, D, hyperparathyroidism and ferritin were present at ≥2 years postoperative. These results emphasise the long‐term need for nutritional assessment, and review of the adequacy, adherence and response to supplementation in bariatric patients. Vitamin D and iPTH, calcium and albumin all play an important role in maintaining bone homeostasis, but in the current study there was negligible albumin abnormalities observed. The initial significant improvement in the mean vitamin D and iPTH levels was similar to other studies which also demonstrated an improvement in vitamin D levels (from 81% to 36% deficiency levels) at 1 year following surgery. 32 This has been attributed to the weight loss and the release of vitamin D from adipose tissue 29 as well as better adherence to recommended vitamin D supplementation. However, over time, vitamin D deficiency of 80% ‐100% and ongoing hyperparathyroidism has been reported in the literature. 6 , 36 These findings not only reinforce the need for routine screening and long term follow up but also highlight the need for population based and individualised treatment recommendations. This aligns with recommendations from the American Society for Metabolic and Bariatric Surgery which has emphasised the need for long term monitoring of vitamin D, iPTH, calcium, and albumin. as well as using a dual‐energy X‐ray absorptiometry scan, 10 as a result of the increased risk of fractures in this population.
In contrast to other reports, 6 folate, Active B12, iron and Hb abnormalities were minimal in our cohort. Several reasons may explain this. Firstly, the dietary contribution of these micronutrients may vary amongst different patient populations, however, this was not investigated in the current study. Secondly, adherence to micronutrient supplementation may also vary amongst patient cohorts. Finally, interpopulation differences in nutrient absorption, metabolism and storage needs should also be considered. The level of abnormality of total protein (which measures the combined amount of two types of proteins, albumin and globulin) and albumin, reflecting the overall nutritional status, were negligible, in this cohort. Protein energy malnutrition is not generally expected following the above mentioned bariatric procedures and nor is it seen in patients who do not experience surgical‐related complications. 9 Similar to other findings, with the improvement of weight loss and its inflammatory processes, C‐reactive protein levels improved over time in the current study. 27
Thiamin levels postsurgery may be of particular interest in this clinical population. A recent systematic review described the incidence of thiamin deficiency to be higher than previously reported within the bariatric surgical population and additionally reported limited studies in this area. 34 Furthermore, current practices of screening and treatment of thiamin deficiency in clinical settings are inconsistent, 35 potentially leading to severe consequences and irreversible neurological side effects. In the current study, thiamin abnormality was present in 2 patients at ≤6‐month postoperative, both of which needed hospital readmission due to foregut symptoms. Only one patient at 1 year following surgery exhibited thiamin abnormality and was clinically symptomatic. These findings are similar to those of Albaugh et al. who found a 3.5% thiamin deficiency in a cohort of 346 patients. 37 However, it is in contrast with others, who have reported a much higher prevalence of thiamin deficiency of up to 29%. 6 , 32 , 38 This difference may be attributed to race with Caucasians experiencing less thiamin deficiency than African Americans or Hispanics. 38
Thiamin deficiency has been reported following all bariatric procedures. 13 , 15 , 16 , 17 , 18 Due to the irreversible and potential life‐threatening consequences of untreated thiamin deficiency, early supplementation, detection and adequate treatment is recommended. In this cohort the patients with thiamin deficiency were reportedly adherent to multivitamin supplementation. In a previous study, where thiamin deficiency was prevalent in 25.7% of patients, no difference was found with adherence to multivitamin supplementation between patients who had thiamin deficiency versus those who did not (70.4%, 84.6%, p = 0.10). 38 However, African American race (odds ratio [OR] 39 3.9, p = 0.019), higher preoperative BMI (OR 1.13, p = 0.001), nausea (OR 3.81, p = 0.02) and vomiting (OR 3.49, p = 0.032) were found to be independent risk factors of developing thiamin deficiency. 38 In the high‐risk group patients, closer monitoring, routine screening of thiamin, and additional thiamin supplementation in the acute postoperative phase may contribute to the prevention, early detection and appropriate treatment of thiamin deficiency.
Another B group vitamin of interest is Vitamin B6 or pyridoxal phosphate, a water‐soluble vitamin with the generic name representing six compounds (vitamers) with vitamin B6 activity. It is a co‐factor for over 100 enzymes involved in amino acid metabolism and neurotransmitter synthesis. It naturally occurs in a variety of food groups such as legumes, nuts, wheat bran, as well as animal sources such as meat. 9 Vitamin B6 has not been well studied in the bariatric population and hence the understanding of its prevalence of deficiency and toxicity remains limited. 9 Recent studies report varying level of vitamin B6 toxicity prior to surgery (21%) and increasing postoperatively (47.5%). 32 The concern with vitamin B6 toxicity is that unlike other water‐soluble B vitamins, chronic administration of high dose supplements can cause severe and progressive sensory neuropathy characterised by ataxia. The severity of symptoms generally appears to be dose dependent, and usually stop with discontinuation of the excess pyridoxine supplements. The incidence of vitamin B6 toxicity in the cohort in the current study was much lower and only seen in 5 patients (2.2%). This may be due to the type and the quantity of the additional B6 supplements contributing to the toxicity. The current study's cohort were taking additional supplements with added vitamin B6 whereas others reported the toxicity to be related to consumption of energy drinks. 40
Given the abnormalities noted with nutrients, vitamin supplementation is routinely provided in this patient cohort. Despite several guidelines and clinician recommendations, the adherence to multivitamin supplementation remains suboptimal and tend to decline over time. 8 , 36 In this cohort 90% of patients reported to be taking supplements, however this reduced to 77% by 2 years and beyond. The adherence to the additional supplements was even lower with 60% of patients taking the additional recommended calcium and vitamin D supplementation in the current study, and this declined over time. Other studies have shown similar findings with 74.1% of patients taking a vitamin D supplement during the first postoperative year and reducing to only 11.1% after 4 years. 36
A recent study including 4614 postoperative bariatric surgical patients used a 24‐item questionnaire to explore the factors affecting adherence to multivitamin supplements. 41 They overall found a negative attitude towards taking supplements with common reported barriers for inconsistent users identified to be: forgetting daily intake (68.3%), gastro‐intestinal side effects (25.6%) and unpleasant taste or smell (22.7%). In contrast those who did not use any multivitamin supplementation reported gastro‐intestinal side effects (58.5%), high costs (13.5%) and the absence of vitamin deficiencies (20.9%) to be the barriers. Furthermore, they found a total of 28.5% of patients to be dissatisfied with the instructions received on multivitamin supplementation use. Hence to optimise adherence to multivitamin supplementation, closer attention to adherence at each consult, consideration of patients' individual preferences, and implementation of a shared decision–making process by the clinicians was recommended.
Consistent with other literature, common gastrointestinal symptoms reported by patients in the current study were reflux, food intolerances, nausea and vomiting. 27 , 42 These mostly resolved over time, likely as the gastrointestinal system evolves and also patients cognitively adapt to their new capacity. In this cohort, the complication rate was 6.5% with 14 patients requiring further investigations or interventions. Only two patients with foregut symptoms who needed hospital interventions had their thiamin level tested. Both had thiamin abnormality and one further developed severe malnutrition, refeeding syndrome, requiring intensive care unit admission and enteral nutrition support for its management. Patients with complications were generally presented to their nearest hospital, which may not routinely care for bariatric surgical patients. Hence thiamin was not assessed for all patients, nor was it supplemented during hospital readmissions. Some of the reasons were the expense of the test as well as the duration of time it takes to receive the results (up to 2 weeks). This not only results in underreporting of thiamin deficiency, but highlights the role of the bariatric specialised clinics in education of staff in other hospitals, in caring for bariatric patients.
The limitations of this study were firstly reliance on the retrospective data available for some aspects of the study as well as also relying on reported data. Secondly, patients were not randomly allocated to each surgical group and the majority of patients had sleeve gastrectomy procedure, hence the results do not translate to other bariatric procedures. Thirdly, only a small number of patients had thiamin levels tested, especially during the acute stages postoperatively and hence this may underreport the prevalence of thiamin deficiency. However, with limited studies published on thiamin levels, this study presents a realistic observation of private bariatric clinics and hence encourages others to reflect on clinical practice, especially given that most bariatric surgery is conducted privately in Australia. 43 Developing a protocol for routine assessment, screening and supplementation of thiamin may also be of benefit for patients presenting with complications leading to foregut symptoms and requiring readmissions.
Bariatric surgery results in durable weight loss, however acute and chronic nutritional concerns remain an issue in the bariatric surgical patient. With weight loss and good adherence to supplementation, some such as vitamin D and iPTH tend to initially improve, however as stores deplete, these become a concern in the longer term. Furthermore, adherence to supplementation tends to reduce over time, increasing the risk of long‐term complications. Patients with foregut symptoms and complications develop diet and multivitamin supplement intolerance, increasing their risk of acute nutritional deficiency, especially thiamin and its related consequences. Thiamin deficiency is however underreported, as its not routinely screened. Hence, the need for prompt assessment and supplementation in the symptomatic bariatric patient, with the aim to prevent severe complications. To increase awareness in health settings that may not routinely care for bariatric surgical patients and hence reduce potential risk, a specific protocol in managing the bariatric complicated patient is recommended.
AUTHOR CONTRIBUTIONS
NZ, LCT, EPN and MB contributed to the conception and design of the study. NZ contributed to acquisition, analysis and interpretation of data, and drafted the manuscript. MB contributed to data analysis, LCT, MB, EPN and MLT contributed to data interpretation. All authors critically revised and gave final approval for the manuscript. The authors acknowledge Assoc Prof Garrett‐ Smith and Dr Steve Leibman for assistance with subject recruitment and Assoc Prof Garrett‐Smith for assistance with deidentification of data prior to analyses.
CONFLICT OF INTEREST
Marijka Batterham and Elizabeth Phillipa Neale are on the Editorial Board of Nutrition & Dietetics. They were excluded from the peer review process and all decision‐making regarding this article. This manuscript has been managed throughout the review process by the Journal's Editor‐in‐Chief. The Journal operates a blinded peer review process and the peer reviewers for this manuscript were unaware of the authors of the manuscript. This process prevents authors who also hold an editorial role to influence the editorial decisions made. The authors declare no other conflicts of interest.
Supporting information
TABLE S1 Anthropometrical outcomes
FIGURE S1 Mean vitamin D levels before and following bariatric surgeries
FIGURE S2 Mean iPTH levels before and following bariatric surgeries
FIGURE S3 Mean Active B12 levels before and following bariatric surgery
FIGURE S4 Mean Folate levels before and following bariatric surgery
FIGURE S5 Mean Total protein levels before and following bariatric surgery
Zarshenas N, Tapsell LC, Batterham M, Neale EP, Talbot ML. Investigating the prevalence of nutritional abnormalities in patients prior to and following bariatric surgery. Nutrition & Dietetics. 2022;79(5):590‐601. doi: 10.1111/1747-0080.12747
Funding information Open access publishing facilitated by University of Wollongong, as part of the Wiley ‐ University of Wollongong agreement via the Council of Australian University Librarians.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683‐2784. [DOI] [PubMed] [Google Scholar]
- 2. Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945‐953. doi: 10.1007/s00125-016-3903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta‐analysis. JAMA. 2004;292:1724‐1737. doi: 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 4. NHMRC . National Health and Medical Research Council. Department of Health and Aging. Australian Dietary Guidelines: Eat for Health. https://www.eatforhealth.gov.au/sites/default/files/files/the_guidelines/n55_australian_dietary_guidelines.pdf (2013).
- 5. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures: 2019 update—cosponsored by American Association of Clinical Endocrinologists/American college of endocrinology, the Obesity Society, American Society for Metabolic & bariatric surgery, obesity medicine association, and American Society of Anesthesiologists—executive summary. Endocr Pract. 2019;25:1346‐1359. doi: 10.4158/gl-2019-0406 [DOI] [PubMed] [Google Scholar]
- 6. Parrott J, Frank L, Rabena R, Craggs‐Dino L, Isom KA, Greiman L. American society for metabolic and bariatric surgery integrated health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2016;13:727‐741. doi: 10.1016/j.soard.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 7. O'Kane M, Parretti HM, Pinkney J, et al. British obesity and metabolic surgery society guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery: 2020 update. Obes Rev. 2020;1‐23:e13087. doi: 10.1111/obr.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zarshenas N, Nacher M, Loi KW, Jorgensen JO. Investigating nutritional deficiencies in a Group of Patients 3 years post laparoscopic sleeve gastrectomy. Obes Surg. 2016;26:2936‐2943. [DOI] [PubMed] [Google Scholar]
- 9. Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. Bariatric nutrition: suggestions for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4:S73‐S108. doi: 10.1016/j.soard.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 10. Kim J, Nimeri A, Khorgami Z, et al. Metabolic bone changes after bariatric surgery: 2020 update, American Society for Metabolic and Bariatric Surgery Clinical Issues Committee position statement. Surg Obes Relat Dis. 2021;12:1‐18. doi: 10.1016/j.soard.2020.09.031 [DOI] [PubMed] [Google Scholar]
- 11. Sechi G, Serra S. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442‐455. [DOI] [PubMed] [Google Scholar]
- 12. Punchai S, Hanipah ZN, Meister KM, Schauer PR, Brethauer SA, Aminian A. Neurologic manifestations of vitamin B deficiency after bariatric surgery. Obes Surg. 2017;27:2079‐2082. doi: 10.1007/s11695-017-2607-8 [DOI] [PubMed] [Google Scholar]
- 13. Stroh C, Meyer F, Manger T. Beriberi, a severe complication after metabolic surgery: review of the literature. Obes Facts. 2014;7:246‐252. doi: 10.1159/000366012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerns JC, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6:147‐153. doi: 10.3945/an.114.007526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen BA, Chen LC. Wernicke's encephalopathy after bariatric surgery with atypical magnetic resonance imaging: a case report. Acta Neurol Taiwan. 2017;26:29‐32. [PubMed] [Google Scholar]
- 16. Angstadt JD, Bodziner RA. Peripheral polyneuropathy from thiamine deficiency following laparoscopic Roux‐en‐Y gastric bypass. Obes Surg. 2005;15:890‐892. doi: 10.1381/0960892054222759 [DOI] [PubMed] [Google Scholar]
- 17. Martines G, Musa N, Aquilino F, Capuano P. Severe neurological complication following adjustable gastric banding. G Chir. 2018;39:92‐96. [PubMed] [Google Scholar]
- 18. Makarewicz W, Kaska L, Kobiela J, et al. Wernicke's syndrome after sleeve gastrectomy. Obes Surg. 2007;17:704‐706. [DOI] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 20. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient: 2013 update—cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & bariatric surgery. Endocr Pract. 2013;19:337‐372. doi: 10.4158/ep12437.Gl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kessler Y, Adelson D, Mardy‐Tilbor L, et al. Nutritional status following one anastomosis gastric bypass. Clin Nutr. 2020;39:599‐605. doi: 10.1016/j.clnu.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 22. Aleman R, Lo Menzo E, Szomstein S, Rosenthal R. Efficiency and risks of one‐anastomosis gastric bypass. Trans Med. 2020;8(Suppl 1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robert M, Espalieu P, Pelascini E, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux‐en‐Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open‐label, non‐inferiority trial. Lancet. 2019;393:1299‐1309. doi: 10.1016/s0140-6736(19)30475-1 [DOI] [PubMed] [Google Scholar]
- 24. Parmar CD, Mahawar KK. One anastomosis (mini) gastric bypass is now an established bariatric procedure: a systematic review of 12,807 patients. Obes Surg. 2018;28:2956‐2967. doi: 10.1007/s11695-018-3382-x [DOI] [PubMed] [Google Scholar]
- 25. Parikh M, Eisenberg D, Johnson J, El‐Chaar M. American Society for Metabolic and Bariatric Surgery review of the literature on one‐anastomosis gastric bypass. Surg Obes Relat Dis. 2018;14:1088‐1092. doi: 10.1016/j.soard.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 26. Toh SY, Zarshenas N, Jorgensen JO. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150‐1156. doi: 10.1016/j.nut.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 27. Zarshenas N, Tapsell LC, Batterham M, Neale E, Talbot M. Changes in anthropometric measures, nutritional indices and gastrointestinal symptoms following one anastomosis gastric bypass (OAGB) compared with roux‐en‐y gastric bypass (RYGB). Obes Surg. 2021;31:2619‐2631. doi: 10.1007/s11695-021-05284-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ABS , 2011. Australian Bureau of Statistics. ABS Cat no. 4719.0. Canberra, 2011–2012.
- 29. Blum M, Dolnikowski G, Seyoum E, et al. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90‐94. doi: 10.1007/s12020-008-9051-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brzozowska M, Sainsbury A, Eisman A, Baldock P, Center J. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013;14:52‐67. doi: 10.1111/j.1467-789X.2012.01050.x [DOI] [PubMed] [Google Scholar]
- 31. Frame‐Peterson L, Megill R, Carobrese S, Schweitzer M. Nutrient deficiencies are common prior to bariatric surgery. Nutr Clin Pract. 2017;32:463‐469. [DOI] [PubMed] [Google Scholar]
- 32. van Rutte PJW, Aarts EO, Smulders JF, Nienhuijs SW. Nutrient deficiencies before and after sleeve gastrectomy. Obes Surg. 2014;24:1639‐1646. doi: 10.1007/s11695-014-1225-y [DOI] [PubMed] [Google Scholar]
- 33. Ben‐Porat T, Elazary R, Yuval JB, Wieder A, Khalaileh A, Weiss R. Nutritional deficiencies after sleeve gastrectomy: can they be predicted preoperatively? Surg Obes Relat Dis. 2015;11:1029‐1036. doi: 10.1016/j.soard.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 34. Aasheim E. Wernicke encephalopathy after bariatric surgery. A Systematic Review. Ann Surg. 2008;248:714‐720. [DOI] [PubMed] [Google Scholar]
- 35. Zarshenas N, Tapsell L, Batterham M, Neale E, Talbot M. Screening and treatment of thiamine deficiency in a sample of multidisciplinary bariatric surgery clinical teams. Obes Surg. 2021;31:4666‐4668. doi: 10.1007/s11695-021-05614-4 [DOI] [PubMed] [Google Scholar]
- 36. Ben‐Porat T, Elazary R, Goldenshluger A, Sherf Dagan S, Mintz Y, Weiss R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy‐are supplements required for a lifetime? Surg Obes Relat Dis. 2017;13:1138‐1144. doi: 10.1016/j.soard.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 37. Albaugh V, Williams B, MAher C, Spann M, English W. Prevalence of thiamine deficiency is significant in patients undergoing primary bariatric surgery. Surg Obes Relat Dis. 2021;17:653‐658. doi: 10.1016/j.soard.2020.11.032 [DOI] [PubMed] [Google Scholar]
- 38. Tang L, Alsulaim HA, Canner JK, Prokopowicz GP, Steele KE. Prevalence and predictors of postoperative thiamine deficiency after vertical sleeve gastrectomy. Surg Obes Relat Dis. 2018;14:943‐950. doi: 10.1016/j.soard.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 39. Maessen DE, Hanssen NM, Lips MA, et al. Energy restriction and Roux‐en‐Y gastric bypass reduce postprandial alpha‐dicarbonyl stress in obese women with type 2 diabetes. Diabetologia. 2016;59(9):2013‐2017. doi: 10.1007/s00125-016-4009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tynes M, Matthias H, Timper K. Regular intake of energy drinks and multivitamin supplements is associated with elevated plasma vitamin B6 levels in post‐bariatric patients. Sci Rep. 2021;11:17830. doi: 10.1038/s41598-021-97205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smelt J, Heusschen L, Theel W, et al. Factors affecting patient adherence to multivitamin intake after bariatric surgery: a multicentre survey study from the patient's perspective. Obes Surg. 2021;31:4316‐4326. doi: 10.1007/s11695-021-05571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zarshenas N, Tapsell LC, Neale EP, Batterham M, Talbot ML. The relationship between bariatric surgery and diet quality: a systematic review. Obes Surg. 2020;30:1768‐1792. doi: 10.1007/s11695-020-04392-9 [DOI] [PubMed] [Google Scholar]
- 43. BSR . Bariatric Surgery Registry Eighth Annual Report: 2019/20. Monash University; 2019/2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Anthropometrical outcomes
FIGURE S1 Mean vitamin D levels before and following bariatric surgeries
FIGURE S2 Mean iPTH levels before and following bariatric surgeries
FIGURE S3 Mean Active B12 levels before and following bariatric surgery
FIGURE S4 Mean Folate levels before and following bariatric surgery
FIGURE S5 Mean Total protein levels before and following bariatric surgery
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


